Evaluation of Hypoglycemic Activity and Sub-Acute Toxicity of the Novel Biochanin A–Chromium(III) Complex
Abstract
:1. Introduction
2. Results
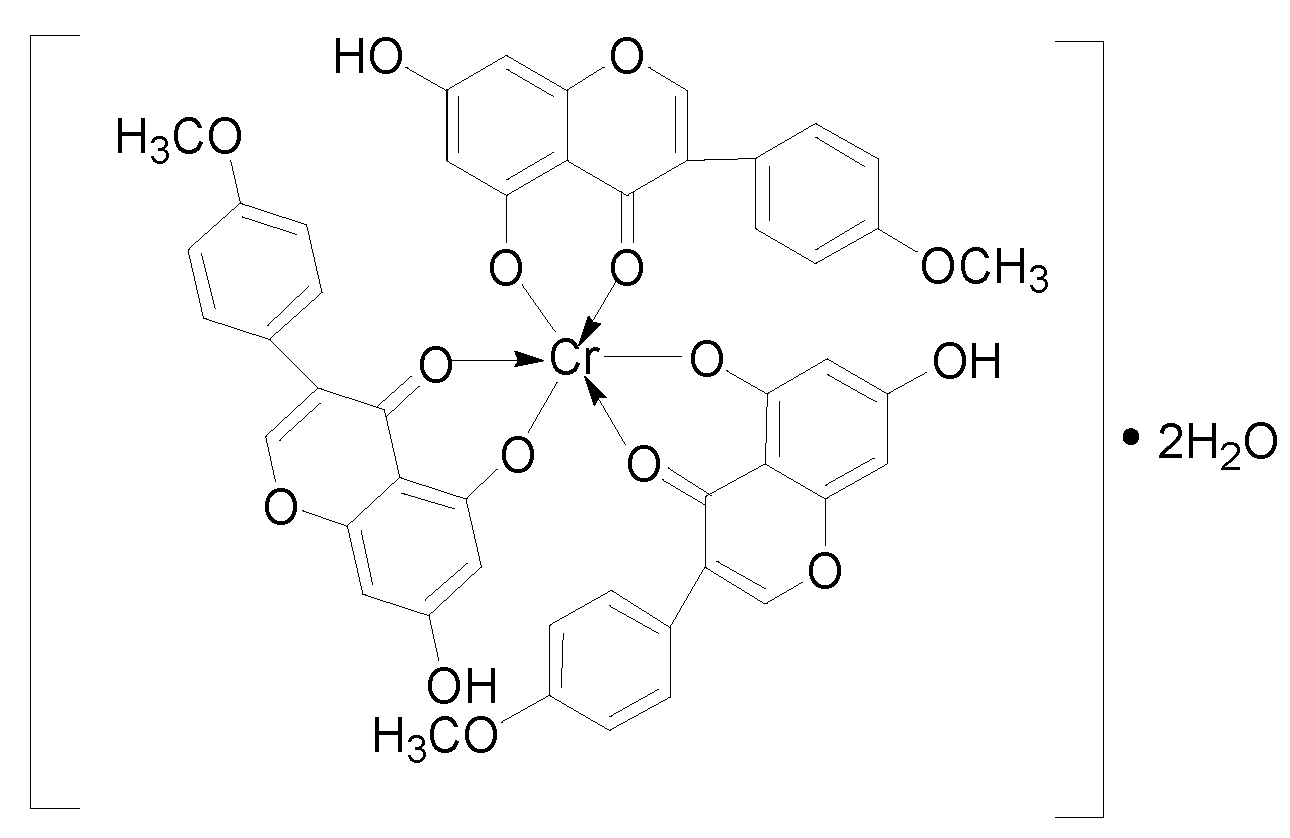
2.1. Synthesis and Structural Characterization of [CrBCA3]
2.1.1. Physicochemical Properties, Elemental Analysis and Mass Spectrometry Analysis
2.1.2. Thermogravimetry (TG) and Differential Thermal Analysis (DTA)
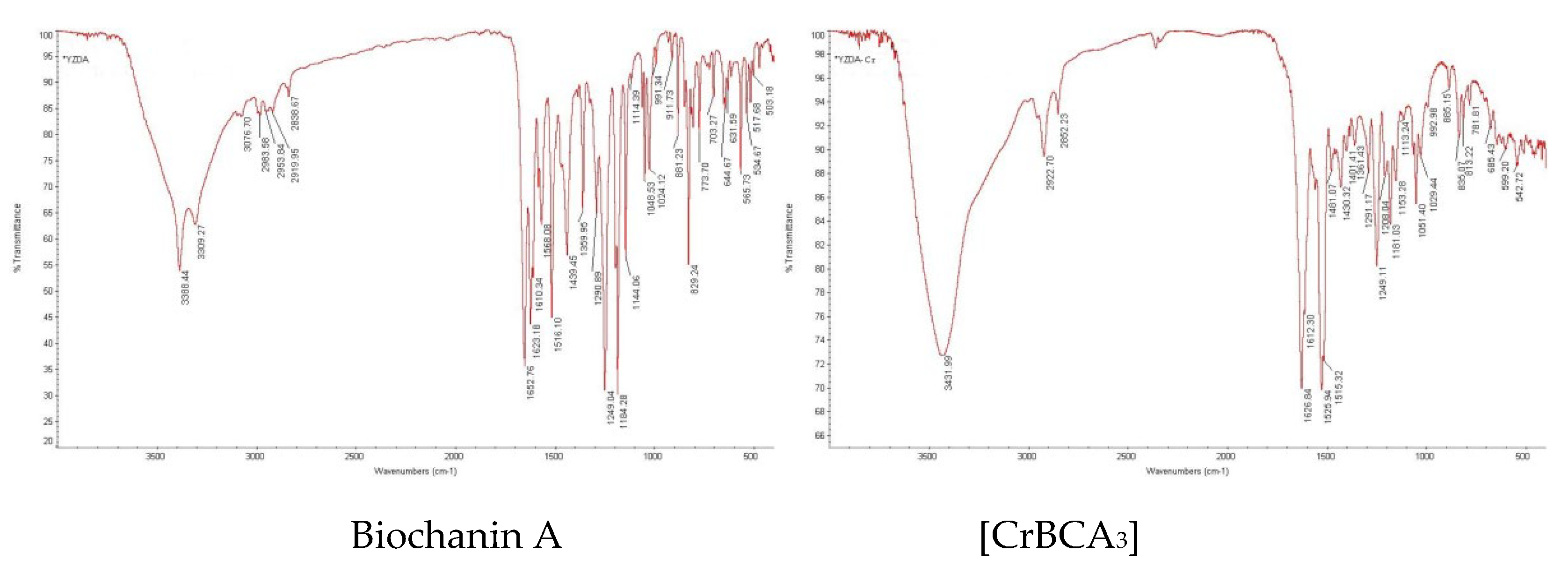
2.1.3. Infrared Spectrum Analysis
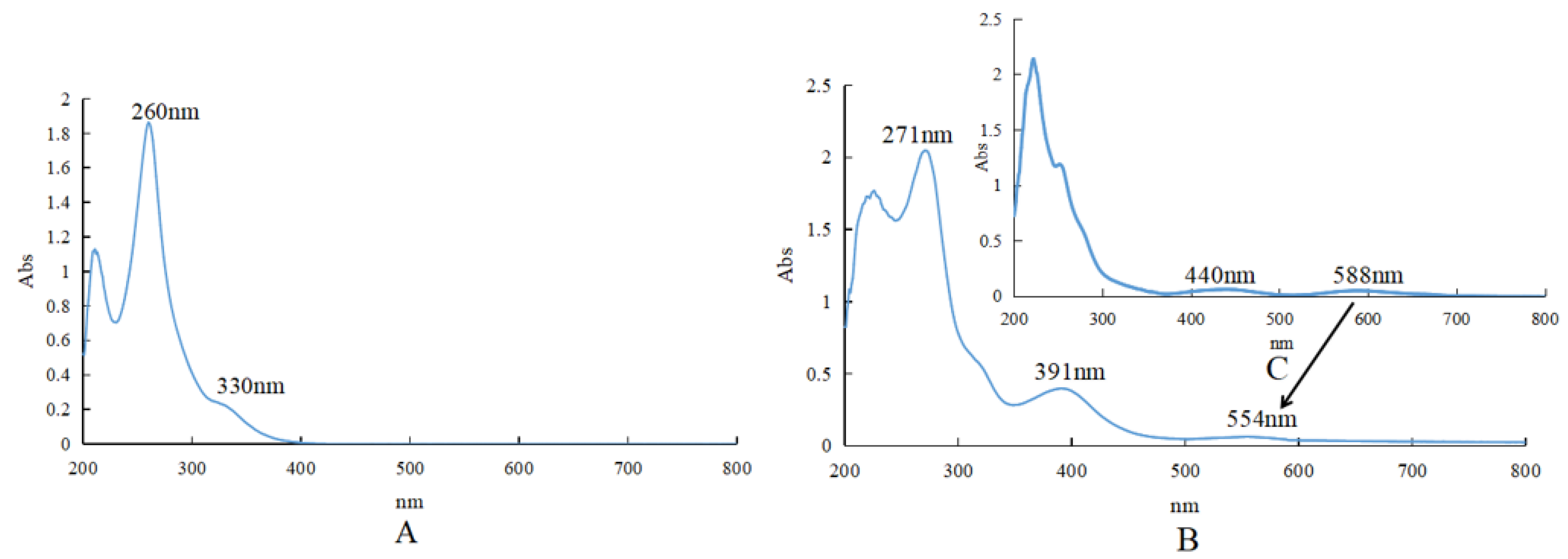
2.1.4. UV Spectrum
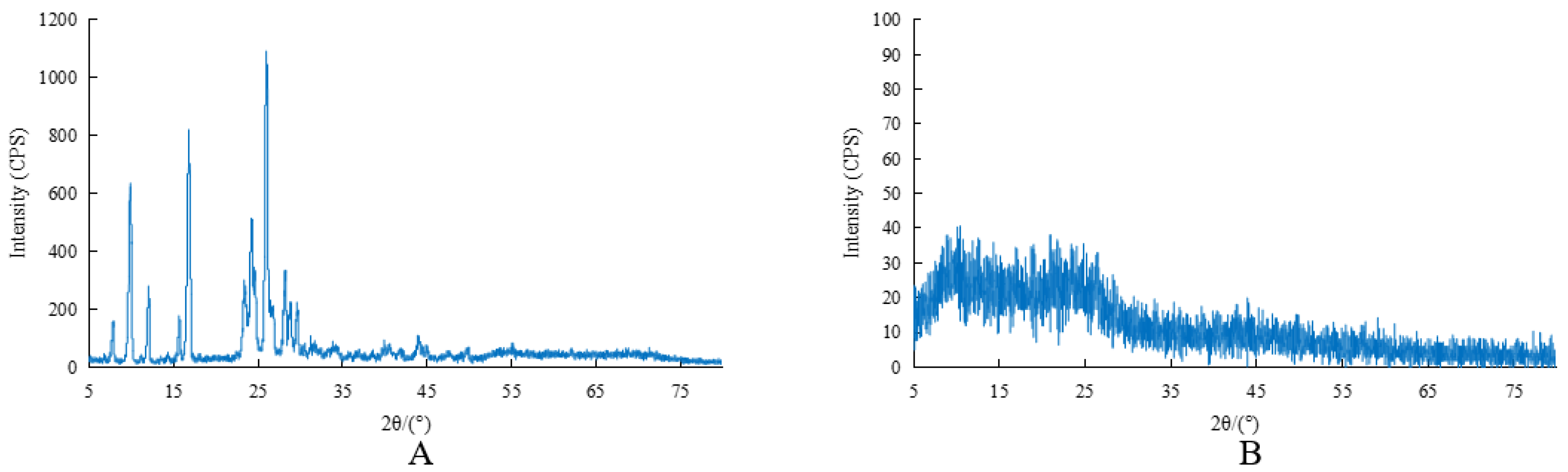
2.1.5. X-ray Powder Diffraction
2.2. Biochemical Results
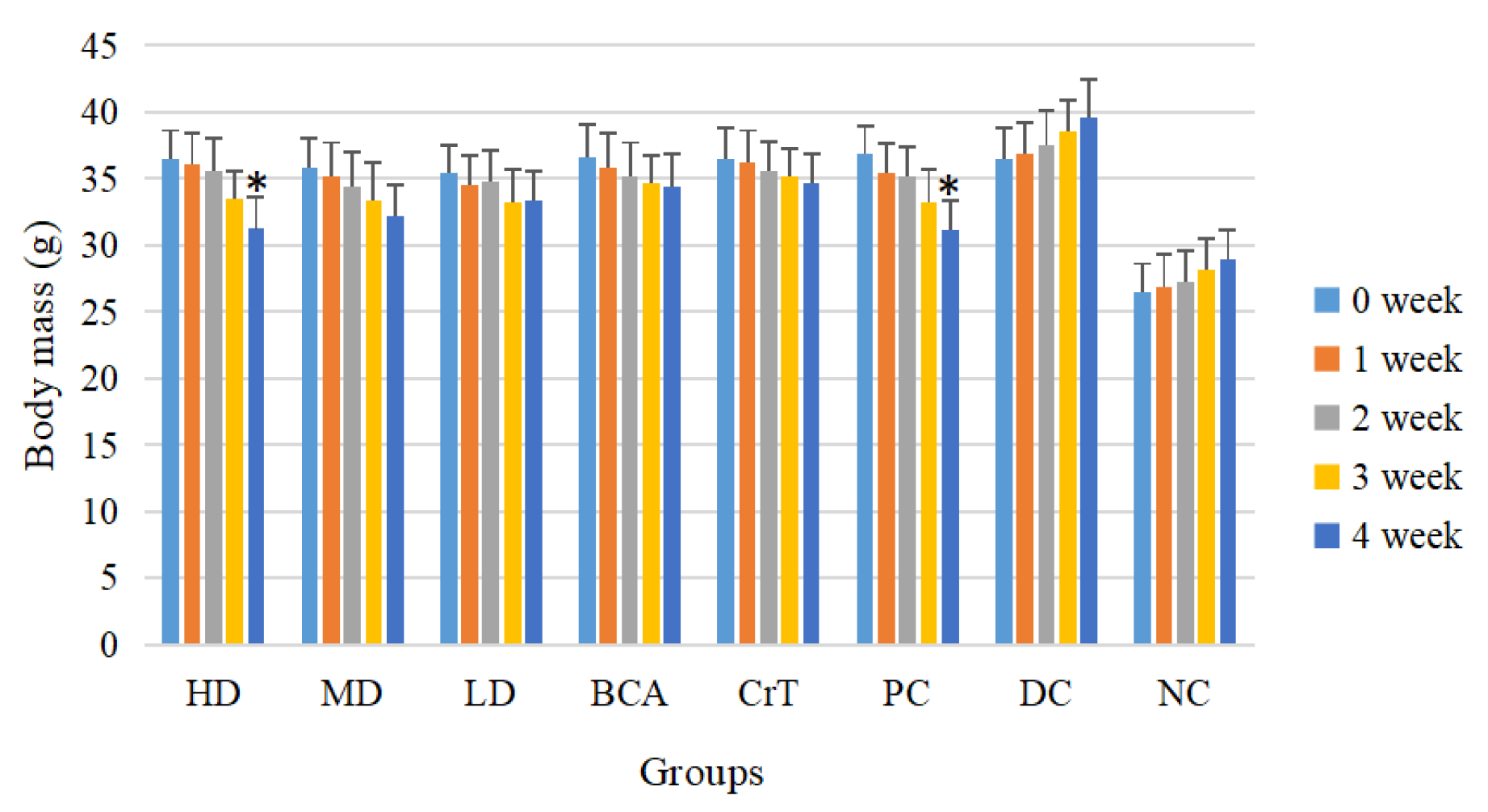
2.2.1. Animal Growth
2.2.2. Determination of Fasting Blood Glucose
2.2.3. Determination of Random Blood Glucose
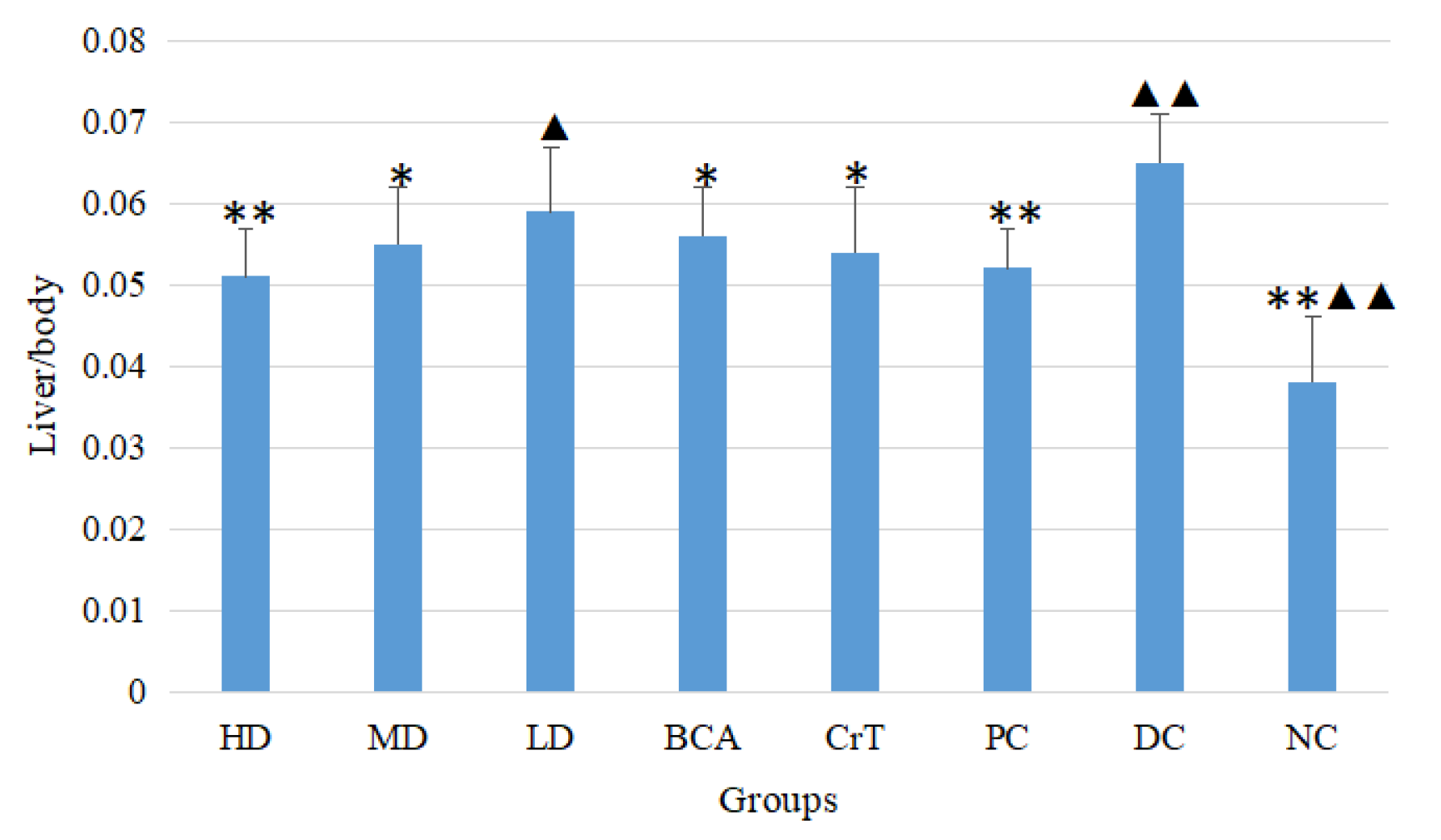
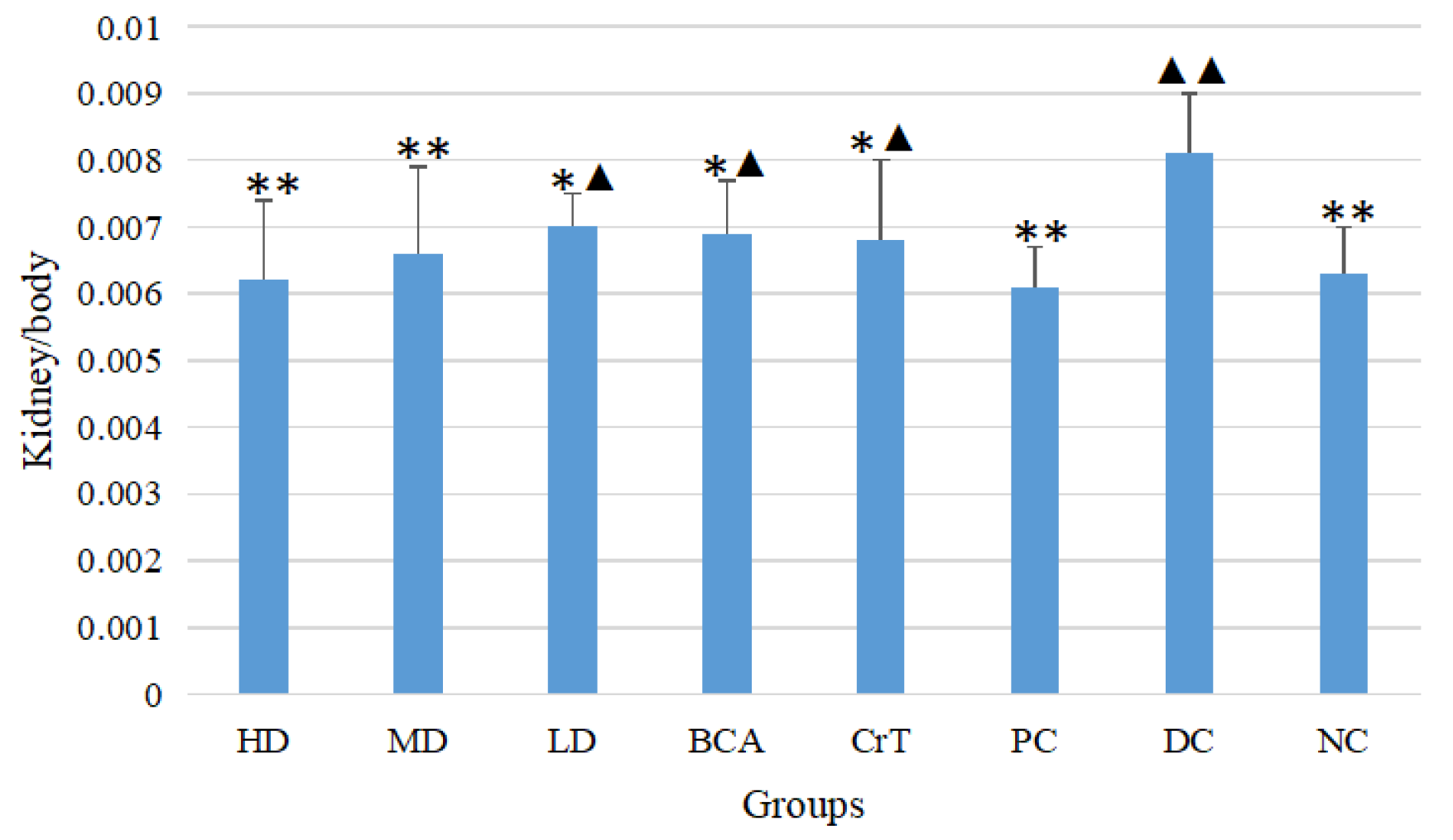
2.2.4. Determination of Liver/Body and Kidney/Body Ratios
2.2.5. Comparison of OGTT
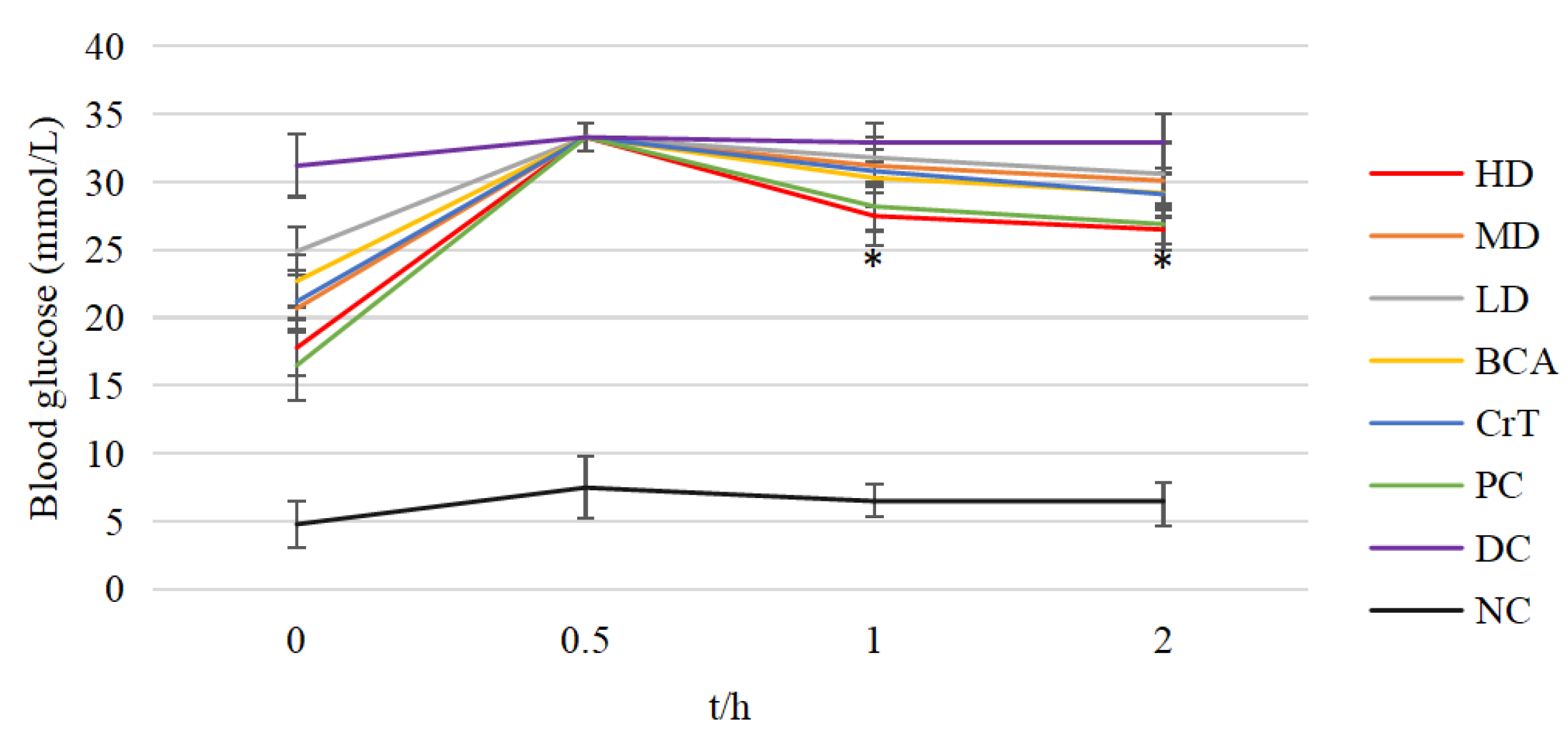
2.2.6. Comparison of ITT
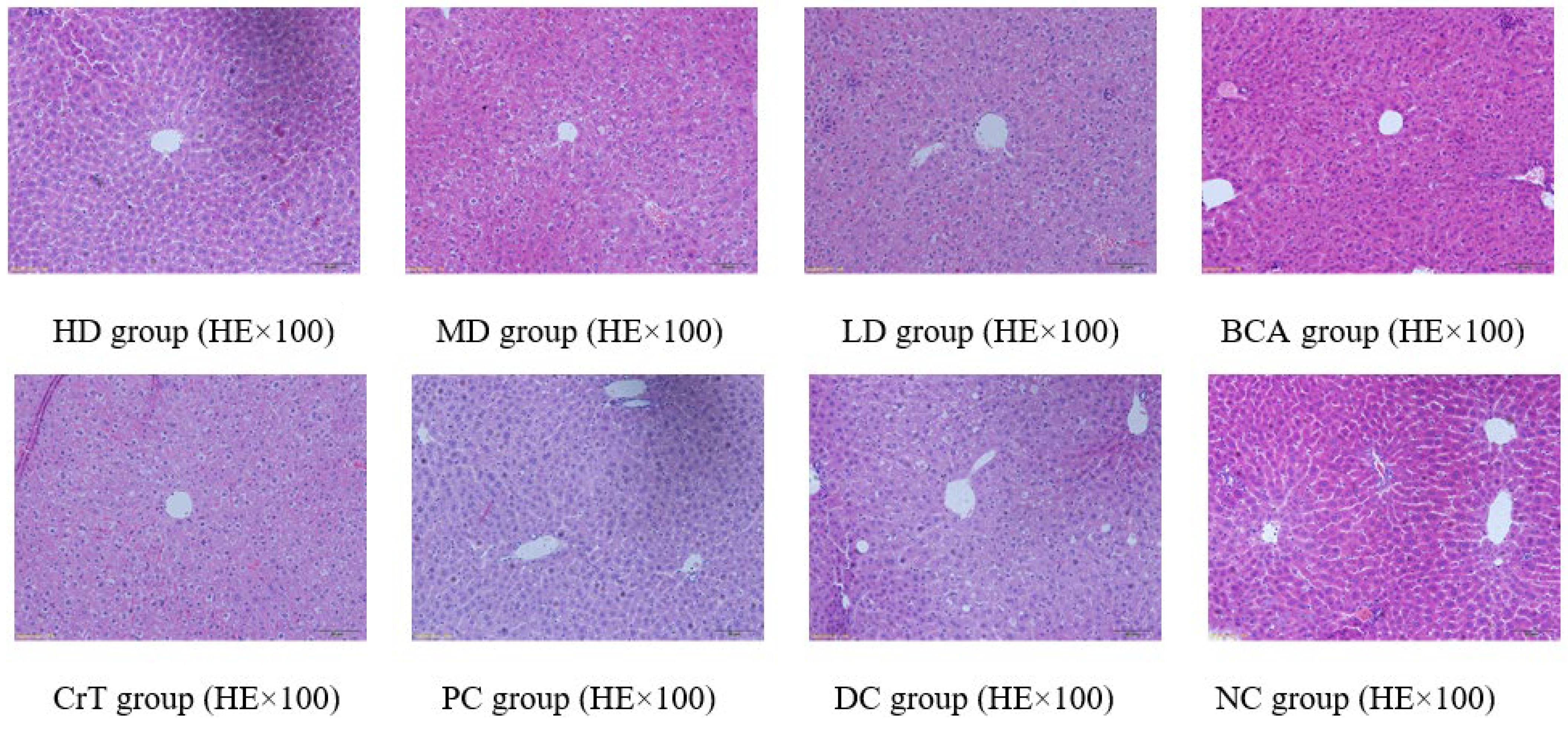
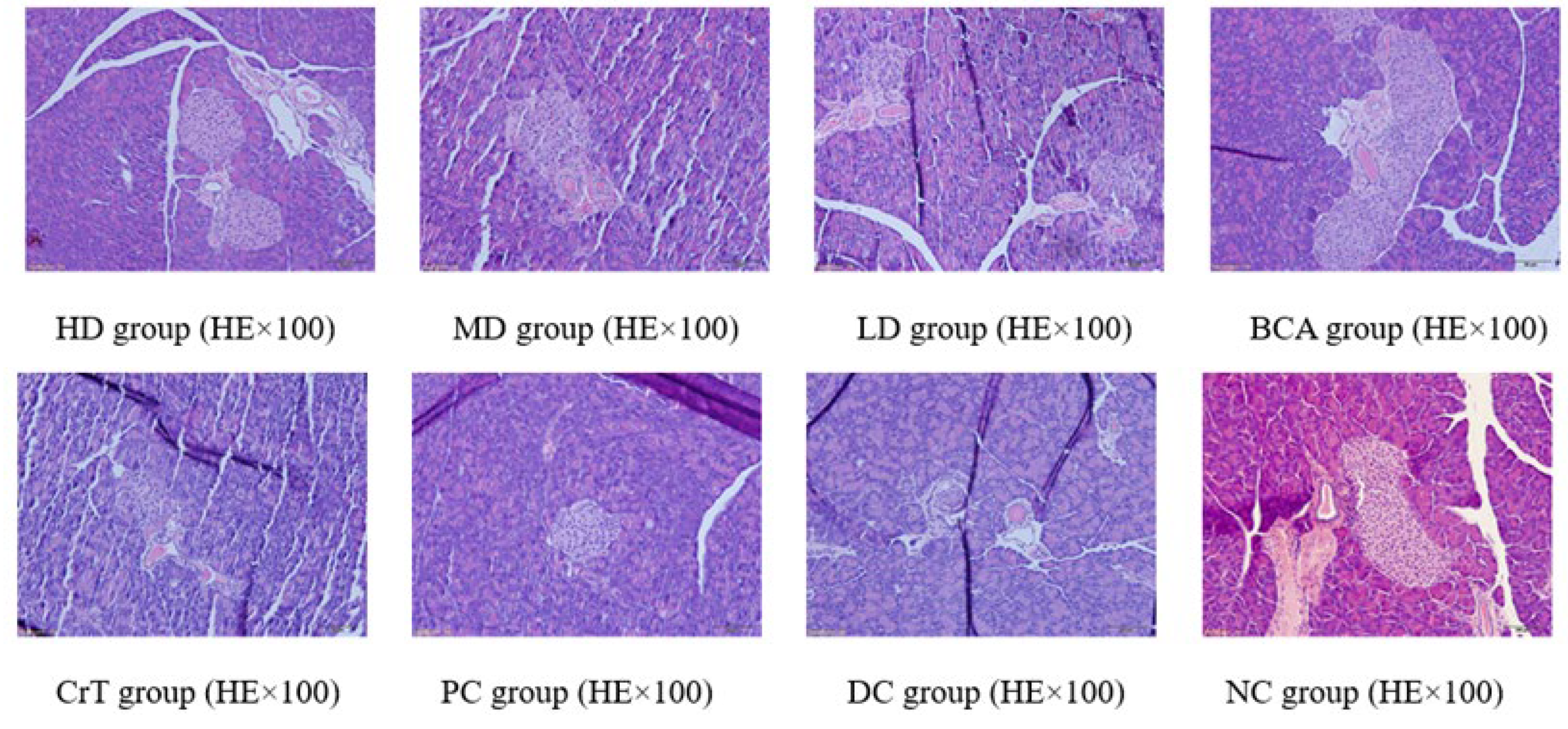
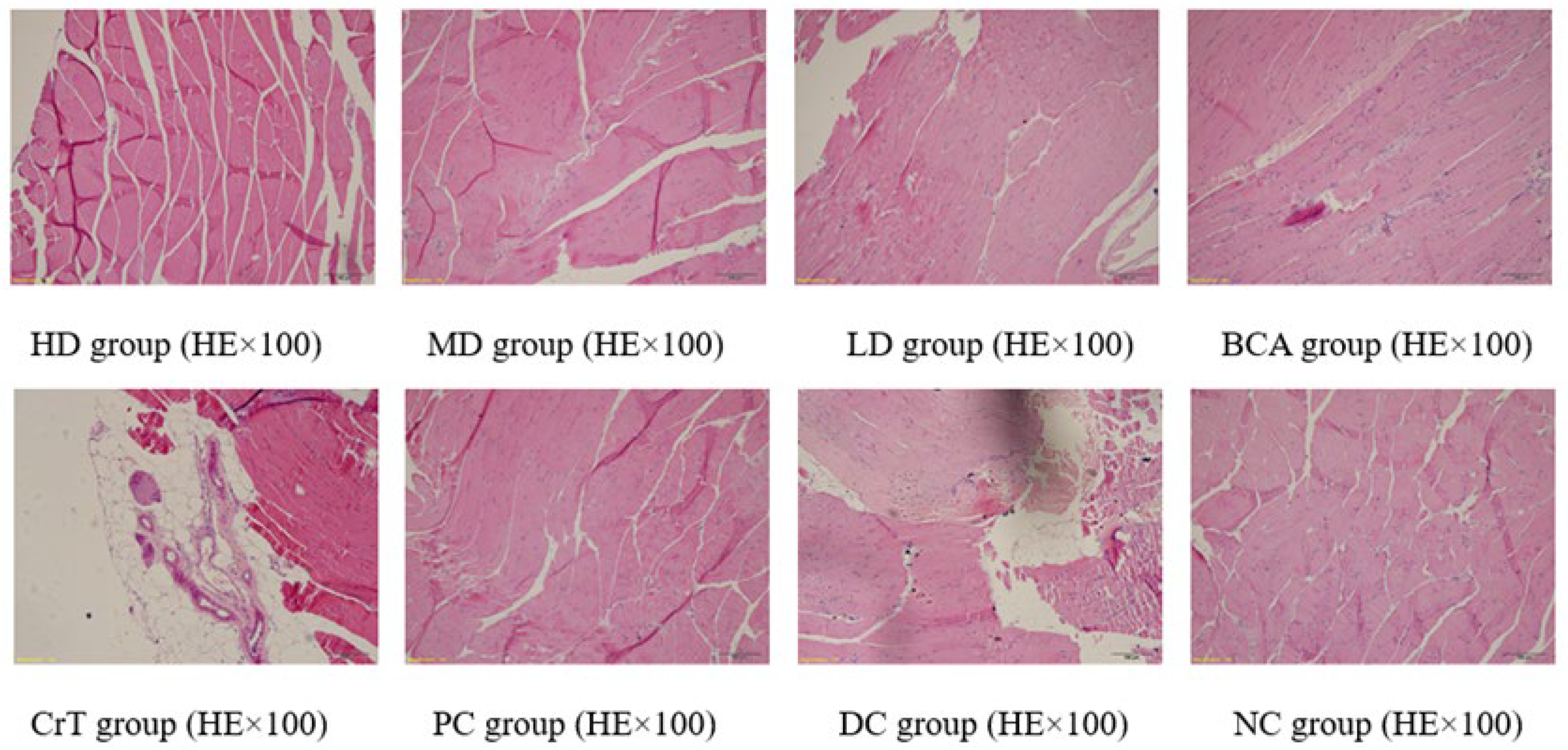
2.2.7. Histopathological Observation
Morphological Changes in Liver Tissue
Morphological Changes in Renal Tissue
Morphological Changes in Pancreatic Tissue
Morphological Changes in Skeletal Muscle Tissue
2.2.8. Determination of Serum Biochemical Indices
2.2.9. Effects on MDA Levels and Antioxidant Enzyme Activities in the Liver
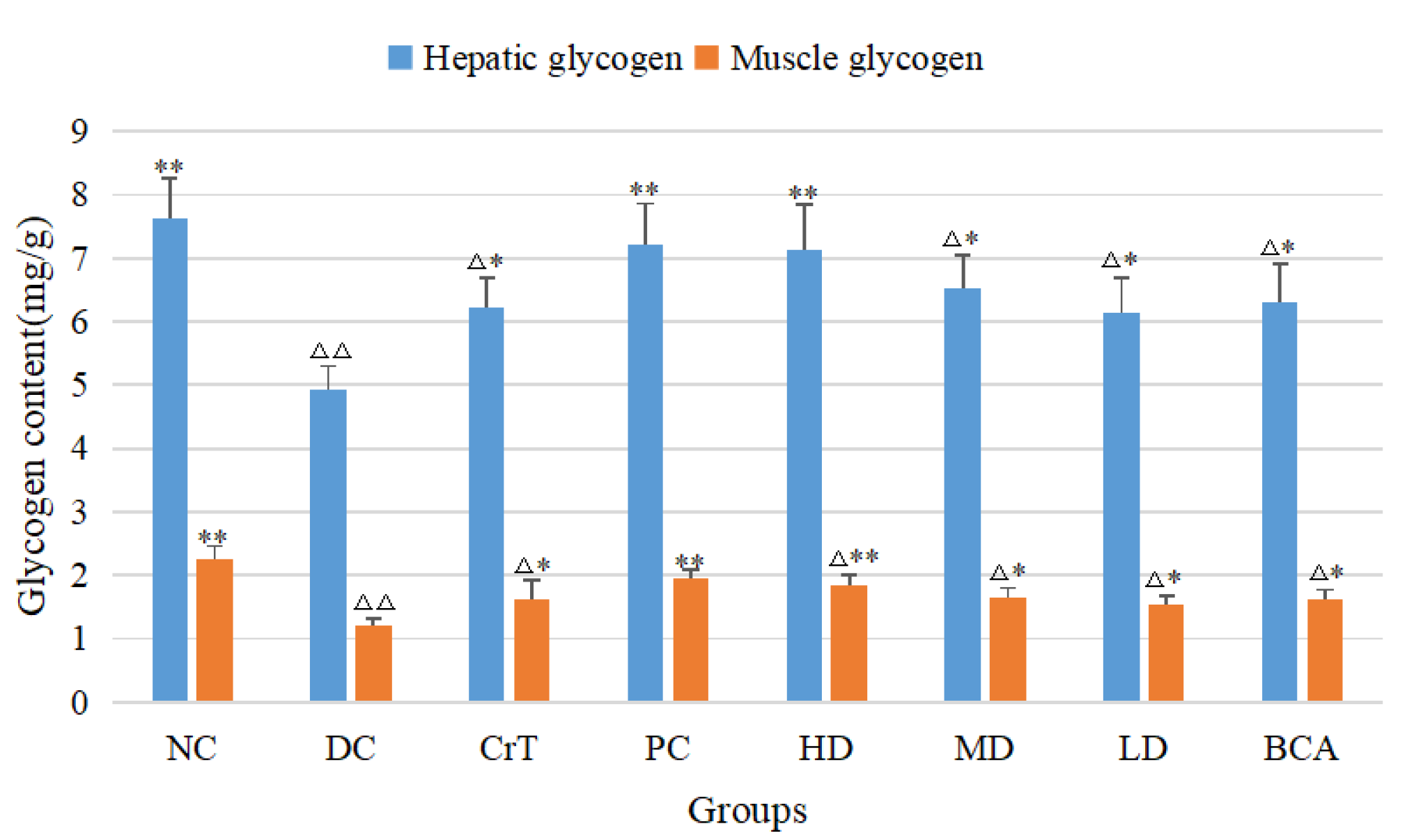
2.2.10. The Effects on Glycogen Levels of db/db Mice
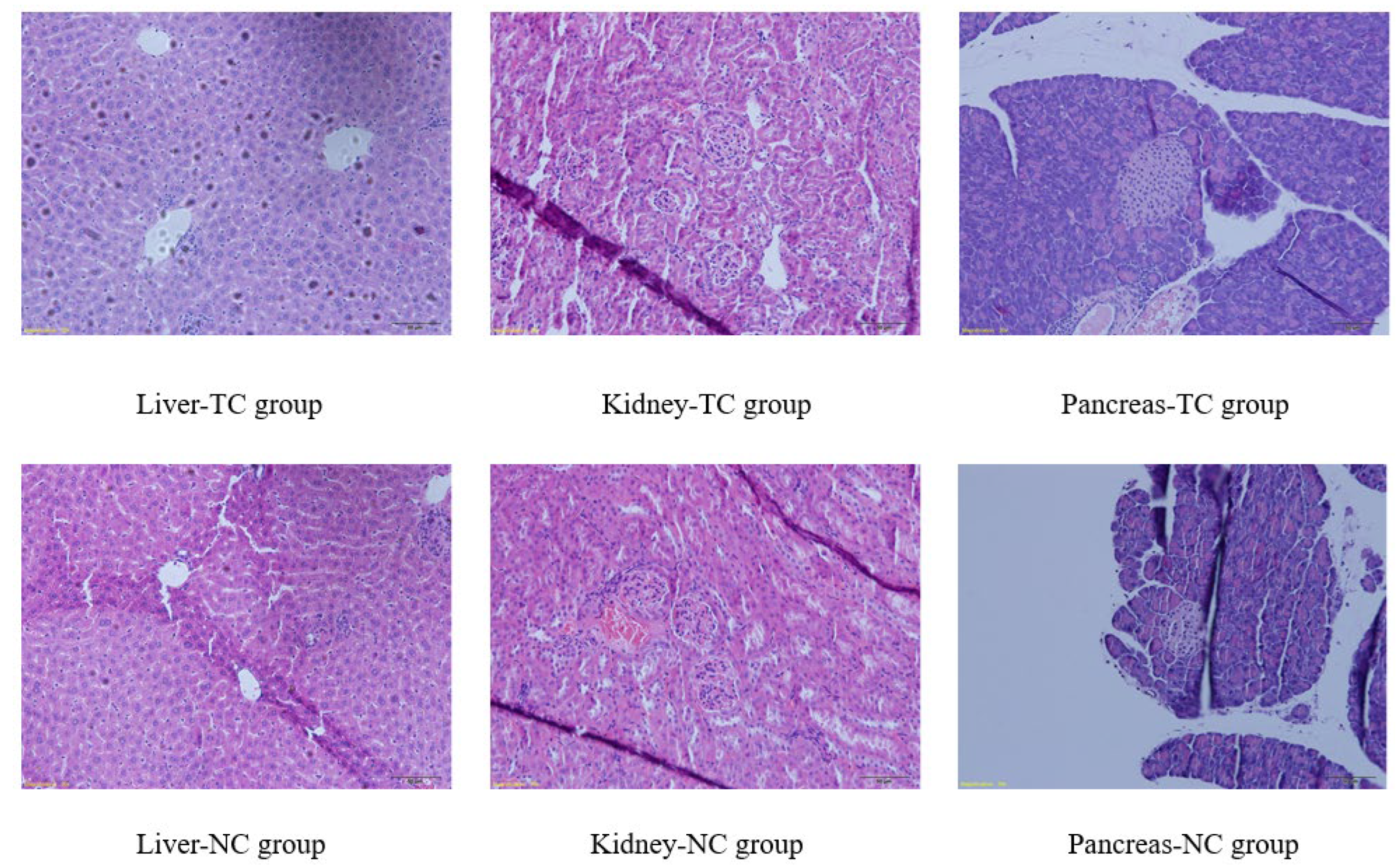
2.3. Sub-Acute Toxicity Study in Mice
3. Materials and Methods
3.1. Chemicals and Instruments [42]
3.2. Experimental Animals [42]
3.3. Synthesis and Structural Characterisation of [CrBCA3]
3.4. Animal Hypoglycemic Experiment Design
3.5. Biochemical Assays [42]
3.6. Sub-Acute Toxicity Test [42]
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiajun, C.; Ying, W.; Tingting, S.; Chaojie, C.; Xingya, W. Research progress on Ganoderma polysaccharide in prevention and treatment of diabetes and its complication. Chin. Tradit. Herb. Drugs 2022, 53, 937–947. [Google Scholar]
- Lixin, G. 2021 annual review of major progress in diabetes. Chin. J. Diabetes Mellit. 2022, 14, 1–8. [Google Scholar]
- Izbicki John, A.; Ball James, W.; Bullen, T.D.; Sutley Stephen, J. Chromium, chromium isotopes and selected trace elements, western Mojave Desert, USA. Appl. Geochem. 2008, 23, 1325–1352. [Google Scholar] [CrossRef]
- Cui, H.; Jiejie, H.; Wei, W.; Zhangrun, H.; Guangsheng, L.; Lijuan, Z.; Xia, Z.; Guangli, Y. Insulin Sensitizing Effects of Oligomannuronate-Chromium(III) Complexes in C2C12 Skeletal Muscle Cells. PLoS ONE 2011, 6, e24598. [Google Scholar]
- Ewelina, K.; Zbigniew, K. Chromium(III) propionate complex supplementation improves carbohydrate metabolism in insulin-resistance rat mode. Food Chem. Toxicol. 2010, 48, 2791–2796. [Google Scholar]
- Yang, X.P.; Palanichamy, K.; Ontko, A.C.; Rao, M.N.A.; Fang, C.X.; Ren, J.; Sreejayan, N. A newly synthetic chromium complex-chromium(phenylalanine)3 improves insulin responsiveness and reduces whole body glucose tolerance. FEBS Lett. 2005, 579, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Gaoyi, T.; Shanshan, Z.; Minhong, Z.; Jinghai, F.; Peng, X.; Jinming, B. Study of Oxidative Damage in Growing-Finishing Pigs with Continuous Excess Dietary Chromium Picolinate Intake. Biol. Trace Elem. Res. 2008, 126, 129–140. [Google Scholar]
- Andersson Maria, A.; Petersson Grawe Kierstin, V.; Karlsson Oskar, M.; Abramsson-Zetterberg Lilianne, A.G.; Hellman Bjorn, E. Evaluation of the potential genotoxicity of chromium picolinate in mammalian cells in vivo and in vitro. Food Chem. Toxicol. 2007, 45, 1097–1110. [Google Scholar] [CrossRef]
- Bailey, M.M.; Boohaker, J.G.; Sawyer, R.D.; Behling, J.E.; Rasco, J.F.; Jernigan, J.J.; Hood, R.D.; Vincent, J.B. Exposure of pregnant mice to chromium picolinate results in skeletal defects in their offspring. Birth Defects Res. B Dev. Reprod. Toxicol. 2006, 77, 244–249. [Google Scholar] [CrossRef]
- Speetjens, J.K.; Collins, R.A.; Vincent, J.B.; Woski, S.A. The nutritional supplement Chromium(III) Tris(picolinate) Cleaves DNA. Chem. Res. Toxicol. 1999, 12, 483–487. [Google Scholar] [CrossRef]
- Whittaker, P.; San, R.H.C.; Clarke, J.J.; Seifried, H.E.; Dunkel, V.C. Mutagencity of chromium picolinate and its components in Salmonella typhimurium and L5178Y mouse lymphoma cells. Food Chem. Toxicol. 2005, 43, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Jinlong, D.; Bin, L.; BinSheng, Y. Synthesis, crystal structure and magnetic properties of trinuclear chromium(III) basic carboxylate assembly:[Cr3O(salH)7(H2O)2] (salH2=salicylic acid), a new member of [Cr3O] family. J. Mol. Struct. 2016, 1116, 311–316. [Google Scholar]
- Bin, L.; Jie, C.; Xiangquan, H.; Yujiao, Z.; Junxia, N.; Binsheng, Y. Synthesis, structure, stability and DNA cleavage activities of three Cr(III) complexes with salicylate and ammonium ligands. Inorg. Chem. Commun. 2015, 52, 27–30. [Google Scholar]
- Tadros, A.M.; Michael, M. Aminopyridine complexes of Cr(III) basic carboxylates as potential polymer precursors: Synthesis, characterization, and crystal structure of [Cr3O(propionate)6 (X-amino pyridine)3] + (X = 3 or 4). Polyhedron 2015, 100, 17–27. [Google Scholar] [CrossRef]
- Liu, B.; Chai, J.; Feng, S.S.; Yang, B.S. Structure, Photochemistry and magnetic properties of tetrahydrogenated Schiff base chromium(III) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 437–443. [Google Scholar] [CrossRef]
- Karthikeyan, K.S.; Rajender, M.; Shilpa, M.; Sastry, K.S. Glucose Tolerance Factor Activity of Binary Amino acid Chromium Complexes in Animals and yeast. Int. J. Bio-Resour. Stress Manag. 2012, 3, 459–463. [Google Scholar]
- Ye, H.; Shen, Z.; Cui, J.; Zhu, Y.; Li, Y.; Chi, Y.; Wang, J.; Wang, P. Hypoglycemic activity and mechanism of the sulfated rhamnose polysaccharides chromium(III) complex in type 2 diabetic mice. Bioorganic Chem. 2019, 88, 102942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, M.; Hong, R.; Chen, H. Preparation of a Momordica charantia L. polysaccharide-chromium(III) complex and its anti-hyperglycemic activity in mice with streptozotocin-induced diabetes. Int. J. Biol. Macromol. 2019, 122, 619–627. [Google Scholar] [CrossRef]
- Bing, L.; Zhenkui, Q.; Xiangmei, L.; Lin, M.; Wanshun, L.; Baoqin, H. Promotion effect of chitooligosaccharides and its derivatives on pancreatic islet cells proliferation and insulin secretion. J. Clin. Rehabil. Tissue Eng. Res. 2009, 13, 513–516. [Google Scholar]
- Pei, C.; Jingqian, W.; Zhenyan, C.; Xidong, M.; Xiuting, Q.; Jinhui, S. Effects of chromium gluconate on levels of four serum hormones and activities of glyco-metabolism-related enzymes in hepatopancreas of koi carp Cyprinus carpio. J. Dalian Ocean. Univ. 2018, 33, 481–486. [Google Scholar]
- Jing, C. Synthesis, Characterization and Activity of Luteolin with Zinc and Chromium Derivatives; Jiamusi University: Jiamusi, China, 2012. [Google Scholar]
- Xiufang, S. Synthesis and Anti-Diabetic Activity of Chromium(III) Carnosinate; Tianjin University: Tianjin, China, 2005. [Google Scholar]
- Mingzhu, H. Synthesis and Hypoglycemic Activity of Complexes of Gallic Acid and Protocatechuic Acid with Chromium; Inner Mongolia University: Huhehaote, Mongolia, 2020. [Google Scholar]
- Weiwei, F. Study on Anti-Hyperglycemic Activity, Mechanism and Preliminary Safety Evaluation of Chromium Malate; Jiangsu University: Zhenjiang, China, 2015. [Google Scholar]
- Occhiuto, F.; Zangla, G.; Samperi, S.; Palumbo, D.R.; Pino, A.; Pasquale, R.D.; Circosta, C. The phytoestrogenic isoflavones from Trifolium pratense L. (Red clover) protects human cortical neurons from glutamatetoxicity. Phytomedicine 2008, 15, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Kale, R.K.; Kar, A. Chemoprevention of mammary tumorigenesis and chemomodulation of the antioxidative enzymes and peroxidative damage in prepubertal Sprague Dawley rats by Biochanin A. Mol. Cell. Biochem. 2008, 312, 1. [Google Scholar] [CrossRef]
- Chan, H.Y.; Wang, H.; Leung, L.K. The red clover (Trifolium pratense) isoflavone biochanin A modulates the biotransformation pathways of 7, 12-dimethylbenz[a]anthracene. Br. J. Nutr. 2003, 90, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mertas, A.; Paradysz, A.; Krol, W. The dietary isoflavone biochanin A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol. Oncol. 2013, 31, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, B.S.; Chun, S.Y.; Park, Y.K.; Kang, K.S.; Kwon, T.G. Apoptotic effects of genistein, biochanin A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J. Korean Med. Sci. 2011, 26, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Compston, J.E.; Day, N.E.; Dowsett, M.; Bingham, S.A. The effects of phytoestrogen isoflavones on bone density in women: A double blind, randomized, placebo-controlled trial. Am. J. Clin. Nut. 2004, 79, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.; Ding, H.Y.; Tsai, P.C.; Wu, J.Y.; Lu, Y.H.; Chang, T.S. In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pratense. Biosci. Biotechnol. Biochem. 2011, 75, 914–918. [Google Scholar] [CrossRef]
- Park, J.; Chung, S.W.; Kim, S.H.; Kim, T.S. Up-regulation of interleukin-4 production via NF-AT/AP-1 activation in T cells by biochanin A, a phytoestrogen and its metabolites. Toxicol. Appl. Pharmacol. 2006, 212, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Sklenickova, O.; Flesar, J.; Kokoska, L.; Vlkova, E.; Halamova, K.; Malik, J. Selective growth inhibitory effect of biochanin A against intestinal tract colonizing bacteria. Molecules 2010, 15, 1270–1279. [Google Scholar] [CrossRef]
- Chen, H.Q.; Jin, Z.Y.; Li, G.H. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 2007, 417, 112–117. [Google Scholar] [CrossRef]
- Harini, R.; Ezhumalai, M.; Pugalendi, K.V. Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur. J. Pharmacol. 2012, 676, 89–94. [Google Scholar] [CrossRef]
- Qiu, L.; Ye, H.; Chen, L.; Hong, Y.; Zhong, F.; Zhang, T. Red Clover Extract Ameliorates Dyslipidemia in Streptozotocin-induced Diabetic C57BL/6 Mice by Activating Hepatic PPARα. Phytother. Res. 2012, 26, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018, 107, 1119–1127. [Google Scholar] [CrossRef]
- Tang, Y.D.; Wang, X.Z.; Zhang, J.J. Research progress in the construction of type II diabetes animal models. Acta Lab. Anim. Sci. Sin. 2020, 28, 870–876. [Google Scholar]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Nirwana, S.I. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat and human. Physiol. Genomics 2007, 29, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; McCue, P.; Dunn, S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Renal. Physiol. 2003, 284, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cao, Y.; Song, G.; Zhao, B.; Ma, Q.; Li, Z.; He, C. Anti-diabetic properties of genistein-chromium(III) complex in db/db diabetic mice and its sub-acute toxicity evaluation in normal mice. J. Trace. Elem. Med. Bio. 2020, 62, 126606. [Google Scholar] [CrossRef]
- SCF (Scientific Committee on Food). Opinion on the Scientific Committee on Food on the Tolerable Upper Intake Level of Trivalent Chromium; SCF/CS/NUT/UPPLEV/67 Final; European Commission: Brussels, Belgium, 2003; p. 18. [Google Scholar]
- Tingting, L.; Dingkun, G.; Sheng, G. Protective Effect of Astaxanthin on Diabetic Nephropathy in db/db Mice. Food Nutr. China 2021, 27, 73–77. [Google Scholar]
- Wenying, Z.; Xiaolin, Y.; Xinzhi, W.; Rui, L.; Junchao, D.; Qian, Z.; Hao, W.; Huimin, B. The Hypoglycemic Effect of Yin Sang Granules on db/db Mice. J. Nanjing Univ. Tradit. Chin. Med. 2017, 33, 252–257. [Google Scholar]
- Sun, J.E.; Ao, Z.H.; Lu, Z.M.; Xu, H.Y.; Zhang, X.M.; Dou, W.F.; Xu, Z.H. Anti-hyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J. Ethnopharmacol. 2008, 118, 7–13. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yi, X.J.; Li, J.C.; Bai, Y.H.; Zhou, H.; Li, J.B.; Pei, L.P. Biological characteristics of spontaneous type 2 diabetes mellitus model db/db mice. Chin. J. Basic Med. Tradit. Chin. Med. 2019, 25, 909–912. [Google Scholar]
- Lu, J.; Liu, J.; Pei, T.X.; Tian, X.M.; Shen, X.P. Study on biological characteristics of spontaneous type 2 diabetic model db/db mice. Drug Eval. Res. 2013, 36, 341–345. [Google Scholar]
- Ewelina, K.; Zbigniew, K. Evaluation of anti-diabetic potential of chromium(III) propionate complex in high-fat diet fed and STZ injected rats. Food Chem. Toxicol. 2011, 49, 3217–3223. [Google Scholar]
- Sharma, S.; Agrawal, R.P.; Choudhary, M.; Jain, S.; Goyal, S.; Agarwal, V. Beneficial effect of chromium supplementation on glucose, HbA1C and lipid variables in individuals with newly onset type-2 diabetes. J. Trace Elem. Med. Biol. 2011, 25, 149–153. [Google Scholar] [CrossRef]
- Kazim, S.; Muhittin, O.; Mehmet, T.; Bilal, U.; Gurkan, C.; Ibrahim, H.O.; Vidyasagar, S.; Vijaya, J.; James, R.K. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: The fat-fed, streptozotocin-treated rat. Metabolism 2007, 56, 1233–1240. [Google Scholar]
- Ali, A.; Shibu, M.A.; Kuo, C.H.; Lo, J.F.; Chen, R.J.; Day, C.H.; Ho, T.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. CHIP-overexpressing Wharton’s jelly-derived mesenchymal stem cells attenuate hyperglycemia-induced oxidative stress-mediated kidney injuries in diabetic rats. Free. Radic. Biol. Med. 2021, 173, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Temiz, M.A.; Okumus, E.; Yaman, T.; Keles, O.F. Mixture of leaf and flower extract of Prunus spinosa L. alleviates hyperglycemia and oxidative stress in streptozotocin-induced diabetic rats. S. Afr. J. Bot. 2021, 141, 145–151. [Google Scholar] [CrossRef]
- Salaj, N.; Kladar, N.; Čonić, B.S.; Jeremić, K.; Hitl, M.; Gavarić, N.; Bozin, B. Traditional multi-herbal formula in diabetes therapy—Antihyperglycemic and antioxidant potential. Arab. J. Chem. 2021, 14, 103347–103357. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Complementary therapies for diabetes: The case for chromium, magnesium, and antioxidants. Arch. Med. Res. 2005, 36, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.; Jiddah-kazeem, B.; Fatoki, T.H.; Ibukun, E.O.; Akinmoladun, A.C. Antioxidant property of Eucalyptus globulus Labill. Extracts and inhibitory activities on carbohydrate metabolizing enzymes related to type-2 diabetes. Biocatal. Agric. Biotechnol. 2021, 36, 102111. [Google Scholar] [CrossRef]
- Isabelle, H.; Rachida, B.; Mireille, O.; Henri, F.; Anne, M.R.; Richard, A.A. Safety of trivalent chromium complexes: No evidence for DNA damage in human HaCaT keratinocytes. Free. Radic. Biol. Med. 2007, 42, 1759–1765. [Google Scholar]
- Nivedita, C.; Zejiao, L. Cr-(III)-organic compounds treatment causes genotoxicity and changes in DNA and protein level in Saccharomyces cerevisiae. Ecotoxicology 2010, 19, 593–603. [Google Scholar]
- Nash, R.J.; Kato, A.; Yu, C.Y.; Fleet, G.W. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Chennaiah, A.; Dahiya, A.; Dubbu, S.; Vankar, Y.D. A Stereoselective Synthesis of an Imino Glycal: Application in the Synthesis of (−)-1-epi-Adenophorine and a Homoiminosugar. Eur. J. Org. Chem. 2018, 46, 6574–6581. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of glycals into vicinal-1,2-diazides and 1,2-(or 2,1)-azidoacetates using hypervalent iodine reagents and Me3SiN3. Application in the synthesis of N-glycopeptides, pseudo-trisaccharides and an iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef] [Green Version]




| C% | H% | Cr% | Molecular Weight | ||||
|---|---|---|---|---|---|---|---|
| test | theory | test | theory | test | theory | test | theory [M-2H2O-H]− |
| 61.43 | 61.47 | 3.93 | 3.95 | 5.53 | 5.55 | 899.8812 | 900.1146 |
| Compound | ν(O-H) | ν(C = O) | ν(C = C) | ν(C-O-C) |
|---|---|---|---|---|
| Biochanin A | 3388 | 1653 | 1623 | 1249 |
| [CrBCA3] | 3432 | 1627 | 1612 | 1249 |
| Groups | n | Fasting Blood Glucose (mmol/L) | ||||
|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | ||
| HD | 7 | 30.1 ± 1.3 | 28.2 ± 2.3 | 24.1 ± 2.6 ** | 20.2 ± 3.2 ** | 17.4 ± 2.1 ** |
| MD | 7 | 30.2 ± 1.2 | 29.5 ± 3.0 | 26.1 ± 2.3 * | 23.3 ± 2.1 **,★ | 20.2 ± 2.4 **,★ |
| LD | 7 | 31.3 ± 2.1 | 30.5 ± 2.3 | 28.2 ± 2.1 *,★ | 26.1 ± 2.3 *,★★ | 24.3 ± 2.1 **,★★ |
| BCA | 7 | 30.3 ± 2.2 | 28.2 ± 2.1 | 26.6 ± 2.3 * | 24.6 ± 2.2 **,★ | 22.4 ± 1.8 **,★★ |
| CrT | 7 | 30.1 ± 2.1 | 29.2 ± 2.9 | 26.5 ± 3.1 * | 23.1 ± 2.3 **,★ | 21.5 ± 2.5 **,★ |
| PC | 7 | 31.4 ± 2.3 | 28.9 ± 3.1 | 24.7 ± 2.3 ** | 20.5 ± 2.1 ** | 16.2 ± 2.2 ** |
| DC | 7 | 30.3 ± 2.1 | 30.5 ± 2.4 | 31.5 ± 3.2 ★★ | 31.8 ± 2.1 ★★ | 31.1 ± 2.9 ★★ |
| NC | 7 | 4.4 ± 0.5 | 4.7 ± 0.8 | 4.5 ± 0.6 | 4.8 ± 0.9 | 4.6 ± 0.9 |
| Groups | n | Random Blood Glucose (mmol/L) | ||||
|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | ||
| HD | 7 | 31.2 ± 2.2 | 29.4 ± 2.3 * | 25.3 ± 3.2 ** | 21.4 ± 3.6 ** | 18.2 ± 4.2 ** |
| MD | 7 | 31.1 ± 1.3 | 29.3 ± 2.5 * | 26.4 ± 3.5 ** | 23.4 ± 2.1 ** | 21.3 ± 3.4 **,★ |
| LD | 7 | 31.4 ± 0.9 | 30.7 ± 2.3 | 28.5 ± 3.5 *,★ | 26.6 ± 3.2 **,★ | 24.9 ± 2.5 **,★★ |
| BCA | 7 | 31.6 ± 1.5 | 30.10 ± 1.2 | 28.2 ± 1.5 *,★ | 26.5 ± 2.5 **,★ | 24.2 ± 2.5 **,★★ |
| CrT | 7 | 31.1 ± 1.3 | 30.3 ± 1.5 | 27.4 ± 2.4 * | 25.1 ± 1.7 **,★ | 22.2 ± 2.5 **,★ |
| PC | 7 | 31.2 ± 1.2 | 29.1 ± 1.5 * | 25.2 ± 2.3 ** | 21.1 ± 1.8 ** | 17.1 ± 3.2 ** |
| DC | 7 | 31.4 ± 1.1 | 32.7 ± 1.6 ★ | 33.1 ± 0.8 ★★ | 32.8 ± 1.5 ★★ | 32.9 ± 1.1 ★★ |
| NC | 7 | 5.4 ± 0.4 | 5.8 ± 0.7 | 6.5 ± 1.2 | 6.2 ± 0.8 | 5.8 ± 0.6 |
| NC Group | DC Group | CrT Group | PC Group | HD Group | MD Group | LD Group | BCA Group | |
|---|---|---|---|---|---|---|---|---|
| TC (mmol/L) | 5.21 ± 0.24 ** | 8.35 ± 0.36 △△ | 6.96 ±0.24△,* | 5.92 ± 0.51 ** | 6.09 ± 0.28 ** | 7.15 ± 0.68 △,* | 7.96 ± 0.71 △△ | 7.27 ± 0.59 △,* |
| TG (mmol/L) | 1.19 ± 0.18 ** | 2.83 ± 0.38 △△ | 2.58 ± 0.45 △△ | 2.21 ± 0.31 △△,* | 2.08 ± 0.46 △△,* | 2.56 ± 0.39 △△ | 2.68 ± 0.42 △△ | 2.55 ± 0.39 △△ |
| HDL-C (mmol/L) | 1.62 ± 0.21 ** | 0.68 ± 0.12 △△ | 0.96 ± 0.13 △△,* | 1.21 ± 0.16 △,** | 1.32 ± 0.21 △,** | 1.01 ± 0.22 △△,* | 0.85 ± 0.22 △△,* | 0.92 ± 0.14 △△,* |
| LDL-C (mmol/L) | 3.59 ± 0.23 ** | 5.16 ± 0.31 △△ | 4.73 ± 0.42 △ | 4.05 ± 0.26 △,* | 4.13 ± 0.32 △,* | 4.69 ± 0.29 △ | 5.08 ± 0.41 △△ | 4.61 ± 0.32 △ |
| GSP (mmol/L) | 3.09 ± 0.23 * | 3.92 ± 0.26 △ | 3.46 ± 0.35 △,* | 3.25 ± 0.29 * | 3.13 ± 0.36 * | 3.45 ± 0.32 △,* | 3.71 ± 0.43 △ | 3.62 ± 0.42 △ |
| NEFA (mmol/L) | 2.15 ± 0.23 ** | 3.76 ± 0.31 △△ | 3.14 ± 0.42 △△,* | 2.82 ± 0.26 △,* | 2.75 ± 0.21 △,* | 3.11 ± 0.28 △△,* | 3.66 ± 0.51 △△ | 3.25 ± 0.36 △△,* |
| NC Group | DC Group | CrT Group | PC Group | HD Group | MD Group | LD Group | BCA Group | |
|---|---|---|---|---|---|---|---|---|
| MDA (nmol/mg) | 3.25 ± 0.41 ** | 4.87 ± 0.62 △△ | 4.19 ± 0.44 △,* | 3.59 ± 0.43 △,** | 3.66 ± 0.41 △,** | 3.98 ± 0.42 △,* | 4.52 ± 0.51 △△ | 4.21 ± 0.53 △,* |
| CAT (U/mg) | 43.18 ± 3.28 ** | 21.42 ± 2.15 △△ | 28.37 ± 2.56 △△,* | 37.34 ± 3.25 △,** | 35.43 ± 3.38 △,** | 30.23 ± 0.48 △△,** | 24.31 ± 0.44 △△ | 28.43 ± 0.26 △△,* |
| SOD (U/mg) | 329.47 ± 9.23 ** | 232.48 ± 7.46 △△ | 265.47 ± 7.39 △△,* | 301.42 ± 8.65 △,** | 297.58 ± 5.89 △,** | 271.44 ± 6.21 △△,* | 255.47 ± 6.82 △△ | 260.58 ± 6.79 △△,* |
| GSH-Px (U/mg) | 621.45 ± 7.39 ** | 424.56 ± 7.56 △△ | 512.51 ± 8.67 △△,** | 602.47 ± 6.25 ** | 593.39 ± 8.21 ** | 524.72 ± 7.85 △△,** | 481.23 ± 8.83 △△,* | 506.28 ± 6.28 △△,** |
| Liver | Kidney | Pancreas | Heart | Thymus | Spleen | |
|---|---|---|---|---|---|---|
| TC group (mg/g) | 61.47 ± 4.22 | 7.24 ± 0.81 | 5.38 ± 0.66 | 6.29 ± 0.59 | 2.24 ± 0.27 | 3.56 ± 0.36 |
| NC group (mg/g) | 61.21 ± 3.14 | 7.05 ± 0.69 | 5.02 ± 0.61 | 6.11 ± 0.68 | 2.12 ± 0.31 | 3.21 ± 0.41 |
| Parameter | Normal Control Group (NC) | Toxicity Group (TC) |
|---|---|---|
| RBG (mmol/L) | 6.14 ± 0.89 | 6.25 ± 0.96 |
| INS (mIU/L) | 11.12 ± 1.16 | 11.34 ± 1.38 |
| TC (mmol/L) | 5.23 ± 0.67 | 5.41 ± 0.58 |
| TG (mmol/L) | 1.21 ± 0.23 | 1.14 ± 0.21 |
| LDL-C (mmol/L) | 3.54 ± 0.21 | 3.65 ± 0.32 |
| HDL-C (mmol/L) | 1.71 ± 0.25 | 1.81 ± 0.23 |
| GSP (μmol/L) | 2.89 ± 0.25 | 2.95 ± 0.31 |
| NEFA (μmol/g) | 1.96 ± 0.14 | 2.14 ± 0.23 |
| MDA (nmol/mg) | 3.22 ± 0.35 | 3.14 ± 0.29 |
| CAT (U/mg) | 43.28 ± 5.11 | 44.25 ± 4.43 |
| SOD (U/mg) | 328.43 ± 13.47 | 326.45 ± 15.78 |
| GSH-Px (U/mg) | 625.37 ± 12.43 | 634.59 ± 12.47 |
| Parameter | Normal Control Group (NC) | Toxicity Group (TC) |
|---|---|---|
| WBC (109/L) | 9.71 ± 0.82 | 9.85 ± 0.96 |
| RBC (1012/L) | 7.53 ± 0.68 | 7.46 ± 0.84 |
| HGB (g/L) | 144.28 ± 9.21 | 149.88 ± 9.08 |
| PLT (109/L) | 628.41 ± 51.26 | 633.48 ± 62.47 |
| ALT (U/L) | 39.59 ± 0.96 | 41.36 ± 0.92 |
| AST (U/L) | 142.58 ± 2.66 | 137.89 ± 2.98 |
| ALP (U/L) | 97.58 ± 1.15 | 95.23 ± 1.35 |
| TP (g/L) | 70.82 ± 0.96 | 72.55 ± 1.19 |
| ALB (g/L) | 31.46 ± 0.96 | 32.44 ± 1.01 |
| BUN (mmol/L) | 6.28 ± 0.29 | 6.13 ± 0.21 |
| CR (μmol/L) | 41.26 ± 1.35 | 42.58 ± 1.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Li, M.; Lou, X.; Zhao, B.; Ma, Q.; Bian, Y.; Mi, X. Evaluation of Hypoglycemic Activity and Sub-Acute Toxicity of the Novel Biochanin A–Chromium(III) Complex. Molecules 2022, 27, 5786. https://doi.org/10.3390/molecules27185786
Li P, Li M, Lou X, Zhao B, Ma Q, Bian Y, Mi X. Evaluation of Hypoglycemic Activity and Sub-Acute Toxicity of the Novel Biochanin A–Chromium(III) Complex. Molecules. 2022; 27(18):5786. https://doi.org/10.3390/molecules27185786
Chicago/Turabian StyleLi, Pengshou, Mengdan Li, Xinhua Lou, Baosheng Zhao, Qixiang Ma, Yumiao Bian, and Xiliang Mi. 2022. "Evaluation of Hypoglycemic Activity and Sub-Acute Toxicity of the Novel Biochanin A–Chromium(III) Complex" Molecules 27, no. 18: 5786. https://doi.org/10.3390/molecules27185786
APA StyleLi, P., Li, M., Lou, X., Zhao, B., Ma, Q., Bian, Y., & Mi, X. (2022). Evaluation of Hypoglycemic Activity and Sub-Acute Toxicity of the Novel Biochanin A–Chromium(III) Complex. Molecules, 27(18), 5786. https://doi.org/10.3390/molecules27185786





