Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production
Abstract
:1. Introduction
2. Experimental
2.1. Materials
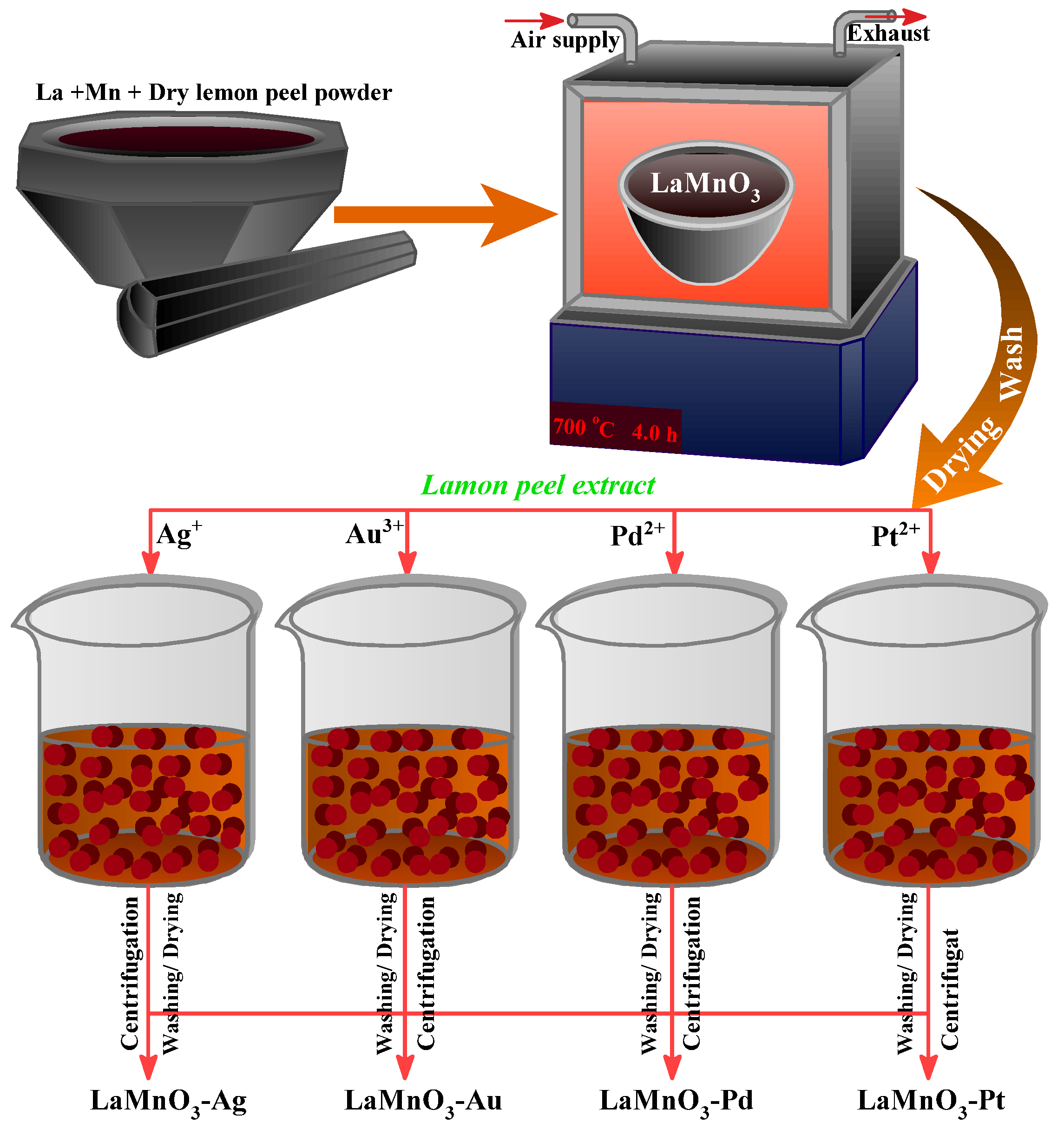
2.2. Preparation of Nanomaterials
2.2.1. Synthesis of LaMnO3 by Green Solid-State Combustion Method
2.2.2. Deposition of Noble Metals
2.3. Characterization of Synthesized Nanomaterials
2.4. Photocatalytic Reforming of Methanol to Hydrogen
3. Results and Discussion
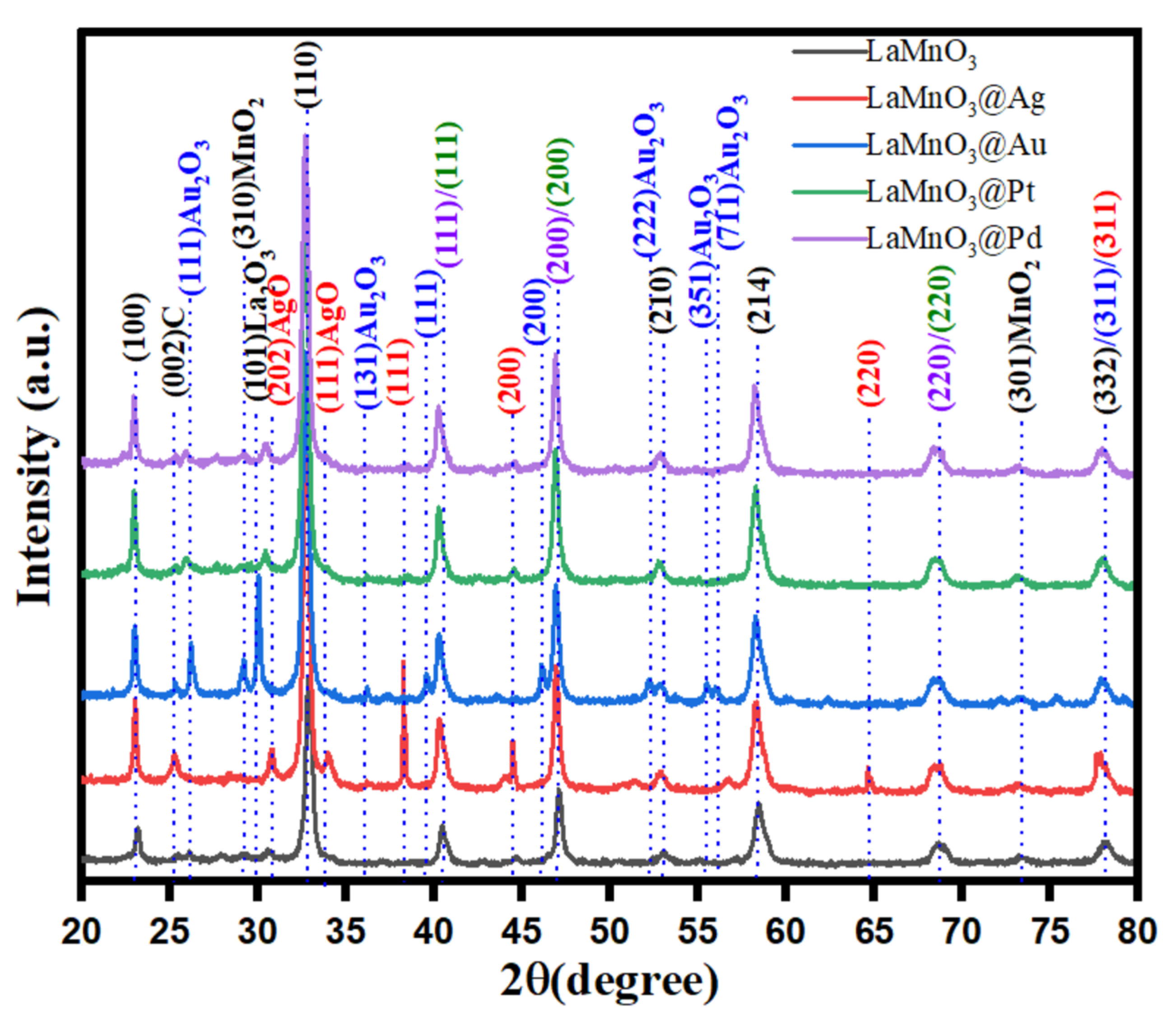
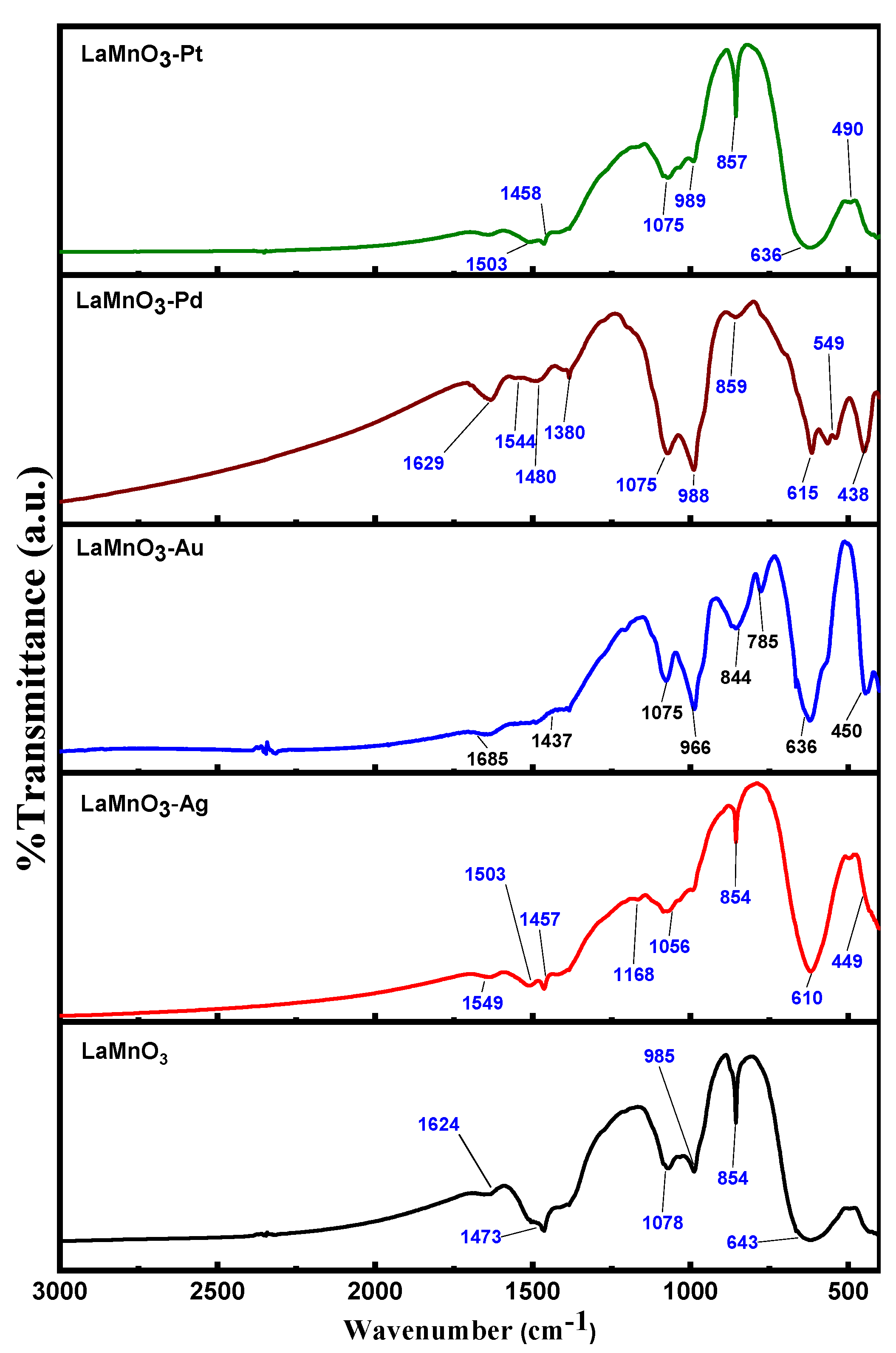
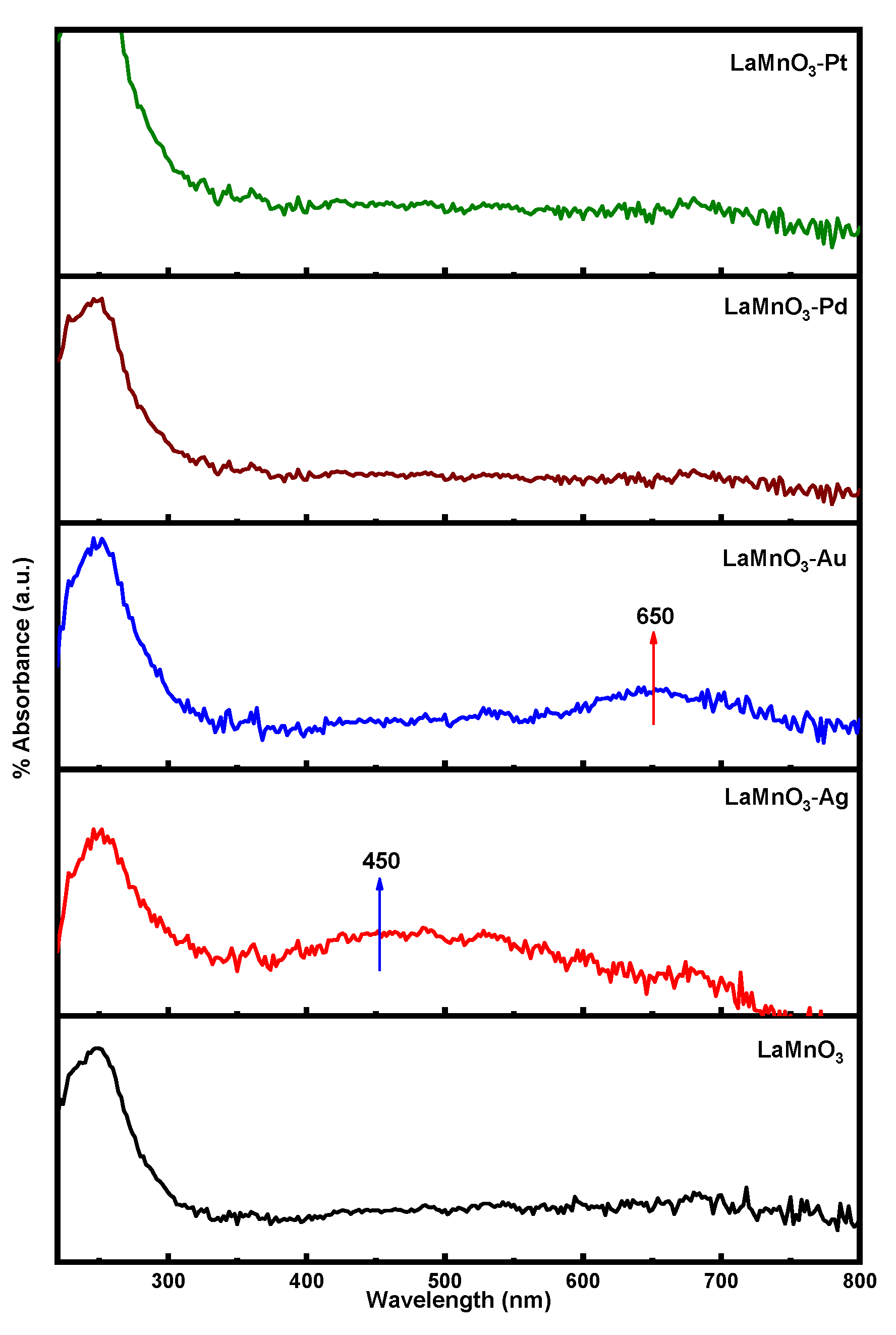
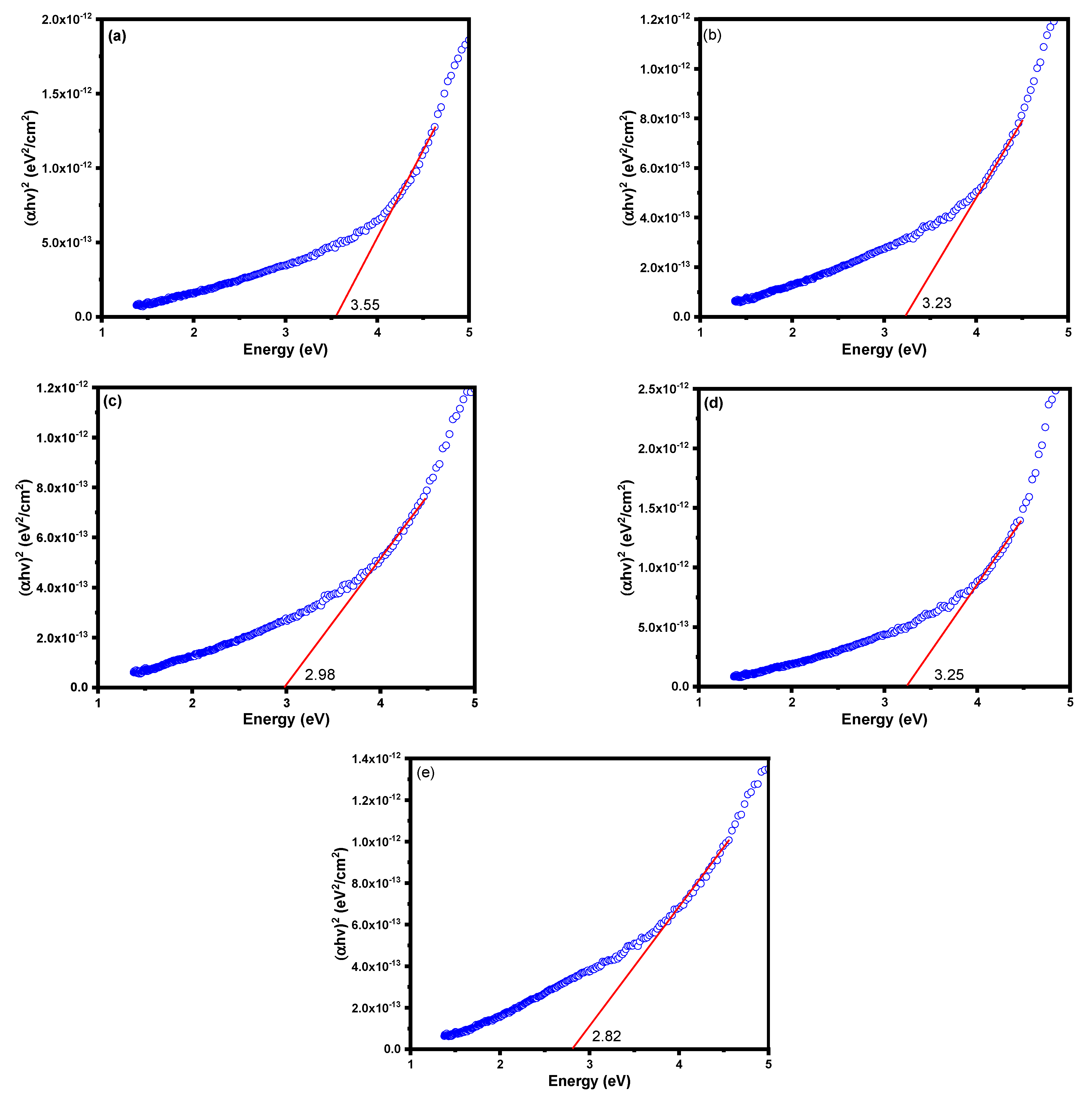
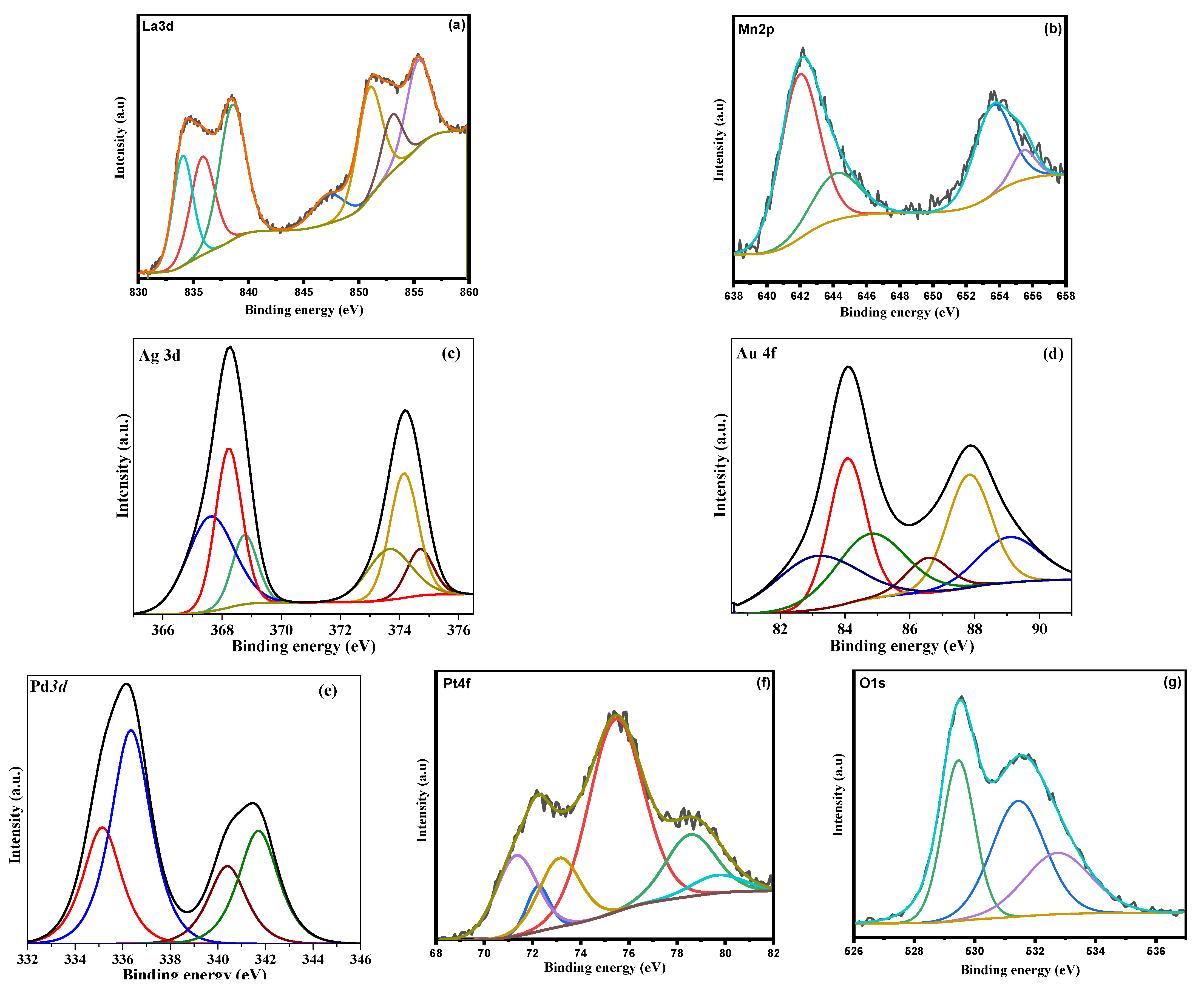
3.1. Characterization of LaMnO3 Nanocomposites
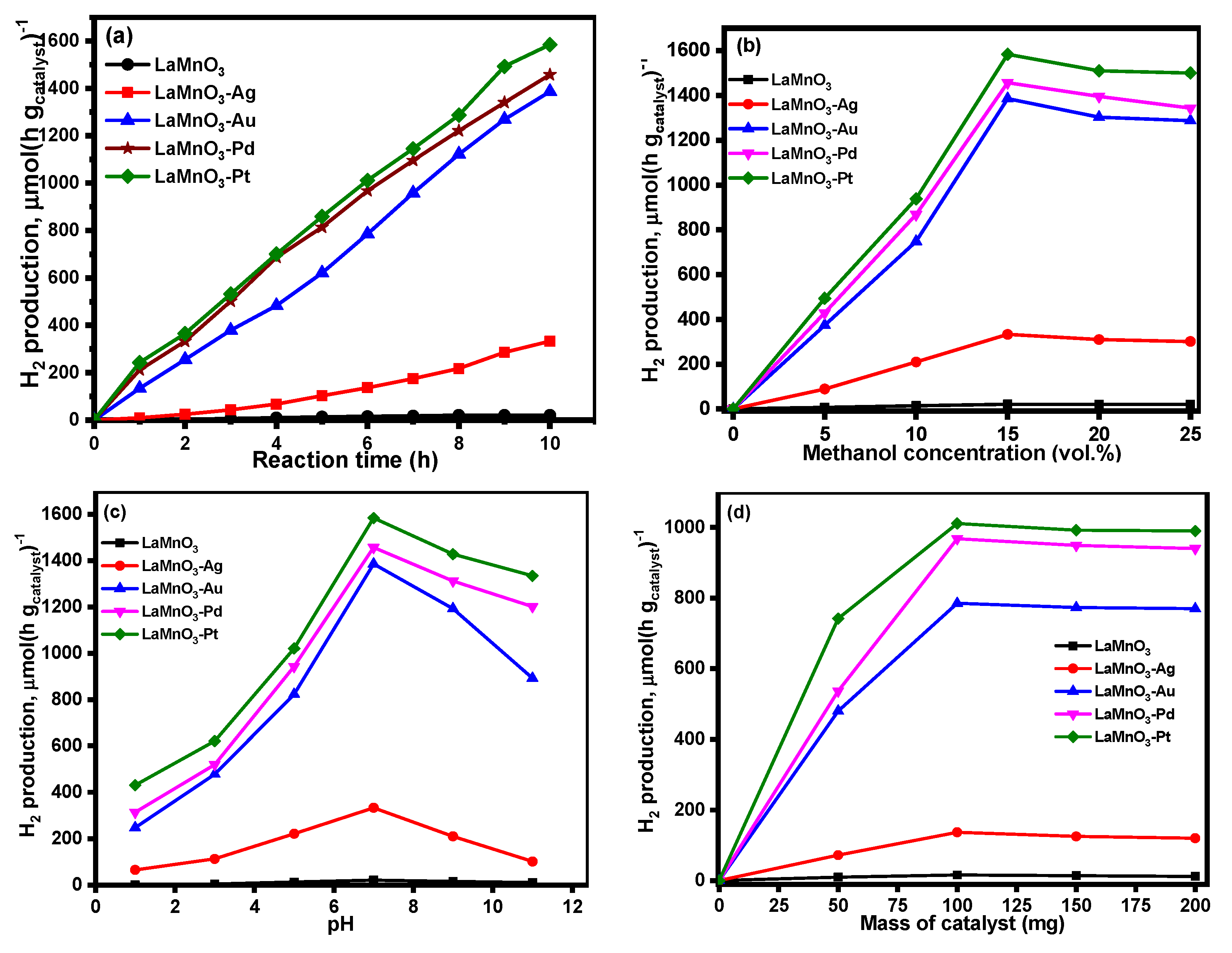
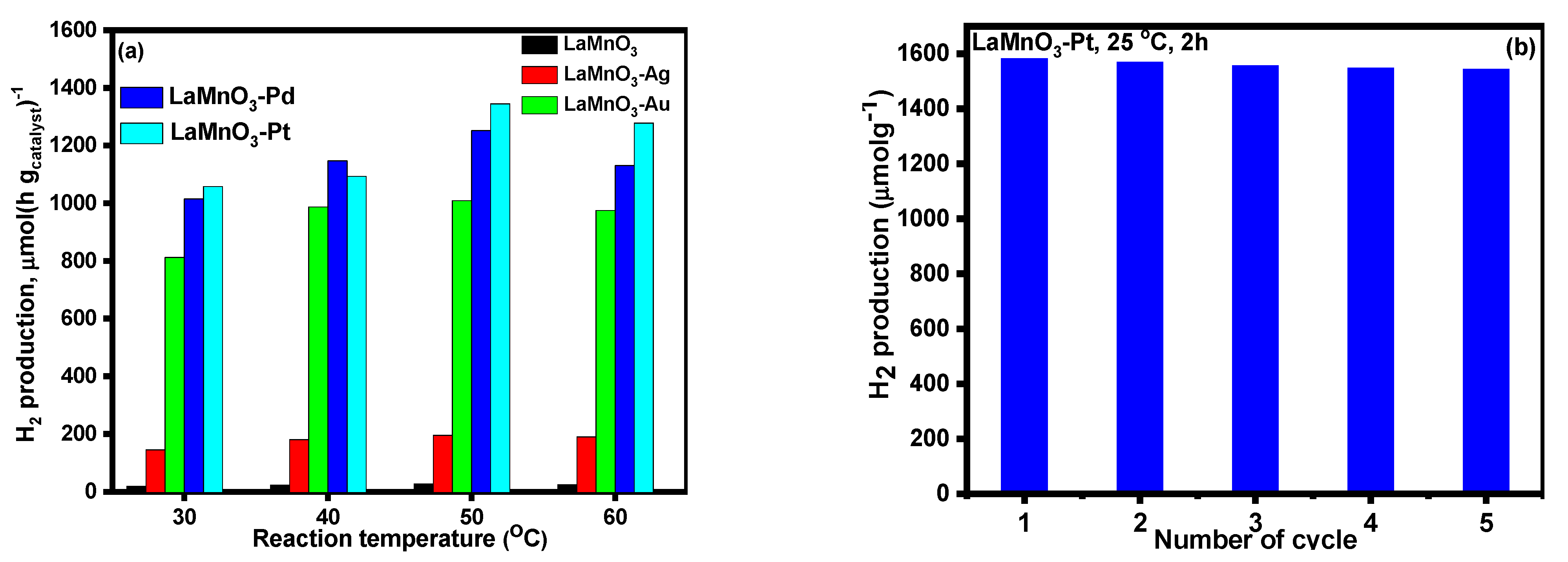
3.2. Photocatalytic Activity of Bulk and Noble Metal Deposited LaMnO3 Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrogen Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Okolie, J.A.; Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy. Int. J. Hydrogen Energy 2021, 46, 8885–8905. [Google Scholar] [CrossRef]
- Ganguly, P.; Harb, M.; Cao, Z.; Cavallo, L.; Breen, A.; Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D Nanomaterials for Photocatalytic Hydrogen Production. ACS Energy Lett. 2019, 4, 1687–1709. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.; Dawood, T.M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Zamani, P.; Higgins, D.C.; Hassan, F.; Fu, X.; Choi, J.-Y.; Hoque, A.; Jiang, G.; Chen, Z. Highly active and porous graphene encapsulating carbon nanotubes as a non-precious oxygen reduction electrocatalyst for hydrogen-air fuel cells. Nano Energy 2016, 26, 267–275. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Manzardo, A.; Ren, J.; Mazzi, A.; Scipioni, A. A grey-based group decision-making methodology for the selection of hydrogen technologies in life cycle sustainability perspective. Int. J. Hydrogen Energy 2012, 37, 17663–17670. [Google Scholar] [CrossRef]
- Abbasi, M.; Farniaei, M.; Rahimpour, M.R.; Shariati, A. Enhancement of Hydrogen Production and Carbon Dioxide Capturing in a Novel Methane Steam Reformer Coupled with Chemical Looping Combustion and Assisted by Hydrogen Perm-Selective Membranes. Energy Fuels 2013, 27, 5359–5372. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Daskalaki, V.M.; Patsoura, A.; Verykios, X.E. Hydrogen Production by Photo-Induced Reforming of Biomass Components and Derivatives at Ambient Conditions. Catal. Lett. 2008, 122, 26–32. [Google Scholar] [CrossRef]
- Silva, C.G.; Sampaio, M.J.; Marques, R.R.; Ferreira, L.A.; Tavares, P.B.; Silva, A.M.; Faria, J.L. Photocatalytic production of hydrogen from methanol and saccharides using carbon nanotube-TiO2 catalysts. Appl. Catal. B Environ. 2015, 178, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-L.; Sakthinathan, S.; Chen, S.-Y.; Yu, B.-S.; Chiu, T.-W.; Dong, C. Hydrogen generation by methanol steam reforming process by delafossite-type CuYO2 nanopowder catalyst. Microporous Mesoporous Mater. 2021, 324, 111305. [Google Scholar] [CrossRef]
- Tountas, A.A.; Peng, X.; Tavasoli, A.V.; Duchesne, P.N.; Dingle, T.L.; Dong, Y.; Hurtado, L.; Mohan, A.; Sun, W.; Ulmer, U.; et al. Towards Solar Methanol: Past, Present, and Future. Adv. Sci. 2019, 6, 1801903. [Google Scholar] [CrossRef]
- Ren, S.J.; Shoemaker, W.R.; Wang, X.F.; Shang, Z.Y.; Klinghoffer, N.; Li, S.G.; Yu, M.; He, X.Q.; White, T.A.; Liang, X.H. Highly active and selective Cu-ZnO based catalyst for methanol and dimethyl ether synthesis via CO2 hydrogenation. Fuel 2019, 239, 1125–1133. [Google Scholar] [CrossRef]
- Perng, S.-W.; Wu, H.-W. Effect of depth and diameter of cylindrical cavity in a plate-type methanol steam reformer on estimated net power of PEMFC. Energy Convers. Manag. 2018, 177, 190–209. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M. Photocatalytic behaviour of nanocarbon–TiO2 composites and immobilization into hollow fibres. Appl. Catal. B Environ. 2013, 142–143, 101–111. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube–TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Ahmmad, B.; Kusumoto, Y.; Somekawa, S.; Ikeda, M. Carbon nanotubes synergistically enhance photocatalytic activity of TiO2. Catal. Commun. 2008, 9, 1410–1413. [Google Scholar] [CrossRef]
- Serra, M.; Albero, J.; García, H. Photocatalytic activity of Au/TiO2 photocatalysts for H2 evolution: Role of the Au nanoparticles as a function of the irradiation wavelength. ChemPhysChem 2015, 16, 1842–1845. [Google Scholar] [CrossRef] [PubMed]
- Al-Azri, Z.H.N.; Al-Oufi, M.; Chan, A.; Waterhouse, G.I.; Idriss, H. Metal Particle Size Effects on the Photocatalytic Hydrogen Ion Reduction. ACS Catal. 2019, 9, 3946–3958. [Google Scholar] [CrossRef]
- Mei, B.; Han, K.; Mul, G. Driving Surface Redox Reactions in Heterogeneous Photocatalysis: The Active State of Illuminated Semiconductor-Supported Nanoparticles during Overall Water-Splitting. ACS Catal. 2018, 8, 9154–9164. [Google Scholar] [CrossRef]
- Nair, V.; Munoz-Batista, M.J.; Fernandez-García, M.; Luque, R.; Colmenares, C.J. Thermo-photocatalysis: Environmental and energy applications. ChemSusChem 2019, 12, 2098–2116. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Li, R.; Sun, X.; Xian, T.; Yang, H. Construction of novel ternary Au/LaFeO3/Cu2O composite photocatalysts for RhB degradation via photo-Fenton catalysis. Mater. Technol. 2021, 36, 603–615. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Agostini, G.; Marini, C.; Kubacka, A.; Fandez-García, M. Hydrogen thermo-photo production using Ru/TiO2: Heat and light synergistic effects. Appl. Catal. B Environ. 2019, 256, 117790. [Google Scholar] [CrossRef]
- Arabi, A.; Fazli, M.; Ehsani, M.H. Synthesis and characterization of calcium-doped lanthanum manganite nanowires as a photocatalyst for degradation of methylene blue solution under visible light irradiation. Bull. Mater. Sci. 2018, 41, 77. [Google Scholar] [CrossRef]
- Xu, J.; Sun, C.; Wang, Z.; Hou, Y.; Ding, Z.; Wang, S. Perovskite Oxide LaNiO3 Nanoparticles for Boosting H2 Evolution over Commercial CdS with Visible Light. Chem. Eur. J. 2018, 24, 18512. [Google Scholar] [CrossRef]
- Panahi, P.N.; Rasoulifard, M.H.; Babaei, S. Photocatalytic activity of cation (Mn) and anion (N) substitution in LaCoO3 nanoperovskite under visible light. Rare Met. 2020, 39, 139–146. [Google Scholar] [CrossRef]
- Bumajdad, A.; Madkour, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef]
- Susanti, Y.D.; Afifah, N.; Saleh, R. Ag modified LaMnO3 nanoparticles for methylene blue degradation via photosonocatalytic activities. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 012037. [Google Scholar] [CrossRef]
- Vo, T.-T.; Dang, C.-H.; Doan, V.-D.; Dang, V.-S.; Nguyen, T.-D. Biogenic Synthesis of Silver and Gold Nanoparticles from Lactuca indica Leaf Extract and Their Application in Catalytic Degradation of Toxic Compounds. J. Inorg. Organomet. Polym. Mater. 2020, 30, 388–399. [Google Scholar] [CrossRef]
- Hernández, E.; Sagredo, V.; Delgado, G. Synthesis and magnetic characterization of LaMnO3 nanoparticles. Rev. Mex. Física 2015, 61, 166–169. [Google Scholar]
- LiK, K.; Li, X.; Zhu, K. Infrared Absorption spectra of Manganese Oxides. J. Appl. Phys 1997, 10, 6943. [Google Scholar]
- Han, X.; Wang, Y.; Hao, H.; Guo, R.; Hu, Y.; Jiang, W. Ce1–xLaxOy solid solution prepared from mixed rare earth chloride for soot oxidation. J. Rare Earths 2016, 34, 590–596. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Park, S.-H.; Lee, S.-C.; Kang, M.; Park, C.-H.; Choung, S.-J. Optical properties of Pt-TiO2 catalyst and photocatalytic activities for benzene decomposition. Korean J. Chem. Eng. 2003, 20, 812–818. [Google Scholar] [CrossRef]
- Chen, H.-W.; Ku, Y.; Kuo, Y.-L. Effect of Pt/TiO2 characteristics on temporal behavior of o-cresol decomposition by visible light-induced photocatalysis. Water Res. 2007, 41, 2069–2078. [Google Scholar] [CrossRef]
- Alshehri, A.; Narasimharao, K. PtOx-TiO2 anatase nanomaterials for photocatalytic reformation of methanol to hydrogen: Effect of TiO2 morphology. J. Mater. Res. Technol. 2020, 9, 14907–14921. [Google Scholar] [CrossRef]
- Natile, M.M.; Ugel, E.; Maccato, C.; Glisenti, A. LaCoO3: Effect of synthesis conditions on properties and reactivity. Appl. Catal. B Environ. 2007, 72, 351–362. [Google Scholar] [CrossRef]
- Tzimpilis, E.; Moschoudis, N.; Stoukides, M.; Bekiaroglou, P. Preparation, active phase composition and Pd content of perovskite-type oxides. Appl. Catal. B Environ. 2008, 84, 607–615. [Google Scholar] [CrossRef]
- Popa, A.; Raita, O.; Stan, M.; Pana, O.; Borodi, G.; Giurgiu, L. Electron Paramagnetic Resonance of Mn-Doped Sn1−xMnxO2 Powders. Appl. Magn. Reson. 2012, 42, 453–462. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134–135, 251–257. [Google Scholar] [CrossRef]
- Weaver, J.F.; Hoflund, G.B. Surface Characterization Study of the Thermal Decomposition of Ag2O. Chem. Mater. 1994, 6, 1693–1699. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Veith, G.M.; Wang, D.; Manzoli, M.; Prati, L.; Hutchings, G.J. Characterisation of gold catalysts. Chem. Soc. Rev. 2016, 45, 4953–4994. [Google Scholar] [CrossRef]
- Yao, W.; Wang, R.; Yang, X. LaCo1−xPdxO3 perovskite-type oxides: Synthesis, characterization and simultaneous removal of NOx and diesel soot. Catal. Lett. 2009, 130, 613–621. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Korzhak, A.V.; Ermokhina, N.I.; Stroyuk, A.L.; Bukhtiyarov, V.K.; Raevskaya, A.E.; Litvin, V.I.; Kuchmiy, S.Y.; Ilyin, V.G.; Manorik, P.A. Photocatalytic hydrogen evolution over mesoporous TiO2/metal nanocomposites. J. Photochem. Photobiol. A Chem. 2008, 198, 126–134. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- Yang, G.; Ding, H.; Chen, D.; Feng, J.; Hao, Q.; Zhu, Y. Construction of urchin-like ZnIn2S4-Au-TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 234, 260–267. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Kuo, D.-H.; Ke, W.-C. Oriented p–n Heterojunction Ag2O/Zn(O,S) Nanodiodes on Mesoporous SiO2 for Photocatalytic Hydrogen Production. ACS Appl. Energy Mater. 2019, 2, 3228–3236. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Kuo, D.-H. Effects of graphene oxide and sacrificial reagent for highly efficient hydrogen production with the costless Zn(O,S) photocatalyst. Int. J. Hydrogen Energy 2019, 44, 29516–29528. [Google Scholar] [CrossRef]


| S. No. | Catalyst | BET Surface Area (m2g−1) | Pore Volume (cm3g−1) | Pore Diameter (Å) |
|---|---|---|---|---|
| 1 | LaMnO3 | 14.6 | 0.010 | 38 |
| 2 | Ag@ LaMnO3 | 12.2 | 0.019 | 43 |
| 3 | Au@ LaMnO3 | 13.5 | 0.015 | 64 |
| 4 | Pd@ LaMnO3 | 12.4 | 0.018 | 61 |
| 5 | Pt@ LaMnO3 | 20.1 | 0.023 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhari, A.H.; Hasan, N.; Radini, I.A.; Narasimharao, K.; Malik, M.A. Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production. Nanomaterials 2022, 12, 2985. https://doi.org/10.3390/nano12172985
Jawhari AH, Hasan N, Radini IA, Narasimharao K, Malik MA. Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production. Nanomaterials. 2022; 12(17):2985. https://doi.org/10.3390/nano12172985
Chicago/Turabian StyleJawhari, Ahmed Hussain, Nazim Hasan, Ibrahim Ali Radini, Katabathini Narasimharao, and Maqsood Ahmad Malik. 2022. "Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production" Nanomaterials 12, no. 17: 2985. https://doi.org/10.3390/nano12172985
APA StyleJawhari, A. H., Hasan, N., Radini, I. A., Narasimharao, K., & Malik, M. A. (2022). Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production. Nanomaterials, 12(17), 2985. https://doi.org/10.3390/nano12172985










