Simple Summary
This study investigated the effects of the interaction of acidification and warming on the photosynthetic apparatus and sterol metabolism of sea anemone Heteractis crispa. Thermal stress is the dominant driver of the deteriorating health of H. crispa, which might be relatively insensitive to the impact of ocean acidification; upregulation of chlorophyll content is suggested as an important strategy for symbionts to adapt to high pCO2. However, warming and acidification (alone or combined) significantly affected the cholesterol or sterol levels. Indeed, environmental changes like warming and acidification will affect the sterol metabolism and health of H. crispa in the coming decades.
Abstract
Ocean acidification and warming are two of the most important threats to the existence of marine organisms and are predicted to co-occur in oceans. The present work evaluated the effects of acidification (AC: 24 ± 0.1 °C and 900 μatm CO2), warming (WC: 30 ± 0.1 °C and 450 μatm CO2), and their combination (CC: 30 ± 0.1 °C and 900 μatm CO2) on the sea anemone, Heteractis crispa, from the aspects of photosynthetic apparatus (maximum quantum yield of photosystem II (PS II), chlorophyll level, and Symbiodiniaceae density) and sterol metabolism (cholesterol content and total sterol content). In a 15-day experiment, acidification alone had no apparent effect on the photosynthetic apparatus, but did affect sterol levels. Upregulation of their chlorophyll level is an important strategy for symbionts to adapt to high partial pressure of CO2 (pCO2). However, after warming stress, the benefits of high pCO2 had little effect on stress tolerance in H. crispa. Indeed, thermal stress was the dominant driver of the deteriorating health of H. crispa. Cholesterol and total sterol contents were significantly affected by all three stress conditions, although there was no significant change in the AC group on day 3. Thus, cholesterol or sterol levels could be used as important indicators to evaluate the impact of climate change on cnidarians. Our findings suggest that H. crispa might be relatively insensitive to the impact of ocean acidification, whereas increased temperature in the future ocean might impair viability of H. crispa.
1. Introduction
As a consequence of human activities and the burning of fossil fuels, atmospheric carbon dioxide (CO2) has increased to its highest level in past decades [], with 30% of the CO2 released into the atmosphere being absorbed by the oceans []. This has disturbed seawater carbonate chemistry and will increase acidity by 0.3–0.4 pH units by the end of the 21st century []. Meanwhile, increased anthropogenic activities have promoted emissions of greenhouse gases (especially CO2) []. Simulations by the Intergovernmental Panel on Climate Change (IPCC) have indicated a 0.3–4.8 °C increase in the average global surface temperature of the ocean by the end of the 21st century []. Marine ecosystems are very sensitive to climate change [], which is happening at an unprecedented pace that may induce adverse effects on marine organisms []. In addition to risks posed by climate change, marine ecosystems are exposed to a suite of local pollutants, including chemical pollutants, petroleum hydrocarbons, and persistent organic pollutants (POPs) [,,,]. Indeed, the combination of climate change stressors (warming, acidification, high ultraviolet radiation, etc.) and local pollutants can increase threats and risks to marine ecosystems [].
Coral reefs are among the world’s most biologically complex ecosystems, with high societal and economic values; however, they are in significant decline globally due, in part, to rapid climatic changes []. Coral cover has declined by approximately 1–5% per year [], and 70% of all tropical reefs could disappear by 2050 []. Ocean acidification and global warming are two of the most important threats to the existence of coral reefs. Studies of the effects of acidification on corals have commonly focused on calcification [,,,,], growth [,,], physiology [,,], and phenotype [,], with only a few studies reporting that acidification is associated with coral bleaching [,]. Indeed, a large body of literature has demonstrated that global warming affects the coral community [,], and its health [,,], metabolism [,,,], and calcification [,].
However, acidification and warming are predicted to co-occur in oceans [], and the combined effects of acidification and global warming might lead to a significant decrease in coral diversity []. Understanding the combined ecological risks of acidification and warming on coral populations is urgently required to predict potential shifts in coral community structure under future global climate change. Accumulating evidence has shown that complicated interactions between these two stressors would have deleterious effects on coral ecosystems [,,,]. Indeed, the combination of warming and acidification has a negative effect on coral calcification [], which likely leads to severely reduced coral diversity [,,]. In addition, the synergistic effects of global warming and acidification are highly variable among and within taxa [,,,,].
Corals and sea anemones belong to the class of anthozoans []. Reef-building corals are less-studied laboratory subjects because they grow slowly and are difficult and costly to maintain. Compared to most corals, sea anemones thrive under a wider array of conditions in the laboratory. Most importantly, their process of endosymbiosis establishment is similar to that of many reef-building corals, highlighting the relevance of the sea anemone as a model organism. The sea anemone, Heteractis crispa, exists widely throughout the tropical and subtropical waters of the Indo-Pacific region, from the eastern coasts of Africa to Polynesia and from southern Japan to Australia and New Caledonia [], providing a habitat for 14 of the 30 species of anemonefishes (also known as clownfish) []. Moreover, H. crispa requires low feeding levels for maximal growth and is highly dependent on nutrients provided by dinoflagellate endosymbionts (Symbiodiniaceae) [,,]. This characteristic is advantageous to studying the interaction between cnidarian hosts and their symbionts in this system. However, there are no data available regarding the responses of H. crispa to the interaction of acidification and global warming.
In this study, we investigated how the individual and combined effects of acidification and warming might affect the phenotype (Symbiodiniaceae density, total chlorophyll, and maximum quantum yield of photosystem II (PS II; Fv/Fm)) of H. crispa. Sterols are vital structural and regulatory components in eukaryotic cells; our previous study found that sterols, especially cholesterol, play an important role in the bleaching process of H. crispa []. Therefore, we used cholesterol and total sterol as important biomolecules to evaluate the sterol metabolic response of H. crispa to climate change [,].
2. Materials and Methods
2.1. Growth Conditions, Acclimation, and Treatments
Sea anemones (H. crispa) were obtained from the Aquarium of Yunlong Town (Haikou, China). The growth conditions and acclimation methods of H. crispa were similar to those in our previous study []. In brief, sea anemones were maintained in artificial 35‰ seawater in a recirculation aquaculture system (120 × 50 × 60 cm) under controlled conditions (24 ± 0.1 °C, 16-h/8-h light/dark cycles with approximately 100 µmol·photons·m−2·s−1 light during light phases). Seawater was prepared using sea salt (Reef crystals, Lorraine, France) and filtered through 1 µm pore size filters. Healthy cultures were transferred into fresh media and acclimated for at least half a month before the experiments were performed. Animals were fed with brine shrimp weekly and then starved for approximately 7 days before the experiments to avoid sample contamination by food metabolites. Sea anemones that were not subjected to stresses served as controls.
To investigate the interactive effects of acidification and warming on H. crispa, we experimentally manipulated the partial pressure of carbon dioxide (pCO2) and temperature within indoor mesocosms. Increased CO2 and temperature levels were chosen based on the IPCC scenario WG RCP 8.5 []. Specifically, conditions used were control conditions (Control: 24 ± 0.1 °C and 450 μatm CO2); acidification conditions (AC: 24 ± 0.1 °C and 900 μatm CO2); warming conditions (WC: 30 ± 0.1 °C and 450 μatm CO2); and combined conditions (CC: 30 ± 0.1 °C and 900 μatm CO2). Briefly, after acclimation, three identical aquariums (three individuals were added to each aquarium) were used for each treatment group. Note that because of limited space, the four treatments did not run concurrently, although we carried out a control treatment and a stress treatment simultaneously each time; thus, the entire research comprised three consecutive trials. In total, 54 H. crispa individuals were measured in this study. Air was bubbled into the control and WC group, while CO2 mixed with air was bubbled into the AC and CC groups, which was controlled using an air and CO2 gas flow adjustment system. The temperature of the seawater was controlled using 300 W submerged heaters (E300, EHEIM, Deizisau, Germany). During the experimental period, the seawater temperature and pH for each treatment group were measured twice a day (Figures S1 and S2). Salinity was measured every day using a multiparameter sensor YSI (YSI, Yellow Springs, OH, USA). Total alkalinity was determined using potentiometric titration (888 Titrando, Metrohm, Riverview, FL, USA) [] and the other carbonate parameters were calculated using CO2SYS software [] (Table S1).
2.2. Tissue Collection and Physiological Measurements
Sea anemone tentacle samples were collected on days 1, 3, 6, 9, and 15 to determine the maximum quantum yield of PS II (Fv/Fm), Symbiodiniaceae density, and the chlorophyll concentration. The sample collection protocol was conducted as described in previous reports [,]. Briefly, 10 tentacles were removed from each animal, and parts of the tentacle (1 cm long) were used for Fv/Fm measurement. The remaining parts were cut lengthwise, scraped to separate the endodermal cell layer, which contains the Symbiodiniaceae, from the epidermal tissue, which were then placed into 5 mL centrifuge tubes containing 3 mL of 0.22 μm-filtered, chilled ddH2O at 4 °C []. The tissues were homogenized for 1 min using a saw-tooth homogenizer. The homogenate was then centrifuged at 5000× g for 15 min to separate the Symbiodiniaceae from the host. The supernatant (host fraction) was used for protein determination according to a previously described method []. The pellet was vortexed for 1 min and resuspended in 4 mL of chilled ddH2O. Next, 500 µL of the resuspended pellet was used to measure symbiont cell density and another 500 µL was used to measure the chlorophyll concentration. The remaining 3 mL of solution was recentrifuged at 13,000× g for 3 min to collect the Symbiodiniaceae cells and stored at −80 °C for subsequent measurement of the sterol content.
Fv/Fm was measured using a chlorophyll fluorimeter (WALZ, Effeltrich, Germany), with a measuring intensity of 4, a saturating intensity of 7, and a gain and damping of 2 []. The Symbiodiniaceae density was normalized to the host protein mass [] and was determined using a 0.1 mm deep Improved Neubauer Hemocytometer (ThermoFisher Scientific, Waltham, MA, USA) by measuring the cell number at defined intervals. Pigment extraction and measurement were performed according to previously published methods []. Methanol was used as the extraction solvent (1 mL for each sample), and extraction was carried out at 60 °C in the dark for 30 min. Extracts were then centrifuged at 5000× g for 15 min to remove algal debris. Pigment absorbances were determined using a spectrophotometer (GeneQuant 1300, Biochrom, Holliston, MA, USA) using light wavelengths of 664 nm and 630 nm for chlorophyll (chl) a and chl c, corrected by subtracting the absorbance value (Abs) at 750 nm. Equations used to determine the pigment concentrations were as follows:
chl a = 11.85 × [(Abs 664 nm − Abs 750 nm) − 0.08 × (Abs 630 nm − Abs 750 nm)];
chl c = 24.52 × [(Abs 630 nm − Abs 750 nm) − 1.67 × (Abs 664 nm − Abs 750 nm).
2.3. Sterol Extraction and Analysis
We used gas chromatography mass spectrometry (GC/MS) analysis to determine the total sterol content and cholesterol content on day 3 and day 15, [,]. Before sterol extraction, Symbiodiniaceae samples were lyophilized. Sterols were extracted and analyzed according to our previous study []. Briefly, a 1 µL portion of each derivatized sample was injected into a GC/MS single quadrupole (GC/MSD) system. The collected data were analyzed using Agilent GC/MSD Productivity ChemStation and AMDIS (Automated Mass spectral Deconvolution and Identification System) software (Agilent, Santa Clara, CA, USA).
2.4. Statistical Analysis
All treatments were replicated at least three times. For all tested parameters, data are represented as the means ± standard deviation (SD). The data’s normality and homogeneity of variance were tested using Shapiro–Wilks and Levene’s tests, respectively. The data showed homogeneity and normal distribution, and were analyzed using analysis of variance (ANOVA). A statistically significant difference was accepted at p < 0.05. In the figures, asterisks (*) indicate p values of 0.05 or less.
3. Results
3.1. Maximum Quantum Yield of Photosystem II and Chlorophyll
The maximum quantum yield of photosystem II (Fv/Fm) differed among the different treatments (Figure 1A). Acidification alone had no apparent deleterious effect on the photochemical efficiency of photosystem II (p > 0.05); indeed, we observed an upward trend in Fv/Fm values over the first 3 days, although the difference was not significant (p > 0.05) (Figure 1A). Similar results were observed in the CC group during the first 3 days. However, after 3 days of exposure in the CC group and 2 days in the WC group, the Fv/Fm values of both treatment groups decreased significantly (p < 0.05) (Figure 1A).
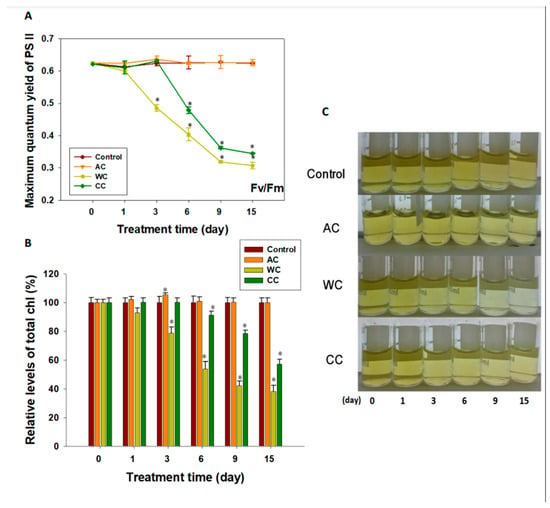
Figure 1.
Consequences of different stresses on the photosynthetic activity and apparatus of the symbionts in H. crispa. (A) Maximum quantum yield of photosystem II (PSII) (n = 3); (B) Total chlorophyll content (n = 3); (C) Crude chlorophyll extract. AC, acidification conditions; WC, warming conditions; CC, combined conditions. Asterisks (*) indicate p-values < 0.05.
Similar to the results observed for Fv/Fm, on day 3 we detected that the level of chlorophyll was significantly higher in the AC group compared with that in the control (p < 0.05) (Figure 1B and Figure S3A). However, total chlorophyll was negatively influenced by warming, being generally lower in the WC and CC groups, with a decrease starting on day 3 and day 6, respectively (p < 0.05) (Figure 1B). No pigment bleaching was observed during the experiment in the AC and CC groups, but was observed in the WC group (Figure 1C).
3.2. Symbiodiniaceae Density
Consistent with the results of the chlorophyll content and Fv/Fm value, acidification alone appeared to have little effect on Symbiodiniaceae density (p > 0.05) (Figure 2). However, the density of symbionts decreased significantly (p < 0.05) after 3 days under both high temperature stress and combined stress (Figure 2). Apparently, warming alone had a greater effect on Symbiodiniaceae density than the combined treatment (p < 0.01).
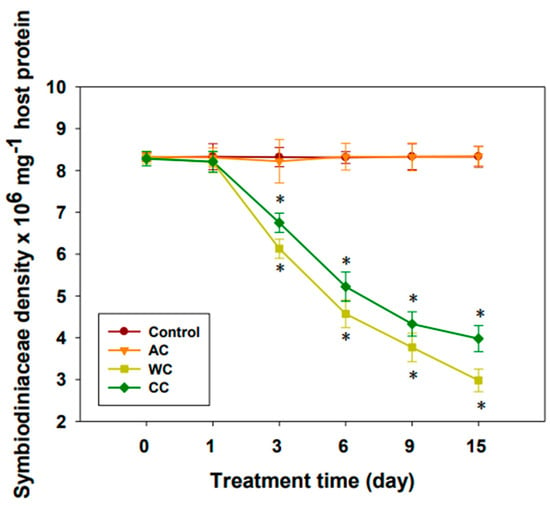
Figure 2.
Consequences of different stresses on Symbiodiniaceae density (n = 3). AC, acidification conditions; WC, warming conditions; CC, combined conditions. Asterisks (*) indicate p-values < 0.05.
3.3. Cholesterol Contents and the Phenotype
Results showed that sterol and cholesterol levels decreased from day 3 and were even lower on day 15 (p < 0.05) under CC and WC treatments (Figure 3A,B; Tables S2 and S3). Although there was no significant change in sterol metabolism in the AC group on day 3 (p > 0.05), it was significantly reduced at the end of the experiment (p < 0.05) (Figure 3A,B; Tables S2 and S3). In agreement with these findings, we found that the health of H. crispa was affected (Figure 3C) by acidification stress, although the photosynthetic apparatus and density of symbionts did not change significantly (Figure 1 and Figure 2). In addition, severe bleaching occurred under WC and CC treatments; the former was more severe, even though we did not detect the color score of the tentacles (Figure 3C).
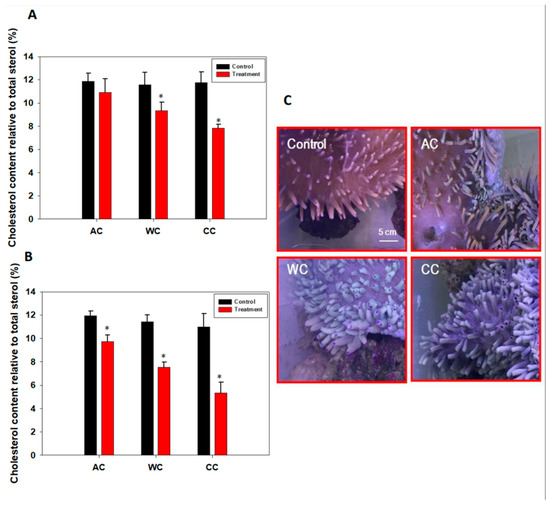
Figure 3.
Changes in levels of symbiont cholesterol and the phenotype of H. crispa. in response to stress. (A) Changes in levels of symbiont cholesterol on day 3 (n = 3); (B) Changes in levels of symbiont cholesterol on day 15 (n = 3); (C) Phenotype of H. crispa in response to stress. AC, acidification conditions; WC, warming conditions; CC, combined conditions. Asterisks (*) indicate p-values < 0.05.
4. Discussion
Coral reefs worldwide have experienced an unprecedented decline over recent decades, primarily because of global climate change []. The combined effects of acidification and global warming might lead to a marked decrease in coral diversity []. To the best of our knowledge, this was the first study to demonstrate how future ocean conditions will impact H. crispa.
In the present study, acidification alone had no apparent effect on the photochemical efficiency of photosystem II. However, we found that on the third day of acidification treatment, there was a slight, but not significant, decrease in Symbiodiniaceae density. Interestingly, we observed an upward trend in Fv/Fm values over the first 3 days, although the difference was not significant. It is possible that the significant increase in the chlorophyll level at this time point was a strategy to compensate for the possible decline in symbiont density []. It is suggested that H. crispa is able to acclimatize to high pCO2 environments [,,,]. In contrast, the chlorophyll content, symbiont cell density, and Fv/Fm value were lower than the control under both warming and combined stress conditions. In addition, it was obvious that the deleterious effects of warming alone on H. crispa were more serious than those of the combined treatment. In contrast, a recent study showed that combined stress had a more negative effect on coral health []. The reason for this difference might be the mentioned focused on calcification of a scleractinian coral. Data have shown that both warming and acidification have adverse effects on coral calcification [,,,]. However, the sea anemone used in this study does not have a calcium carbonate skeleton. This further illustrates that H. crispa is able to acclimatize to high pCO2 environments, and that increasing chlorophyll levels is an important strategy by which symbionts adapt to high pCO2. However, upregulation of chlorophyll levels had little effect on the environmental adaption of H. crispa after warming stress. Therefore, the combined effects of acidification and global warming might lead to severe destruction of H. crispa, despite the stimulating effect of acidification alone on H. crispa’s primary productivity [,].
After 15 days of exposure, pCO2 did not cause a significant bleaching response in H. crispa (Figure 3C), as found for the sea anemones Anemonia viridis [] and Entacmaea quadricolor [], which suggested that H. crispa might be relatively insensitive to the impact of ocean acidification [,]. However, warming, alone or in combination with acidification, caused severe bleaching by the end of the experiment. In addition, warming alone was more harmful to sea anemones than the combined treatment, which supports the view that thermal stress is the dominant driver of deteriorating health []. This also supported the view that the benefits of acidification had a little effect on coral survival after thermal stress because of bleaching []. Generally, warming, alone or in combination with acidification, causes more serious damage to H. crispa than acidification alone [,,,].
Sterols are vital membrane components in all eukaryotic cells []. In corals, it has been suggested that sterols are originally synthesized by the symbiotic Symbiodiaceae and not by the host corals [,]. Indeed, sterols play an important role in the bleaching process of cnidarians [,]. In particular, a lack of cholesterol is a significant feature of sea anemone bleaching []. In the present study, we used the cholesterol content and the total sterol content as important indicators and explored the effects of different stresses on the health of H. crispa. The results showed that acidification alone significantly affected the cholesterol and total sterol levels. As expected, sterol metabolism was also seriously affected by warming and the combination of acidification and warming. These results imply that although no significant changes in the photosynthetic apparatus were observed during acidification alone, the cholesterol and total sterol contents were affected significantly. Indeed, integration of metabolite and physiological data has a high predictive power to define organism performance [,]. In general, cholesterol or sterol is essential to the healthy growth of H. crispa and could be used as an important indicator in the scientific evaluation of the impact of climate change on cnidarians [,].
5. Conclusions
Acidification alone had no apparent effect on the maximum quantum yield of PS II and Symbiodiniaceae density, but did affect sterol levels. Upregulation of the chlorophyll content is suggested as an important strategy for symbionts to adapt to high pCO2. However, after warming stress, the benefits of high pCO2 had little effect on stress tolerance in H. crispa. Indeed, thermal stress is the dominant driver of deteriorating health of H. crispa. The cholesterol and total sterol contents could be used as important indicators to evaluate the impact of climate change on cnidarians. These findings expand our understanding of the responses of marine species to climate change. More importantly, they provide a scientific basis to predict the impact of environmental changes on corals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12172259/s1, Figure S1: Temperature for each treatment over the 15-day experiment; Figure S2: pH for each treatment over the 15-day experiment. Figure S3: Pigment contents of the symbionts in H. crispa under the indicated stress conditions (n = 3); Table S1: Seawater carbonate chemistry under experimental conditions; Table S2: Changes in the levels of symbiont sterols in response to stress on day 3; Table S3: Changes in the levels of symbiont sterols in response to stress on day 15.
Author Contributions
Conceptualization, J.J.; methodology, J.J. and L.Y.; resources, J.J. and Q.Y.; investigation, Y.W., W.T. and C.C.; physiological and sterol analysis, Y.W., W.T. and C.C.; data curation, J.J., L.Y. and Q.Y.; writing—original draft preparation, Y.W. and W.T.; writing—review and editing, J.J. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Middle-aged and Young Teachers’ Basic Ability Promotion Project of Guangxi (grant no. 2021KY0056) and Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education, China (grant no. ERESEP2021Z10).
Institutional Review Board Statement
The experimental procedures in this study complied withanimal welfare and research laws in China and were approved by the Ethics Committee of Guangxi Normal University (No. 202208-006).
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank all participants in this work, including staff at the College of Life Sciences, Guangxi Normal University and staff at the Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paraschiv, S.; Paraschiv, L.S. Trends of carbon dioxide (CO2) emissions from fossil fuels combustion (coal, gas and oil) in the EU member states from 1960 to 2018. Energy Rep. 2020, 6, 237–242. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Aristegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.S.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Portner, H.O., Roberts, D.C., Masson Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegria, A., Nicolai, M., Okem, A., et al., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
- Jackson, R.B.; Le Quere, C.; Andrew, R.M.; Canadell, J.G.; Peters, G.P.; Roy, J.; Wu, L. Warning signs for stabilizing global CO2 emissions. Environ. Res. Lett. 2017, 12, 110202. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, N., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Assan, D.; Kuebutornye, F.K.A.; Mustapha, U.F.; Chen, H.P.; Li, G.L. Effects of climate change on marine organisms. Am. J. Clim. Chang. 2020, 9, 204–216. [Google Scholar] [CrossRef]
- Martino, C.; Byrne, M.; Roccheri, M.C.; Chiarelli, R. Interactive effects of increased temperature and gadolinium pollution in Paracentrotus lividus sea urchin embryos: A climate change perspective. Aquat. Toxicol. 2021, 232, 105750. [Google Scholar] [CrossRef]
- Flores, F.; Marques, J.A.; Uthicke, S.; Fisher, R.; Patel, F.; Kaserzon, S.; Negri, A.P. Combined effects of climate change and the herbicide diuron on the coral acropora millepora. Mar. Pollut. Bull. 2021, 169, 112582. [Google Scholar] [CrossRef]
- Nordborg, F.M.; Jones, R.J.; Oelgemoller, M.; Negri, A.P. The effects of ultraviolet radiation and climate on oil toxicity to coral reef organisms—A review. Sci. Total Environ. 2020, 720, 137486. [Google Scholar] [CrossRef]
- Kibria, G.; Nugegoda, D.; Rose, G.; Haroon, A.K.Y. Climate change impacts on pollutants mobilization and interactive effects of climate change and pollutants on toxicity and bioaccumulation of pollutants in estuarine and marine biota and linkage to seafood security. Mar. Pollut. Bull. 2021, 167, 112364. [Google Scholar] [CrossRef]
- Suggett, D.J.; Smith, D.J. Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob. Chang. Biol. 2020, 26, 68–79. [Google Scholar] [CrossRef]
- Roth, M.S. The engine of the reef: Photobiology of the coral-algal symbiosis. Front. Microbiol. 2014, 5, 422. [Google Scholar] [PubMed]
- Elias, S.A. Loss of Coral Reefs. In Encyclopedia of the Anthropocene; Dellasala, D.A., Goldstein, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–258. [Google Scholar]
- Tambutte, E.; Venn, A.A.; Holcomb, M.; Segonds, N.; Techer, N.; Zoccola, D.; Tambutte, S. Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat. Commun. 2015, 6, 7368. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.I.; Teneva, L.; Okamoto, D.K.; Schneider, K.; Caldeira, K.; Miard, T.; Chai, A.; Marker, M.; Dunbar, R.B.; Mitchell, B.G.; et al. Living coral tissue slows skeletal dissolution related to ocean acidification. Nat. Ecol. Evol. 2019, 3, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Wouters, J.; Yasuda, N. Seasonal calcification of the coral Acropora digitifera from a subtropical marginal Okinawa reef under ocean acidification. Coral Reefs 2019, 38, 443–454. [Google Scholar] [CrossRef]
- Van der Zande, R.M.; Achlatis, M.; Bender-Champ, D.; Kubicek, A.; Dove, S.; Hoegh-Guldberg, O. Paradise lost: End-of-century warming and acidification under business-as-usual emissions have severe consequences for symbiotic corals. Glob. Chang. Biol. 2020, 26, 2203–2219. [Google Scholar] [CrossRef] [PubMed]
- Tansik, A.L.; Hopkinson, B.M.; Meile, C. Inorganic carbon fluxes and perturbations by ocean acidification estimated using a data-constrained, process-based model of coral physiology. Mar. Biol. 2021, 168, 116. [Google Scholar] [CrossRef]
- Guo, W.F.; Bokade, R.; Cohen, A.L.; Mollica, N.R.; Leung, M.; Barainard, R.E. Ocean acidification has impacted coral growth on the Great Barrier Reef. Geophys. Res. Lett. 2020, 47, e2019GL086761. [Google Scholar] [CrossRef]
- Albright, R.; Takeshita, Y.; Koweek, D.A.; Ninokawa, A.; Wolfe, K.; Rivlin, T.; Nebuchina, Y.; Young, J.; Caldeira, K. Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature 2018, 555, 516. [Google Scholar] [CrossRef]
- Eyre, B.D.; Cyronak, T.; Drupp, P.; De Carlo, E.H.; Sachs, J.P.; Andersson, A.J. Coral reefs will transition to net dissolving before end of century. Science 2018, 359, 908–911. [Google Scholar] [CrossRef]
- Hennige, S.J.; Wicks, L.C.; Kamenos, N.A.; Perna, G.; Findlay, H.S.; Roberts, J.M. Hidden impacts of ocean acidification to live and dead coral framework. Proc. R. Soc. B 2015, 282, 20150990. [Google Scholar] [CrossRef]
- Rivest, E.B.; Chen, C.S.; Fan, T.Y.; Li, H.H.; Hofmann, G.E. Lipid consumption in coral larvae differs among sites: A consideration of environmental history in a global ocean change scenario. Proc. R. Soc. B-Biol. Sci. 2017, 284, 20162825. [Google Scholar] [CrossRef] [PubMed]
- Biscere, T.; Zampighi, M.; Lorrain, A.; Jurriaans, S.; Foggo, A.; Houlbreque, F.; Rodolfo-Metalpa, R. High pCO2 promotes coral primary production. Biol. Lett. 2019, 15, 20180777. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.L. Components of a Flexible Phenotype in Two Species of Scleractinian Coral under Ocean Acidification. Ph.D. Thesis, California State University, Northridge, CA, USA, 2017. [Google Scholar]
- Liew, Y.J.; Zoccola, D.; Li, Y.; Tambutte, E.; Venn, A.A.; Michell, C.T.; Gui, G.; Deutekom, E.S.; Kaandorp, J.A.; Voolstra, C.R.; et al. Epigenome-associated phenotypic acclimatization to ocean acidification in a reef-building coral. Sci. Adv. 2018, 24, eaar8028. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; HoeghGuldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed]
- Kavousi, J.; Tanaka, Y.; Nishida, K.; Suzuki, A.; Nojiri, Y.; Nakamura, T. Colony-specific calcification and mortality under ocean acidification in the branching coral Montipora digitata. Mar. Environ. Res. 2016, 119, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Harii, S.; Hongo, C.; Ishihara, M.; Ide, Y.; Kayanne, H. Impacts of multiple disturbances on coral communities at Ishigaki Island, Okinawa, Japan, during a 15 year survey. Mar. Ecol. Prog. Ser. 2014, 509, 171–180. [Google Scholar] [CrossRef]
- Jaroensutasinee, K.; Somchuea, S.; Jaroensutasinee, M. Coral and reef fish community recovery following the 2010 extreme ocean warming event (mass bleaching event) at Thailand. J. Anim. Behav. Biometeorol. 2021, 9, 21004. [Google Scholar] [CrossRef]
- Goyen, S.; Camp, E.F.; Fujise, L.; Lloyd, A.; Nitschke, M.R.; Lajeunensse, T.; Kahlke, T.; Ralph, P.J.; Suggett, D. Mass coral bleaching of P. versipora in Sydney Harbour driven by the 2015–2016 heatwave. Coral Reefs 2019, 38, 815–830. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, J.; Zhao, H.; Han, X.; Xiang, Y.; Zhou, W. Clade-specific sterol metabolites in dinoflagellate endosymbionts are associated with coral bleaching in response to environmental cues. mSystems 2020, 5, e00765-20. [Google Scholar] [CrossRef]
- Pryor, S.H.; Andrews, L.; Kelaher, B.P.; Tagliafico, A.; Scott, A. Ocean temperature, but not acidification, causes sea anemone bleaching under a near-future climate scenario. Coral Reefs 2021, 40, 355–364. [Google Scholar]
- Hillyer, K.E.; Dias, D.A.; Lutz, A.; Wilkinson, S.P.; Roessner, U.; Davy, S.K. Metabolite profiling of symbiont and host during thermal stress and bleaching in the coral Acropora aspera. Coral Reefs 2016, 36, 105–118. [Google Scholar] [CrossRef]
- Roach, T.; Dilworth, J.; Christian, M.H.; Jones, D.; Quinn, R.A.; Drury, C. Metabolomic signatures of coral bleaching history. Nat. Ecol. Evol. 2021, 5, 495–503. [Google Scholar] [CrossRef]
- Williams, A.; Chiles, E.N.; Conetta, D.; Pathmanathan, J.S.; Cleves, P.A.; Putnam, H.M.; Su, X.Y.; Bhattacharya, D. Metabolomic shifts associated with heat stress in coral holobionts. Sci. Adv. 2021, 7, eabd4210. [Google Scholar] [CrossRef] [PubMed]
- Bove, C.B.; Umbanhowar, J.; Castillo, K.D. Meta-analysis reveals reduced coral calcification under projected ocean warming but not under acidification across the Caribbean sea. Front. Mar. Sci. 2020, 7, 127. [Google Scholar] [CrossRef]
- Schoepf, V.; D’Olivo, J.P.; Rigal, C.; Jung, E.M.; McCulloch, M.T. Heat stress differentially impacts key calcification mechanisms in reef-building corals. Coral Reefs 2021, 40, 459–471. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; Death, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Guillermic, M.; Cameron, L.P.; Corte, I.D.; Misra, S.; Bijma, J.; de Beer, D.; Reymond, C.E.; Westphal, H.; Ries, J.B.; Eagle, R.A. Thermal stress reduces pocilloporid coral resilience to ocean acidification by impairing control over calcifying fluid chemistry. Sci. Adv. 2021, 7, eaba9958. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Silva, C.L.; Sarmento, V.C.; Santos, P.J.P. Climate change scenarios of increased CO2 and temperature affect a coral reef peracarid (Crustacea) community. Mar. Environ. Res. 2021, 173, 105518. [Google Scholar] [CrossRef]
- Kayal, E.; Roure, B.; Philippe, H.; Collins, A.G.; Lavrov, D.V. Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC Evol. Biol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Harrison, P.L. Embryonic and larval development of the host sea anemones Entacmaea quadricolor and Heteractis crispa. Biol. Bull. 2007, 213, 110–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ollerton, J.; McCollin, D.; Fautin, D.G.; Allen, G.R. Finding NEMO: Nestedness engendered by mutualistic organization in anemonefish and their hosts. Proc. R. Soc. B 2007, 274, 591–598. [Google Scholar] [CrossRef]
- Scott, A. Effects of feeding on the growth rates of captive-bred Heteractis crispa: A popular marine ornamental for aquariums. Bull. Mar. Sci. 2012, 88, 81–87. [Google Scholar] [CrossRef]
- Scott, A.; Dixson, D.L. Reef fishes can recognize bleached habitat during settlement: Sea anemone bleaching alters anemonefish host selection. Proc. R. Soc. B-Biol. Sci. 2016, 283, 20152694. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Ndobe, S.; Yasir, I.; Ambo-Rappe, R.; Jompa, J. Banggai cardinalfish and its microhabitats in a warming world: A preliminary study. IOP Conf. Ser. Earth Environ. Sci. 2019, 253, 012021. [Google Scholar] [CrossRef]
- Hill, L.J.; Paradas, W.C.; Willemes, M.J.; Pereira, M.G.; Salomon, P.S.; Mariath, R.; Moura, R.L.; Atella, G.C.; Farina, M.; Amado-Filho, G.M.; et al. Acidification-induced cellular changes in Symbiodinium isolated from Mussismilia braziliensis. PLoS ONE 2019, 14, e0220130. [Google Scholar] [CrossRef] [PubMed]
- Kelaher, B.P.; Coleman, M.A.; Bishop, M.J. Ocean warming, but not acidification, accelerates seagrass decomposition under near future climate scenarios. Mar. Ecol. Prog. Ser. 2018, 605, 103–110. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.W.R. Program Developed for CO2 System Calculation; ORNL/CDIAC-105; Carbon Dioxide Information Analysis Center: Oak Ridge, TN, USA, 1998.
- Cleves, P.A.; Krediet, C.J.; Lehnert, E.M.; Onishi, M.; Pringle, J.R. Insights into coral bleaching under heat stress from analysis of gene expression in a sea anemone model system. Proc. Natl. Acad. Sci. USA 2020, 117, 28906–28917. [Google Scholar] [CrossRef]
- Molina, V.H.; Castillo-Medina, R.E.; Thome, P.E. Experimentally induced bleaching in the Sea Anemone Exaiptasia supports glucose as a main metabolite associated with its symbiosis. J. Mar. Biol. 2017, 2017, 3130723. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y. Metabolite profiling of Breviolum minutum in response to acidification. Aquat. Toxicol. 2019, 213, 105215. [Google Scholar] [CrossRef]
- Ventura, P.; Jarrold, M.D.; Merle, P.L.; Barnay-Verdier, S.; Zamoum, T.; Rodolfo-Metalpa, R.; Calosi, P.; Furla, P. Resilience to ocean acidification: Decreased carbonic anhydrase activity in sea anemones under high pCO2 conditions. Mar. Ecol. Prog. Ser. 2016, 559, 257–263. [Google Scholar] [CrossRef][Green Version]
- Hoadley, K.D.; Pettay, D.T.; Dodge, D.; Warner, M.E. Contrasting physiological plasticity in response to environmental stress within different cnidarians and their respective symbionts. Coral Reefs 2016, 35, 529–542. [Google Scholar] [CrossRef]
- Noonan, S.H.C.; Fabricius, K.E. Ocean acidification affects productivity but not the severity of thermal bleaching in some tropical corals. ICES J. Mar. Sci. 2016, 73, 715–726. [Google Scholar] [CrossRef]
- Davies, S.W.; Ries, J.B.; Marchetti, A.; Castillo, K.D. Symbiodinium functional diversity in the coral Siderastrea siderea is influenced by thermal stress and reef environment, but not ocean acidification. Front. Mar. Sci. 2018, 5, 150. [Google Scholar] [CrossRef]
- Rivest, E.B.; Kelly, M.W.; DeBiasse, M.B.; Hofmann, G.E. Host and symbionts in Pocillopora damicornis larvae display different transcriptomic responses to ocean acidification and warming. Front. Mar. Sci. 2018, 5, 186. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, A.; Deng, X.; Zhou, W.; Gan, Q.; Lu, Y. How Symbiodiniaceae meets the challenges of life during coral bleaching. Coral Reefs 2021, 40, 1339–1353. [Google Scholar] [CrossRef]
- Sogin, E.M.; Putnam, H.M.; Anderson, P.E.; Gates, R.D. Metabolomic signatures of increases in temperature and ocean acidification from the reef-building coral, Pocillopora damicornis. Metabolomics 2016, 12, 71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).