Role of SrCO3 on Photocatalytic Performance of SrTiO3-SrCO3 Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure, Morphology, and Textural Properties
2.2. Optical Properties
2.3. Photocatalytic Results
2.3.1. Photocatalytic Diclofenac Degradation
2.3.2. Photocatalytic CO2 Reduction Experiments
3. Experimental Materials and Methods
3.1. Chemicals
3.2. Material Synthesis
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solís, R.R.; Bedia, J.; Rodríguez, J.J.; Belver, C. A review on alkaline earth metal titanates for applications in photocatalytic water purification. Chem. Eng. J. 2021, 409, 128110. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.-I.; Eda, T.; Hanaya, M. Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Wu, G.; Li, P.; Xu, D.; Luo, B.; Hong, Y.; Shi, W.; Liu, C. Hydrothermal synthesis and visible-light-driven photocatalytic degradation for tetracycline of Mn-doped SrTiO3 nanocubes. Appl. Surf. Sci. 2015, 333, 39–47. [Google Scholar] [CrossRef]
- Chou, H.-L.; Hwang, B.-J.; Sun, C.-L. Catalysis in Fuel Cells and Hydrogen Production. In New and Future Developments in Catalysis: Batteries, Hydrogen Storage and Fuel Cells; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 219–263. [Google Scholar]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Canu, G.; Buscaglia, V. Hydrothermal synthesis of strontium titanate: Thermodynamic considerations, morphology control and crystallisation mechanisms. CrystEngComm 2017, 19, 3867–3891. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; Tong, Z.; Nan, Y.; Jiang, Z. Fabrication of bimodal-pore SrTiO3 microspheres with excellent photocatalytic performance for Cr(VI) reduction under simulated sunlight. J. Hazard. Mater. 2016, 312, 45–54. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, J.; Li, Y.; Zhao, W.; Zeng, Y.; Wang, C. Photocatalytic CO2 reduction over SrTiO3: Correlation between surface structure and activity. Appl. Surf. Sci. 2018, 447, 627–635. [Google Scholar] [CrossRef]
- Sakata, Y.; Miyoshi, Y.; Maeda, T.; Ishikiriyama, K.; Yamazaki, Y.; Imamura, H.; Ham, Y.; Hisatomi, T.; Kubota, J.; Yamakata, A.; et al. Photocatalytic property of metal ion added SrTiO3 to overall H2O splitting. Appl. Catal. A Gen. 2016, 521, 227–232. [Google Scholar] [CrossRef]
- Huang, S.-T.; Lee, W.W.; Chang, J.-L.; Huang, W.-S.; Chou, S.-Y.; Chen, C.-C. Hydrothermal synthesis of SrTiO3 nanocubes: Characterization, photocatalytic activities, and degradation pathway. J. Taiwan Inst. Chem. Eng. 2014, 45, 1927–1936. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, K.; Wang, J. Perovskites for photovoltaics: A combined review of organic–inorganic halide perovskites and ferroelectric oxide perovskites. J. Mater. Chem. A 2015, 3, 18809–18828. [Google Scholar] [CrossRef]
- Ricci, D.; Bano, G.; Pacchioni, G.; Illas, F. Electronic structure of a neutral oxygen vacancy in SrTiO3. Phys. Rev. B 2003, 68, 224105. [Google Scholar] [CrossRef]
- Raisch, C.; Chassé, T.; Langheinrich, C.; Chassé, A. Preparation and investigation of the A-site and B-site terminated SrTiO3(001) surface: A combined experimental and theoretical x-ray photoelectron diffraction study. J. Appl. Phys. 2012, 112, 073505. [Google Scholar] [CrossRef]
- Shao, K.; Wang, Y.; Iqbal, M.; Lin, L.; Wang, K.; Zhang, X.; He, M.; He, T. Modification of Ag nanoparticles on the surface of SrTiO3 particles and resultant influence on photoreduction of CO2. Appl. Surf. Sci. 2018, 434, 717–724. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Wei, Y.; Xiong, J.; Zhao, Y.; Zhao, Z.; Liu, J.; Li, J. Multifunctional photocatalysts of Pt-decorated 3DOM perovskite-type SrTiO3 with enhanced CO2 adsorption and photoelectron enrichment for selective CO2 reduction with H2O to CH4. J. Catal. 2019, 377, 309–321. [Google Scholar] [CrossRef]
- Lv, X.; Lam, F.L.-Y.; Hu, X. Developing SrTiO3/TiO2 heterostructure nanotube array for photocatalytic fuel cells with improved efficiency and elucidating the effects of organic substrates. Chem. Eng. J. 2022, 427, 131602. [Google Scholar] [CrossRef]
- Ganapathy, M.; Hsu, Y.; Thomas, J.; Chen, L.-Y.; Chang, C.-T.; Alagan, V. Preparation of SrTiO3/Bi2S3 heterojunction for efficient photocatalytic hydrogen production. Energy Fuels 2021, 35, 14995–15004. [Google Scholar] [CrossRef]
- Jin, S.; Dong, G.; Luo, J.; Ma, F.; Wang, C. Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst. Appl. Catal. B Environ. 2018, 227, 24–34. [Google Scholar] [CrossRef]
- Pan, X.; Chen, X.; Yi, Z. Photocatalytic oxidation of methane over SrCO3 decorated SrTiO3 nanocatalysts via a synergistic effect. Phys. Chem. Chem. Phys. 2016, 18, 31400–31409. [Google Scholar] [CrossRef]
- Deng, Y.; Shu, S.; Fang, N.; Wang, R.; Chu, Y.; Liu, Z.; Cen, W. One-pot synthesis of SrTiO3-SrCO3 heterojunction with strong interfacial electronic interaction as a novel photocatalyst for water splitting to generate H2. Chin. Chem. Lett. 2022, in press. [Google Scholar] [CrossRef]
- Márquez-Herrera, A.; Ovando-Medina, V.M.; Castillo-Reyes, B.E.; Melendez-Lira, M.; Zapata-Torres, M.; Saldana, N. A novel synthesis of SrCO3–SrTiO3 nanocomposites with high photocatalytic activity. J. Nanopart. Res. 2014, 16, 2804. [Google Scholar] [CrossRef]
- Arai, T.; Sato, S.; Kajino, T.; Morikawa, T. Solar CO2 reduction using H2O by a semiconductor/metal-complex hybrid photocatalyst: Enhanced efficiency and demonstration of a wireless system using SrTiO3 photoanodes. Energy Environ. Sci. 2013, 6, 1274–1282. [Google Scholar] [CrossRef]
- Ali, K.; Bahadur, A.; Jabbar, A.; Iqbal, S.; Bashir, M.I. Synthesis, structural, dielectric and magnetic properties of CuFe2O4/MnO2 nanocomposite. J. Magn. Magn. Mater. 2017, 434, 30–36. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, L.; Li, Y.; Yang, R.; Qu, J.; Li, Y.; Li, X. Synthesis and high photocatalytic hydrogen production of SrTiO3 nanoparticles from water splitting under UV irradiation. J. Power Sources 2008, 183, 701–707. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Han, Y.; Chen, B.; Li, X. Formation mechanisms of SrTiO3 nanoparticles under hydrothermal conditions. Mater. Sci. Eng. B 2004, 110, 11–17. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Wang, L.; Xue, D.; Yin, S. Preparation and photocatalytic properties of strontium titanate powders via sol–gel process. J. Cryst. Growth 2009, 311, 746–748. [Google Scholar] [CrossRef]
- Wang, T.X.; Shuang, Z.L.; Jun, C. Molten salt synthesis of SrTiO3 nanocrystals using nanocrystalline TiO2 as a precursor. Powder Technol. 2011, 205, 289–291. [Google Scholar] [CrossRef]
- Hou, D.; Hu, X.; Ho, W.; Hu, P.; Huang, Y. Facile fabrication of porous Cr-doped SrTiO3 nanotubes by electrospinning and their enhanced visible-light-driven photocatalytic properties. J. Mater. Chem. A 2015, 3, 3935–3943. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Thomson Brooks/Cole: Belmont, MA, USA, 2004. [Google Scholar]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.; Chen, W. A review of synthesis and morphology of SrTiO3 for energy and other applications. Int. J. Energy Res. 2019, 43, 5151–5174. [Google Scholar] [CrossRef]
- Lim, P.F.; Leong, K.H.; Sim, L.C.; Oh, W.-D.; Chin, Y.H.; Saravanan, P.; Dai, C. Mechanism insight of dual synergistic effects of plasmonic Pd-SrTiO3 for enhanced solar energy photocatalysis. Appl. Phys. A 2020, 126, 550. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynskaa, A. TiO2/SrTiO3 and SrTiO3 microspheres decorated with Rh, Ru or Pt nanoparticles: Highly UV–vis responsible photoactivity and mechanism. J. Catal. 2017, 350, 159–173. [Google Scholar] [CrossRef]
- Marchelek, M.; Grabowska, E.; Klimczuk, T.; Lisowski, W.; Giamello, E.; Zaleska-Medynska, A. Studies on novel BiyXz-TiO2/SrTiO3 composites: Surface properties and visible light-driven photoactivity. Appl. Surf. Sci. 2018, 435, 1174–1186. [Google Scholar] [CrossRef]
- Lencka, M.M.; Riman, R.E. Hydrothermal synthesis of perovskite materials: Thermodynamic modeling and experimental verification. Ferroelectrics 1994, 151, 159–164. [Google Scholar] [CrossRef]
- Kalyani, V.; Vasile, B.S.; Ianculescu, A.; Testino, A.; Carino, A.; Buscaglia, M.T.; Buscaglia, V.; Nanni, P. Hydrothermal Synthesis of SrTiO3: Role of Interfaces. Cryst. Growth Des. 2015, 15, 5712–5725. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Ren, Z.; Ahmad, Z.; Li, X.; Han, G. Synthesis of porous CaTiO3 nanotubes with tunable hollow structures via single-nozzle electrospinning. Mater. Lett. 2015, 152, 82–85. [Google Scholar] [CrossRef]
- Jiang, D.; Sun, X.; Wu, X.; Shi, L.; Du, F. Hydrothermal synthesis of single-crystal Cr-doped SrTiO3 for efficient visible-light responsive photocatalytic hydrogen evolution. Mater. Res. Express 2019, 7, 015047. [Google Scholar] [CrossRef]
- Ramos, B.; Ookawara, S.; Matsushita, Y.; Yoshikawa, S. Low-cost polymeric photocatalytic microreactors: Catalyst deposition and performance for phenol degradation. J. Environ. Chem. Eng. 2014, 2, 1487–1494. [Google Scholar] [CrossRef]
- Kutty, T.; Vivekanandan, R.; Murugaraj, P. Precipitation of rutile and anatase (Tio12) fine powders and their conversion to MTiO3 (M = Ba, Sr, Ca) by the hydrothermal method. Mater. Chem. Phys. 1988, 19, 533–546. [Google Scholar] [CrossRef]
- Van Benthem, K.; Elsässer, C.; French, R.H. Bulk electronic structure of SrTiO3: Experiment and theory. J. Appl. Phys. 2001, 90, 6156–6164. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.M.; Liu, G.G.; Chen, X.L.; Jiang, K. Photodegradation of diclofenac in aqueous solution by simulated sunlight irradiation: Kinetics, thermodynamics and pathways. Water Sci. Technol. 2017, 75, 2163–2170. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Nakamura, Y.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: Laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009, 43, 351–362. [Google Scholar] [CrossRef]
- Vu, V.T.; Bartling, S.; Peppel, T.; Lund, H.; Kreyenschulte, C.; Rabeah, J.; Moustakas, N.G.; Surkus, A.-E.; Ta, H.D.; Steinfeldt, N. Enhanced photocatalytic performance of polymeric carbon nitride through combination of iron loading and hydrogen peroxide treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 589, 124383. [Google Scholar] [CrossRef]
- Villamena, F.A. EPR Spin Trapping, in Reactive Species Detection in Biology—From Fluorescence to Electron Paramagnetic Resonance Spectroscopy; Elsevier: Amesterdam, The Netherlands, 2017; pp. 163–202. [Google Scholar]
- Vione, D.; Khanra, S.; Man, S.C.; Maddigapu, P.R.; Das, R.; Arsene, C.; Olariu, R.-I.; Maurino, V.; Minero, C. Inhibition vs. enhancement of the nitrate-induced phototransformation of organic substrates by the •OH scavengers bicarbonate and carbonate. Water Res. 2009, 43, 4718–4728. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Thomas, J. Photochemical reduction of carbonate to formaldehyde on TiO2 powder. Chem. Phys. Lett. 1983, 99, 7–10. [Google Scholar] [CrossRef]
- Bhatt, C.R.; Jain, J.C.; Edenborn, H.M.; McIntyre, D.L. Mineral carbonate dissolution with increasing CO2 pressure measured by underwater laser induced breakdown spectroscopy and its application in carbon sequestration. Talanta 2019, 205, 120170. [Google Scholar] [CrossRef]
- Staykov, A.; Fukumori, S.; Yoshizawa, K.; Sato, K.; Ishihara, T.; Kilnerad, J. Interaction of SrO-terminated SrTiO3 surface with oxygen, carbon dioxide, and water. J. Mater. Chem. A 2018, 6, 22662–22672. [Google Scholar] [CrossRef]
- Kosmulski, M. pH-dependent surface charging and points of zero charge. IV. Update and new approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, P.; Zhang, W.; Gong, S.; Zhu, L.; Xu, J.; Rao, F.; Xie, X.; Zhu, G. Constructing SrCO3/SrTiO3 nanocomposites with highly selective photocatalytic CO2-to-CO reduction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129686. [Google Scholar] [CrossRef]
- Gyulavári, T.; Dusnoki, D.; Márta, V.; Yadav, M.; Abedi, M.; Sápi, A.; Kukovecz, Á.; Kónya, Z.; Pap, Z. Dependence of Photocatalytic Activity on the Morphology of Strontium Titanates. Catalysts 2022, 12, 523. [Google Scholar] [CrossRef]
- Handoko, C.T.; Moustakas, N.G.; Peppel, T.; Springer, A.; Oropeza, F.E.; Huda, A.; Bustan, M.D.; Bambang, Y.; Gulo, F.; Strunk, J. Characterization and effect of Ag (0) vs. Ag (I) species and their localized plasmon resonance on photochemically inactive TiO2. Catalysts 2019, 9, 323. [Google Scholar] [CrossRef] [Green Version]
- Pougin, A.; Dilla, M.; Strunk, J. Identification and exclusion of intermediates of photocatalytic CO2 reduction on TiO2 under conditions of highest purity. Phys. Chem. Chem. Phys. 2016, 18, 10809–10817. [Google Scholar] [CrossRef]
- Dimitrijevic, N.M.; Vijayan, B.K.; Poluektov, O.G.; Rajh, T.; Gray, K.A.; He, H.; Zapol, P. Role of water and carbonates in photocatalytic transformation of CO2 to CH4 on titania. J. Am. Chem. Soc. 2011, 133, 3964–3971. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Strunk, J. Requirements for efficient metal oxide photocatalysts for CO2 reduction. In Metal Oxides in Energy Technologies; Wu, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 275–301. [Google Scholar]
- Moustakas, N.G. Strategic design and evaluation of metal oxides for photocatalytic CO2 reduction. In Materials Science in Photocatalysis; García-López, E.I., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 255–265. [Google Scholar]
- Sipos, P.; May, P.M.; Hefter, G.T. Carbonate removal from concentrated hydroxide solutions. Analyst 2000, 125, 955–958. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 02, 154–160. [Google Scholar] [CrossRef]
- Marschall, R.; Mukherji, A.; Tanksale, A.; Sun, C.; Smith, S.C.; Wang, L.; Lu, M. Preparation of new sulfur-doped and sulfur/nitrogen co-doped CsTaWO6 photocatalysts for hydrogen production from water under visible light. J. Mater. Chem. 2011, 21, 8871–8879. [Google Scholar] [CrossRef]
- Mei, B.; Pougin, A.; Strunk, J. Influence of photodeposited gold nanoparticles on the photocatalytic activity of titanate species in the reduction of CO2 to hydrocarbons. J. Catal. 2013, 306, 184–189. [Google Scholar] [CrossRef]
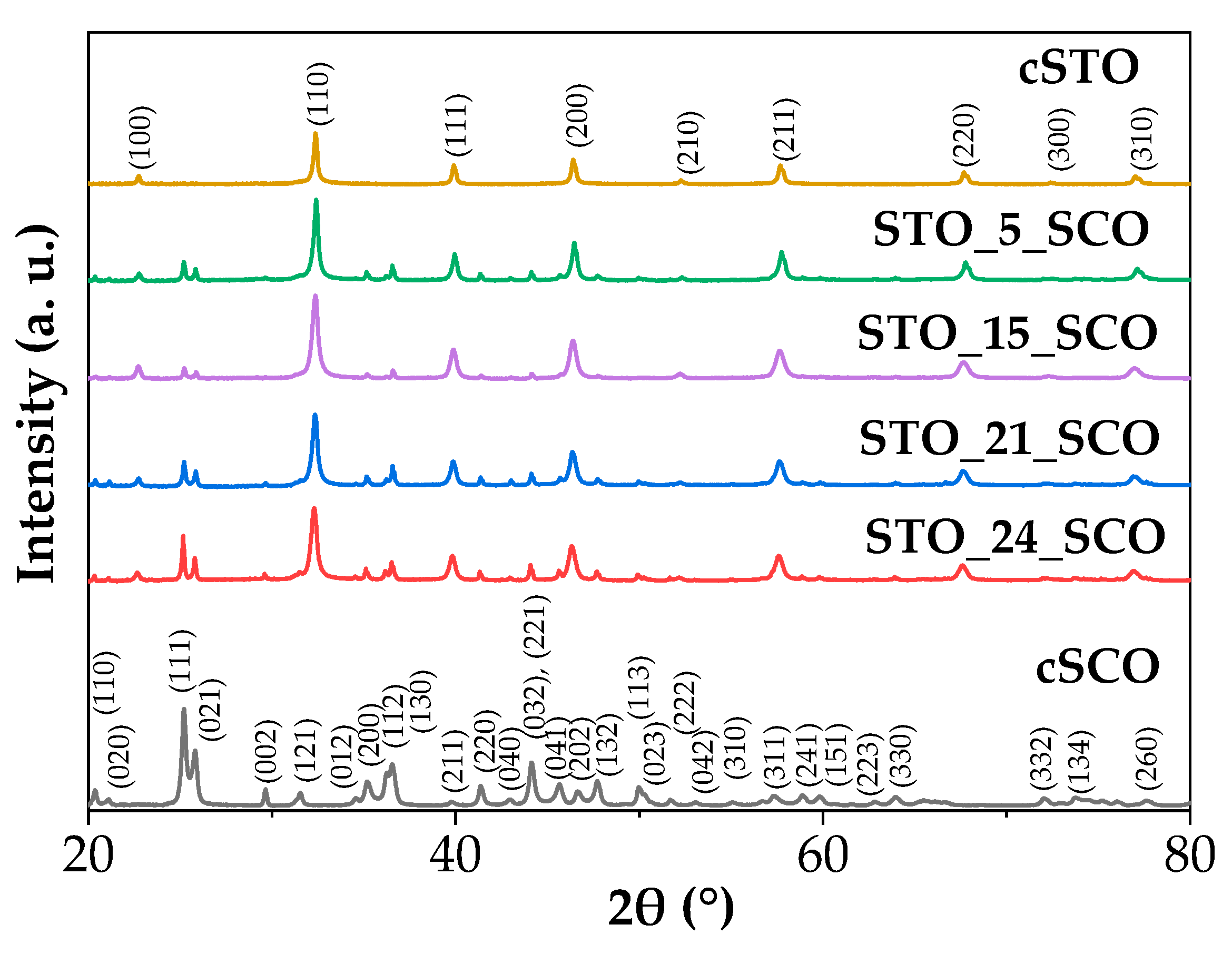


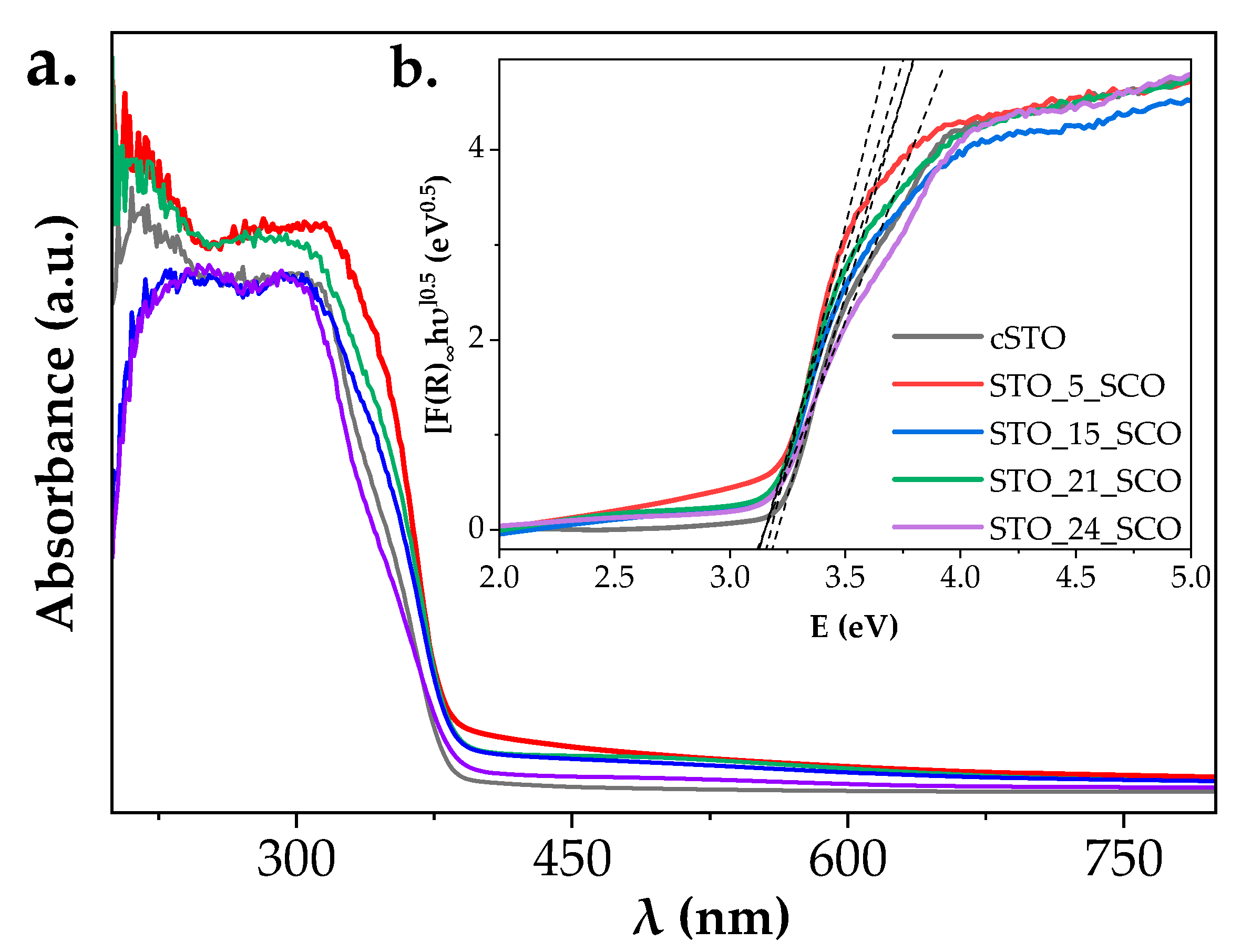


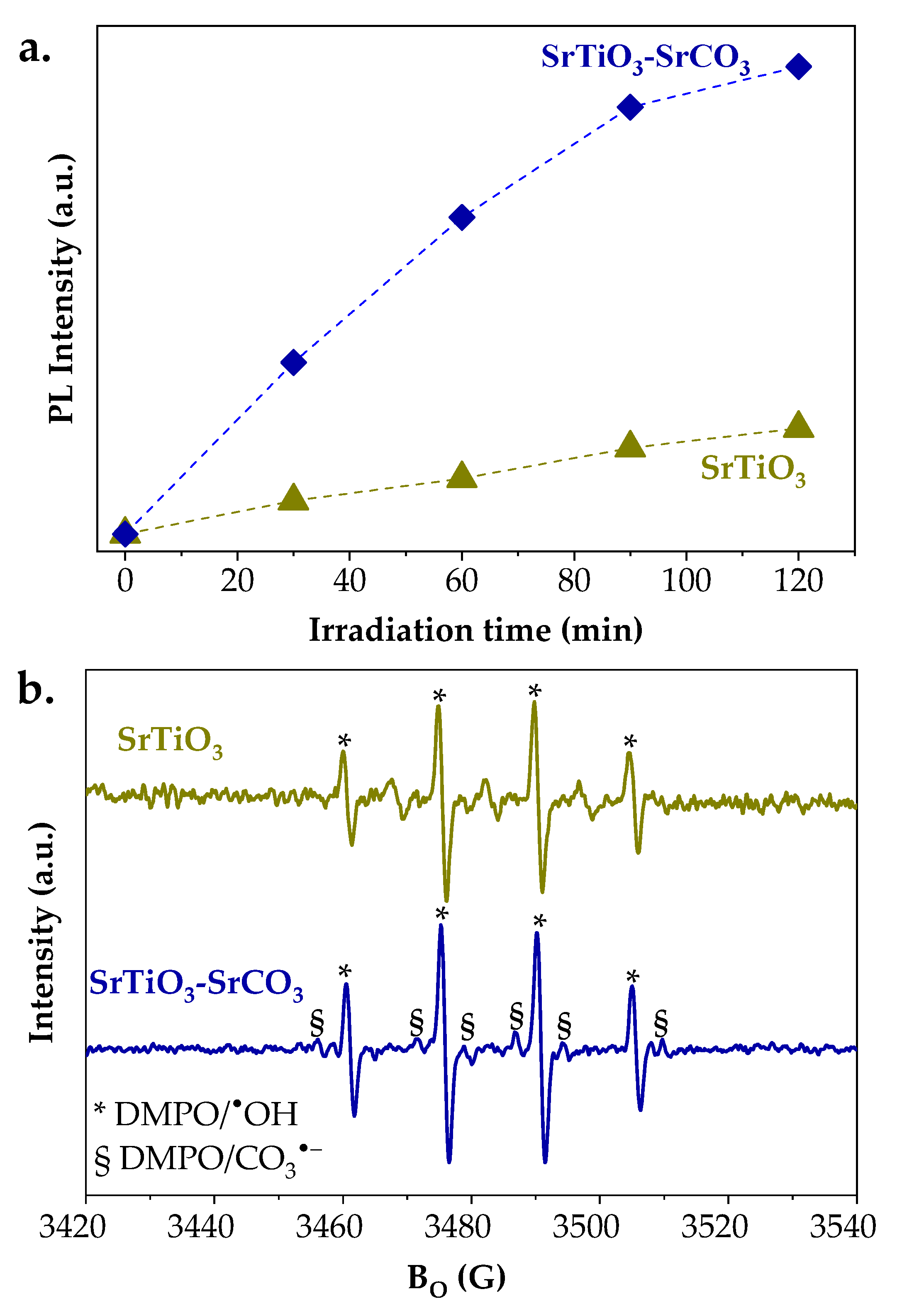
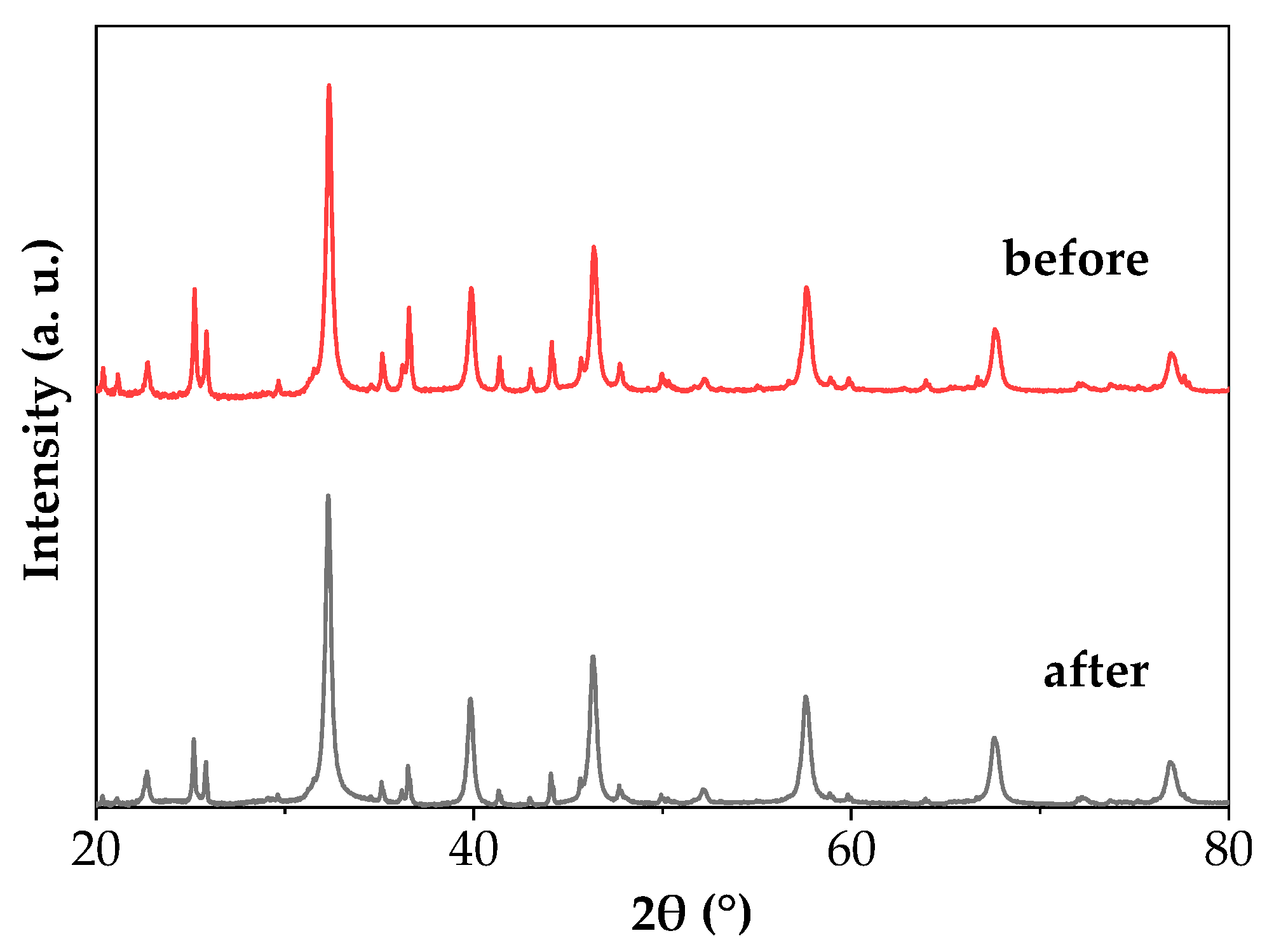

| No. | Samples | Strategy/Observation | PCSSTO (nm) | PCSSCO (nm) |
|---|---|---|---|---|
| 1 | cSTO | — | 28 | — |
| 2 | STO_5_SCO | Preliminary purification of KOH and Schlenk line technique | 18 | 49 |
| 3 | STO_15_SCO | Starting synthesis | 25 | 55 |
| 4 | STO_21_SCO | rn(Sr2+:Ti4+) = 1.25 | 20 | 54 |
| 5 | STO_24_SCO | Modification of Ti4+ source | 19 | 57 |
| Samples | SSA-BET (m2∙g−1) | ΔEg (eV) | λthres (nm) | Ethres (eV) |
|---|---|---|---|---|
| cSTO | 15 | 3.23 | 374 | 3.32 |
| STO_5_SCO | 16 | 3.18 | 371 | 3.34 |
| STO_15_SCO | 45 | 3.16 | 378 | 3.28 |
| STO_21_SCO | 33 | 3.18 | 379 | 3.27 |
| STO_24_SCO | 41 | 3.16 | 377 | 3.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boga, B.; Steinfeldt, N.; Moustakas, N.G.; Peppel, T.; Lund, H.; Rabeah, J.; Pap, Z.; Cristea, V.-M.; Strunk, J. Role of SrCO3 on Photocatalytic Performance of SrTiO3-SrCO3 Composites. Catalysts 2022, 12, 978. https://doi.org/10.3390/catal12090978
Boga B, Steinfeldt N, Moustakas NG, Peppel T, Lund H, Rabeah J, Pap Z, Cristea V-M, Strunk J. Role of SrCO3 on Photocatalytic Performance of SrTiO3-SrCO3 Composites. Catalysts. 2022; 12(9):978. https://doi.org/10.3390/catal12090978
Chicago/Turabian StyleBoga, Bíborka, Norbert Steinfeldt, Nikolaos G. Moustakas, Tim Peppel, Henrik Lund, Jabor Rabeah, Zsolt Pap, Vasile-Mircea Cristea, and Jennifer Strunk. 2022. "Role of SrCO3 on Photocatalytic Performance of SrTiO3-SrCO3 Composites" Catalysts 12, no. 9: 978. https://doi.org/10.3390/catal12090978
APA StyleBoga, B., Steinfeldt, N., Moustakas, N. G., Peppel, T., Lund, H., Rabeah, J., Pap, Z., Cristea, V.-M., & Strunk, J. (2022). Role of SrCO3 on Photocatalytic Performance of SrTiO3-SrCO3 Composites. Catalysts, 12(9), 978. https://doi.org/10.3390/catal12090978










