The Short-Term Effects of Amendments on Nematode Communities and Diversity Patterns under the Cultivation of Miscanthus × giganteus on Marginal Land
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Field
2.2. Soil Characteristics
2.3. Nematode Analysis
2.3.1. Nematode Sampling
2.3.2. Nematode Isolation and Identification
2.3.3. Nematode Fauna Analysis Based on Life Traits and Feeding Habitats
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, R.A.; Sumbul, A.; Rizvi, R.; Mahmood, I. Organic Soil Amendments: Potential Tool for Soil and Plant Health Management. In Plant Health Under Biotic Stress: Volume 1: Organic Strategies; Ansari, R.A., Mahmood, I., Eds.; Springer: Singapore, 2019; pp. 1–35. ISBN 9789811360435. [Google Scholar]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Popławska, A. The Possibility of Using Sewage Sludge for Energy Crop Cultivation Exemplified by Reed Canary Grass and Giant Miscanthus. Soil Sci. Annu. 2019, 70, 21–33. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Załuski, D.; Sokólski, M. The Effect of Sewage Sludge Fertilization on the Biomass Yield of Giant Miscanthus and the Energy Balance of the Production Process. Energy 2020, 206, 118189. [Google Scholar] [CrossRef]
- Alasmary, Z.; Hettiarachchi, G.M.; Roozeboom, K.L.; Davis, L.C.; Erickson, L.E.; Pidlisnyuk, V.; Stefanovska, T.; Trögl, J. Phytostabilization of a Contaminated Military Site Using Miscanthus and Soil Amendments. J. Environ. Qual. 2021, 50, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.A.; Ostle, N.; Bardgett, R.D.; Hopkins, D.W.; Vanbergen, A.J. Biochar in Bioenergy Cropping Systems: Impacts on Soil Faunal Communities and Linked Ecosystem Processes. GCB Bioenergy 2013, 5, 81–95. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Newton, R.A.; Mamirova, A. Miscanthus Biochar Value Chain-A Review. J. Environ. Manag. 2021, 290, 112611. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic Amendments Drive Shifts in Microbial Community Structure and Keystone Taxa Which Increase C Mineralization across Aggregate Size Classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, C.S.K.; Ji, X.; Rainey, T.J. Returning Biochar to Fields: A Review. Appl. Soil Ecol. 2017, 116, 1–11. [Google Scholar] [CrossRef]
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.-P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent Developments in Understanding Biochar’s Physical–Chemistry. Agronomy 2021, 11, 615. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and Limitations of Biochar Amendment in Agricultural Soils: A Review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of Microbial Communities to Biochar-Amended Soils: A Critical Review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Krzyżaniak, M.; Stolarski, M.J.; Warmiński, K. Life Cycle Assessment of Giant Miscanthus: Production on Marginal Soil with Various Fertilisation Treatments. Energies 2020, 13, 1931. [Google Scholar] [CrossRef]
- Winkler, B.; Mangold, A.; von Cossel, M.; Clifton-Brown, J.; Pogrzeba, M.; Lewandowski, I.; Iqbal, Y.; Kiesel, A. Implementing Miscanthus into Farming Systems: A Review of Agronomic Practices, Capital and Labour Demand. Renew. Sustain. Energy Rev. 2020, 132, 110053. [Google Scholar] [CrossRef]
- Ziadi, N.; Gagnon, B.; Nyiraneza, J. Crop Yield and Soil Fertility as Affected by Papermill Biosolids and Liming By-Products. Can. J. Soil Sci. 2013, 93, 319–328. [Google Scholar] [CrossRef]
- Mozaffari, M.; Hays, H.C. Effect of a Newly Developed Pelleted Papermill Biosolids on Crop and Soil. J. Agric. Chem. Environ. 2019, 9, 1. [Google Scholar] [CrossRef]
- Dalzell, C.G. Purpose-Grown Biomass Crops: Efficient Production and Real-World Verification. Master’s Thesis, Saint Mary’s University, Halifax, NS, Canada, 2021. [Google Scholar]
- Kowalska, A.; Grobelak, A.; Almås, Å.R.; Singh, B.R. Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review. Energies 2020, 13, 5813. [Google Scholar] [CrossRef]
- Kvak, V.; Stefanovska, T.; Pidlisnyuk, V.; Alasmary, Z.; Kharytonov, M. The Long-Term Assessment of Miscanthus × gigantheus Cultivation in the Forest-Steppe Zone of Ukraine. INMATEH-Agric. Eng. 2018, 54, 113–120. [Google Scholar]
- Wang, C.; Kong, Y.; Hu, R.; Zhou, G. Miscanthus: A Fast-Growing Crop for Environmental Remediation and Biofuel Production. GCB Bioenergy 2021, 13, 58–69. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Testa, G.; Monti, A.; Alexopoulou, E.; Christou, M. 1-The Importance of Perennial Grasses as a Feedstock for Bioenergy and Bioproducts. In Perennial Grasses for Bioenergy and Bioproducts; Alexopoulou, E., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–33. ISBN 978-0-12-812900-5. [Google Scholar]
- Pidlisnyuk, V.; Shapoval, P.; Zgorelec, Ž.; Stefanovska, T.; Zhukov, O. Multiyear Phytoremediation and Dynamic of Foliar Metal(Loid)s Concentration during Application of Miscanthus × giganteus Greef et Deu to Polluted Soil from Bakar, Croatia. Environ. Sci. Pollut. Res. 2020, 27, 31446–31457. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Herts, A.; Khomenchuk, V.; Mamirova, A.; Kononchuk, O.; Ust’ak, S. Dynamic of Morphological and Physiological Parameters and Variation of Soil Characteristics during Miscanthus × giganteus Cultivation in the Diesel-Contaminated Land. Agronomy 2021, 11, 798. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Burdová, H.; Trubač, J.; Štojdl, J.; Kuráň, P.; Kříženecká, S.; Machová, I.; Kubát, K.; Popelka, J.; Malinská, H.A.; et al. Enhanced Carbon Sequestration in Marginal Land Upon Shift towards Perennial C4 Miscanthus × giganteus: A Case Study in North-Western Czechia. Agronomy 2021, 11, 293. [Google Scholar] [CrossRef]
- Neher, D.A. Role of Nematodes in Soil Health and Their Use as Indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar] [PubMed]
- Liu, X.; Zhang, D.; Li, H.; Qi, X.; Gao, Y.; Zhang, Y.; Han, Y.; Jiang, Y.; Li, H. Soil Nematode Community and Crop Productivity in Response to 5-Year Biochar and Manure Addition to Yellow Cinnamon Soil. BMC Ecol. 2020, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T. Nematode Indicators of Organic Enrichment. J. Nematol. 2006, 38, 3–12. [Google Scholar] [PubMed]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Holajjer, P.; Kamra, A.; Singh, P. Influence of Nematode-Bacterial Interactions on N and P Mineralisation in Soil and on Decomposition of Crop Residues during Aerobic Composting. Appl. Ecol. Environ. Res. 2016, 14, 283–299. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological Importance of Soil Bacterivores for Ecosystem Functions. Plant Soil 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.; Wang, X.; Chen, L.; Liu, M.; Li, H.; Sun, B. Nematodes and Microbial Community Affect the Sizes and Turnover Rates of Organic Carbon Pools in Soil Aggregates. Soil Biol. Biochem. 2018, 119, 22–31. [Google Scholar] [CrossRef]
- Jagodič, A.; Trdan, S.; Laznik, Ž. Entomopathogenic Nematodes: Can We Use the Current Knowledge on Belowground Multitrophic Interactions in Future Plant Protection Programmes?–Review. Plant Prot. Sci. 2019, 55, 243–254. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera—An Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- McSorley, R. Overview of Organic Amendments for Management of Plant-Parasitic Nematodes, with Case Studies from Florida. J. Nematol. 2011, 43, 69–81. [Google Scholar]
- Herren, G.L.; Habraken, J.; Waeyenberge, L.; Haegeman, A.; Viaene, N.; Cougnon, M.; Reheul, D.; Steel, H.; Bert, W. Effects of Synthetic Fertilizer and Farm Compost on Soil Nematode Community in Long-Term Crop Rotation Plots: A Morphological and Metabarcoding Approach. PLoS ONE 2020, 15, e0230153. [Google Scholar] [CrossRef]
- Lazarova, S.; Coyne, D.; Rodríguez, M.G.; Peteira, B.; Ciancio, A. Functional Diversity of Soil Nematodes in Relation to the Impact of Agriculture—A Review. Diversity 2021, 13, 64. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Nguyen, P.T.K.; Araki, M.; Perry, R.N.; Ba Tran, L.; Minh Chau, K.; Min, Y.Y.; Toyota, K. Effects of Cropping Systems and Soil Amendments on Nematode Community and Its Relationship with Soil Physicochemical Properties in a Paddy Rice Field in the Vietnamese Mekong Delta. Appl. Soil Ecol. 2020, 156, 103683. [Google Scholar] [CrossRef]
- Pulavarty, A.; Egan, A.; Karpinska, A.; Horgan, K.; Kakouli-Duarte, T. Plant Parasitic Nematodes: A Review on Their Behaviour, Host Interaction, Management Approaches and Their Occurrence in Two Sites in the Republic of Ireland. Plants 2021, 10, 2352. [Google Scholar] [CrossRef]
- Zasada, I.A.; Halbrendt, J.M.; Kokalis-Burelle, N.; LaMondia, J.; McKenry, M.V.; Noling, J.W. Managing Nematodes without Methyl Bromide. Annu. Rev. Phytopathol. 2010, 48, 311–328. [Google Scholar] [CrossRef]
- Rosskopf, E.; Di Gioia, F.; Hong, J.C.; Pisani, C.; Kokalis-Burelle, N. Organic Amendments for Pathogen and Nematode Control. Annu. Rev. Phytopathol. 2020, 58, 277–311. [Google Scholar] [CrossRef]
- Mennan, S.; Melakeberhan, H. Effects of Biosolid Amendment on Populations of Meloidogyne Hapla and Soils with Different Textures and pHs. Bioresour. Technol. 2010, 101, 7158–7164. [Google Scholar] [CrossRef]
- Zasada, I.; Rogers, S.; Sardanelli, S. Application of Alkaline-Stabilised Biosolids for Meloidogyne Incognita Suppression in Microplots. Nematology 2007, 9, 123–129. [Google Scholar] [CrossRef]
- Zasada, I.A.; Avendano, F.; Li, Y.C.; Logan, T.; Melakeberhan, H.; Koenning, S.R.; Tylka, G.L. Potential of an Alkaline-Stabilized Biosolid to Manage Nematodes: Case Studies on Soybean Cyst and Root-Knot Nematodes. Plant Dis. 2008, 92, 4–13. [Google Scholar] [CrossRef]
- Yeates, G.W.; Speir, T.W.; Taylor, M.D.; Clucas, L.; Schaik, A.P. Nematode Responses to Biosolids Incorporation in Five Soil Types. Biol. Fertil. Soils 2006, 42, 550–555. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Li, Q.; Liang, W.-J.; Zhang, M.; Bao, X.-L.; Xie, Z.-B. Soil Nematode Response to Biochar Addition in a Chinese Wheat Field. Pedosphere 2013, 23, 98–103. [Google Scholar] [CrossRef]
- Wanjiku Kamau, J.; Biber-Freudenberger, L.; Lamers, J.P.A.; Stellmacher, T.; Borgemeister, C. Soil Fertility and Biodiversity on Organic and Conventional Smallholder Farms in Kenya. Appl. Soil Ecol. 2019, 134, 85–97. [Google Scholar] [CrossRef]
- George, C.; Kohler, J.; Rillig, M.C. Biochars Reduce Infection Rates of the Root-Lesion Nematode Pratylenchus penetrans and Associated Biomass Loss in Carrot. Soil Biol. Biochem. 2016, 95, 11–18. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Hu, F.; Ran, W.; Shen, Q.; Li, H.; Whalen, J.K. Carbon-Rich Organic Fertilizers to Increase Soil Biodiversity: Evidence from a Meta-Analysis of Nematode Communities. Agric. Ecosyst. Environ. 2016, 232, 199–207. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of Nematode Suppression by Organic Soil Amendments—A Review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Huang, W.; Ji, H.; Gheysen, G.; Debode, J.; Kyndt, T. Biochar-Amended Potting Medium Reduces the Susceptibility of Rice to Root-Knot Nematode Infections. BMC Plant Biol. 2015, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, N.; Viaene, N.; Vandecasteele, B.; D’Hose, T.; Debode, J.; Cremelie, P.; De Tender, C.; Moens, M. Traditional and New Soil Amendments Reduce Survival and Reproduction of Potato Cyst Nematodes, except for Biochar. Appl. Soil Ecol. 2016, 107, 191–204. [Google Scholar] [CrossRef]
- Harel, Y.M.; Kolton, M.; Elad, Y.; Rav-David, D.; Cytryn, E.; Borenshtein, M.; Shulchani, R.; Graber, E.R. Biochar Impact on Plant Development and Disease Resistance in Pot Trials. IOBCWPRS Bull 2012, 78, 141–147. [Google Scholar]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; d’Errico, G.; Crasto, A.; Mazzei, P.; Piccolo, A.; Incerti, G.; Woo, S.L.; et al. Biochars from Olive Mill Waste Have Contrasting Effects on Plants, Fungi and Phytoparasitic Nematodes. PLoS ONE 2018, 13, e0198728. [Google Scholar] [CrossRef]
- Huebner, R.A.; Rodriguez-Kabana, R.; Patterson, R.M. Hemicellulosic Waste and Urea for Control of Plant Parasitic Nematodes: Effect on Soil Enzyme Activities. Nematropica 1983, 13, 37–54. [Google Scholar]
- Min, Y.Y.; Toyota, K.; Sato, E.; Takada, A. Effects of Anaerobically Digested Slurry on Meloidogyne incognita and Pratylenchus penetrans in Tomato and Radish Production. Appl. Environ. Soil Sci. 2011, 2011, e528712. [Google Scholar] [CrossRef]
- DSTU 4287:2004. Soil Quality. Sampling. DP “UkrNDNC”: Kyiv, Ukraine, 2005; p. 9.
- DSTU:ISO:10390:2001. Soil Quality. Determination of pH. DP “UkrNDNC”: Kyiv, Ukraine, 2002; p. 10.
- 275/1998 Sb. Release of the Ministry of Agriculture Related the Agrochemical Testing of the Soils and Evaluation the Land Properties of the Forest Land (with Amendments 335/2017 Sb). Ministry of Agriculture: Nové Město, Czech Republic, 2017.
- Němeček, J.; Mühlhanselová, M.; Macků, J.; Vokoun, J.; Vavříček, D.; Novák, P. Czech Taxonomic Soil Classification System, 2nd ed.; Czech University of Life Sciences in Prague: Prague, Czech Republic, 2011; ISBN 978-80-213-2155-7. [Google Scholar]
- DSTU:7632-2014; Soils. Method for Determination of Organic Matter. DP “UkrNDNC”: Kyiv, Ukraine, 2014; p. 15.
- DSTU:4115:2002; Soils. Determination of Mobile Phosphorus and Potassium Compounds by the Modified Chirikov’s Method. DP “UkrNDNC”: Kyiv, Ukraine, 2003; p. 12.
- DSTU:7861:2015; Soil Quality. Determination of Exchanges Calcium, Magnesium, Sodium and Potassium in Soil according to Shollenberger in NSC ISSAR Named after Sokolovsky Modification. DP “UkrNDNC”: Kyiv, Ukraine, 2016; p. 12.
- Szczygieł, A. Application of the Centrifugal Method for Extraction of Nematodes from Soil. J. Prog. Agric. Sci. 1971, 12, 169–179. [Google Scholar]
- Seinhorst, J.W. Killing Nematodes for Taxonomic Study with Hot f.a. 4:1. Nematologica 1966, 12, 178–178a. [Google Scholar] [CrossRef]
- Brzeski, M.W. Nematodes of Tylenchina in Poland and Temperate Europe; Muzeum i Instytutu Zoologii, Polska Akademia Nauk (MiIZ PAN): Warsaw, Poland, 1998; ISBN 83-85192-84-0. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary (Nematoda Errantia). In Pedozoologica Hungarica; Csuzdi, C., Mahunka, S., Eds.; Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2007; Volume II, ISBN 963. [Google Scholar]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An Automated Calculation System for Nematode-Based Biological Monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Prentice, I.C. A Theory of Gradient Analysis. In Advances in Ecological Research; Begon, M., Fitter, A.H., Ford, E.D., Macfadyen, A., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 18, pp. 271–317. [Google Scholar]
- Stefanovska, T.; Skwiercz, A.; Zouhar, M.; Pidlisnyuk, V.; Zhukov, O. Plant-Feeding Nematodes Associated with Miscanthus × giganteus and Their Use as Potential Indicators of the Plantations’ State. Int. J. Environ. Sci. Technol. 2021, 18, 57–72. [Google Scholar] [CrossRef]
- Tabarant, P.; Villenave, C.; Risede, J.-M.; Roger-Estrade, J.; Thuries, L.; Dorel, M. Effects of Four Organic Amendments on Banana Parasitic Nematodes and Soil Nematode Communities. Appl. Soil Ecol. 2011, 49, 59–67. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, H.; Chen, L.; Yuan, Y.; Fang, H.; Luan, L.; Chen, Y.; Wang, X.; Liu, M.; Li, H.; et al. Nematodes and Microorganisms Interactively Stimulate Soil Organic Carbon Turnover in the Macroaggregates. Front. Microbiol. 2018, 9, 2803. [Google Scholar] [CrossRef]
- Liang, W.; Lou, Y.; Li, Q.; Zhong, S.; Zhang, X.; Wang, J. Nematode Faunal Response to Long-Term Application of Nitrogen Fertilizer and Organic Manure in Northeast China. Soil Biol. Biochem. 2009, 41, 883–890. [Google Scholar] [CrossRef]
- Bouwman, L.A.; Zwart, K.B. The Ecology of Bacterivorous protozoans and Nematodes in Arable Soil. Agric. Ecosyst. Environ. 1994, 51, 145–160. [Google Scholar] [CrossRef]
- Pan, F.; Han, X.; Li, N.; Yan, J.; Xu, Y. Effect of Organic Amendment Amount on Soil Nematode Community Structure and Metabolic Footprints in Soybean Phase of a Soybean-Maize Rotation on Mollisols. Pedosphere 2020, 30, 544–554. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Fan, W.; He, R.; Yao, Y.; Sun, L.; Zhao, X.; Wu, J. Comparative Effects of Different Organic Materials on Nematode Community in Continuous Soybean Monoculture Soil. Appl. Soil Ecol. 2018, 125, 12–17. [Google Scholar] [CrossRef]
- Nieminen, J.K.; Räisänen, M. Effects of Sewage Sludge Addition to Norway Spruce Seedlings on Nitrogen Availability and Soil Fauna in Clear-Cut Areas. Environ. Pollut. 2013, 178, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Creamer, R.E.; Rimmer, D.L.; Black, H.I.J. Do Elevated Soil Concentrations of Metals Affect the Diversity and Activity of Soil Invertebrates in the Long-Term? Soil Use Manag. 2008, 24, 37–46. [Google Scholar] [CrossRef]
- Kapusta, P.; Szarek-Łukaszewska, G.; Stefanowicz, A.M. Direct and Indirect Effects of Metal Contamination on Soil Biota in a Zn–Pb Post-Mining and Smelting Area (S Poland). Environ. Pollut. 2011, 159, 1516–1522. [Google Scholar] [CrossRef]
- Chauvin, C.; Trambolho, M.; Hedde, M.; Makowski, D.; Cérémonie, H.; Jimenez, A.; Villenave, C. Soil Nematodes as Indicators of Heavy Metal Pollution: A Meta-Analysis. Open J. Soil Sci. 2020, 10, 579–601. [Google Scholar] [CrossRef]
- Gupta, D.; Bhandari, S.; Bhusal, D.R. Variation of Nematode Indices under Contrasting Pest Management Practices in a Tomato Growing Agro-Ecosystem. Heliyon 2019, 5, e02621. [Google Scholar] [CrossRef]
- Ortiz, V.; Phelan, S.; Mullins, E. A Temporal Assessment of Nematode Community Structure and Diversity in the Rhizosphere of Cisgenic phytophthora Infestans-Resistant Potatoes. BMC Ecol. 2016, 16, 55. [Google Scholar] [CrossRef]
- Odum, E.P. Trends Expected in Stressed Ecosystems. BioScience 1985, 35, 419–422. [Google Scholar] [CrossRef]
- Bongers, T.; van der Meulen, H.; Korthals, G. Inverse Relationship between the Nematode Maturity Index and Plant Parasite Index under Enriched Nutrient Conditions. Appl. Soil Ecol. 1997, 6, 195–199. [Google Scholar] [CrossRef]
- Čermák, V.; Gaar, V.; Háněl, L.; Široká, K. Composition and Vertical Distribution of Free Living and Plant Parasitic Nematodes in Hop Gardens in the Czech Republic. Helminthologia 2011, 48, 124–136. [Google Scholar] [CrossRef]
- Peralta, G.; Schon, N.L.; Dickie, I.A.; St. John, M.G.; Orwin, K.H.; Yeates, G.W.; Peltzer, D.A. Contrasting Responses of Soil Nematode Communities to Native and Non-Native Woody Plant Expansion. Oecologia 2019, 190, 891–899. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S.; Subedi, A. Sensitivity of Nematode Community Analysis to Agricultural Management Practices and Inoculation with Local Effective Microorganisms in the Southeastern United States. Soil Syst. 2019, 3, 41. [Google Scholar] [CrossRef]
- Wang, Y.M.; Guan, P.T.; Chen, J.W.; Li, Z.X.; Yang, Y.R.; Wang, P. A Comparison of Soil Nematode Community Structure and Environmental Factors along Fen-Bush-Forest Succession in a Peatland, Northeastern China. Glob. Ecol. Conserv. 2021, 28, e01679. [Google Scholar] [CrossRef]
- Abrams, B.I.; Mitchell, M.J. Role of Nematode-Bacterial Interactions in Heterotrophic Systems with Emphasis on Sewage Sludge Decomposition. Oikos 1980, 35, 404–410. [Google Scholar] [CrossRef]
- Tejada, M. Application of Different Organic Wastes in a Soil Polluted by Cadmium: Effects on Soil Biological Properties. Geoderma 2009, 153, 254–268. [Google Scholar] [CrossRef]
- Martinez, J.G.; dos Santos, G.; Derycke, S.; Moens, T. Effects of Cadmium on the Fitness of, and Interactions between, Two Bacterivorous Nematode Species. Appl. Soil Ecol. 2012, 56, 10–18. [Google Scholar] [CrossRef]
- Ferris, H. Form and Function: Metabolic Footprints of Nematodes in the Soil Food Web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A Critical Review of the Possible Adverse Effects of Biochar in the Soil Environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar Boosts Tropical but Not Temperate Crop Yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar Induced Soil Microbial Community Change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Fox, A.; Kwapinski, W.; Griffiths, B.S.; Schmalenberger, A. The Role of Sulfur- and Phosphorus-Mobilizing Bacteria in Biochar-Induced Growth Promotion of Lolium perenne. FEMS Microbiol. Ecol. 2014, 90, 78–91. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota–A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Watzinger, A.; Feichtmair, S.; Kitzler, B.; Zehetner, F.; Kloss, S.; Wimmer, B.; Zechmeister-Boltenstern, S.; Soja, G. Soil Microbial Communities Responded to Biochar Application in Temperate Soils and Slowly Metabolized 13C-Labelled Biochar as Revealed by 13C PLFA Analyses: Results from a Short-Term Incubation and Pot Experiment. Eur. J. Soil Sci. 2014, 65, 40–51. [Google Scholar] [CrossRef]
- Azeem, M.; Hale, L.; Montgomery, J.; Crowley, D.; McGiffen, M.E., Jr. Biochar and Compost Effects on Soil Microbial Communities and Nitrogen Induced Respiration in Turfgrass Soils. PLoS ONE 2020, 15, e0242209. [Google Scholar] [CrossRef]
- Bruun, S.; EL-Zehery, T. Biochar Effect on the Mineralization of Soil Organic Matter. Pesqui. Agropecuária Bras. 2012, 47, 665–671. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, Y.-F. Kinetics of C Mineralization of Biochars in Three Excessive Compost-Fertilized Soils: Effects of Feedstocks and Soil Properties. Agronomy 2020, 10, 1749. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Sánchez-Moreno, S. Biochar Addition Rate Determines Contrasting Shifts in Soil Nematode Trophic Groups in Outdoor Mesocosms: An Appraisal of Underlying Mechanisms. Appl. Soil Ecol. 2021, 158, 103788. [Google Scholar] [CrossRef]
- Cole, E.J.; Barker, A.V.; Zandvakili, O.R.; Sadeghpour, A.; Xing, B.; Hashemi, M.; Allan-Perkins, E.; Jung, G. Soil Nutrient and Nematode Community Changes in Response to Hardwood Charcoal Application. Commun. Soil Sci. Plant Anal. 2021, 52, 917–925. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of Organic Soil Amendments and Soil Organisms in the Biological Control of Plant-Parasitic Nematodes: A Review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Litterick, A.M.; Harrier, L.; Wallace, P.; Watson, C.A.; Wood, M. The Role of Uncomposted Materials, Composts, Manures, and Compost Extracts in Reducing Pest and Disease Incidence and Severity in Sustainable Temperate Agricultural and Horticultural Crop Production—A Review. Crit. Rev. Plant Sci. 2004, 23, 453–479. [Google Scholar] [CrossRef]
- Jaffee, B.A.; Ferris, H.; Scow, K.M. Nematode-Trapping Fungi in Organic and Conventional Cropping Systems. Phytopathology 1998, 88, 344–350. [Google Scholar] [CrossRef]
- Porazinska, D.L.; Duncan, L.W.; McSorley, R.; Graham, J.H. Nematode Communities as Indicators of Status and Processes of a Soil Ecosystem Influenced by Agricultural Management Practices. Appl. Soil Ecol. 1999, 13, 69–86. [Google Scholar] [CrossRef]
- McSorley, R.; Gallaher, R. Cultural Practices Improve Crop Tolerance to Nematodes. Nematropica 1995, 25, 53–60. [Google Scholar]
- Kimpinski, J.; Gallant, C.E.; Henry, R.; Macleod, J.A.; Sanderson, J.B.; Sturz, A.V. Effect of Compost and Manure Soil Amendments on Nematodes and on Yields of Potato and Barley: A 7-Year Study. J. Nematol. 2003, 35, 289–293. [Google Scholar]
- Bélair, G.; Tremblay, N. The Influence of Chitin-Urea Amendments Applied to an Organic Soil on a Meloidogyne hapla Population and on the Growth of Greenhouse Tomato. Phytoprotection 1995, 76, 75–80. [Google Scholar] [CrossRef]
- Stefanovska, T.; Skwiercz, A.; Flis, Ł.; Pidlisnyuk, V.; Zouhar, M. First Record of the Ectoparasitic Nematode Amplimerlinius macrurus (Nematoda: Tylenchida) on the Perennial Grass Miscanthus × giganteus (Angiosperms: Poaceae) in Ukraine. J. Nematol. 2021, 53, e2021-24. [Google Scholar] [CrossRef]
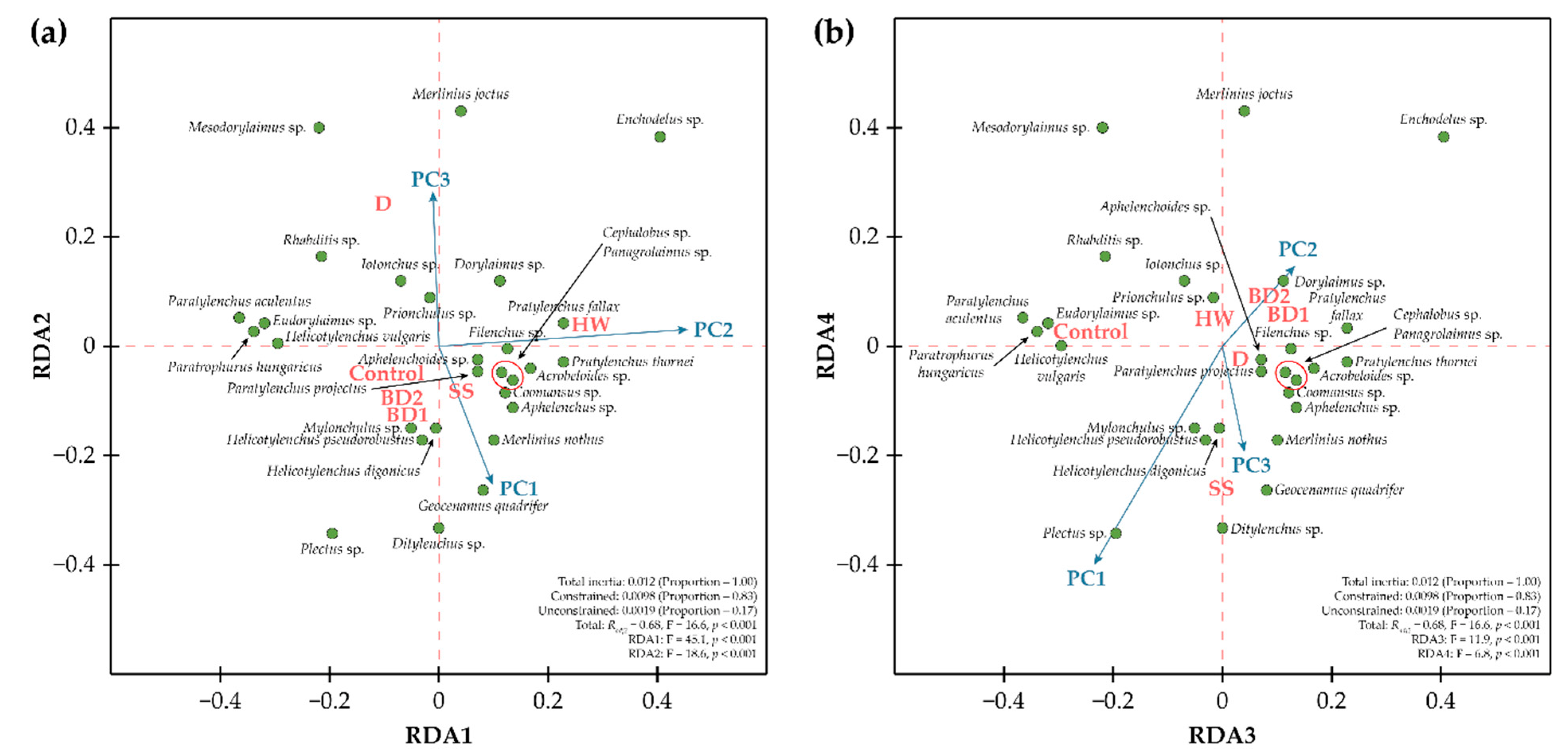
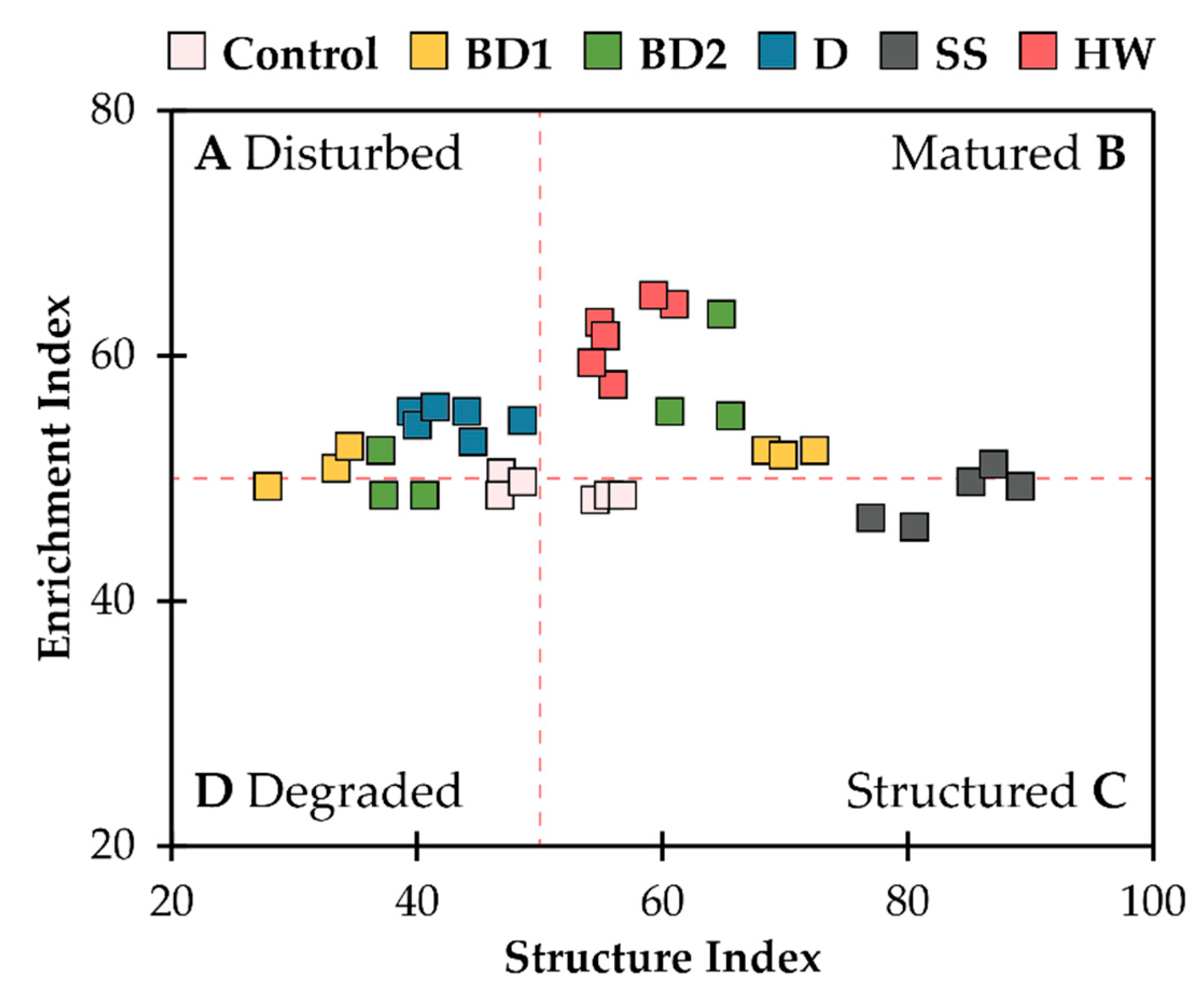
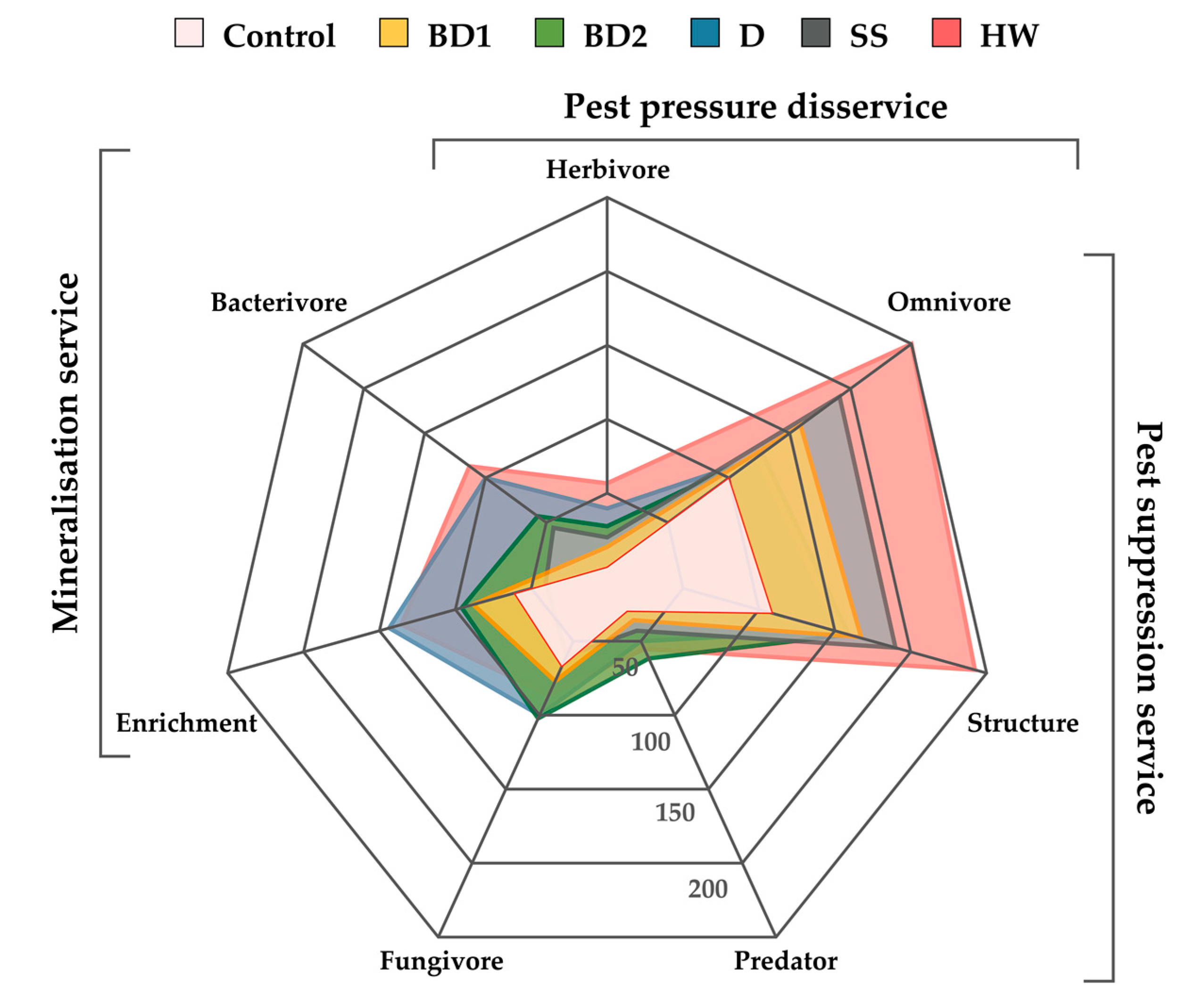
| Treatment | Description |
|---|---|
| Control | No soil amendment |
| Biochar, dose 1 (BD1) | Biochar produced by pyrolysis from the sludge of the wastewater treatment plant in Brno (WWTP), single dose (5%) |
| Biochar, dose 2 (BD2) | Biochar produced by pyrolysis from the sludge of the WWTP in Brno, double dose (10%) |
| Sewage sludge (SS) | Anaerobically stabilised sewage sludge from WWTP in Udlice, Chomutov region |
| Biogas digestate (D) | Anaerobically stabilised and dehydrated material from the waste biogas plant, Ahníkov, Chomutov region |
| Hemicellulose waste (HW) | Hemicellulose waste from paper manufacture, Chomutov region |
| Properties | Unit | Soil Amendments | |||
|---|---|---|---|---|---|
| BD | SS | D | HW | ||
| N | % DM | 2.44 ± 0.03 | 2.99 ± 0.11 | 2.03 ± 0.07 | 5.51 ± 0.04 |
| P | % DM | 2.88 ± 0.03 | 1.98 ± 0.07 | 0.43 ± 0.01 | 0.90 ± 0.03 |
| K | % DM | 0.32 ± 0.03 | 0.22 ± 0.03 | 2.93 ± 0.07 | 0.75 ± 0.02 |
| Ca | % DM | 2.56 ± 0.06 | 2.19 ± 0.19 | 1.65 ± 0.23 | 4.47 ± 0.04 |
| Mg | % DM | 0.43 ± 0.02 | 0.45 ± 0.01 | 0.49 ± 0.05 | 0.32 ± 0.01 |
| Na | % DM | 0.19 ± 0.01 | 0.09 ± 0.01 | 0.24 ± 0.01 | 0.06 ± 0.01 |
| Ash, 550 °C | % | 69.1 ± 0.10 | 50.6 ± 1.20 | 36.2 ± 2.10 | 27.8 ± 0.20 |
| Cox | % | 15.5 ± 0.01 | 24.7 ± 0.60 | 31.9 ± 1.00 | 36.1 ± 0.10 |
| C:N | - | 6.33 ± 0.07 | 8.26 ± 0.11 | 15.5 ± 0.74 | 6.55 ± 0.07 |
| Parameters | Unit | Value | Measuring Standard |
|---|---|---|---|
| pH (KCl) | - | 4.9 ± 0.2 | DSTU ISO 10390:2001 [56] |
| pH (H2O) | - | 5.7 ± 0.2 | DSTU ISO 10390:2001 [56] |
| Organic matter | % | 4.6 ± 0.3 | DSTU 7632:2014 [59] |
| Available P | mg kg−1 | 50.6 ± 2.0 | DSTU 4115:2002 [60] |
| Available K | mg kg−1 | 315 ± 9.8 | DSTU 4115:2002 [60] |
| Available Ca | mg kg−1 | 1 769 ± 80.5 | DSTU 7861:2015 [61] |
| Available Mg | mg kg−1 | 258 ± 7.3 | DSTU 7861:2015 [61] |
| Effect | Wilks-λ | F-Ratio | Effect df | Error df | p-Value | Effect Size (η2) |
|---|---|---|---|---|---|---|
| Intercept | 0.0000028 | 19,658.4 | 18 | 1.00 | 0.01 | 1.000 |
| Sampling time (T) * | 0.0000062 | 22.2 | 36 | 2.00 | 0.04 | 0.998 |
| Amendment type (A) ** | 0.0000000 | 70.1 | 90 | 9.36 | <0.001 | 0.999 |
| T × A | 0.0000000 | 2.0 | 180 | 29.71 | 0.02 | 0.922 |
| Taxon | Pf2/Pf1 | Pi/Pf2 | BD1 | BD2 | D | SS | HW | Radj2 |
|---|---|---|---|---|---|---|---|---|
| Herbivores | ||||||||
| Paratylenchus projectus (ec) | – | – | – | ↓ | ↓ | ↓ | ↓ | 0.30 |
| Paratylenchus aculentus (ec) | – | – | ↓ | ↓ | ↓ | ↓ | ↓ | 0.89 |
| Geocenamus quadrifer (ec) | – | – | ↓ | ↓ | ↓ | ↓ | ↓ | 0.77 |
| Merlinius nothus (ec) | – | – | – | ↓ | – | ↓ | ↓ | 0.66 |
| Merlinius joctus (ec) | – | – | – | – | ↓ | ↓ | ↓ | 0.81 |
| Helicotylenchus digonicus (sen) | – | – | – | ↓ | ↓ | – | ↓ | 0.53 |
| Helicotylenchus pseudorobustus (sen) | – | – | ↓ | ↓ | ↓ | – | ↓ | 0.48 |
| Helicotylenchus vulgaris (sen) | – | – | ↓ | ↓ | ↓ | ↓ | ↓ | 0.88 |
| Paratrophurus hungaricus (ec) | – | – | ↓ | ↓ | ↓ | ↓ | ↓ | 0.76 |
| Pratylenchus fallax (men) | – | – | – | – | ↑ | – | ↑ | 0.07 |
| Pratylenchus thornei (men) | – | – | – | – | – | – | – | –0.27 |
| Bacterivores | ||||||||
| Acrobeloides sp. | – | – | – | – | – | ↓ | ↓ | 0.26 |
| Cephalobus sp. | – | ↓ | – | – | – | ↓ | ↓ | 0.68 |
| Panagrolaimus sp. | – | ↓ | – | – | ↑ | ↓ | ↓ | 0.65 |
| Plectus sp. | – | – | ↓ | ↓ | ↑ | – | ↓ | 0.74 |
| Rhabditis sp. | – | – | ↓ | ↓ | – | ↓ | ↓ | 0.68 |
| Fungivores | ||||||||
| Aphelenchoides sp. | – | – | ↓ | – | ↓ | ↓ | ↑ | 0.23 |
| Aphelenchus sp. | – | ↓ | – | ↓ | – | ↓ | ↓ | 0.60 |
| Ditylenchus sp. | – | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | 0.90 |
| Filenchus sp. | ↑ | ↓ | – | – | – | ↓ | ↓ | 0.88 |
| Omnivores | ||||||||
| Dorylaimus sp. | ↑ | ↓ | – | – | ↑ | – | ↑ | 0.87 |
| Eudorylaimus sp. | – | – | – | – | ↓ | – | – | 0.07 |
| Mesodorylaimus sp. | – | – | – | – | – | ↓ | ↓ | –0.23 |
| Predators | ||||||||
| Enchodelus sp. | – | – | ↑ | ↑ | – | – | – | 0.72 |
| Coomansus sp. | – | – | – | – | – | ↑ | ↑ | 0.02 |
| Iotonchus sp. | – | – | – | – | – | – | – | –0.24 |
| Mylonchulus sp. | – | – | ↓ | ↓ | ↓ | ↓ | ↓ | 0.60 |
| Prionchulus sp. | – | – | – | – | – | – | – | –0.14 |
| Variable | PC1, λ = 16.3 (48.1%) | PC2, λ = 8.8 (25.8%) | PC3, λ = 3.0 (8.9%) | PC4, λ = 2.3 (6.8%) |
|---|---|---|---|---|
| Maturity Index (MI) | 0.97 | – | – | – |
| Maturity Index 2–5 | 0.95 | – | – | – |
| Sigma Maturity Index [67] | 0.99 | – | – | – |
| Plant Parasitic Index (PPI) | 0.45 | 0.42 | – | – |
| PPI/MI | −0.95 | – | – | – |
| Channel Index | 0.49 | −0.76 | – | – |
| Basal Index | −0.86 | −0.44 | – | – |
| Enrichment Index | −0.40 | 0.83 | – | – |
| Structure Index | 0.93 | – | – | – |
| Total biomass, mg | – | 0.94 | – | – |
| Total number, ind | −0.63 | – | −0.48 | 0.51 |
| Composite footprint | – | 0.92 | – | – |
| Enrichment footprint | −0.78 | 0.46 | – | – |
| Structure footprint | – | 0.92 | – | – |
| Herbivore footprint | −0.44 | – | −0.51 | 0.64 |
| Fungivore footprint | −0.73 | – | −0.54 | – |
| Bacterivore footprint | −0.47 | 0.68 | – | 0.33 |
| Predator footprint | 0.34 | – | – | 0.75 |
| Omnivore footprint | – | 0.93 | – | – |
| Herbivores, % of total | 0.80 | – | – | – |
| Fungivores, % of total | −0.84 | – | – | −0.37 |
| Fungivores, % of FLNs | −0.80 | −0.47 | – | – |
| Bacterivores, % of total | −0.81 | – | 0.39 | – |
| Bacterivores, % of FLNs | −0.78 | – | 0.43 | – |
| Predators, % of total | 0.69 | −0.47 | – | 0.36 |
| Predators, % of FLNs | 0.78 | −0.36 | – | 0.38 |
| Omnivores, % of total | 0.71 | 0.56 | – | – |
| Omnivores, % of FLNs | 0.86 | 0.35 | – | – |
| Migratory endoparasites, % of herbivores | 0.76 | 0.35 | – | – |
| Semi-endoparasites, % of herbivores | – | −0.74 | – | – |
| Ectoparasites, % of herbivores | −0.90 | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanovska, T.; Skwiercz, A.; Pidlisnyuk, V.; Zhukov, O.; Kozacki, D.; Mamirova, A.; Newton, R.A.; Ust’ak, S. The Short-Term Effects of Amendments on Nematode Communities and Diversity Patterns under the Cultivation of Miscanthus × giganteus on Marginal Land. Agronomy 2022, 12, 2063. https://doi.org/10.3390/agronomy12092063
Stefanovska T, Skwiercz A, Pidlisnyuk V, Zhukov O, Kozacki D, Mamirova A, Newton RA, Ust’ak S. The Short-Term Effects of Amendments on Nematode Communities and Diversity Patterns under the Cultivation of Miscanthus × giganteus on Marginal Land. Agronomy. 2022; 12(9):2063. https://doi.org/10.3390/agronomy12092063
Chicago/Turabian StyleStefanovska, Tatyana, Andrzej Skwiercz, Valentina Pidlisnyuk, Oleksandr Zhukov, Dawid Kozacki, Aigerim Mamirova, Robert Ato Newton, and Sergey Ust’ak. 2022. "The Short-Term Effects of Amendments on Nematode Communities and Diversity Patterns under the Cultivation of Miscanthus × giganteus on Marginal Land" Agronomy 12, no. 9: 2063. https://doi.org/10.3390/agronomy12092063
APA StyleStefanovska, T., Skwiercz, A., Pidlisnyuk, V., Zhukov, O., Kozacki, D., Mamirova, A., Newton, R. A., & Ust’ak, S. (2022). The Short-Term Effects of Amendments on Nematode Communities and Diversity Patterns under the Cultivation of Miscanthus × giganteus on Marginal Land. Agronomy, 12(9), 2063. https://doi.org/10.3390/agronomy12092063









