Phytostimulation and Synergistic Antipathogenic Effect of Tagetes erecta Extract in Presence of Rhizobacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Physico-Chemical Characterization of Plant Extract
2.1.1. Extraction Procedure
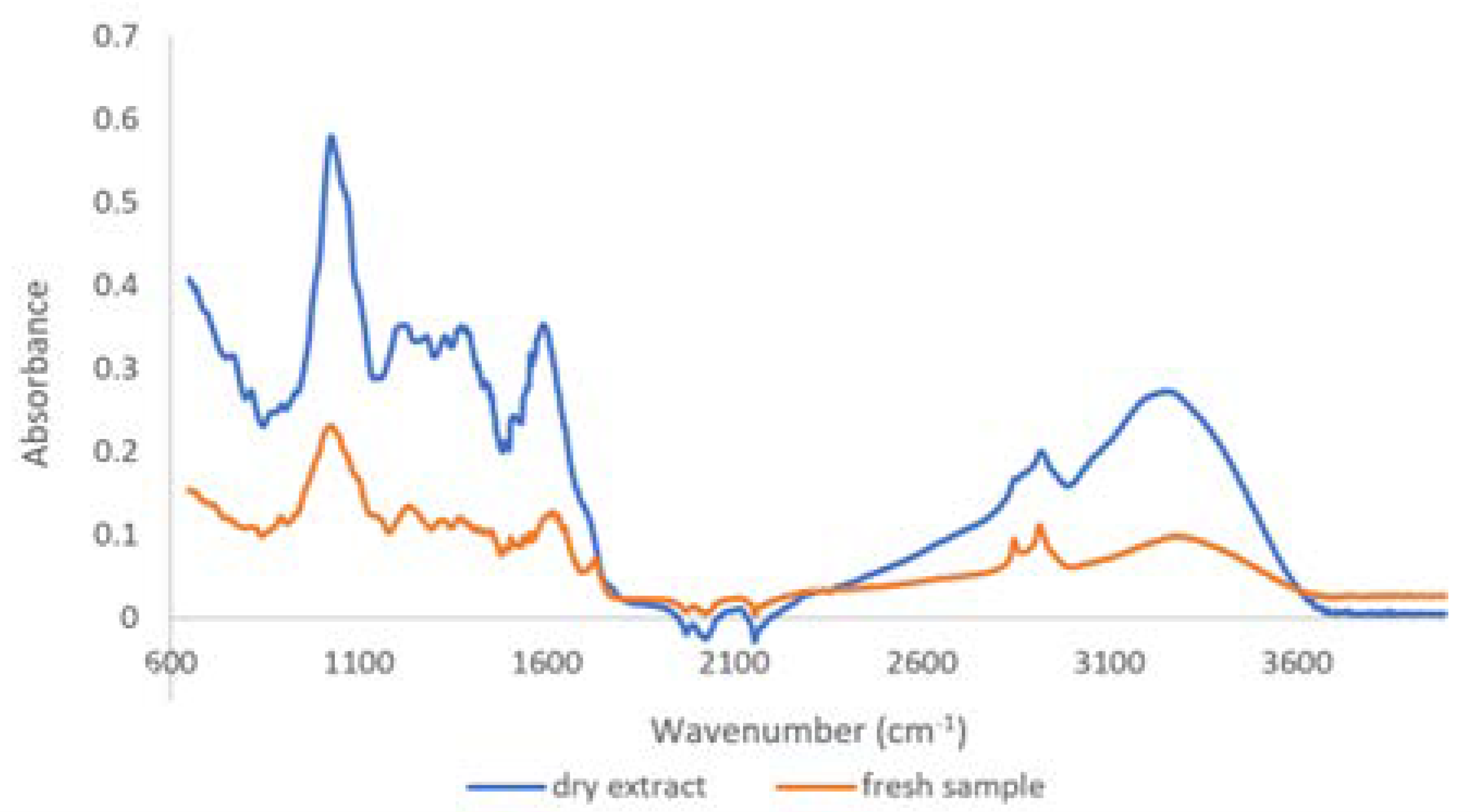
2.1.2. Qualitative Analysis by ATR-FTIR
2.1.3. Determination of Phenol Content
2.1.4. Determination of Flavonoid Content
2.1.5. Determination of Reducing Sugar Content
2.1.6. Ultra-High-Performance Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry
2.2. Antioxidant Activity
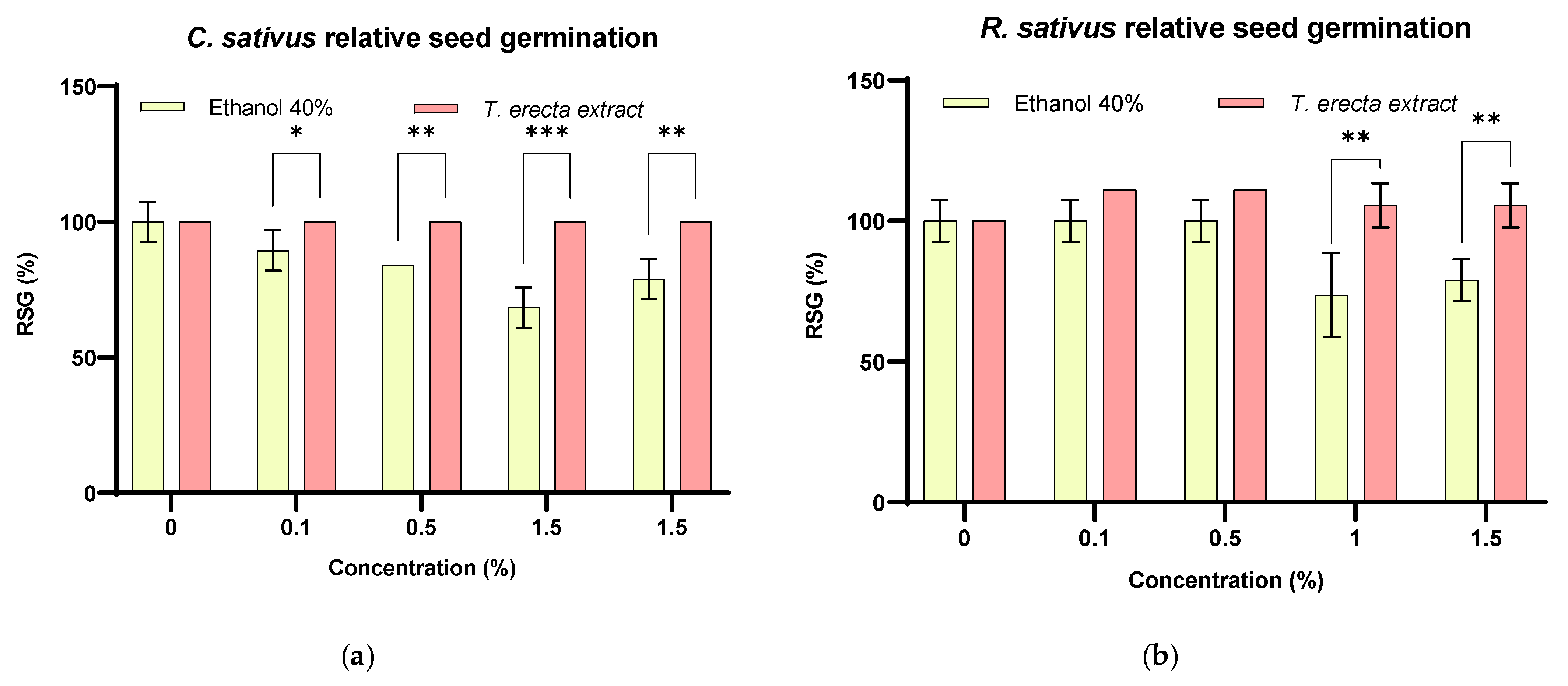
2.3. Seed Germination Bioassay
2.4. Modulators of the Microorganisms Growth
2.4.1. Prebiotic Activity on Bacillus sp.
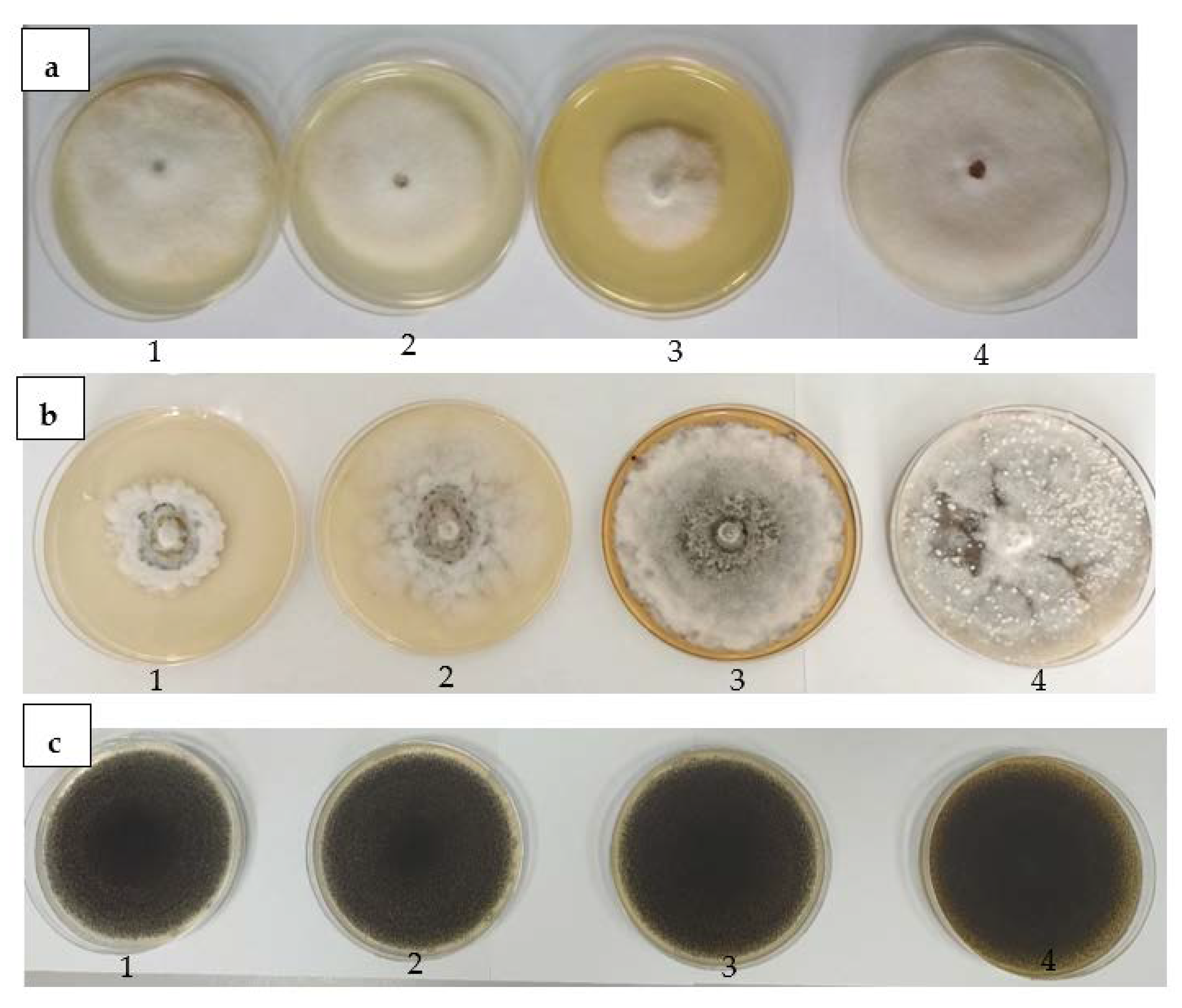
2.4.2. Antifungal Activity against Three Pathogenic Fungi
2.4.3. Synergistic Effect between T. erecta Extract and Rhizobacteria
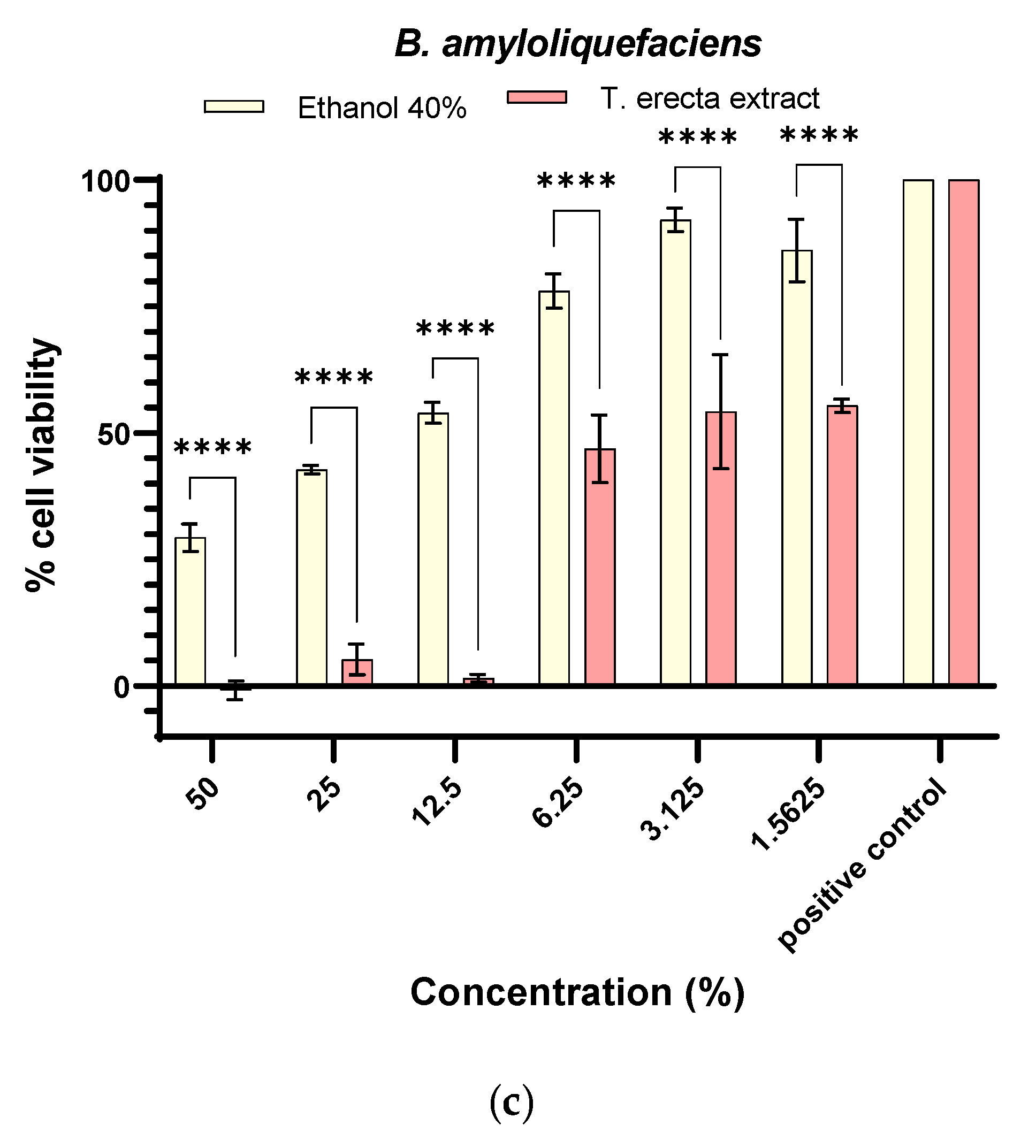
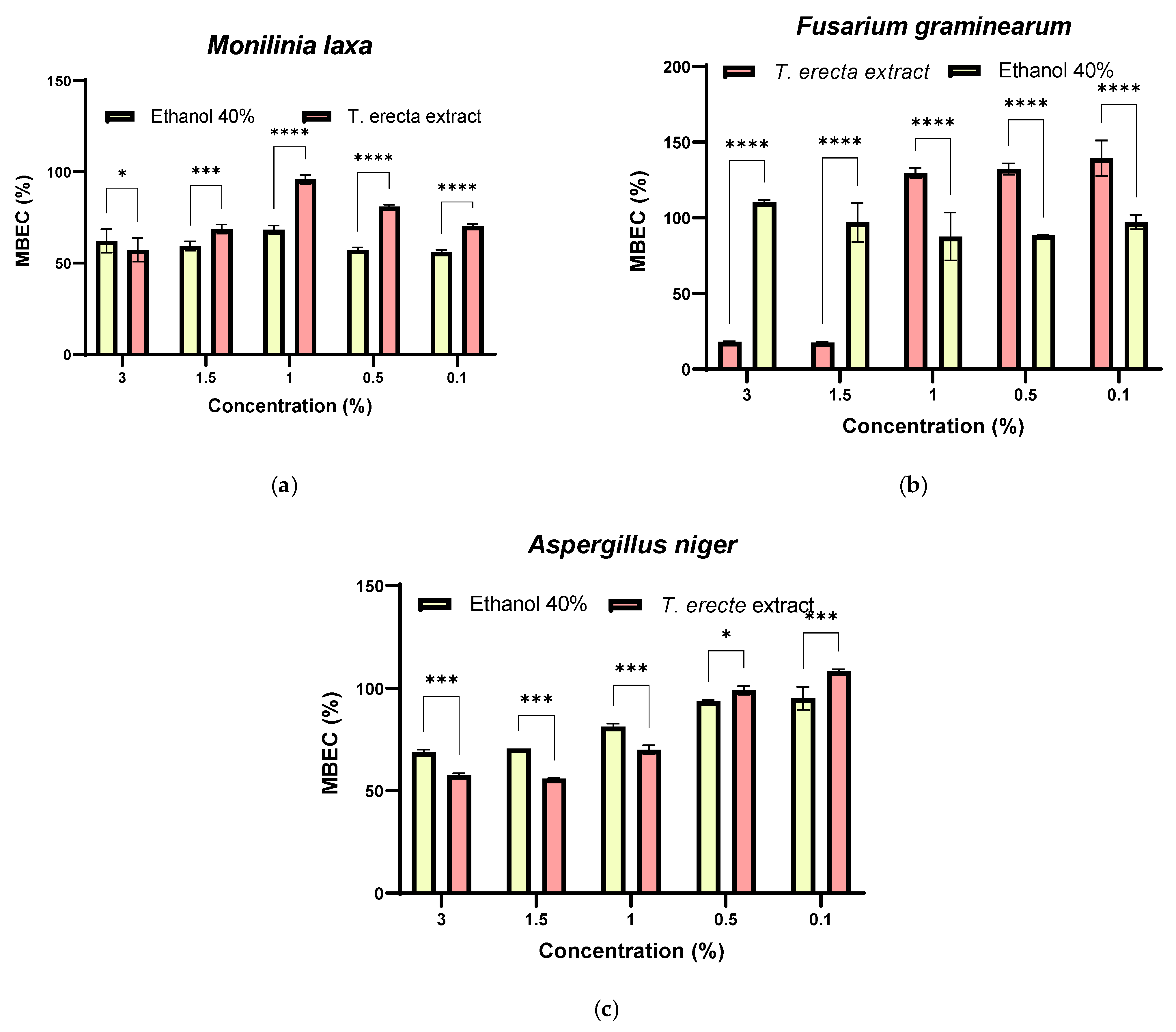
2.4.4. Antibiofilm Activity
2.5. Statistical Analysis
3. Results and Discussions
3.1. Physico-Chemical Characterization of T. erecta Extract
3.2. Antioxidant Activity
3.3. Optimization of T. erecta Extract Concentration as Prebiotic
3.4. T. erecta Extract as a Phytostimulant
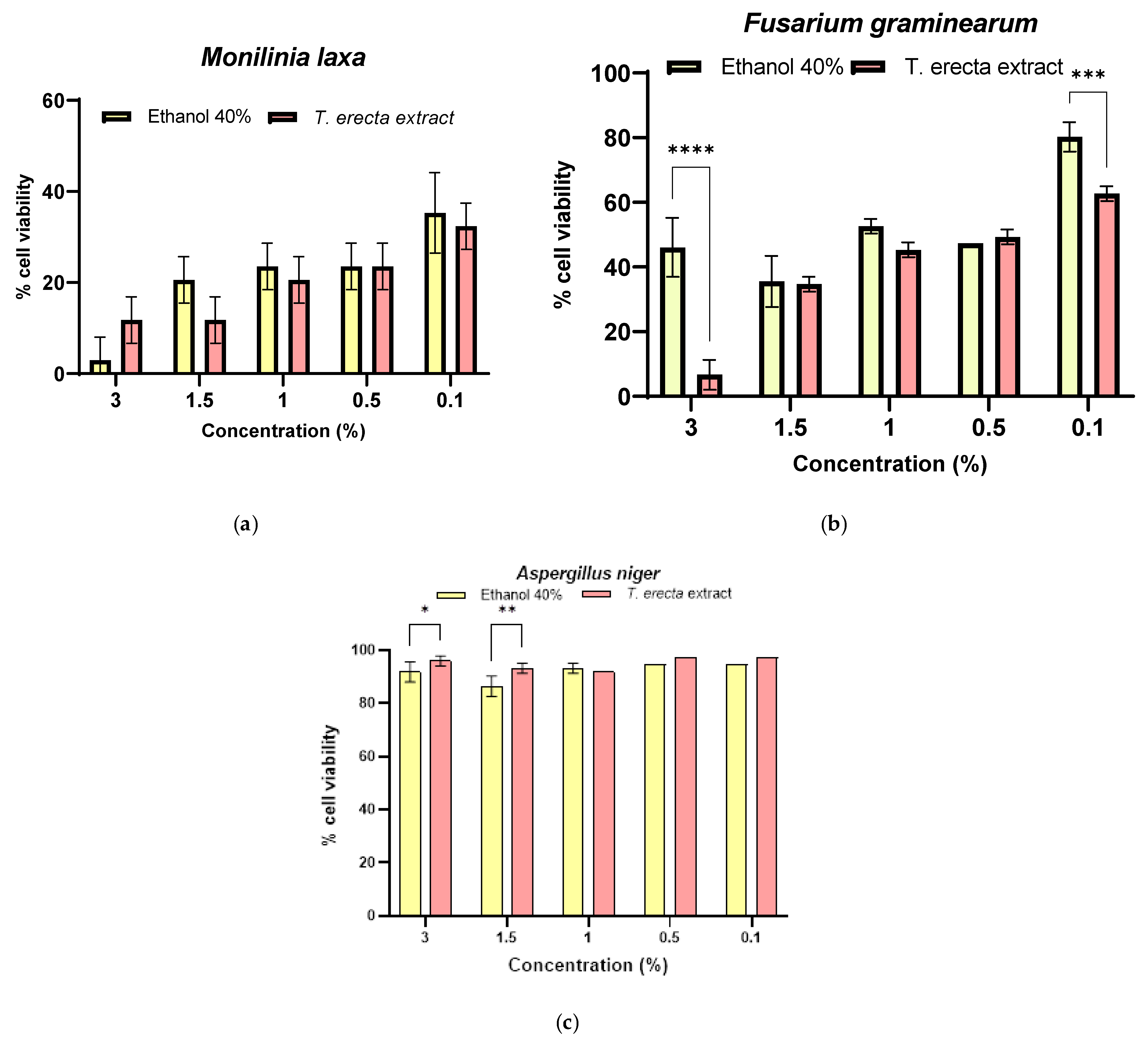
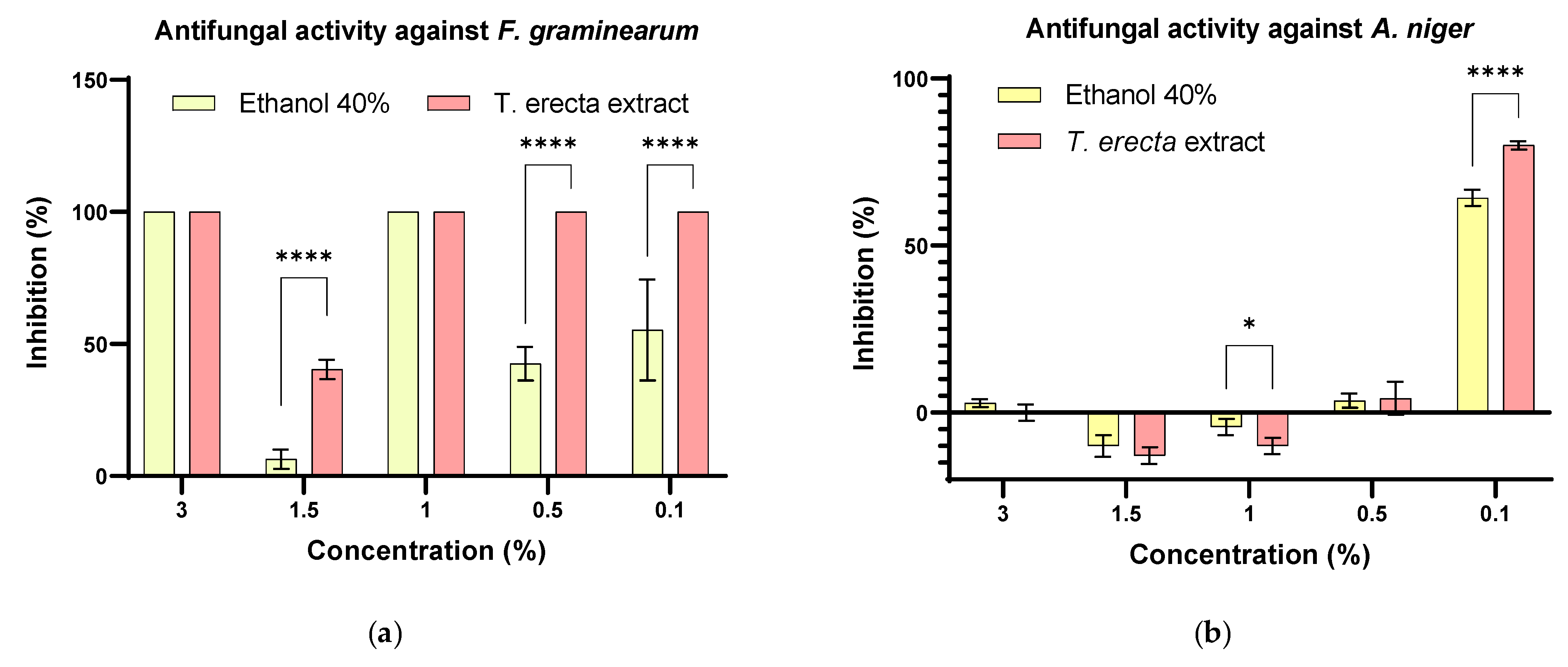
3.5. Antifungal Activity of T. erecta Extract
3.6. Synergistic Antifungal Activity between Bacillus sp. and T. erecta Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Pandey, A.K. Enhancement of Plant Nutrient Uptake by Bacterial Biostimulants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 435–456. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.-M. Allelopathic and Autotoxic Effects of Medicago Sativa—Derived Allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef]
- Mihalache, G.; Mihasan, M.; Zamfirache, M.M.; Stefan, M.; Raus, L. Phosphate Solubilizing Bacteria from Runner Bean Rhizosphere and Their Mechanism of Action. Rom. Biotechnol. Lett. 2018, 23, 13853–13861. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Alexander, S.A.; Waldenmaier, C.M. Suppression of Pratylenchus Penetrans Populations in Potato and Tomato Using African Marigolds. J. Nematol. 2002, 34, 130–134. [Google Scholar]
- Natarajan, N.; Cork, A.; Boomathi, N.; Pandi, R.; Velavan, S.; Dhakshnamoorthy, G. Cold Aqueous Extracts of African Marigold, Tagetes erecta for Control Tomato Root Knot Nematode, Meloidogyne Incognita. Crop Prot. 2006, 25, 1210–1213. [Google Scholar] [CrossRef]
- Benítez-García, I.; Vanegas-Espinoza, P.E.; Meléndez-Martínez, A.J.; Heredia, F.J.; Paredes-López, O.; del Villar-Martínez, A.A. Callus Culture Development of Two Varieties of Tagetes erecta and Carotenoid Production. Electron. J. Biotechnol. 2014, 17, 107–113. [Google Scholar] [CrossRef]
- Rajkumari, J.; Choudhury, Y.; Bhattacharjee, K.; Pandey, P. Rhizodegradation of Pyrene by a Non-Pathogenic Klebsiella Pneumoniae Isolate Applied with Tagetes erecta L. and Changes in the Rhizobacterial Community. Front. Microbiol. 2021, 12, 593023. [Google Scholar] [CrossRef]
- Ullah, Z.; Abbas, S.J.; Naeem, N.; Lutfullah, G.; Malik, T.; Khan, M.A.U.; Khan, I. Effect of Indolebutyric Acid (IBA) and Naphthaleneacetic Acid (NAA) Plant Growth Regulaters on Mari Gold (Tagetes erecta L.). Afr. J. Agric. Res. 2013, 8, 4015–4019. [Google Scholar]
- Niu, Y.; Han, S.; Wu, Z.; Pan, C.; Wang, M.; Tang, Y.; Zhang, Q.; Tan, G.; Han, B. A Push–Pull Strategy for Controlling the Tea Green Leafhopper (Empoasca flavescens F.) Using Semiochemicals from Tagetes erecta and Flemingia macrophylla. Pest Manag. Sci. 2022, 78, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus subtilis: A Plant-Growth Promoting Rhizobacterium that also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal Biofilms: Targets for the Development of Novel Strategies in Plant Disease Management. Front. Microbiol. 2017, 8, 654. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Geana, E.-I.; Tutunaru, O.; Pircalabioru, G.G.; Zgura, I.; Chifiriuc, M.C. Valorization of Gleditsia triacanthos Invasive Plant Cellulose Microfibers and Phenolic Compounds for Obtaining Multi-Functional Wound Dressings with Antimicrobial and Antioxidant Properties. Int. J. Mol. Sci. 2020, 22, 33. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Kassuya, C.A.L.; Neto, F.F.; Volobuff, C.R.F.; Iriguchi, E.K.K.; Vieira, M.D.C.; Foglio, M.A. The Flavonoid Content and Antiproliferative, Hypoglycaemic, Anti-Inflammatory and Free Radical Scavenging Activities of Annona dioica St. Hill. BMC Complement. Altern. Med. 2013, 13, 14. [Google Scholar] [CrossRef]
- Khatri, D.; Chhetri, S.B.B. Reducing Sugar, Total Phenolic Content, and Antioxidant Potential of Nepalese Plants. BioMed Res. Int. 2020, 2020, 7296859. [Google Scholar] [CrossRef]
- Corbu, V.M.; Gheorghe, I.; Marinaș, I.C.; Geană, E.I.; Moza, M.I.; Csutak, O.; Chifiriuc, M.C. Demonstration of Allium sativum Extract Inhibitory Effect on Biodeteriogenic Microbial Strain Growth, Biofilm Development, and Enzymatic and Organic Acid Production. Molecules 2021, 26, 7195. [Google Scholar] [CrossRef]
- Madhu, G.; Bose, V.C.; Aiswaryaraj, A.S.; Maniammal, K.; Biju, V. Defect Dependent Antioxidant Activity of Nanostructured Nickel Oxide Synthesized through a Novel Chemical Method. Colloids Surf. A: Physicochem. Eng. Asp. 2013, 429, 44–50. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Determination of Antioxidants by a Novel On-Line HPLC-Cupric Reducing Antioxidant Capacity (CUPRAC) Assay with Post-Column Detection. Anal. Chim. Acta 2010, 674, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mitelut, A.C.; Popa, E.M. Seed Germination Bioassay for Toxicity Evaluation of Different Composting Biodegradable Materials. Rom. Biotechnol. Lett. 2011, 16, 121–129. [Google Scholar]
- Milon, A.R.; Chang, S.W.; Ravindran, B. Biochar Amended Compost Maturity Evaluation Using Commercial Vegetable Crops Seedlings through Phytotoxicity Germination Bioassay. J. King Saud Univ. -Sci. 2022, 34, 101770. [Google Scholar] [CrossRef]
- Fan, B.; Blom, J.; Klenk, H.-P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an “Operational Group B. amyloliquefaciens” within the B. subtilis Species Complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef]
- Gutiérrez-Mañero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The Plant-Growth-Promoting Rhizobacteria Bacillus pumilus and Bacillus licheniformis Produce High Amounts of Physiologically Active Gibberellins. Physiol. Plant. 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Buleandra, M.; Badea, I.A.; Tihauan, B.M.; Marutescu, L.; Angheloiu, M.; Matei, E.; Chifiriuc, M.C. Chemical Composition, Antipathogenic and Cytotoxic Activity of the Essential Oil Extracted from Amorpha fruticosa Fruits. Molecules 2021, 26, 3146. [Google Scholar] [CrossRef]
- Javed, S.; Shoaib, A.; Mahmood, Z.; Mushtaq, S.; Iftikhar, S. Analysis of Phytochemical Constituents of Eucalyptus citriodora L. Responsible for Antifungal Activity against Post-Harvest Fungi. Nat. Prod. Res. 2012, 26, 1732–1736. [Google Scholar] [CrossRef]
- Lee, H.-A.; Kim, J.-H. Isolation of Bacillus amyloliquefaciens Strains with Antifungal Activities from Meju. Prev. Nutr. Food Sci. 2012, 17, 64–70. [Google Scholar] [CrossRef][Green Version]
- Kaboré, D.; Gagnon, M.; Roy, D.; Sawadogo-Lingani, H.; Diawara, B.; LaPointe, G. Rapid Screening of Starter Cultures for Maari Based on Antifungal Properties. Microbiol. Res. 2018, 207, 66–74. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Byrne, H.J.; Heredia, F.J. Study of Phenolic Extractability in Grape Seeds by Means of ATR-FTIR and Raman Spectroscopy. Food Chem. 2017, 232, 602–609. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Infrared Spectroscopy in the Study of Edible Oils and Fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Extraction and Characterization of Cellulose Microfibers from Agricultural Wastes of Onion and Garlic. J. Nat. Fibers 2018, 15, 465–473. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Cozzolino, R.; Malorni, L.; Martignetti, A.; Picariello, G.; Siano, F.; Forte, G.; de Giulio, B. Comparative Analysis of Volatile Profiles and Phenolic Compounds of Four Southern Italian Onion (Allium cepa L.) Landraces. J. Food Compos. Anal. 2021, 101, 103990. [Google Scholar] [CrossRef]
- Fernández, K.; Agosin, E. Quantitative Analysis of Red Wine Tannins Using Fourier-Transform Mid-Infrared Spectrometry. J. Agric. Food Chem. 2007, 55, 7294–7300. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline Structure Analysis of Cellulose Treated with Sodium Hydroxide and Carbon Dioxide by Means of X-Ray Diffraction and FTIR Spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, W.; Jiang, J.; Yuan, F.; Gao, Y. Subcritical Water Extraction and Antioxidant Activity Evaluation with On-Line HPLC-ABTS·+ Assay of Phenolic Compounds from Marigold (Tagetes erecta L.) Flower Residues. J. Food Sci. Technol. 2014, 52, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, X.; He, W.-H.; Xu, H.-G.; Yuan, F.; Gao, Y.-X. Investigation into the Antioxidant Activity and Chemical Composition of Alcoholic Extracts from Defatted Marigold (Tagetes erecta L.) Residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef]
- de Matos, Y.M.L.S.; Vasconcelos, D.L.M.; Barreto, A.C.H.; Rocha, J.E.; de Araújo-Neto, J.B.; Campina, F.F.; da Silva, M.M.C.; al Yafawi, T.T.; Sobral-Souza, C.E.; Pinheiro, J.C.A.; et al. Protection against the Phytotoxic Effect of Mercury Chloride by Catechin and Quercetin. J. Chem. 2022, 2022, 3770935. [Google Scholar] [CrossRef]
- El-Soud, W.A.; Hegab, M.M.; AbdElgawad, H.; Zinta, G.; Asard, H. Ability of Ellagic Acid to Alleviate Osmotic Stress on Chickpea Seedlings. Plant Physiol. Biochem. 2013, 71, 173–183. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.; Bektaşoğlu, B.; Berker, K.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Qiao, J.; Yu, X.; Liang, X.; Liu, Y.; Borriss, R.; Liu, Y. Addition of Plant-Growth-Promoting Bacillus subtilis PTS-394 on Tomato Rhizosphere Has No Durable Impact on Composition of Root Microbiome. BMC Microbiol. 2017, 17, 131. [Google Scholar] [CrossRef]
- Dame, Z.T.; Rahman, M.; Islam, T. Bacilli as Sources of Agrobiotechnology: Recent Advances and Future Directions. Green Chem. Lett. Rev. 2021, 14, 246–271. [Google Scholar] [CrossRef]
- Barnawal, D.; Maji, D.; Bharti, N.; Chanotiya, C.S.; Kalra, A. ACC Deaminase-Containing Bacillus subtilis Reduces Stress Ethylene-Induced Damage and Improves Mycorrhizal Colonization and Rhizobial Nodulation in Trigonella foenum-graecum Under Drought Stress. J. Plant Growth Regul. 2013, 32, 809–822. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant Growth-Promoting Rhizobacteria Inoculation to Enhance Vegetative Growth, Nitrogen Fixation and Nitrogen Remobilisation of Maize under Greenhouse Conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sheng, J.; Chen, L.; Men, Y.; Gan, L.; Guo, S.; Shen, L. Bacterial Community Compositions of Tomato (Lycopersicum esculentum Mill.) Seeds and Plant Growth Promoting Activity of ACC Deaminase Producing Bacillus subtilis (HYT-12-1) on Tomato Seedlings. World J. Microbiol. Biotechnol. 2014, 30, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 Increases Water Use Efficiency via Growth Stimulation in Both Normal and Drought Conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef]
- Dursyn, A.; Ekinci, M.; Donmez, M.F. Effects of Foliar Application of Plant Growth Promoting Bacterium on Chemical Contents, Yield and Growth of Tomato (Lycopersicon esculentum L.) and Cucumber (Cucumis sativus L.). Pak. J. Bot. 2010, 42, 3349–3356. [Google Scholar]
- Chowdhury, S.P.; Hartmann, A.; Gao, X.; Borriss, R. Biocontrol Mechanism by Root-Associated Bacillus amyloliquefaciens FZB42 —A Review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef]
- Latifian, E.; Otur, C.; Abanoz-Secgin, B.; Arslanoglu, S.F.; Kurt-Kizildogan, A. Evaluation of Antimicrobial Activity in Extracts of Different Parts of Three Tagetes Species. Turk. J. Field Crops 2021, 26, 117–122. [Google Scholar] [CrossRef]
- Huang, R.; Feng, H.; Xu, Z.; Zhang, N.; Liu, Y.; Shao, J.; Shen, Q.; Zhang, R. Identification of Adhesins in Plant Beneficial Rhizobacteria Bacillus velezensis SQR9 and Their Effect on Root Colonization. Mol. Plant-Microbe Interact. 2022, 35, 64–72. [Google Scholar] [CrossRef]
- Townsley, L.; Yannarell, S.M.; Huynh, T.N.; Woodward, J.J.; Shank, E.A. Cyclic Di-AMP Acts as an Extracellular Signal That Impacts Bacillus subtilis Biofilm Formation and Plant Attachment. MBio 2018, 9, e00341-18. [Google Scholar] [CrossRef]
- Li, F.; Tang, M.; Tang, X.; Sun, W.; Gong, J.; Yi, Y. Bacillus subtilis—Arabidopsis thaliana: A Model Interaction System for Studying the Role of Volatile Organic Compounds in the Interchange between Plants and Bacteria. Botany 2019, 97, 661–669. [Google Scholar] [CrossRef]
- Cho, W.-M.; Ravindran, B.; Kim, J.K.; Jeong, K.-H.; Lee, D.J.; Choi, D.-Y. Nutrient Status and Phytotoxicity Analysis of Goat Manure Discharged from Farms in South Korea. Environ. Technol. 2017, 38, 1191–1199. [Google Scholar] [CrossRef]
- Ravindran, B.; Mupambwa, H.A.; Silwana, S.; Mnkeni, P.N.S. Assessment of Nutrient Quality, Heavy Metals and Phytotoxic Properties of Chicken Manure on Selected Commercial Vegetable Crops. Heliyon 2017, 3, e00493. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Santos, P.C.; Santos, V.H.M.; Mecina, G.F.; Andrade, A.R.; Fegueiredo, P.A.; Moraes, V.M.O.; Silva, L.P.; Silva, R.M.G. Phytotoxicity of Tagetes erecta L. and Tagetes patula L. on Plant Germination and Growth. South Afr. J. Bot. 2015, 100, 114–121. [Google Scholar] [CrossRef]
- Hulot, J.F.; Hiller, N. Exploring the Benefits of Biocontrol for Sustainable Agriculture—A Literature Review on Biocontrol in Light of the European Green Deal; Institute for European Environmental Policy: Brussels, Belgium, 2021. [Google Scholar]
- Janakiev, T.; Berić, T.; Stević, T.; Stanković, S.; Bačić, J.; Majstorović, H.; Fira, D.; Dimkić, I. The Microbiome of the ‘Williams’ Pear Variety Grown in the Organic Orchard and Antifungal Activity by the Autochthonous Bacterial and Yeast Isolates. Microorganisms 2022, 10, 1282. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Niu, C.-W.; Chen, X.-Y.; Guo, L.-Y. Monilinia Species Associated with Brown Rot of Cultivated Apple and Pear Fruit in China. Plant Dis. 2016, 100, 2240–2250. [Google Scholar] [CrossRef]
- Gautam, A.K.; Sharma, S.; Avasthi, S.; Bhadauria, R. Diversity, Pathogenicity and Toxicology of A. niger: An Important Spoilage Fungi. Res. J. Microbiol. 2011, 6, 270–280. [Google Scholar] [CrossRef]
- Akhtar, N.; Shoaib, A.; Munir, S.; Ali, A.; Khurshid, S. Isolation, Identification and Enzyme Production Profile of A. niger. J. Anim. Plant Sci. 2014, 24, 1438–1443. [Google Scholar]
- Soares, C.; Calado, T.; Venâncio, A. Mycotoxin Production by Aspergillus niger Aggregate Strains Isolated from Harvested Maize in Three Portuguese Regions. Rev. Iberoam. De Micol. 2013, 30, 9–13. [Google Scholar] [CrossRef]
- Larmour, R.; Marchant, R. The Induction of Conidiation in Fusarium culmorum Grown in Continuous Culture. J. Gen. Microbiol. 1977, 99, 49–58. [Google Scholar] [CrossRef]
- You, M.; Wickramaratne, D.B.M.; Silva, G.L.; Chai, H.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Kinghorn, A.D.; Pezzuto, J.M. (-)-Roemerine, an Aporphine Alkaloid from Annona Senegalensis That Reverses the Multidrug-Resistance Phenotype with Cultured Cells. J. Nat. Prod. 1995, 58, 598–604. [Google Scholar] [CrossRef]
- Meschini, S.; Marra, M.; Calcabrini, A.; Federici, E.; Galeffi, C.; Arancia, G. Voacamine, a Bisindolic Alkaloid from Peschiera Fuchsiaefolia, Enhances the Cytotoxic Effect of Doxorubicin on Multidrug-Resistant Tumor Cells. Int. J. Oncol. 2003, 23, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Anurag, A.; Fatima, Z.; Hameed, S. Natural Phenolic Compounds, A Potential Antifungal Agent; Mendez-Vilas, A., Ed.; Microbial pathogens and strategies for combating them: Science, technology and education; Formatex Research Center: Badajoz, Spain, 2013; pp. 1189–1195. [Google Scholar]
- Shukla, A.; Dwivedi, S.K. Antifungal Approach of Phenolic Compounds against Fusarium udum and Fusarium oxysporum f.sp.ciceri. Afr. J. Agric. Res. 2013, 8, 596–600. [Google Scholar]
- Patel, R.; Pandya, J.; Rajpurohit, N.; Chaudhary, R.; Bhalakiya, H. Antifungal Activity of Tithonia rotundifolia Blake and Tagetes erecta L against Aspergillus niger. Int. J. Creat. Res. Thoughts 2020, 8, 4193–4198. [Google Scholar]
- Nabti, E.H.; Mokrane, N.; Ghoul, M.; Manyani, H.; Dary, M.; Megias, M.G. Isolation and Characterization of Two Halophilic Bacillus (B. licheniformis and Bacillus sp.) with Antifungal Activity. J. Ecol. Health Environ. 2013, 1, 13–17. [Google Scholar] [CrossRef]
- Ali, S.; Hameed, S.; Shahid, M.; Iqbal, M.; Lazarovits, G.; Imran, A. Functional Characterization of Potential PGPR Exhibiting Broad-Spectrum Antifungal Activity. Microbiol. Res. 2020, 232, 126389. [Google Scholar] [CrossRef]
- Tendulkar, S.R.; Saikumari, Y.K.; Patel, V.; Raghotama, S.; Munshi, T.K.; Balaram, P.; Chattoo, B.B. Isolation, Purification and Characterization of an Antifungal Molecule Produced by Bacillus licheniformis BC98, and Its Effect on Phytopathogen Magnaporthe Grisea. J. Appl. Microbiol. 2007, 103, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.-L.; Peng, S.; Chen, L.-L.; Liu, Y.; Yan, C.; Zhu, F. Identification and Characterization of a Serine Protease from Bacillus licheniformis W10: A Potential Antifungal Agent. Int. J. Biol. Macromol. 2020, 145, 594–603. [Google Scholar] [CrossRef]
- Mardanova, A.M.; Fanisovna Hadieva, G.; Tafkilevich Lutfullin, M.; Valer’evna Khilyas, I.; Farvazovna Minnullina, L.; Gadelevna Gilyazeva, A.; Mikhailovna Bogomolnaya, L.; Rashidovna Sharipova, M. Bacillus subtilis Strains with Antifungal Activity against the Phytopathogenic Fungi. Agric. Sci. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Cui, T.-B.; Chai, H.-Y.; Jiang, L.-X. Isolation and Partial Characterization of an Antifungal Protein Produced by Bacillus licheniformis BS-3. Molecules 2012, 17, 7336–7347. [Google Scholar] [CrossRef]


| Parameter | T. erecta Extract Values |
|---|---|
| TPC (mg GAE/mL) | 15.12 ± 0.27 |
| TFC (mg QE/mL) | 2.01 ± 0.03 |
| % Flavonoids from total phenols | 13.29 |
| Reducing sugar content (mg D-glucose/mL) | 5.97 ± 0.48 |
| No | Compound | Retention Time [min] | Accurate Mass [M-H]− | Mass Fragments | Concentration (mg/L) |
|---|---|---|---|---|---|
| 1 | Gallic acid | 1.99 | 169.0131 | 125.0231 | 6.16 |
| 2 | 3,4-Dihydroxybenzoic acid | 4.18 | 153.0181 | 109.0281 | 20.50 |
| 3 | 4-Hydroxybenzoic acid | 6.47 | 137.0231 | 93.0331 | 3.13 |
| 4 | Chlorogenic acid | 7.91 | 353.0876 | 191.0553 | 11.85 |
| 5 | Syringic acid | 8.24 | 197.0447 | 182.0212, 166.9976, 153.0547, 138.0311, 123.0075 | 30.71 |
| 6 | Caffeic acid | 8.01 | 179.0340 | 135.044 | 1.27 |
| 7 | Vanillic acid | 8.44 | 167.0339 | 152.0105, 124.0154, 111.0075, 139.0025, 95.0125 | 0.75 |
| 8 | p-Coumaric acid | 8.69 | 163.0389 | 119.0489 | 0.50 |
| 9 | t-Ferulic acid | 8.90 | 193.0498 | 178.0262, 134.0361 | 0.51 |
| 10 | Ellagic acid | 9.69 | 300.9988 | 300.9990 | 26.80 |
| 11 | Cinnamic acid | 10.34 | 147.0441 | 119.0489, 103.0387 | 0.36 |
| 12 | Abscisic acid | 10.04 | 263.1288 | 179.9803, 191.9454 | 0.03 |
| Ʃ phenolic acids (mg/L) | 102.57 | ||||
| 13 | Quercetin | 10.69 | 301.0354 | 151.0226, 178.9977, 121.0282, 107.0125 | 383.74 |
| 14 | Hesperidin | 9.29 | 609.1824 | 377.0876 | 0.14 |
| 15 | Rutin | 8.99 | 609.1464 | 3345.0614 | 0.07 |
| 16 | Kaempferol | 11.04 | 285.0403 | 151.0389, 117.0180 | 0.31 |
| 17 | Isorhamnetin | 11.78 | 315.0512 | 300.0276 | 6.49 |
| 18 | Apigenin | 11.80 | 269.0454 | 117.0333, 151.0027, 107.0126 | 0.21 |
| 19 | Pinocembrin | 12.56 | 255.0660 | 213.0551, 151.0026, 107.0125 | 0.11 |
| 20 | Chrysin | 13.40 | 253.0503 | 143.0491, 145.0284, 107.0125, 209.0603, 63.0226, 65.0019 | 0.14 |
| 21 | Galangin | 13.62 | 269.0454 | 169.0650, 143.0491 | 0.09 |
| 22 | Myricetin | 8.70 | 317.0304 | 178.9986, 164.9263, 151.0036, 137.0244, 107.0125 | 13.64 |
| Ʃ flavonoids (mg/L) | 404.97 | ||||
| 23 | t-Resveratrol | 9.55 | 227.0707 | 185.0813, 143.0337 | 0.04 |
| 24 | Caffeic Acid Phenethyl Ester | 13.18 | 283.0975 | 174.9551, 112.9843 | 0.07 |
| Parameter | Antioxidant Activity |
|---|---|
| CUPRAC (mM Trolox/mL) | 65.81 ± 1.51 |
| FRAP (mM Trolox/mL) | 22.36 ± 1.47 |
| TEAC (mM Trolox/mL) | 20.64 ± 0.93 |
| DPPH (mM Trolox/mL) | 19.37 ± 0.09 |
| PGPR Strains | Sample | Diameter Zone |
|---|---|---|
| Inhibition (mm) | ||
| B. subtilis | T, erecta extract | 13.33 ± 1.15 |
| Ethanol 40% | 10.33 ± 1.15 | |
| p-value | <0.01 | |
| B. ligheniformis | T, erecta extract | 12.33 ± 1.15 |
| Ethanol 40% | 9.66 ± 0.58 | |
| p-value | <0.05 | |
| B. amyloliquefaciens | T, erecta extract | 13.33 ± 1.15 |
| Ethanol 40% | 9.33 ± 1.15 | |
| p-value | <0.01 |
| Concentrations (%) | Sample | B. subtilis | B. licheniformis | B. amyloliquefaciens |
|---|---|---|---|---|
| MBEC (%) | MBEC (%) | MBEC (%) | ||
| MIC/2 (6.25%) | T, erecta extract | 47.22 ± 5.97 | 157.98 ± 24.54 | 82.93 ± 13.07 |
| Ethanol 40% | 133.5 ± 34.29 | 64.89 ± 1.04 | 132.51 ± 2.93 | |
| p-value (vs. ethanol 40%) p-value (vs. positive control) | <0.001 <0.01 | <0.0001 <0.01 | <0.05 >0.05 | |
| MIC/4 (3.125%) | T, erecta extract | 32.04 ± 0.48 | 220.54 ± 7.69 | 137.67 ± 20.52 |
| Ethanol 40% | 135.0 ± 31.11 | 216.24 ± 14.28 | 105.18 ± 6.01 | |
| p-value (vs. ethanol 40%) p-value (vs. positive control) | <0.0001 <0.001 | >0.05 <0.0001 | >0.05 >0.05 | |
| MIC/8 (1.56%) | T, erecta extract | 97.67 ± 17.55 | 224.67 ± 38.57 | 138.15 ± 21.98 |
| Ethanol 40% | 268.0 ± 44.55 | 106.13 ± 10.91 | 132.98 ± 3.58 | |
| p-value (vs. ethanol 40%) p-value (vs. positive control) | <0.0001 >0.05 | <0.0001 <0.0001 | >0.05 >0.05 |
| Concentrations (%) | Sample | F. graminearum | M. laxa | A. niger |
|---|---|---|---|---|
| Inhibition (%) | Inhibition (%) | Inhibition (%) | ||
| 0.5% | T, erecta extract | 27.08 ± 2.95 | 52.29 ± 2.60 | 21.18 ± 0.00 |
| Ethanol 40% | 0.00 ± 0.00 | 32.11 ± 2.60 | 26.47 ± 4.16 | |
| p-value (vs. ethanol 40%) | <0.01 | <0.05 | >0.05 | |
| p-value (vs. positive control) | <0.0001 | <0.0001 | <0.0001 | |
| 1% | T, erecta extract | 37.50 ± 5.89 | 38.53 ± 3.89 | 19.71 ± 0.42 |
| Ethanol 40% | 0.00 ± 0.00 | 10.09 ± 2.60 | 26.76 ± 0.42 | |
| p-value (vs. ethanol 40%) | <0.05 | <0.05 | <0.01 | |
| p-value (vs. positive control) | <0.0001 | <0.0001 | <0.0001 | |
| 5% | T, erecta extract | 54.17 ± 5.89 | 13.76 ± 2.60 | 23.82 ± 0.42 |
| Ethanol 40% | 19.71 ± 0.42 | 11.01 ± 1.30 | 34.18 ± 1.66 | |
| p-value (vs. ethanol 40%) | <0.05 | >0.05 | <0.05 | |
| p-value (vs. positive control) | <0.0001 | <0.0001 | <0.0001 |
| Sample | Concentrations (%) | F. graminearum | M. laxa | A. niger | |
|---|---|---|---|---|---|
| Diameter Zone Inhibition (mm) | Diameter Zone Inhibition (mm) | Diameter Zone Inhibition (mm) | |||
| B. licheniformis strain | T, erecta extract | 1.5% | 15.67 ± 0.58 | Complete inhibition | Resistant |
| Ethanol 40% | 11.33 ± 1.15 | Complete inhibition | Resistant | ||
| p-value | <0.01 | - | - | ||
| T, erecta extract | 3% | 28.33 ± 2.08 | Complete inhibition | 17.67 ± 1.53 | |
| B. subtilis strain | Ethanol 40% | 20.67 ± 1.53 | Complete inhibition | 14.33 ± 0.58 | |
| p-value | <0.01 | - | <0.05 | ||
| T, erecta extract | 1.5% | 26.67 ± 1.53 | Complete inhibition | 19.67 ± 1.53 | |
| Ethanol 40% | 25.33 ± 1.53 | Complete inhibition | 17.33 ± 2.52 | ||
| p-value | >0.05 | - | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perisoara, A.; Marinas, I.C.; Geana, E.I.; Constantin, M.; Angheloiu, M.; Pirvu, L.; Cristea, S. Phytostimulation and Synergistic Antipathogenic Effect of Tagetes erecta Extract in Presence of Rhizobacteria. Horticulturae 2022, 8, 779. https://doi.org/10.3390/horticulturae8090779
Perisoara A, Marinas IC, Geana EI, Constantin M, Angheloiu M, Pirvu L, Cristea S. Phytostimulation and Synergistic Antipathogenic Effect of Tagetes erecta Extract in Presence of Rhizobacteria. Horticulturae. 2022; 8(9):779. https://doi.org/10.3390/horticulturae8090779
Chicago/Turabian StylePerisoara, Alina, Ioana Cristina Marinas, Elisabeta Irina Geana, Mariana Constantin, Marian Angheloiu, Lucia Pirvu, and Stelica Cristea. 2022. "Phytostimulation and Synergistic Antipathogenic Effect of Tagetes erecta Extract in Presence of Rhizobacteria" Horticulturae 8, no. 9: 779. https://doi.org/10.3390/horticulturae8090779
APA StylePerisoara, A., Marinas, I. C., Geana, E. I., Constantin, M., Angheloiu, M., Pirvu, L., & Cristea, S. (2022). Phytostimulation and Synergistic Antipathogenic Effect of Tagetes erecta Extract in Presence of Rhizobacteria. Horticulturae, 8(9), 779. https://doi.org/10.3390/horticulturae8090779







