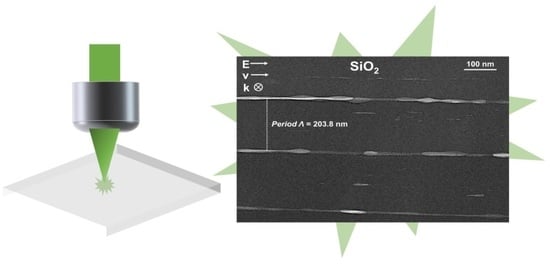
On the Formation of Nanogratings in Commercial Oxide Glasses by Femtosecond Laser Direct Writing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
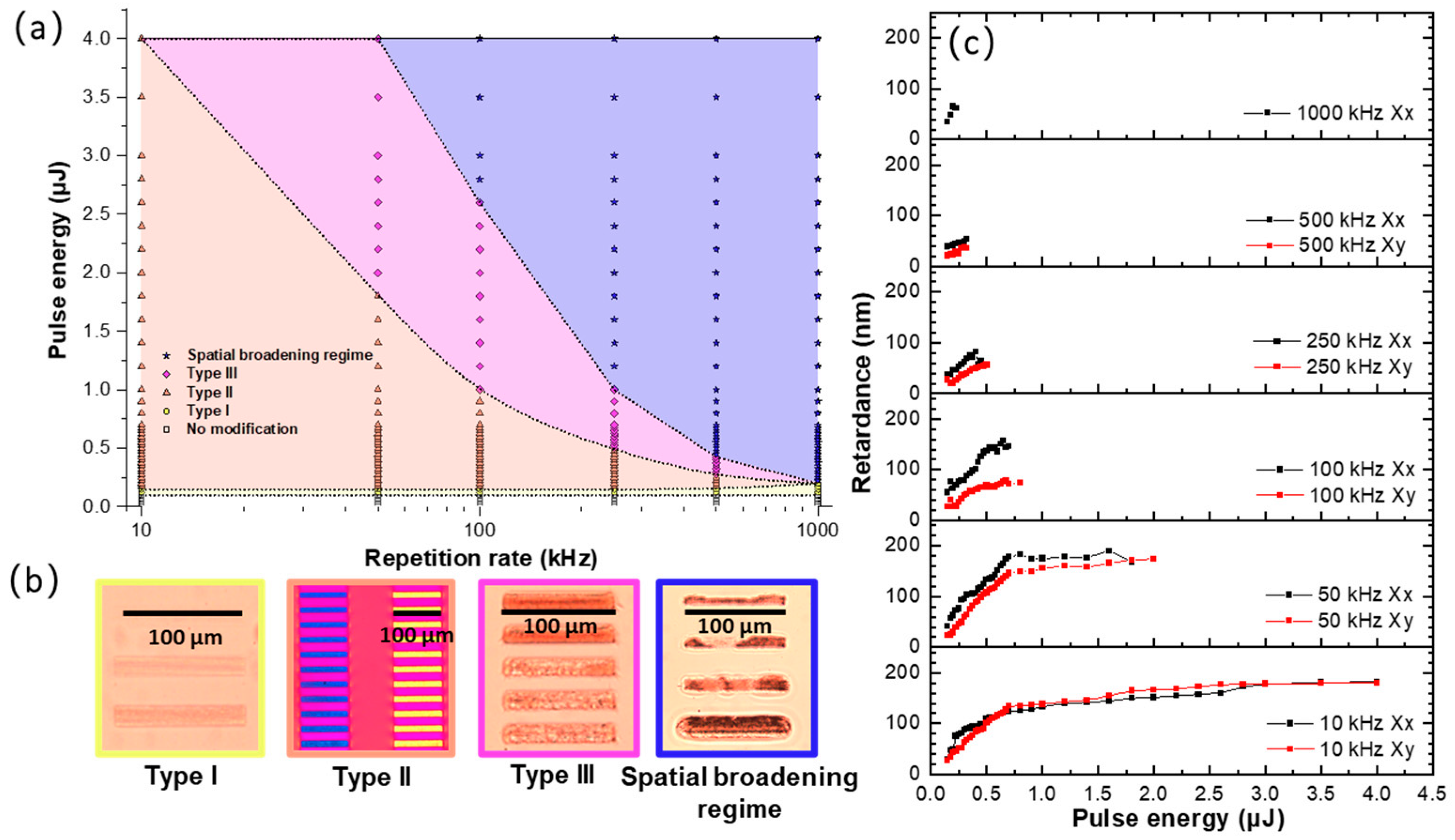
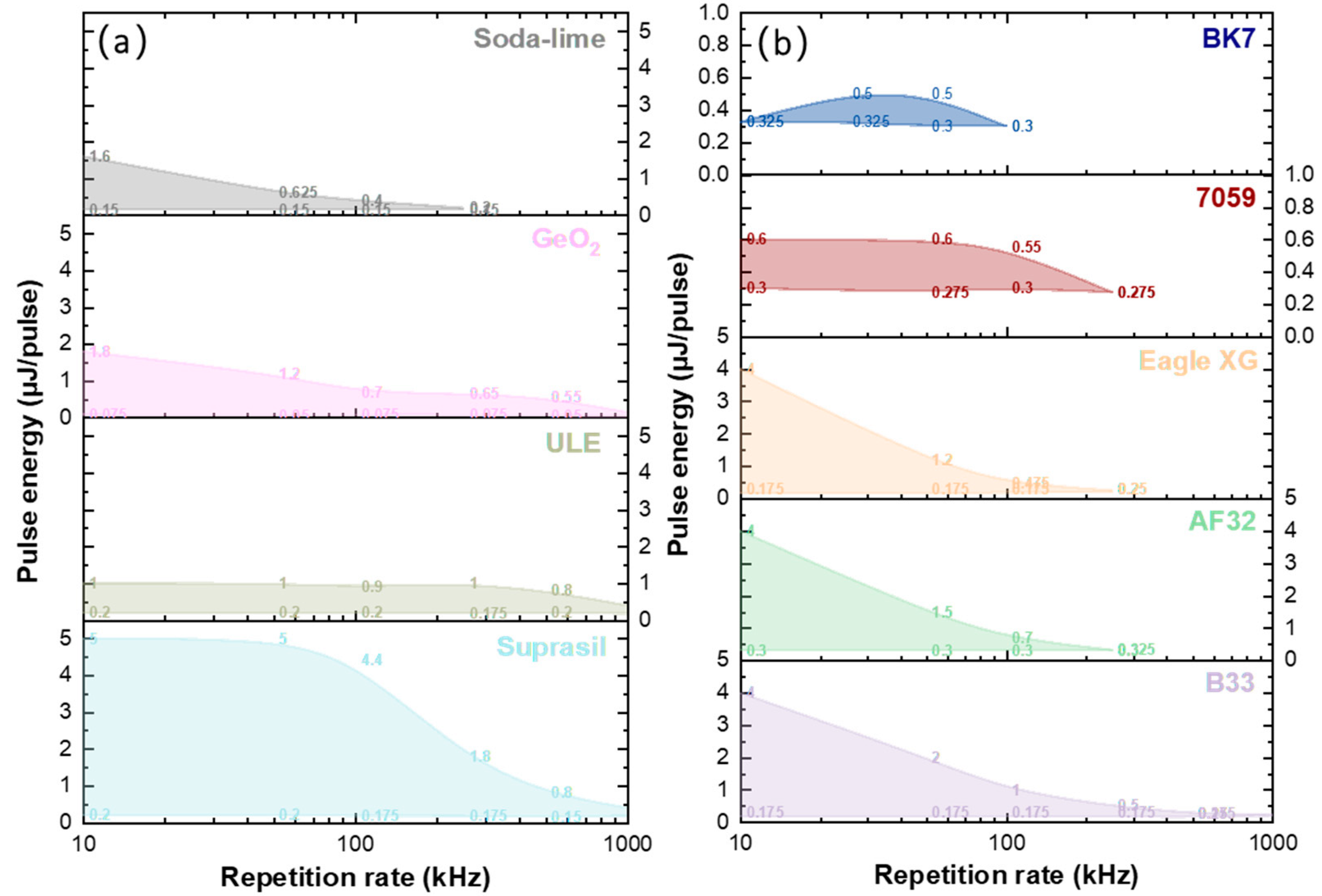
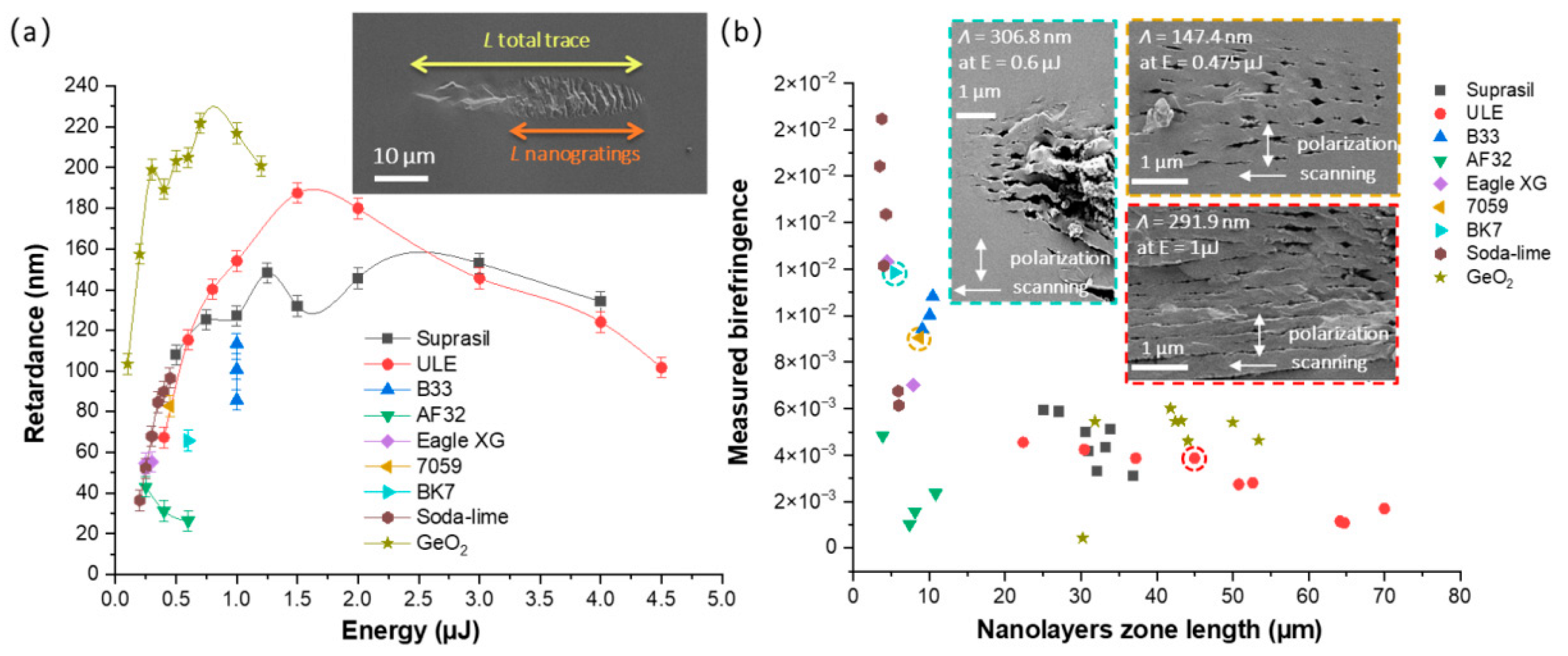
3.1. Study of Type II Modifications Windows in the Different Glasses
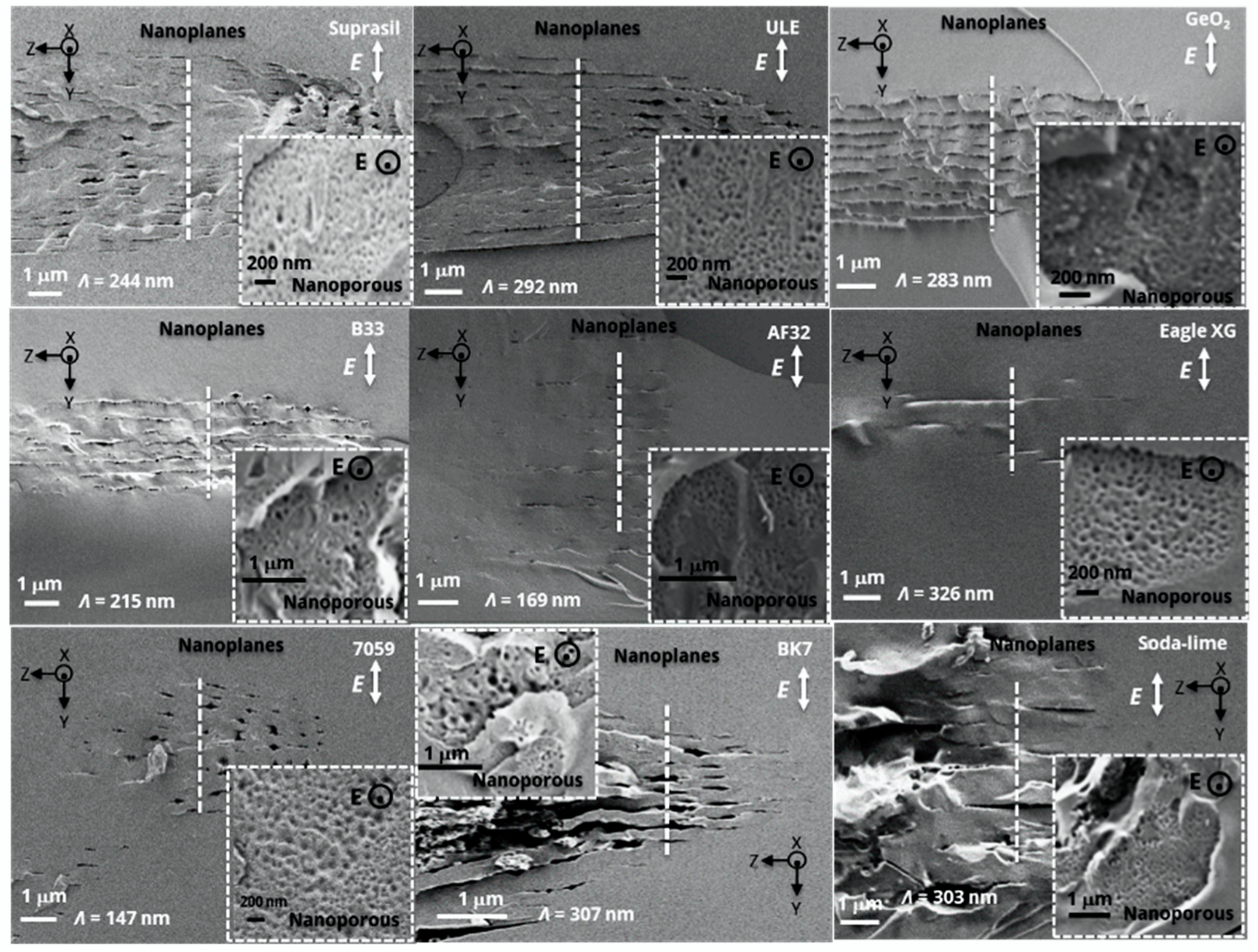
3.2. Study of Type II Modifications Origin in the Different Glasses
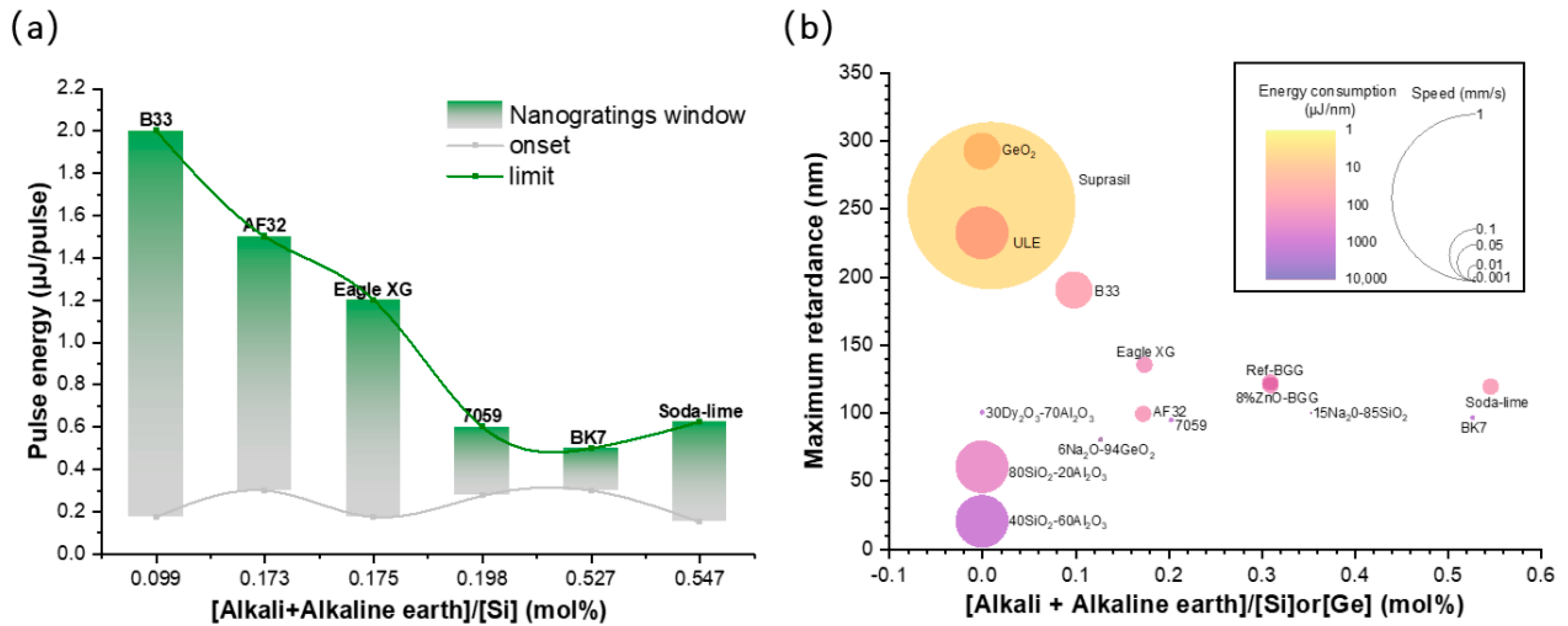
3.3. Influence of Alkali Content on NG Windows in Different Glasses
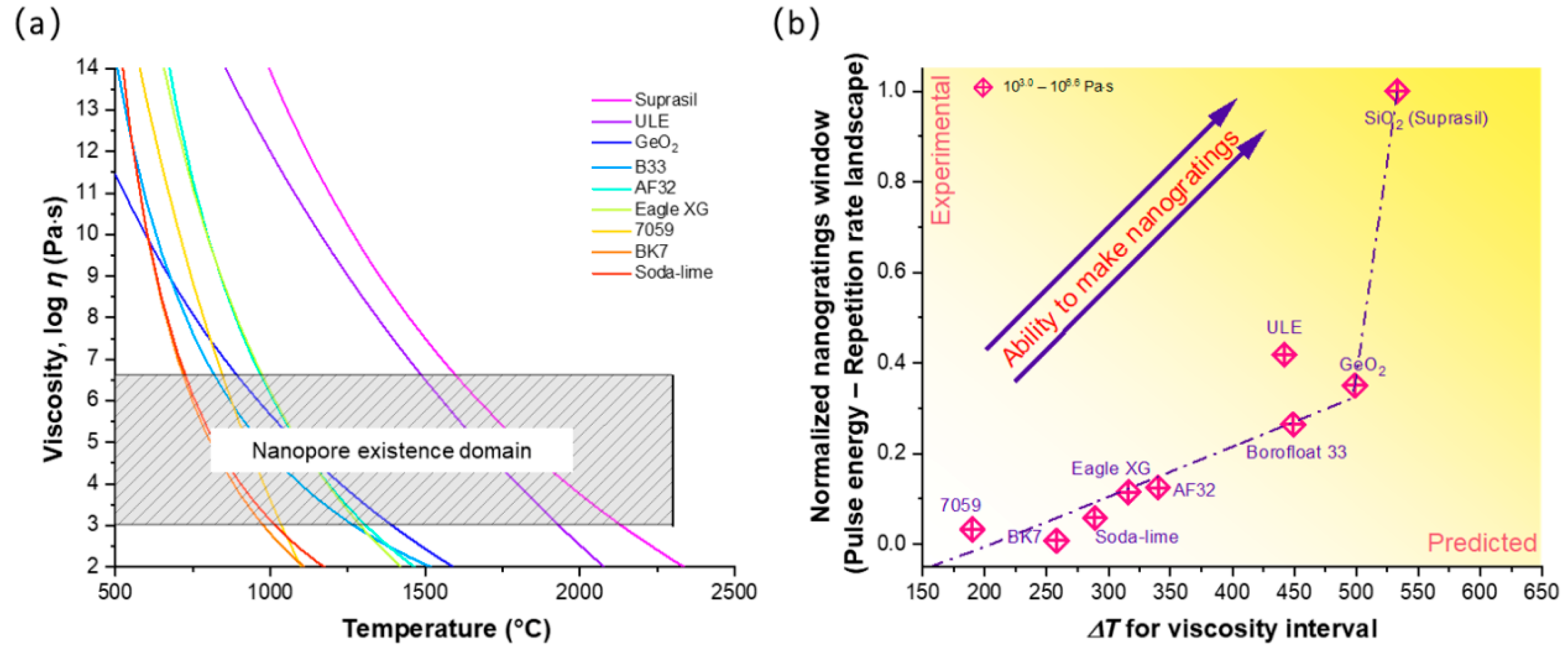
4. Discussion on Nanopores Formation and Erasure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shimotsuma, Y.; Kazansky, P.G.; Qiu, J.; Hirao, K. Self-organized nanogratings in glass irradiated by ultrashort light pulses. Phys. Rev. Lett. 2003, 91, 247405. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liao, Y.; He, F.; Zeng, B.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Tuning etch selectivity of fused silica irradiated by femtosecond laser pulses by controlling polarization of the writing pulses. J. Appl. Phys. 2011, 109, 053114. [Google Scholar] [CrossRef]
- Liao, Y.; Shen, Y.; Qiao, L.; Chen, D.; Cheng, Y.; Sugioka, K.; Midorikawa, K. Femtosecond laser nanostructuring in porous glass with sub-50 nm feature sizes. Opt. Lett. 2013, 38, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Hnatovsky, C.; Taylor, R.S.; Simova, E.; Rajeev, P.P.; Rayner, D.M.; Bhardwaj, V.R.; Corkum, P.B. Fabrication of microchannels in glass using focused femtosecond laser radiation and selective chemical etching. Appl. Phys. A 2006, 84, 47–61. [Google Scholar] [CrossRef]
- Beresna, M.; Gecevičius, M.; Kazansky, P.G.; Taylor, T.; Kavokin, A.V. Exciton mediated self-organization in glass driven by ultrashort light pulses. Appl. Phys. Lett. 2012, 101, 053120. [Google Scholar] [CrossRef]
- Liao, Y.; Cheng, Y.; Liu, C.; Song, J.; He, F.; Shen, Y.; Chen, D.; Xu, Z.; Fan, Z.; Wei, X.; et al. Direct laser writing of sub-50 nm nanofluidic channels buried in glass for three-dimensional micro-nanofluidic integration. Lab Chip 2013, 13, 1626–1631. [Google Scholar] [CrossRef]
- Taylor, R.; Hnatovsky, C.; Simova, E. Applications of femtosecond laser induced self-organized planar nanocracks inside fused silica glass. Laser Photon. Rev. 2008, 2, 26–46. [Google Scholar] [CrossRef]
- Lancry, M.; Poumellec, B.; Canning, J.; Cook, K.; Poulin, J.-C.; Brisset, F. Ultrafast nanoporous silica formation driven by femtosecond laser irradiation. Laser Photon. Rev. 2013, 7, 953–962. [Google Scholar] [CrossRef]
- Lancry, M.; Zimmerman, F.; Desmarchelier, R.; Tian, J.; Brisset, F.; Nolte, S.; Poumellec, B. Nanogratings formation in multicomponent silicate glasses. Appl. Phys. B 2016, 122, 66. [Google Scholar] [CrossRef]
- Lancry, M.; Canning, J.; Cook, K.; Heili, M.; Neuville, D.R.; Poumellec, B. Nanoscale femtosecond laser milling and control of nanoporosity in the normal and anomalous regimes of GeO2-SiO2 glasses. Opt. Mater. Express 2016, 6, 321–330. [Google Scholar] [CrossRef]
- Rudenko, A.; Colombier, J.P.; Itina, T.E. Nanopore-mediated ultrashort laser-induced formation and erasure of volume nanogratings in glass. Phys. Chem. Chem. Phys. 2018, 20, 5887–5899. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Cavillon, M.; Poumellec, B.; Pugliese, D.; Janner, D.; Lancry, M. Application and validation of a viscosity approach to the existence of nanogratings in oxide glasses. Opt. Mater. 2022, 130, 112576. [Google Scholar] [CrossRef]
- Poumellec, B.; Lancry, M.; Chahid-Erraji, A.; Kazansky, P.G. Modification thresholds in femtosecond laser processing of pure silica: Review of dependencies on laser parameters. Opt. Mater. Express 2011, 1, 766–782. [Google Scholar] [CrossRef]
- Shimotsuma, Y.; Kubota, S.; Murata, A.; Kurita, T.; Sakakura, M.; Miura, K.; Lancry, M.; Poumellec, B. Tunability of form birefringence induced by femtosecond laser irradiation in anion-doped silica glass. J. Am. Ceram. Soc. 2017, 100, 3912–3919. [Google Scholar] [CrossRef]
- Zimmermann, F.; Lancry, M.; Plech, A.; Richter, S.; Babu, B.H.; Poumellec, B.; Tünnermann, A.; Nolte, S. Femtosecond laser written nanostructures in Ge-doped glasses. Opt. Lett. 2016, 41, 1161–1164. [Google Scholar] [CrossRef]
- Asai, T.; Shimotsuma, Y.; Kurita, T.; Murata, A.; Kubota, S.; Sakakura, M.; Miura, K.; Brisset, F.; Poumellec, B.; Lancry, M. Systematic control of structural changes in GeO2 glass induced by femtosecond laser direct writing. J. Am. Ceram. Soc. 2015, 98, 1471–1477. [Google Scholar] [CrossRef]
- Zhang, F.; Cerkauskaite, A.; Drevinskas, R.; Kazansky, P.G.; Qiu, J. Microengineering of optical properties of GeO2 glass by ultrafast laser nanostructuring. Adv. Opt. Mater. 2017, 5, 1700342. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Dong, G.; Qiu, J. Embedded nanogratings in germanium dioxide glass induced by femtosecond laser direct writing. J. Opt. Soc. Am. B 2014, 31, 860–864. [Google Scholar] [CrossRef]
- Wortmann, D.; Gottmann, J.; Brandt, N.; Horn-Solle, H. Micro- and nanostructures inside sapphire by fs-laser irradiation and selective etching. Opt. Express 2008, 16, 1517–1522. [Google Scholar] [CrossRef]
- Shimotsuma, Y.; Hirao, K.; Qiu, J.R.; Miura, K. Nanofabrication in transparent materials with a femtosecond pulse laser. J. Non-Cryst. Solids 2006, 352, 646–656. [Google Scholar] [CrossRef]
- Zhang, F.; Nie, Z.; Huang, H.; Ma, L.; Tang, H.; Hao, M.; Qiu, J. Self-assembled three-dimensional periodic micro-nano structures in bulk quartz crystal induced by femtosecond laser pulses. Opt. Express 2019, 27, 6442–6450. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Miese, C.; Döring, S.; Zimmermann, F.; Withford, M.J.; Tünnermann, A.; Nolte, S. Laser induced nanogratings beyond fused silica—periodic nanostructures in borosilicate glasses and ULE™. Opt. Mater. Express 2013, 3, 1161–1166. [Google Scholar]
- Zimmermann, F.; Plech, A.; Richter, S.; Tünnermann, A.; Nolte, S. Ultrashort laser pulse induced nanogratings in borosilicate glass. Appl. Phys. Lett. 2014, 104, 211107. [Google Scholar] [CrossRef]
- Fedotov, S.S.; Lipat’ev, A.S.; Lotarev, S.V.; Sigaev, V.N. Local formation of birefringent structures in alkali-silicate glass by femtosecond laser beam. Glass Ceram. 2017, 74, 227–229. [Google Scholar] [CrossRef]
- Lotarev, S.; Fedotov, S.; Lipatiev, A.; Presnyakov, M.; Kazansky, P.; Sigaev, V. Light-driven nanoperiodical modulation of alkaline cation distribution inside sodium silicate glass. J. Non-Cryst. Solids 2018, 479, 49–54. [Google Scholar] [CrossRef]
- Desmarchelier, R.; Poumellec, B.; Brisset, F.; Mazerat, S.; Lancry, M. In the heart of femtosecond laser induced nanogratings: From porous nanoplanes to form birefringence. World J. Nano Sci. Eng. 2015, 5, 115–125. [Google Scholar] [CrossRef]
- Liao, Y.; Ni, J.; Qiao, L.; Huang, M.; Bellouard, Y.; Sugioka, K.; Cheng, Y. High-fidelity visualization of formation of volume nanogratings in porous glass by femtosecond laser irradiation. Optica 2015, 2, 329–334. [Google Scholar] [CrossRef]
- Liao, Y.; Pan, W.J.; Cui, Y.; Qiao, L.L.; Bellouard, Y.; Sugioka, K.; Cheng, Y. Formation of in-volume nanogratings with sub-100-nm periods in glass by femtosecond laser irradiation. Opt. Lett. 2015, 40, 3623–3626. [Google Scholar] [CrossRef]
- Čerkauskaite, A. Ultrafast Laser Nanostructuring for Photonics and Information Technology. Ph.D. Thesis, University of Southampton, Southampton, UK, 2018. [Google Scholar]
- Cerkauskaite, A.; Drevinskas, R.; Rybaltovskii, A.O.; Kazansky, P.G. Ultrafast laser-induced birefringence in various porosity silica glasses: From fused silica to aerogel. Opt. Express 2017, 25, 8011–8021. [Google Scholar] [CrossRef]
- Bricchi, E.; Klappauf, B.G.; Kazansky, P.G. Form birefringence and negative index change created by femtosecond direct writing in transparent materials. Opt. Lett. 2004, 29, 119–121. [Google Scholar] [CrossRef]
- Muzi, E.; Cavillon, M.; Lancry, M.; Brisset, F.; Que, R.; Pugliese, D.; Janner, D.; Poumellec, B. Towards a rationalization of ultrafast laser-induced crystallization in lithium niobium borosilicate glasses: The key role of the scanning speed. Crystals 2021, 11, 290. [Google Scholar] [CrossRef]
- Eaton, S.M.; Zhang, H.; Ng, M.L.; Li, J.; Chen, W.-J.; Ho, S.; Herman, P.R. Transition from thermal diffusion to heat accumulation in high repetition rate femtosecond laser writing of buried optical waveguides. Opt. Express 2008, 16, 9443–9458. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Heinrich, M.; Döring, S.; Tünnermann, A.; Nolte, S.; Peschel, U. Nanogratings in fused silica: Formation, control, and applications. J. Laser Appl. 2012, 24, 042008. [Google Scholar] [CrossRef]
- Mauclair, C.; Zamfirescu, M.; Colombier, J.P.; Cheng, G.; Mishchik, K.; Audouard, E.; Stoian, R. Control of ultrafast laser-induced bulk nanogratings in fused silica via pulse time envelopes. Opt. Express 2012, 20, 12997–13005. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, M.; Lei, Y.; Wang, L.; Yu, Y.H.; Kazansky, P.G. Ultralow-loss geometric phase and polarization shaping by ultrafast laser writing in silica glass. Light Sci. Appl. 2020, 9, 15. [Google Scholar] [CrossRef]
- Zhang, F.; Tu, Z.; Du, X.; Zhang, H.; Qiu, J. Femtosecond laser induced migration of alkali ions in calcium silicate glasses. Mater. Lett. 2014, 137, 92–95. [Google Scholar] [CrossRef]
- Fernandez, T.T.; Sakakura, M.; Eaton, S.M.; Sotillo, B.; Siegel, J.; Solis, J.; Shimotsuma, Y.; Miura, K. Bespoke photonic devices using ultrafast laser driven ion migration in glasses. Prog. Mater Sci. 2018, 94, 68–113. [Google Scholar] [CrossRef]
- Mori, S.; Kurita, T.; Shimotsuma, Y.; Sakakura, M.; Miura, K. Nanogratings embedded in Al2O3-Dy2O3 glass by femtosecond laser irradiation. J. Laser Micro Nanoeng. 2016, 11, 87–90. [Google Scholar] [CrossRef]
- Lotarev, S.V.; Fedotov, S.S.; Kurina, A.I.; Lipatiev, A.S.; Sigaev, V.N. Ultrafast laser-induced nanogratings in sodium germanate glasses. Opt. Lett. 2019, 44, 1564–1567. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Cicconi, M.R.; Tsuji, Y.; Shimizu, M.; Shimotsuma, Y.; Miura, K.; Peng, G.D.; Neuville, D.R.; Poumellec, B.; et al. Femtosecond laser direct writing in SiO2-Al2O3 binary glasses and thermal stability of Type II permanent modifications. J. Am. Ceram. Soc. 2020, 103, 4286–4294. [Google Scholar] [CrossRef]
- Yao, H.; Zaiter, R.; Cavillon, M.; Sapaly, B.; Calzavara, F.; Delullier, P.; Cardinal, T.; Dai, Y.; Poumellec, B.; Lancry, M. Photosensitivity of barium germano-gallate glasses under femtosecond laser direct writing for Mid-IR applications. Ceram. Int. 2021, 47, 34235–34241. [Google Scholar]
- Yao, H.; Zaiter, R.; Cavillon, M.; Delullier, P.; Lu, B.; Cardinal, T.; Dai, Y.; Poumellec, B.; Lancry, M. Formation of nanogratings driven by ultrafast laser irradiation in mid-IR heavy oxide glasses. Ceram. Int. 2022, in press.
- Buschlinger, R.; Nolte, S.; Peschel, U. Self-organized pattern formation in laser-induced multiphoton ionization. Phys. Rev. B 2014, 89, 184306. [Google Scholar]
- Rudenko, A.; Colombier, J.-P.; Itina, T.E. From random inhomogeneities to periodic nanostructures induced in bulk silica by ultrashort laser. Phys. Rev. B 2016, 93, 075427. [Google Scholar]
- Cavillon, M.; Wang, Y.; Poumellec, B.; Brisset, F.; Lancry, M. Erasure of nanopores in silicate glasses induced by femtosecond laser irradiation in the Type II regime. Appl. Phys. A 2020, 126, 876. [Google Scholar]
| Commercial Glass Name | Type | Chemical Composition (mol%) |
|---|---|---|
| SuprasilCG | Silica | 100 SiO2 |
| ULE | Titanium silicate | 94.25 SiO2, 5.75 TiO2 |
| B33 | Free/low alkali aluminoborosilicate | 81 SiO2, 2 Al2O3, 13 B2O3, 4 Na2O/K2O |
| AF32 | 66.43 SiO2, 11.28 Al2O3, 10.73 B2O3, 5.30 CaO, 4.63 MgO, 1.36 BaO, others < 1 | |
| Eagle XG | 65.71 SiO2, 11.10 Al2O3, 11.65 B2O3, 8.64 CaO, 2.28 MgO, others < 1 | |
| 7059 | 63 SiO2, 8.5 Al2O3, 16 B2O3, 12.5 BaO | |
| BK7 | Alkali borosilicate | 69.13 SiO2, 10.75 B2O3, 3.07 BaO, 10.40 Na2O, 6.29 K2O, others < 1 |
| Soda-lime | Soda-lime silicate | 72.6 SiO2, 13 Na2O, 8.8 CaO, 4.3 MgO, 0.6 Al2O3, 0.3 K2O, 0.2 SO3, 0.1 Fe2O3 |
| GeO2 | Germania | 100 GeO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Cavillon, M.; Pugliese, D.; Janner, D.; Poumellec, B.; Lancry, M. On the Formation of Nanogratings in Commercial Oxide Glasses by Femtosecond Laser Direct Writing. Nanomaterials 2022, 12, 2986. https://doi.org/10.3390/nano12172986
Xie Q, Cavillon M, Pugliese D, Janner D, Poumellec B, Lancry M. On the Formation of Nanogratings in Commercial Oxide Glasses by Femtosecond Laser Direct Writing. Nanomaterials. 2022; 12(17):2986. https://doi.org/10.3390/nano12172986
Chicago/Turabian StyleXie, Qiong, Maxime Cavillon, Diego Pugliese, Davide Janner, Bertrand Poumellec, and Matthieu Lancry. 2022. "On the Formation of Nanogratings in Commercial Oxide Glasses by Femtosecond Laser Direct Writing" Nanomaterials 12, no. 17: 2986. https://doi.org/10.3390/nano12172986