Compatibility of Personalized Formulations in Cleoderm™, A Skin Rebalancing Cream Base for Oily and Sensitive Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Reference Standards, and Materials
2.2. Equipment
2.3. Chromatographic Determinations
2.4. Forced Degradation Studies: Characteristics Indicating Stability
- Dilution in acid (0.1 M HCl);
- Dilution in base (0.1 M NaOH);
- Dilution in hydrogen peroxide (H2O2);
- Exposure to ultraviolet light at 365 nm (for 24 h);
- Heating at 70 °C (for 24 h).
2.5. Validation of the HPLC Methods
2.6. Preparation of API Cream Samples for the Compatibility Study
- The required quantity of each ingredient for the total amount to be prepared was calculated.
- Each ingredient was accurately weighed.
- The API was placed in an adequate EMP jar, and combined with a small amount of Levigant, according to each API’s properties.
- The Cleoderm™ was further added into the mixture, and the formulation was mixed using an electronic mixing device (FagronLab™ EMP, Scheßlitz, Germany) for 4 min at a medium mixing speed.
- The product was then passed through a roll mill (FagronLab™ TRM Ointment Mill, Saint Paul, MN, USA) thrice.
- The final product was packaged in airless precise-dose, light-resistant bottles and labeled.
- The creams were then immediately assayed at T = 0 and stored at room temperature (15–30 °C) for the duration of the study.
2.7. Compatibility Study: Physico-Chemical Evaluation
2.8. Compatibility Study: Microbiological Evaluation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A Review of Diagnosis and Treatment of Acne in Adult Female Patients. Int. J. Women’s Dermatol. 2018, 4, 56–71. [Google Scholar] [CrossRef]
- Bhate, K.; Williams, H.C. Epidemiology of Acne Vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef]
- Abokwidir, M.; Feldman, S.R. Rosacea Management. Ski. Appendage Disord. 2016, 2, 26–34. [Google Scholar] [CrossRef]
- Rivero, A.L.; Whitfeld, M. An Update on the Treatment of Rosacea. Aust. Prescr. 2018, 41, 20–24. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Anti-Aging Strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the Treatment of Skin Aging: An Overview of Clinical Efficacy and Safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Polonini, H.; Zander, C.; Radke, J. CleodermTM Clarifying Cream: A Novel, Topical Vehicle Using Plant-Based Excipients and Actives Targeting Acne and Oily Skin. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 381–399. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [Green Version]
- Afzali, B.M.; Yaghoobi, E.; Yaghoobi, R.; Bagherani, N.; Dabbagh, M.A. Comparison of the Efficacy of 5% Topical Spironolactone Gel and Placebo in the Treatment of Mild and Moderate Acne Vulgaris: A Randomized Controlled Trial. J. Dermatolog. Treat. 2012, 23, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Gruber, D.M.; Sator, M.O.; Joura, E.A.; Kokoschka, E.M.; Heinze, G.; Huber, J.C. Topical Cyproterone Acetate Treatment in Women with Acne: A Placebo- Controlled Trial. Arch. Dermatol. 1998, 134, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Önder, M. An Investigation of Efficacy of Topical Niacinamide for the Treatment of Mild and Moderate Acne Vulgaris. J. Turk. Acad. Dermatol. 2008, 2, 4–7. [Google Scholar]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B Vitamin That Improves Aging Facial Skin Appearance. Dermatol. Surg. 2005, 31, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Riegler, E.; Hönigsmann, H.; Farokhnia, S.; Schmidt, B. Effects and Side-Effects of 2% Progesterone Cream on the Skin of Peri- and Postmenopausal Women: Results from a Double-Blind, Vehicle-Controlled, Randomized Study. Br. J. Dermatol. 2005, 153, 626–634. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Binder, M.; Macheiner, W.; Kainz, C.; Gitsch, G.; Bieglmayer, C. Treatment of Skin Ageing Symptoms in Perimenopausal Females with Estrogen Compounds. A Pilot Study. Maturitas 1994, 20, 25–30. [Google Scholar] [CrossRef]
- Atefi, N.; Dalvand, B.; Ghassemi, M.; Mehran, G.; Heydarian, A. Therapeutic Effects of Topical Tranexamic Acid in Comparison with Hydroquinone in Treatment of Women with Melasma. Dermatol. Ther. 2017, 7, 417–424. [Google Scholar] [CrossRef]
- Drugs.Com. Available online: https://www.drugs.com/ (accessed on 25 August 2022).
- Polonini, H.C.; Soldati, P.P.; Oliveira, M.A.L.D.; Brandão, M.A.F.; Chaves, M.d.G.M.; Raposo, N.R.B. Transdermal formulations containing human sexual steroids: Development and validation of methods and in vitro drug release. Quim. Nova 2014, 37, 720–727. [Google Scholar] [CrossRef]
- Ferreira, A.O.; Polonini, H.C.; Silva, S.L.; Patrício, F.B.; Brandão, M.A.F.; Raposo, N.R.B. Feasibility of Amlodipine Besylate, Chloroquine Phosphate, Dapsone, Phenytoin, Pyridoxine Hydrochloride, Sulfadiazine, Sulfasalazine, Tetracycline Hydrochloride, Trimethoprim and Zonisamide in SyrSpend® SF PH4 Oral Suspensions. J. Pharm. Biomed. Anal. 2016, 118, 105–112. [Google Scholar] [CrossRef]
- De Almeida, P.A.; Alves, M.C.; Polonini, H.C.; Dutra, L.S.; Leite, M.N.; Raposo, N.R.B.; Ferreira, A.D.O.; BrandÃo, M.A.F. New HPLC Method for Quality Control of β-Escin in Aesculus Hippocastanum L. Hydroalcoholic Extract. Lat. Am. J. Pharm. 2013, 32, 1082–1087. [Google Scholar]
- Polonini, H.C.; Bastos, C.D.A.; Oliveira, M.A.L.D.; Silva, C.G.A.D.; Collins, C.H.; Brandão, M.A.Ô.F.; Raposo, N.R.B. In Vitro Drug Release and Ex Vivo Percutaneous Absorption of Resveratrol Cream Using HPLC with Zirconized Silica Stationary Phase. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 947–948, 23–31. [Google Scholar] [CrossRef]
- Guy, R.C. International Conference on Harmonisation. Encycl. Toxicol. Third Ed. 2014, 2, 1070–1072. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention <51> Antimicrobial Effectiveness Testing. In United States Pharmacopeia 40—National Formulary 35; 2020.
- Pong, A.; Raghavarao, D. Comparison of Bracketing and Matrixing Designs for a Two-Year Stability Study. J. Biopharm. Stat. 2000, 10, 217–228. [Google Scholar] [CrossRef]
- Conference, I.; Harmonisation, O.N.; Technical, O.F.; For, R.; Of, R.; For, P. International Conference on Harmonisation: Guidance on Q1D Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products; Availability. Notice. Fed. Regist. 2003, 68, 2339–2340. [Google Scholar]
- United States Pharmacopeial Convention <795> Pharmaceutical Compounding—Nonsterile Preparations. In United States Pharmacopeia 43—National Formulary 38; 2020.
- Majekodunmi, B.D.; Lau-Cam, C.A.; Nash, R.A. Stability of Benzoyl Peroxide in Aromatic Ester-Containing Topical Formulations. Pharm. Dev. Technol. 2007, 12, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Shatalebi, M.A.; Roostaei, M. Preparation and Physicochemical Evaluation of Benzoyl Peroxide 5% Foamable Emu Oil Emulsion. Jundishapur J. Nat. Pharm. Prod. 2015, 10, 3–8. [Google Scholar] [CrossRef]
- Langlah, N.; Pinsuwan, S.; Amnuaikit, T. Preparation and Physicochemical Study of Liposomes Containing Nicotinamide. In Proceedings of the 2012 7th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Kyoto, Japan, 5–8 March 2012; pp. 537–541. [Google Scholar] [CrossRef]
- Jeon, J.S.; Lee, M.J.; Yoon, M.H.; Park, J.A.; Yi, H.; Cho, H.J.; Shin, H.C. Determination of Arbutin, Niacinamide, and Adenosine in Functional Cosmetic Products by High-Performance Liquid Chromatography. Anal. Lett. 2014, 47, 1650–1660. [Google Scholar] [CrossRef]
- Ilic, D.; Cvetkovic, M.; Tasic-Kostov, M. Emulsions with Alkyl Polyglucosides as Carriers for Off-Label Topical Spironolactone–Safety and Stability Evaluation. Pharm. Dev. Technol. 2021, 26, 373–379. [Google Scholar] [CrossRef]
- Allen, L.V.; Erickson, M.A. Stability of Ketoconazole, Metolazone, Metronidazole, Procainamide Hydrochloride, and Spironolactone in Extemporaneously Compounded Oral Liquids. Am. J. Health Pharm. 1996, 53, 2073–2078. [Google Scholar] [CrossRef]
- Tirnaksiz, F.; Kayiş, A.; Çelebi, N.; Adişen, E.; Erel, A. Preparation and Evaluation of Topical Microemulsion System Containing Metronidazole for Remission in Rosacea. Chem. Pharm. Bull. 2012, 60, 583–592. [Google Scholar] [CrossRef]
- Basu Sarkar, A.; Kandimalla, A. Chemical Stability of Progesterone in Compounded Topical Preparations Using PLO Transdermal Cream and HRT Cream Base over a 90-Day Period at Two Controlled Temperatures. J. Steroids Horm. Sci. 2013, 04, 2–4. [Google Scholar] [CrossRef]
- Gatti, R.; Gioia, M.G.; Cavrini, V. Analysis and Stability Study of Retinoids in Pharmaceuticals by LC with Fluorescence Detection. J. Pharm. Biomed. Anal. 2000, 23, 147–159. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Škufca, P.; Kristl, A.; Roškar, R. Retinoid Stability and Degradation Kinetics in Commercial Cosmetic Products. J. Cosmet. Dermatol. 2021, 20, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
| Active Pharmaceutical Ingredient | Concentrations Tested (mg/g) | Pharmaceutical Indication * |
|---|---|---|
| Benzoyl peroxide | 2.5% and 10.0% | Antibacterial drug, commonly used to treat acne |
| Cyproterone acetate | 0.5% and 2.0% | Steroid hormone used (in combination or not with other substances) to treat women with severe acne and symptoms of androgenization |
| Estriol | 0.1% and 1.0% | Estrogenic hormone used to improve general skin condition |
| Metronidazole | 0.75% and 5.0% | Antibiotic drug, used to treat a wide variety of skin infections |
| Niacinamide | 1.0% and 5.0% | Form of Vitamin B3, which can improve general skin condition and hydration when used topically |
| Progesterone | 0.5% and 2.0% | A steroid hormone used to improve general skin condition |
| Retinoic acid | 0.025% and 0.1% | Morphogen derived from retinol (Vitamin A), commonly used for treating severe acne |
| Spironolactone | 1.0% and 5.0% | Anti-androgen drug, used topically for treating hormonal acne |
| Tranexamic acid | 1.0% and 5.0% | A synthetic amino acid lysine derivate, which can act as a brightening agent to reduce dark spots and improve hyperpigmentation |
| Active Pharmaceutical Ingredient | Mobile Phase Composition | Working Concentration (µg/mL) | Column | Flux (mL/min) | Ultraviolet Detection Wavelength (nm) |
|---|---|---|---|---|---|
| Benzoyl peroxide | Acetonitrile and water (750:250, v/v) | 250.0, in acetonitrile; 20 µL injection | C18(L1), 4.6 mm × 250 mm; at 45 °C | 1.0 | 254 |
| Cyproterone acetate | Water, methanol, and acetonitrile (40:40:20, v/v/v) | 100.0, in methanol; 20 µL injection | C18(L1), 4.6 mm × 125 mm; at 40 °C | 1.5 | 282 |
| Estriol | Ethanol and water (60:40, v/v) | 40.0, in ethanol; 10 µL injection | C18(L1), 4.6 mm × 250 mm; at 30 °C | 0.3 | 205 |
| Metronidazole | Acetonitrile and Solution A (glacial acetic acid and water, 40:60, v/v) (40:60, v/v) | 20.0, in methanol; 10 µL injection | C18(L1), 4.6 mm × 250 mm; at 30 °C | 1.0 | 316 |
| Niacinamide | Methanol, acetic acid, and sulfonate buffer (27:1:73, v/v/v) | 100.0, in water; 20 µL injection | C18(L1), 4.6 mm × 150 mm; at 25 °C | 1.0 | 280 |
| Progesterone | Ethanol and water (65:35, v/v) | 100.0, in ethanol; 20 µL injection | C18(L1), 4.6 mm × 250 mm; at 45 °C | 1.2 | 254 |
| Retinoic acid | Methanol, water, and glacial acetic acid (80:20:0.5, v/v/v) | 10.0, in methanol; 50 µL injection | C18(L1), 4.6 mm × 150 mm; at 25 °C | 2.0 | 353 |
| Spironolactone | Water, phosphoric acid, methanol, and acetonitrile (435:2.7:50:515, v/v/v/v) | 100.0, in water; 20 µL injection | C18(L1), 4.6 mm × 250 mm; at 25 °C | 1.0 | 254 |
| Tranexamic acid | Phosphate buffer pH 2.5 and methanol (60:40, v/v) | 100.0, in ultra-purified water; 100 µL injection | C18(L1), 4.6 mm × 250 mm; at 35 °C | 1.0 | 220 |
| Active Pharmaceutical Ingredient | Linearity | Specificity | Precision | Accuracy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range (µg/mL) | Analytical Curve | R2 | ANOVA Significance of Regression (F) | LOD (µg/mL) | LOQ (µg/mL) | Discrepancy (%) | Repeatability (CV, %) | Intermediate Precision (CV, %) | Recovery (%) | |
| Benzoyl peroxide | 180.60–335.40 | y = 369,637.54x − 2,191,586.24 | 0.9988 | 5507.09 | 0.01 | 0.02 | 1.82 | 1.76 | 1.69 | 99.75 |
| Cyproterone acetate | 72.80–135.20 | y = 481,222.28x + 5,470,613.44 | 0.9917 | 774.07 | 0.01 | 0.02 | 0.86 | 0.99 | 0.98 | 98.04 |
| Estriol | 28.28–52.52 | y = 3,451,831.25x − 8,500,128.91 | 0.9951 | 1315.26 | 0.01 | 0.03 | 1.78 | 0.65 | 2.08 | 99.91 |
| Metronidazole | 14.01–26.03 | y = 95.83x − 29.50 | 0.9904 | 660.16 | 0.06 | 0.17 | 0.60 | 3.32 | 4.84 | 100.85 |
| Niacinamide | 72.24–134.16 | y = 58,009.28x + 197,874.53 | 0.9988 | 5547.81 | 0.03 | 0.09 | 1.17 | 0.15 | 0.59 | 99.52 |
| Progesterone | 70.56–131.04 | y = 27.36x + 40.39 | 0.9983 | 3845.49 | 0.97 | 0.32 | 0.85 | 0.56 | 0.81 | 99.74 |
| Retinoic acid | 7.49–13.91 | y = 330.11x + 191.91 | 0.9961 | 1641.15 | 0.12 | 0.37 | 1.81 | 1.17 | 2.57 | 101.09 |
| Spironolactone | 70.14–130.26 | y = 41.21x − 35.69 | 0.9993 | 9420.07 | 0.13 | 0.39 | 0.44 | 0.72 | 1.38 | 99.50 |
| Tranexamic acid | 145.60–270.40 | y = 31,315.34x − 82,589.51 | 0.9988 | 5499.49 | 20.01 | 6.60 | 1.52 | 1.87 | 2.91 | 99.85 |
| Active Pharmaceutical Ingredient | HCl (%d) | NaOH (%d) | UV (%d) | Heat (%d) | H2O2 (%d) |
|---|---|---|---|---|---|
| Benzoyl peroxide | 29.99 | −99.54 | 6.09 | −93.40 | 22.10 |
| Cyproterone acetate | −6.38 | −99.34 | 8.11 | 6.61 | 5.58 |
| Estriol | 147.94 | 163.85 | 3.23 | 609.02 | 12.57 |
| Metronidazole | 11.57 | −99.97 | 16.86 | −6.67 | 12.29 |
| Niacinamide | −92.17 | −86.23 | 25.70 | −6.11 | −3.23 |
| Progesterone | 8.91 | −68.25 | −0.89 | 11.36 | 4.29 |
| Retinoic acid | −31.76 | 0.99 | −47.85 | −27.78 | −7.05 |
| Spironolactone | −1.22 | −96.74 | −9.42 | −2.79 | 6.98 |
| Tranexamic acid | −5.54 | −78.56 | −8.78 | −7.17 | 3.54 |
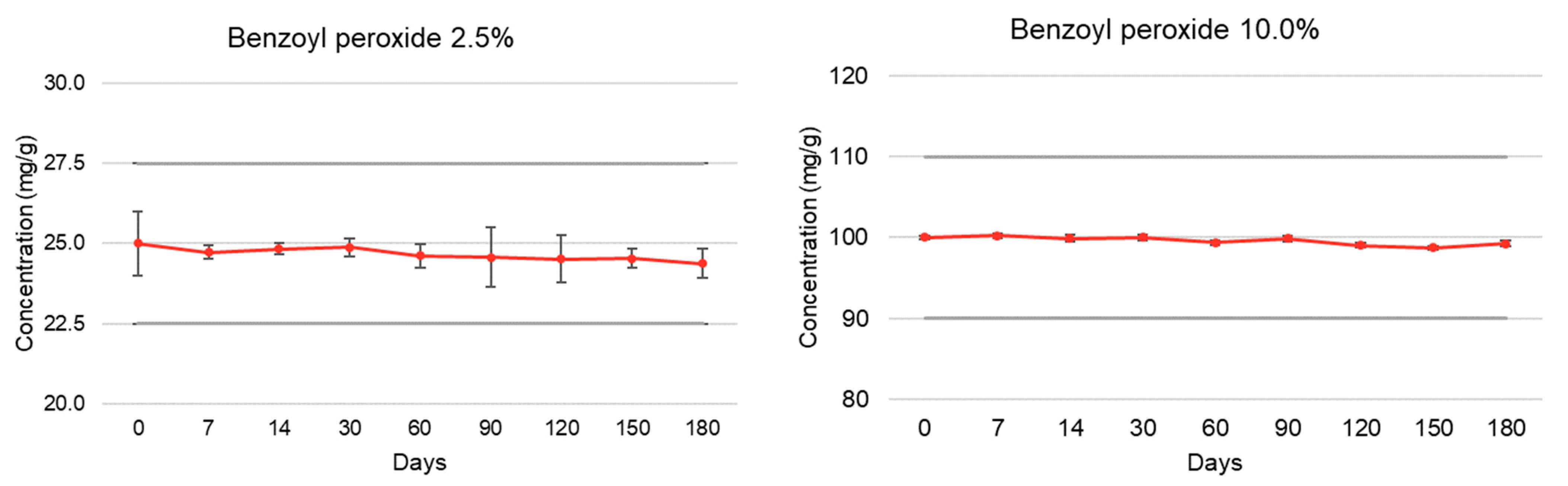
| Active Pharmaceutical Ingredient | Elapsed Time (Days) | % Recovery (Room Temperature, 15–30 °C) | |||
|---|---|---|---|---|---|
| Low Concentration | pH | High Concentration | pH | ||
| Benzoyl peroxide | T = 0 | 100.00 ± 1.00 | 4.82 | 100.00 ± 0.19 | 4.72 |
| (2.5% and 10.0%) | T = 7 | 98.92 ± 0.20 | 4.64 | 100.22 ± 0.26 | 4.67 |
| T = 14 | 99.33 ± 0.17 | 4.63 | 99.90 ± 0.43 | 4.70 | |
| T = 30 | 99.50 ± 0.28 | 4.65 | 100.00 ± 0.30 | 4.69 | |
| T = 60 | 98.48 ± 0.37 | 4.68 | 99.36 ± 0.28 | 4.52 | |
| T = 90 | 98.30 ± 0.94 | 4.67 | 99.88 ± 0.35 | 4.48 | |
| T = 120 | 98.06 ± 0.74 | 4.63 | 99.03 ± 0.30 | 4.51 | |
| T = 150 | 98.15 ± 0.30 | 4.66 | 98.72 ± 0.19 | 4.50 | |
| T = 180 | 97.52 ± 0.46 | 4.65 | 99.23 ± 0.37 | 4.51 | |
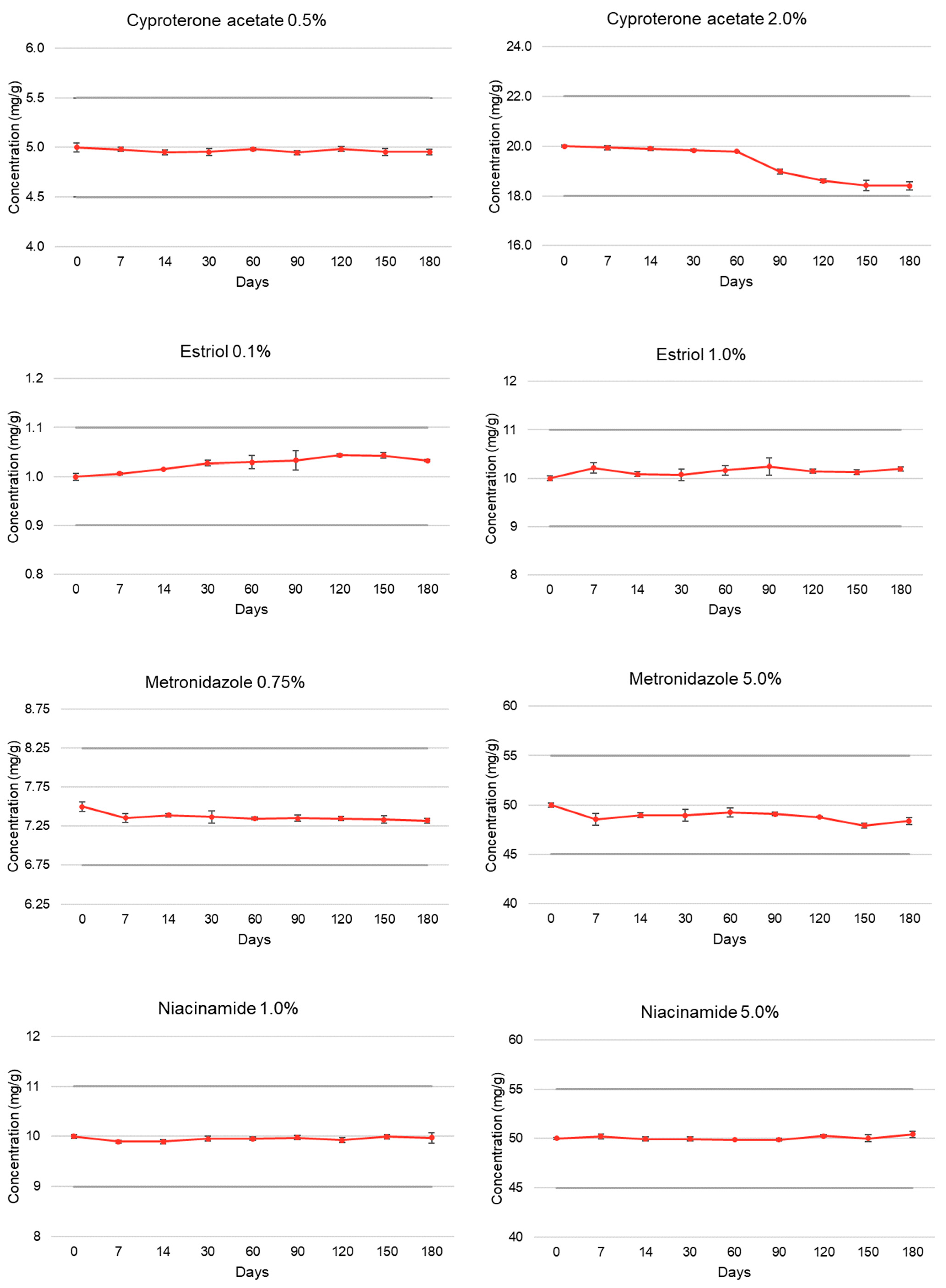
| Cyproterone acetate | T = 0 | 100.00 ± 0.85 | 5.01 | 100.00 ± 0.22 | 5.01 |
| (0.5% and 2.0%) | T = 7 | 99.60 ± 0.44 | 5.06 | 99.73 ± 0.44 | 5.03 |
| T = 14 | 99.06 ± 0.52 | 5.04 | 99.51 ± 0.36 | 5.01 | |
| T = 30 | 99.11 ± 0.67 | 5.07 | 99.19 ± 0.24 | 5.06 | |
| T = 60 | 99.65 ± 0.26 | 4.98 | 98.94 ± 0.07 | 4.92 | |
| T = 90 | 98.95 ± 0.40 | 4.44 | 94.91 ± 0.52 | 4.94 | |
| T = 120 | 99.69 ± 0.47 | 4.99 | 93.06 ± 0.41 | 4.94 | |
| T = 150 | 99.09 ± 0.77 | 4.99 | 92.13 ± 1.13 | 4.94 | |
| T = 180 | 99.10 ± 0.52 | 4.99 | 92.09 ± 0.90 | 4.94 | |
| Estriol | T = 0 | 100.00 ± 0.70 | 5.15 | 100.00 ± 0.53 | 5.13 |
| (0.1% and 1.0%) | T = 7 | 100.63 ± 0.21 | 5.09 | 102.13 ± 1.09 | 5.05 |
| T = 14 | 101.53 ± 0.06 | 5.15 | 100.84 ± 0.44 | 5.20 | |
| T = 30 | 102.74 ± 0.59 | 5.07 | 100.72 ± 1.18 | 5.11 | |
| T = 60 | 102.94 ± 1.33 | 5.13 | 101.67 ± 0.94 | 5.17 | |
| T = 90 | 103.31 ± 1.88 | 5.14 | 102.43 ± 1.73 | 5.15 | |
| T = 120 | 104.32 ± 0.27 | 5.17 | 101.48 ± 0.40 | 5.09 | |
| T = 150 | 104.30 ± 0.53 | 5.06 | 101.24 ± 0.49 | 5.06 | |
| T = 180 | 103.23 ± 0.20 | 5.09 | 101.95 ± 0.43 | 5.05 | |
| Metronidazole | T = 0 | 100.00 ± 0.82 | 5.13 | 100.00 ± 0.41 | 5.14 |
| (0.75% and 5.0%) | T = 7 | 98.07 ± 0.75 | 5.16 | 97.08 ± 1.26 | 5.15 |
| T = 14 | 98.57 ± 0.31 | 5.21 | 97.89 ± 0.49 | 5.23 | |
| T = 30 | 98.25 ± 1.08 | 5.18 | 97.88 ± 1.24 | 5.20 | |
| T = 60 | 98.00 ± 0.19 | 5.12 | 98.49 ± 0.92 | 5.11 | |
| T = 90 | 98.06 ± 0.56 | 5.08 | 98.18 ± 0.37 | 5.11 | |
| T = 120 | 98.00 ± 0.34 | 5.09 | 97.51 ± 0.14 | 5.11 | |
| T = 150 | 97.80 ± 0.64 | 5.10 | 95.80 ± 0.56 | 5.13 | |
| T = 180 | 97.62 ± 0.45 | 5.12 | 96.72 ± 0.76 | 5.12 | |
| Niacinamide | T = 0 | 100.00 ± 0.37 | 5.38 | 100.00 ± 0.21 | 5.39 |
| (1.0% and 5.0%) | T = 7 | 98.99 ± 0.27 | 5.22 | 100.42 ± 0.53 | 5.50 |
| T = 14 | 98.95 ± 0.48 | 5.24 | 99.90 ± 0.39 | 5.51 | |
| T = 30 | 99.57 ± 0.43 | 5.26 | 99.91 ± 0.42 | 5.52 | |
| T = 60 | 99.55 ± 0.34 | 5.22 | 99.72 ± 0.12 | 5.42 | |
| T = 90 | 99.76 ± 0.46 | 5.23 | 99.76 ± 0.32 | 5.50 | |
| T = 120 | 99.26 ± 0.52 | 5.25 | 100.54 ± 0.21 | 5.51 | |
| T = 150 | 99.94 ± 0.45 | 5.22 | 100.05 ± 0.67 | 5.53 | |
| T = 180 | 99.72 ± 1.05 | 5.25 | 100.84 ± 0.57 | 5.50 | |
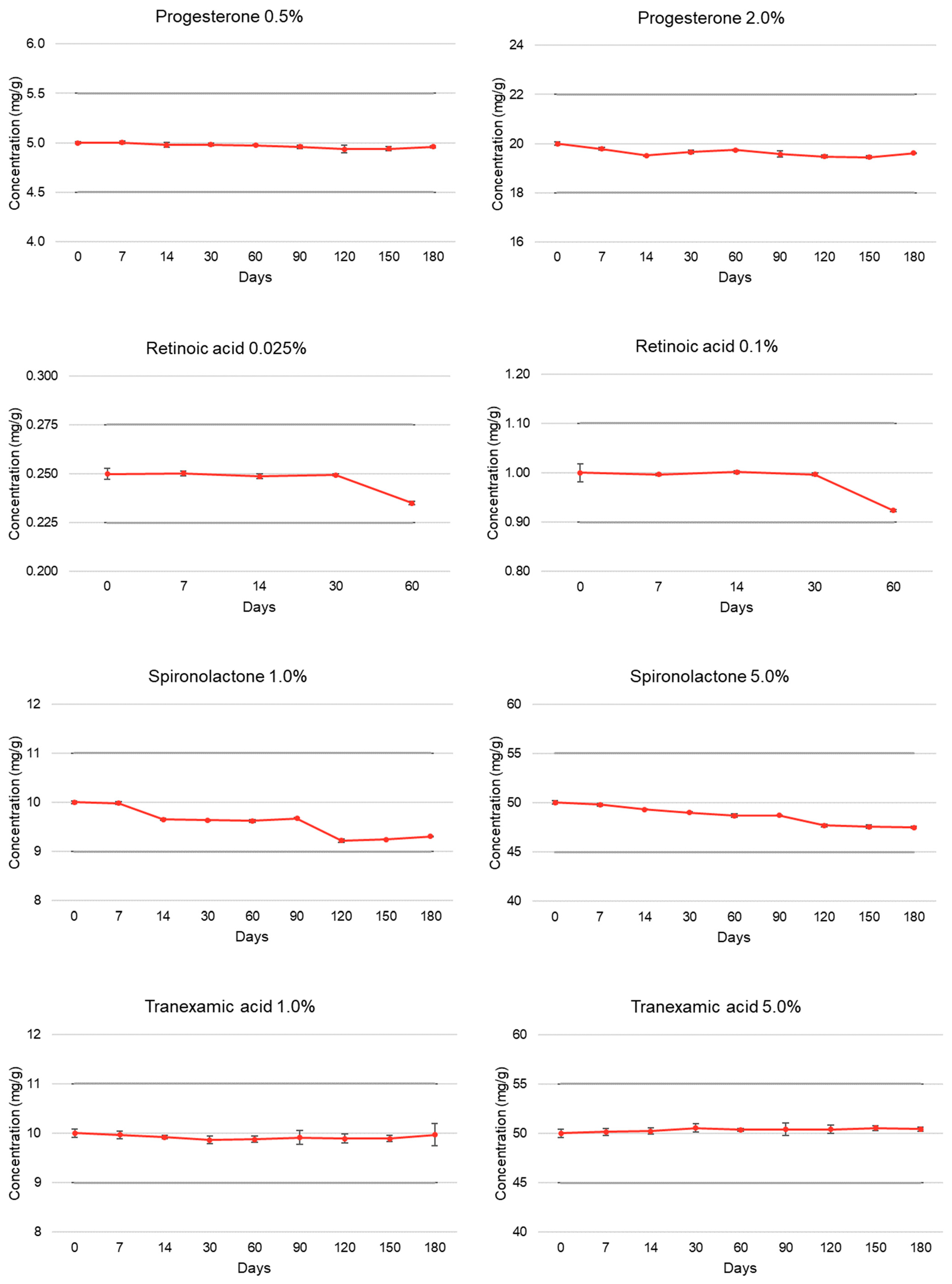
| Progesterone | T = 0 | 100.00 ± 0.26 | 5.18 | 100.00 ± 0.32 | 4.98 |
| (0.5% and 2.0%) | T = 7 | 100.05 ± 0.27 | 5.16 | 98.91 ± 0.30 | 4.97 |
| T = 14 | 99.58 ± 0.49 | 5.18 | 97.58 ± 0.19 | 4.97 | |
| T = 30 | 99.64 ± 0.31 | 5.23 | 98.31 ± 0.37 | 4.55 | |
| T = 60 | 99.48 ± 0.13 | 5.21 | 98.71 ± 0.11 | 4.94 | |
| T = 90 | 99.22 ± 0.34 | 5.13 | 97.87 ± 0.64 | 4.95 | |
| T = 120 | 98.73 ± 0.76 | 5.14 | 97.41 ± 0.31 | 4.91 | |
| T = 150 | 98.82 ± 0.38 | 5.12 | 97.24 ± 0.32 | 4.93 | |
| T = 180 | 99.18 ± 0.20 | 4.96 | 98.09 ± 0.06 | 5.06 | |
| Retinoic acid | T = 0 | 100.00 ± 1.11 | 5.09 | 100.00 ± 1.81 | 5.10 |
| (0.025% and 0.1%) | T = 7 | 100.08 ± 0.44 | 4.94 | 99.64 ± 0.19 | 5.05 |
| T = 14 | 99.49 ± 0.52 | 4.91 | 100.16 ± 0.31 | 5.03 | |
| T = 30 | 99.78 ± 0.24 | 4.90 | 99.70 ± 0.27 | 5.04 | |
| T = 60 | 94.00 ± 0.36 * | 5.01 | 92.35 ± 0.28 * | 4.90 | |
| Spironolactone | T = 0 | 100.00 ± 0.28 | 5.12 | 100.00 ± 0.36 | 5.08 |
| (1.0% and 5.0%) | T = 7 | 99.79 ± 0.29 | 5.15 | 99.60 ± 0.32 | 5.04 |
| T = 14 | 96.52 ± 0.26 | 5.12 | 98.63 ± 0.09 | 5.09 | |
| T = 30 | 96.39 ± 0.13 | 5.13 | 98.02 ± 0.10 | 5.07 | |
| T = 60 | 96.21 ± 0.31 | 5.06 | 97.34 ± 0.36 | 5.09 | |
| T = 90 | 96.74 ± 0.10 | 5.07 | 97.39 ± 0.12 | 5.05 | |
| T = 120 | 92.19 ± 0.35 | 5.09 | 95.39 ± 0.30 | 5.03 | |
| T = 150 | 92.46 ± 0.12 | 5.07 | 95.13 ± 0.36 | 5.05 | |
| T = 180 | 93.10 ± 0.06 | 5.09 | 94.96 ± 0.21 | 5.06 | |
| Tranexamic acid | T = 0 | 100.00 ± 0.87 | 5.91 | 100.00 ± 0.85 | 6.38 |
| (1.0% and 5.0%) | T = 7 | 99.68 ± 0.77 | 5.83 | 100.31 ± 0.68 | 6.52 |
| T = 14 | 99.20 ± 0.38 | 5.79 | 100.45 ± 0.64 | 6.45 | |
| T = 30 | 98.61 ± 0.76 | 5.69 | 101.11 ± 0.79 | 6.43 | |
| T = 60 | 98.78 ± 0.64 | 5.59 | 100.75 ± 0.28 | 6.43 | |
| T = 90 | 99.14 ± 1.38 | 5.51 | 100.83 ± 1.31 | 6.41 | |
| T = 120 | 98.92 ± 0.97 | 5.53 | 100.82 ± 0.88 | 6.46 | |
| T = 150 | 98.90 ± 0.65 | 5.49 | 101.05 ± 0.53 | 6.44 | |
| T = 180 | 99.69 ± 2.25 | 5.55 | 100.87 ± 0.40 | 6.41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonini, H.; Marianni, B.; Taylor, S.; Zander, C. Compatibility of Personalized Formulations in Cleoderm™, A Skin Rebalancing Cream Base for Oily and Sensitive Skin. Cosmetics 2022, 9, 92. https://doi.org/10.3390/cosmetics9050092
Polonini H, Marianni B, Taylor S, Zander C. Compatibility of Personalized Formulations in Cleoderm™, A Skin Rebalancing Cream Base for Oily and Sensitive Skin. Cosmetics. 2022; 9(5):92. https://doi.org/10.3390/cosmetics9050092
Chicago/Turabian StylePolonini, Hudson, Bruna Marianni, Sarah Taylor, and Clark Zander. 2022. "Compatibility of Personalized Formulations in Cleoderm™, A Skin Rebalancing Cream Base for Oily and Sensitive Skin" Cosmetics 9, no. 5: 92. https://doi.org/10.3390/cosmetics9050092




