Abstract
There is an urgent need for improvements in animal production, particularly for ruminants, such that more sustainable and efficient processes are developed for obtaining more nutritious and efficient feeds. Filamentous fungi can add value to residual plant biomass, and may also have the potential to produce metabolites and enrich plant biomasses used in animal nutrition, converting them into nutraceutical sources. Thus, in this work, filamentous fungal fermentation of ruminant feed biomasses commonly used in Brazil was performed, and the enrichment for bioactive metabolites was tested. For this, Fistulina hepatica, Ganoderma lucidum, Pleurotus pulmonarius, Panus lecomtei, and Aspergillus terreus were grown for 28 days on different substrates: starchy grains- (sorghum, oat, and corn), fibrous substrates (coast-cross, rice husk, and moringa plant) and protein-rich substrates (cottonseed cake and pigeon pea plant). Fermented substrates were evaluated for laccase activity, crude protein, β-glucan, and lovastatin content. The highest growth rate was observed for G. lucidum in oat substrate (OT-01) (0.708 ± 0.035 cm/day) and F. hepatica in oat + coast-cross + pigeon pea treatment (OT-10) (0.607 ± 0.012 cm/day). High laccase activity was observed for P. lecomtei grown in starchy grain + moringa + pigeon pea substrate, reaching an activity of 416.8 ± 20.28 U/g. A. terreus growth in ST-09 (sorghum + pigeon pea) showed higher protein (15.3 ± 0.46%), β-glucan (503.56 ± 8.6 mg/g) and lovastatin (1.10 ± 0.17 mg/g) content compared to untreated substrates. These results demonstrate that filamentous fungi are an alternative for nutraceutical enrichment of ruminant feed biomasses. To the best of our knowledge, this is the first report in which P. lecomtei and F. hepatica are evaluated for their ability to be cultivated in ruminant feed substrates from Brazil.
1. Introduction
As one of the top beef producers in the world, Brazil has been making an effort to change the conventional livestock production chain toward more sustainable processes [1]. Some processes have been adopted to reduce the impact of animal production on the environment, such as using degraded land for raising cattle [2] and integrated crop-livestock production systems [3]. However, better livestock management using good quality feed may be more efficient for increasing animal productivity and environmental protection [4].
The chemical modification of plant biomass by fungal fermentation has been used to improve the nutritional value of residual biomasses (e.g., rice straw, wheat straw, corncob, stover and straw) to use in livestock feeding. Biological treatment of plant biomass with filamentous fungi to reduce fiber recalcitrance and improve carbohydrate digestibility has been extensively reported [5,6]. Filamentous fungi, yeast or their extracts have also shown benefits for the ruminant livestock sector, such as modulation of the rumen microbiota [7], weight gain [8], reduction in methane production [9], increase in productivity, and immunomodulation [10].
Demands for the reduction in the use of antibiotics for livestock farming have increased interest in the use of nutraceuticals as a potential alternative for therapeutic use and prevention of diseases [11]. However, there is a need for additional studies before commercial production and animal use [12,13]. Among metabolites are enzymes that have been shown to be capable of increasing nutrient availability in complex feed [14]. Furthermore, for ruminants, white rot fungi laccase has been used as an exogenous agent able to enhance the digestibility of crop residues for heifer feeding [13]. In addition, it modulates the rumen microbiota increasing animal productivity [15]. Furthermore, filamentous fungi/yeast polysaccharides, such as β-glucans, are known to assist nutrient utilization efficiency by ruminants [16] and can activate the animal immune system [17]. β-glucan has been considered a potential alternative for antibiotics [18]. Lovastatin is another fungal metabolite that has been reported as a selective methanogenic archaea controller, with the potential for reducing methane production [19].
A complete filamentous fungi fermented feed, rich in nutraceuticals, can favor the efficiency of biomass use by the animal, and reduce the need for antibiotics. To obtain such feed, it is necessary to establish fermentation conditions, such as the substrate formulation and fungal species. Furthermore, for the fermentation process to be commercially accessible and efficient, biomass and fungal species easily found in each region should be prioritized. In this work, a screening of Brazilian filamentous fungi grown in commonly used ruminant feed biomasses was performed to evaluate the production of fungal bioactive metabolites that can potentially benefit animal production. The evaluated filamentous fungi were able to increase the potential nutraceutical value of substrates by producing laccase, β-glucan, lovastatin, and by concentrating crude protein.
2. Materials and Methods
2.1. Microorganisms and Biomasses
The fungi Pleurotus ostreatus BRM 055505, Fistulina hepatica BRM 047114, Panus lecomtei BRM 044603, and Pleurotus pulmonarius BRM 055674 were obtained from the Collection of Microorganisms and Microalgae Applied to Agroenergy and Biorefineries from Embrapa Agroenergy (CMMAABio), Brasilia, Brazil. The strain of Aspergillus terreus ATCC 20542 was kindly provided by the Molecular Biology Department of the University of Brasilia–UnB, Brazil. These filamentous fungi strains were previously studied for the biotransformation of residual biomass, and showed high biodetoxifying activity of toxic molecules, such as gossypol and phorbol ester, with potential use in animal feed [20,21]. All fungal species were initially grown on PDA (Potato Dextrose Agar) medium at 28 °C and stored at 4 °C for later use. Spawns of each fungus were prepared by inoculating the PDA cultured mycelium in autoclaved (121 °C, 1 atm for 1 h) humid wheat grain. The fungi were grown for 30 days at room temperature (i.e., 25–30 °C).
Most of the biomasses used as substrates in fermentation screening are commonly used for animal nutrition in Brazil. Two of them are known as plant sources of protein (i.e., cotton seed cake (Gossypium sp.), and pigeon pea (Cajanus cajan)), three are sources of grain starch (i.e., sorghum (Sorghum sp.), oat (Avena sativa) and corn (Zea mays)), and three are rich in cellulose/lignin (i.e., coast-cross (Cynodon dactylon (L.) pers), rice husk, and moringa (Moringa oleifera)). Moringa (leaves/pods) have also been considered an alternative protein source for animal nutrition [22]. Cottonseed cake, rice husk, pigeon pea, and moringa trees were obtained at agroindustrial or family farms in the Federal District of Brazil. Table 1 shows the nitrogen content of each biomass used for substrate formulation.

Table 1.
Mean value nitrogen content of each biomass used for substrate formulation.
For substrates preparation, the entire moringa and pigeon pea plants, including leaves, branches, pods, and stems, were mechanically reduced in size using a Trapp® (Jaragua, SC, BR) TRF 400 shredder fit with a 10 mm sieve, and dried under the sun.
2.2. Substrate Preparation, Fungi Cultivation, and Growth Measurement
To prepare the substrates, the biomasses used were divided into 3 groups according to their main nutritional characteristics: source of starch (i.e., sorghum, oat, and corn grains), forage sources of cellulose/lignin (i.e., coast-cross, rice straw and moringa), and source of protein (i.e., cottonseed cake, pigeon pea).
Before the preparation of substrates, in order to correct the biomass moisture content to approximately 70%, all grains and rice husks (which are more resistant to absorbing water) were soaked in water for 12 h at room temperature. The water was removed, and only the soaked grains and husks were used for fungi growth. All other materials (i.e., coast-cross, moringa, pigeon pea and cottonseed cake, which easily absorb water) were wet for 1 h before substrate preparation.
Substrates, prepared as described above, were aliquoted into 800 mL cylindrical glass jars (12 cm high) in a total of four experimental treatments, in triplicates: (1) only starchy grain; (2) starchy grain + lignin/cellulose sources (m/m); (3) starchy grain + protein source (m/m), and (4) starchy grain + protein + lignin/cellulose sources (m/m/m). The use of grains for the preparation of substrates was prioritized because they are cheap and commonly available in the central region of Brazil, thus making the fermentation process more accessible. Experimental treatments used are summarized in Table 2.

Table 2.
Plant biomass substrates used as animal feed sources of starch (sorghum, corn, and oat grains), lignin/cellulose (coast-cross, rice husk, and moringa) and protein (cottonseed cake, pigeon pea) that were tested for filamentous fungi growth.
The glass jars containing each substrate were decontaminated by hot water vapor (100 °C) for 12 h. After cooling down, 40 g of each fungi spawn was inoculated into the different substrates. The inoculated jars were maintained at room temperature for 28 days. Mycelium growth measurement was performed every 7 days (0, 7, 14, 21 and 28 days). After 28 days, substrates that were completely colonized by fungi were selected for further analyses. Mycelial growth rate was calculated by measuring total mycelial growth (cm) and dividing this number by the time (in days) each fungus took to colonize the entire substrate, in triplicates.
2.3. Laccase Activity
The selected fermented substrates were submitted to enzyme extraction with cold water (5 mL, m/v) and agitation for 1 h at 200 rpm. Laccase activity in the filtered (Whatman No. 1) extract was detected as previously described [23,24] by oxidation of 5 mM 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and absorbance measurement at 420 nm. All assays were performed using a UV-Vis spectrophotometer. An enzyme unit (U) was defined as the amount of enzyme required to oxidize 1 µmol of ABTS per min [25]. The fungus exhibiting the highest laccase activity was selected for further analyses.
2.4. Protein Quantification
Total nitrogen (N) was determined according to [26,27]. Each sample was weighed in a tin capsule (8 × 5 mm), and analyzed by an elemental analyzer (Perkin Elmer, PE 2400 series II) after combustion. Crude protein content was then calculated by multiplying the Nitrogen value by a factor of 6.25.
2.5. β-Glucan Quantification
Total glucans were extracted using a solvent accelerated extractor (Thermo Scientific, ASE 350, Waltham, MA, USA) with water extraction at 200 °C. β-glucan was quantified according to the Mushroom and Yeast β-Glucan Assay Procedure (Megazyme International Ireland Limited, Wicklow, UK, 2021) [28]. Measurement of α- and total-glucans were performed using a UV Spectrophotometer at 510 nm. The β-glucan content was obtained by subtraction of α-glucan from total-glucan.
2.6. Lovastatin Quantification
Lovastatin was extracted using acetonitrile solvent (1:10, m/v) followed by agitation at 200 rpm and 40 °C for 2 h. After centrifugation at 20,590× g for 10 min at 25 °C, the supernatant was analyzed according to [29] for detection of β-hydroxy acid and lactone lovastatin form, with modifications. Briefly, separation of sample components by liquid chromatography (UHPLC) was performed in an Acquity UPLC® HSS T 3 column (2.1 × 150 mm × 1.8 µm) maintained at 25 °C, with a flow of 0.400 µL/min using a mobile phase of acetonitrile (A) and water (B), both acidified with 0.1% formic acid. Gradient elution occurred with the following programming: from 00 to 08 min (60% A and 40% B), from 08 to 12 min (80% A and 20% B), and from 12 to 15 min (60% A and 40% B). The Waters-Acquity I-Class Ultra Performance Liquid Chromatograph (UPLC®) composed of a binary pump, PDA detector and column oven coupled to a Waters-Xevo TQDWaters-Acquity® mass spectrometer (MS) was used to detect and quantify lovastatin. ESI (electrospray ionization) operated in positive ionic mode with argon as the collision gas and nitrogen as the auxiliary gas. For detection in MS, the following parameters were used: capillary voltage equal to 3.0 kV, desolvation temp. 400 °C, desolvation gas 700 L/h; gas cone 20 L/h; MRM monitoring mode with mass transition from 405.32 to 199.2 and 405.32 to 285.2 for the two forms of lovastatin; 20 V cone voltage; 3 V collision energy. To obtain the acid form of lovastatin, the lactone standard was treated with 0.1 M NaOH (v/v) and incubated for 1 h at 50 °C in a water bath.
2.7. Statistical Analysis
The results obtained (expressed as mean ± standard deviation) were subjected to analysis of variance of the means, and then were grouped by the ScottKnott or Tukey’s test at 5% probability using the SISVAR® 5.6 (Lavras, MG, Brazil) software.
3. Results and Discussion
3.1. Fungi Growth Rate
From a total of 180 fungi solid fermentations (5 filamentous fungal strains × 36 substrates), only 39 substrates were fully colonized by the fungi within 28 days. Fungi with growth rates above 0.5 cm/day colonized the substrates in less than 28 days; on the other hand, those that had growth rates below 0.4 cm/day did not fully colonize the substrate in the same period (Table 3). Fungi growth time is one of the most important factors in the fermentation process because it affects the chemical composition and nutrients of the biomass [30]. The fungi growth rate and metabolite production vary according to biomass composition (i.e., C:N ratio, minerals present, moisture content), fungus strain and fermentation conditions (temperature, oxygenation, pH) [31].

Table 3.
Mycelial growth rate of filamentous fungi in substrates commonly used as animal feed biomass. The experiment was performed in triplicate and values shown are the mean in cm/day ± standard deviation.
For ruminant feeding, the ideal fermentation process should prioritize microorganisms that reduce the recalcitrance of the biomass (i.e., lignin selective) and keep the carbohydrates [32] in a short fermentation period [33]. In the same period, the fungus must also enrich the biomass with metabolites to modulate the rumen microbiota [34]. A longer fermentation period leads to greater lignin degradation and increases metabolite production by the fungi; however, the consumption of cellulose and hemicellulose is higher as well [30]. Therefore, an intermediate cultivation time, optimized for each species and substrate used must be identified. The results presented in this work show that 28 days of fermentation works well for the filamentous fungi used for cultivation, similar to [30]; however, further optimization can be achieved by evaluating different fermentation conditions.
At 28 days of cultivation, P. lecomtei BRM 044603, G. lucidum BRM 055670 13, F. hepatica BRM 047114, and P. pulmonarius BRM 055674 strains completely colonized 11, 13, 3 and 9 different substrates, respectively. As shown in Table 3, each evaluated strain presented growth rate peculiarities whenever the substrate composition was modified. F. hepatica BRM 047114 was able to fully grow only in substrate containing oat grains: OT-03, OT-07 and OT-10, reaching a growth rate of 0.607 ± 0.012, 0.584 ± 0,030 and 0.55 ± 0.035 cm/day, respectively. However, it was not able to grow mycelium on ST-06 and CT-01 substrates. The G. lucidum BRM 055670 strain showed highest growth rate in the oat substrate (OT-01) reaching 0.7 ± 0.035 cm/day, it also showed superior growth on substrates OT-07, OT-03 and CT-07, with a growth rate of 0.58 ± 0.017, 0.55 ± 0,147 and 0.55 ± 0.04 cm/day, respectively. However, G. lucidum BRM055670 did not grow on the substrates ST-04, CT-05, CT-06, OT-05 and OT-06. P. pulmonarius BRM 055674 and P. lecomtei BRM 044603 were able to fully grow on corn, oat, and sorghum-based substrates. Only P. pulmonarius BRM055674 was able to grow mycelium in all media, even though for most of the substrate colonization was incomplete. P. lecomtei BRM044603 did not grow on ST-08 and OT-08, both containing a mixture of starchy grain, moringa, and cottonseed cake. From these results, it is clear that filamentous fungi are able to grow on different biomasses used as feed for ruminants; however, the growth rate depends on substrate composition.
White-rot basidiomycetes such as P. lecomtei, P. pulmonarius, G. lucidum, and brown-rot basidiomycetes such as F. hepatica, are specialized in growing on residual lignocellulosic biomass. They have been successfully grown for 15 days on residual biomass, cottonseed and Jatropha curcas cake, with the aim of reducing levels of toxic antinutritional components, enabling their use in animal nutrition after this treatment [20,21].
A. terreus ATCC 20542 was also cultivated on all substrates, but due to its high sporulation rate, there was no uniform growth on the substrates which precluded an accurate measurement of its growth rate. However, it was possible to observe total colonization of the substrate by A. terreus ATCC 20542 in the formulations ST-09, CT-01, and OT-01. A. terreus strains are known as carbohydrate enzyme producers [31]; however, it does not seem to produce ligninases (laccase) without medium supplementation with inducers [35]. Thus, its growth rate is expected to be higher on a medium with a higher carbohydrate content, such as a grain/starch-based substrate.
3.2. Laccase Activity
The interaction between fungi and fermentation duration affects enzyme activity, with longer fermentations seeming to benefit activity with a plateau being reached at 28 days for filamentous fungi grown on corn straw [30]. For animal nutrition, the activity of filamentous fungi enzymes, including laccases, can increase residual biomass digestibility and modulates gas production [36]. Ruminant nutrition with exogenously added enzymes has also been reported as having the potential for increasing animal productivity [13,14]. Increased performance and nutrient digestibility in bulls feeding with laccase (0.4 g/kg D.M., 40 U/g) were observed by [15]. The increase in feed digestion and productivity by adding enzymes exogenously may have various mechanisms: exogenous enzymes act in synergy with endogenous ruminal microbial enzymes, a decrease of ruminal pH benefits of fibrolytic bacteria, attachment of ruminal bacteria to the feed fibers, and modulation of the ruminal microbial population [37].
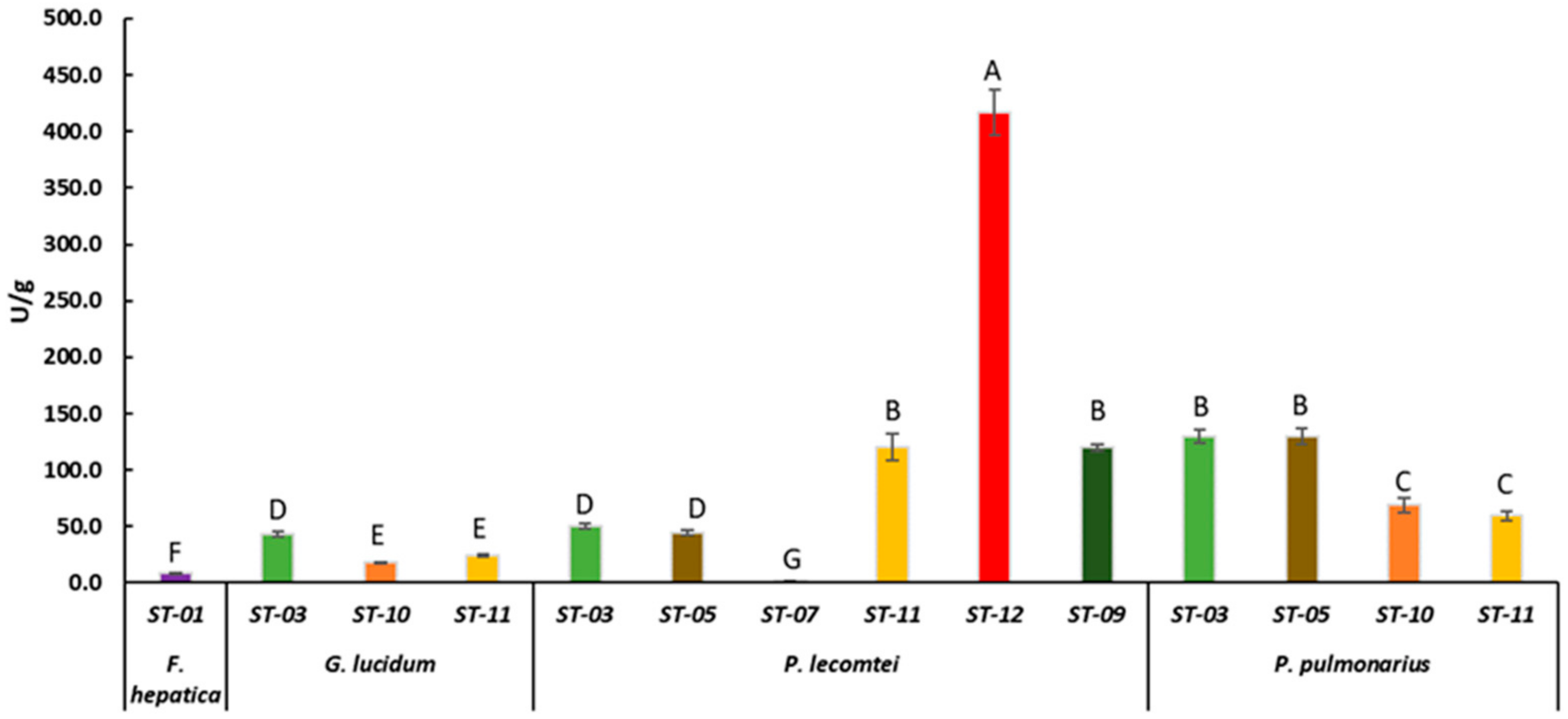
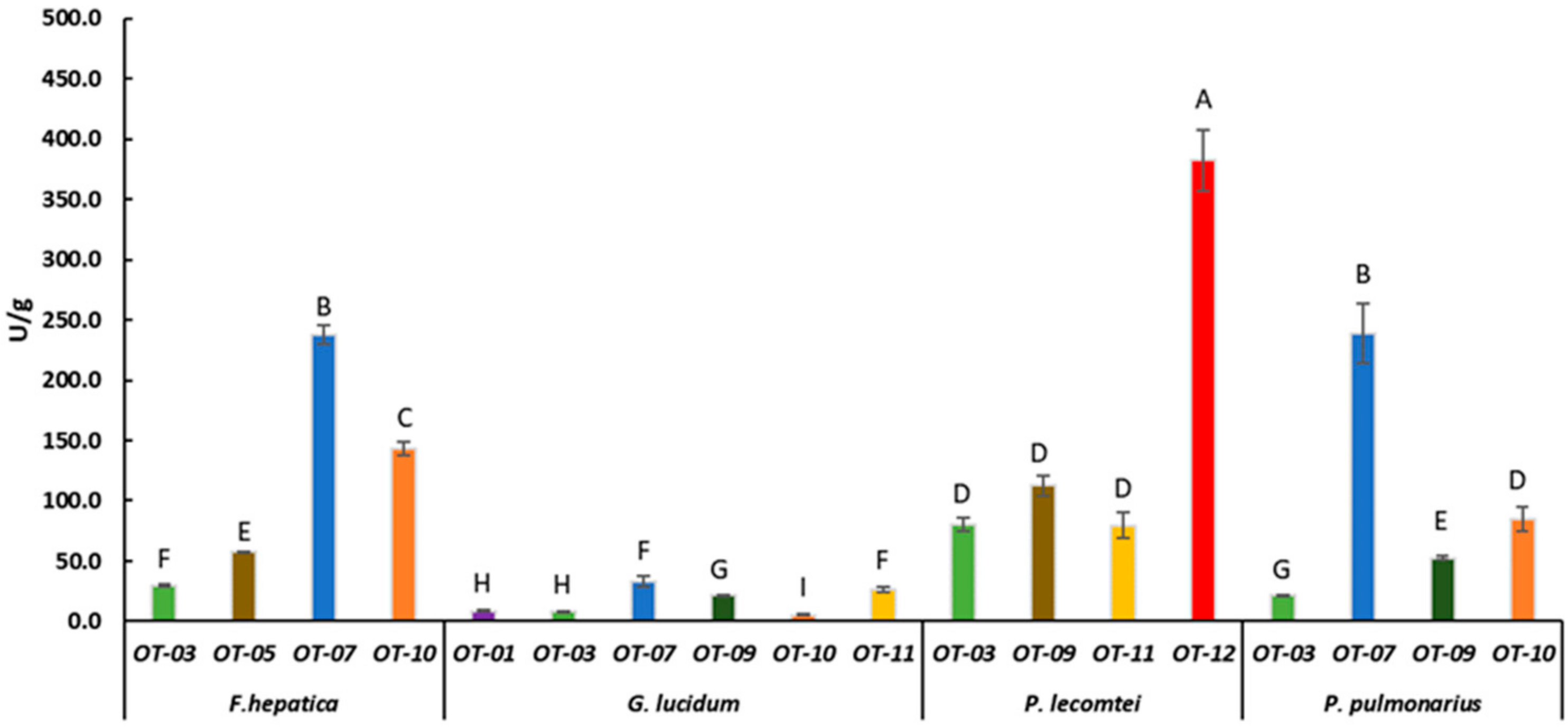
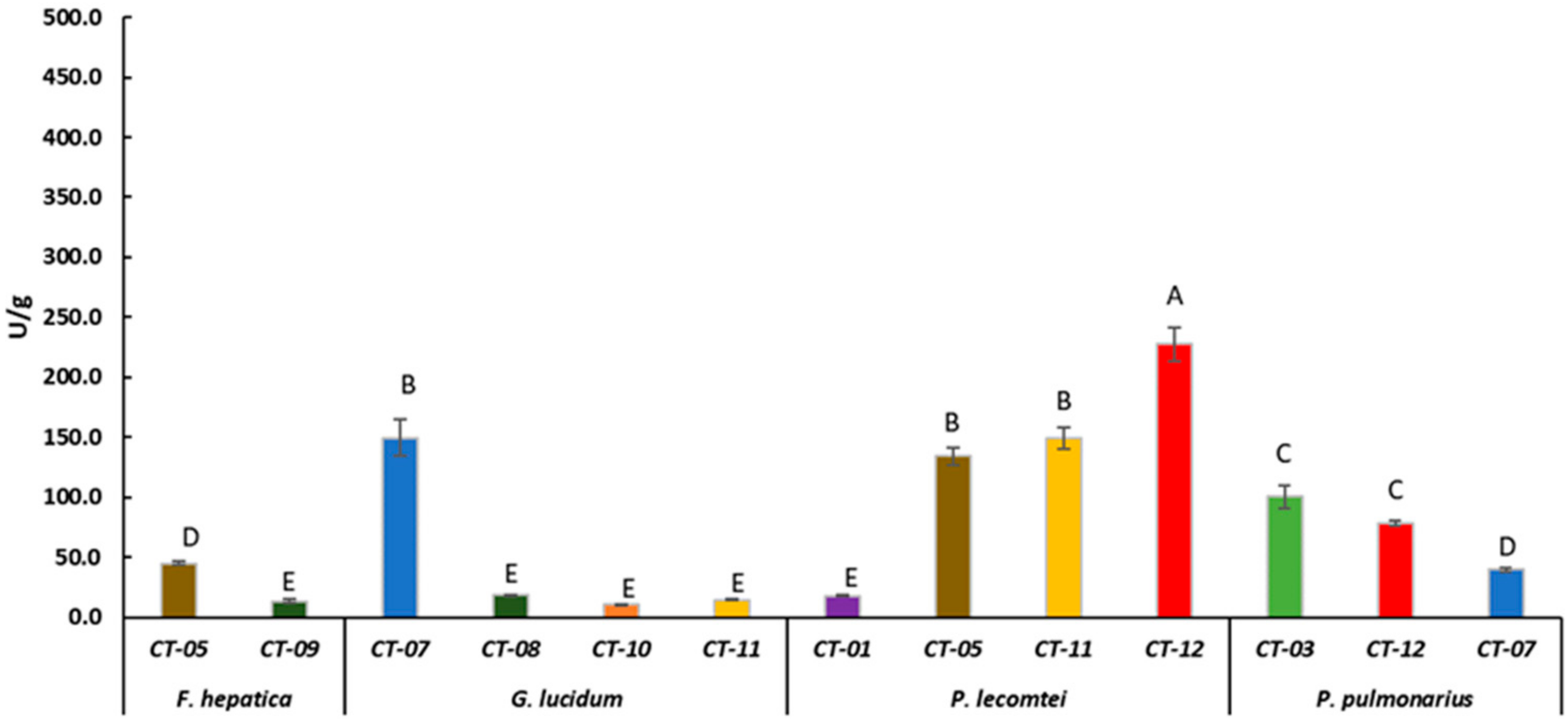
Laccase activity was evaluated in the fermented substrates that showed the best fungal growth rate. P. lecomtei BRM 044603 stood out when grown on substrates based on grain, moringa and pigeon pea, ST-12, OT-12 and CT-12, with laccase enzymatic activity of 416.8 ± 20.28, 382.6 ± 25.37 and 227.6 ± 14.12 U/g, respectively (Figure 1, Figure 2 and Figure 3). When cultivated in submerged fermentation, P. lecomtei BRM 044603 also showed great potential in terms of laccase activity, reaching 799.8 ± 42.0 U/mL when using oil palm decanter cake as the carbon source [38]; however, when growing on mandarin peels, laccase activity of P. lecomtei BCC 903 decreased dramatically to 6.4 ± 1.0 U/mL [39]. These contrasting results corroborate that the production of enzymes by the fungus is strain and carbon source dependent.
Figure 1.
Laccase activity of F. hepatica BRM 047114, G. lucidum BRM 055670, P. lecomtei BRM 044603 and P. pulmonarius BRM 055674 after 28 days cultivation on starchy substrates based on sorghum grain. ST = Substrates based on sorghum grain (Table 1). Mean values of triplicates followed by the same letter do not differ according to the ScottKnott test at a 5% significance level. Bars are standard deviations.
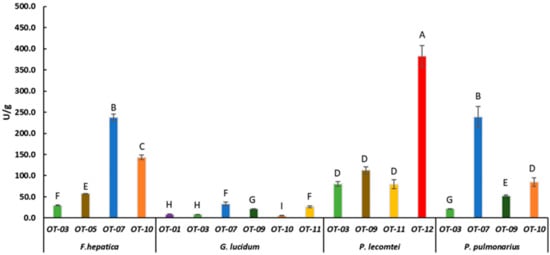
Figure 2.
Laccase activity of F. hepatica BRM 047114, G. lucidum BRM 055670, P. lecomtei BRM 044603 and P. pulmonarius BRM 055674 after 28 days cultivation on substrates based on oat grain (starch). OT- Substrates based on oat grain (Table 1). Mean value of experiment performed in triplicate followed by the same letter does not differ according to ScottKnott’s test at the 5% significance level.
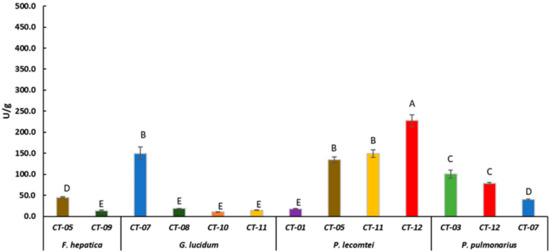
Figure 3.
Laccase activity of F. hepatica BRM 047114, G. lucidum BRM 055670, P. lecomtei BRM 044603 and P. pulmonarius BRM 055674 after 28 days cultivation on substrates based on corn grain (starch). CT- Substrates based on corn grain (Table 1). Mean value of experiment performed in triplicate followed by the same letter does not differ according to ScottKnott’s test at the 5% significance level.
P. pulmonarius BRM 055674 and F. hepatica BRM 047114 presented laccase activity of 239.2 ± 24.8 and 237.7 ± 7.8 U/g, respectively, when grown in substrate based on oat and moringa (OT-07) (Figure 2). The production of laccase by P. pulmonarius is also influenced by the composition of the culture medium, as observed when comparing the activity in the different fermented biomasses (Figure 1, Figure 2 and Figure 3). Similar data were reported by [40], who found laccase activity of 103.50 U/mL produced by P. pulmonarius BPSM 10, and 349.5 U/mL when the medium (malt extract) was supplemented with xylidine. It has also been reported that copper (CuSO4) supplementation increases laccase activity produced by P. pulmonarius CCB-19 from 270 to 1.420 U/L in solid fermentation with corncob [41]. High moisture content also affects laccase production by these fungi [42]. F. hepatica is not commonly used for fermentation since it belongs to a group of brown rot fungi in a lifestyle transition, being more specialized in a non-enzymatic process for polysaccharide consumption [43]; however, F. hepatica also produces lignin-modification enzymes such as aryl alcohol oxidase [44], and lignin and manganese peroxidases [45]. Therefore, it is possible that wood fiber decay by F. hepatica may not be exclusively due to a laccase-based process [46].
Even though G. lucidum presented the best growth rate on the OT-01 substrate, its laccase production in this substrate was only 8.7 ± 0.6 U/g (Figure 2). It also presented laccase activity below 45 U/g in all evaluated substrates based on sorghum, oat, or corn (Figure 1, Figure 2 and Figure 3), except in CT-07 (149.4 ± 15.13 U/g) (Figure 3). When in submerged cultivation using corncob as substrate, G. lucidum presented laccase activity of 68.75 U/mL in only 5 days of incubation [47]. Inducers, such as ethanol, gallic acid, and copper (CuSO4), may increase laccase activity by G. lucidum as well [48]. In solid-state fermentation, laccase activity by G. lucidum is also induced by adding ferulic acid (144.62 ± 38.52 U/g) and copper (149.89 ± 9.98 U/g) to Pinus taeda sawdust [49].
The selected substrates colonized by A. terreus ATCC 20542 were also evaluated for laccase activity; however, none of the selected fermented substrates were able to induce laccase enzyme activity. When grown on wheat bran solid-state fermentation, A. terreus S-19 was reported to sow partially purified laccase activity of 91.08 U/L [50]. Copper (Cu2+), ABTS, Zn, guaiacol and ferulic acid also have been shown to be inducers of laccase genes (Lcc) in A. terreus KC 462061 [35].
3.3. Total Protein and β-Glucan Content
Total protein content was analyzed for selected filamentous fungi fermented substrates which presented the best fungal growth rates and/or laccase production. Most filamentous fungi were able to statistically concentrate protein in the substrates after 28 days of cultivation, except for P. lecomtei in OT-12 and ST-03, P. pulmonarius BRM 055674 in OT-07 and G. lucidum in OT-01, for which there was a not statistically significant increase in crude protein concentration when compared to controls. A. terreus ATCC 20542 was able to concentrate almost four times the protein content in substrate based on sorghum and pigeon pea (ST-09), it also was able to concentrate two times the protein content on oat grain (OT-01), and the same fold concentration was observed for P. lecomtei BRM044603 in substrate ST-09 (Table 4). The increase in protein content by filamentous fungi fermentation has been reported for several agroindustrial residues, such as brewer-spent grain, grape bagasse [51], banana leaves, rice straw, corn cob, sugarcane bagasse [52], corn stover [6], olive cake [25], purple field corn residue [53], and wheat straw [54]. Moreover, filamentous fungi not only concentrate protein in fermented substrates, but also modify the protein profile, increasing essential amino acids such as lysin, arginine, methionine, isoleucine, phenylalanine, valine, and threonine [6,33,55].

Table 4.
Total crude protein and β-glucan content in the filamentous fungi fermented and untreated substrates commonly used as animal feed biomass.
P. pulmonarius fruit body is an edible mushroom rich in crude protein (7.88 ± 0.038/100 g), carbohydrate (60.8 ± 3.27/100 g) and crude fiber (11.54 ± 0.396/100 g) according to [56]; however, these values may vary according to substrate composition, and fermentation conditions [57]. P. pulmonarius has also been reported as being able to improve residual biomass crude protein such as for orange waste [58], sorghum stover [59] and rice straw [60]. However, depending on fermentation conditions, no change in crude protein content has also been reported, such as for fermentation in wheat straw performed by [61]. This corroborates the result obtained for P. pulmonarius BRM055674 in OT-07. G. lucidum, which is mainly consumed for medicinal purposes, even when used as an animal feed additive [62,63], also has demonstrated improvement in the residual biomass crude protein content. Misra [64] reported a protein content increment on mustard straw after 21 days of G. lucidum incubation, and Han [65] observed an increment from 11.0 to 16.5% in protein content in cornmeal. A. terreus is an excellent protein producer, to the point of being considered a single-cell protein microorganism producer for animal nutrition. Different biomasses have been evaluated with the obtention of varying yields of crude protein by A. terreus: rice bran, wheat bran, eichornia and banana peel (total amino acids of 37.5% after fermentation in rice bran) [66], broken rice (utilized 0.344 g protein/gram cell to form 0.622 g protein/gram cell) [67], bagasse substrate (an increase from 14.3 to 20.6% of crude protein) [68], and cellulose (protein content ranged from 23 to 38%) [69]. P. lecomtei and F. hepatica have not been well evaluated for animal feed applications; however, according to [21], both fungi have the potential to increase protein content on Jatropha curcas cake to 30.0 ± 3.9 and 28.3 ± 1.5/100 g (D.W.), respectively, and the ability to biodetoxify phorbol esters. Furthermore, wild F. hepatica mushroom has a protein content of 22.6 ± 0.20 g/100 g (D.W.) [70]. Fungal protein may be an important alternative source of protein for ruminant nutrition with the potential for replacing plant-derived protein sources.
The glucan content in filamentous fungi is also influenced by the fermentation conditions, the substrate used, and the specific fungus strain [71]. A. terreus ATCC 20542 was able to significantly increase β-glucans on substrate AT-01 and ST-09 (490.99 ± 123.9, 503.356 ± 8.6 mg/g (D.W.), but when cultivated on corn grain (CT-01), no changes in polysaccharide content were observed. For P. lecomtei, variation also happened, with no changes on most substrates, except on CT-11, CT-12 and ST-07, which decreased β-glucans, and a slight increase of this carbohydrate was shown on ST-03. For P. pulmonarius, the two substrates analyzed, OT-07 and CT-07, an increase in β-glucan on the former and degradation on the latter were observed. G. lucidum consumed β-glucan on both analyzed fermented substrates (OT-01 and CT-07) (Table 4).
For P. pulmonarius, different β-glucan content has been reported depending on the fermentation process and fungus structure. For example, P. pulmonarius commercial mushroom has been reported to produce 17.466 ± 0.610/100 g (D.W.) of β-glucan [72], while mycelium grown on galactose medium presented 0.42 g/L of β-glucan with a fungal biomass of 1.08 g/L (approximately 39/100 g of fungal biomass). A variation of 16.7 to 9.75 mg/100 g of crude endo-polysaccharide extracted from the mycelium of irradiated and non-irradiated P. pulmonarius, respectively, has also been reported for submerged culture conditions [73]. Chemical variation between the vegetative and reproductive structures of P. pulmonarius also has been reported for other compounds [74].
The growth of G. lucidum in residual biomass is evaluated as a promising product for use in animal feed with nutraceutical properties, with the potential for reducing the use of antibiotics. High levels of β-glucans in soybean residue (234.09 mg/g) and soybean hulls (180.32 mg/g), were achieved by [75], corroborating that fungal biomass nutrients can be used to enrich animal feed. The authors of [76] also valorized industrial residues (beech sawdust, wheat straw, two-phase olive mill waste, and olive pruning residues) by cultivating G. lucidum. The β-glucans content after fermentation ranged from 35.83 ± 2.05 to 43.10 ± 6.38 g/kg (D.W.), regardless of the substrate used, and there was no statistically significant difference.
Even though there are few studies on F. hepatica β-glucans, there is a patent that described it as a possible non-commercial basidiomycete source for obtaining β-glucan [77]. A. terreus is also a potential source of bioactive polysaccharides [78]. Reports have demonstrated that the production of exopolysaccharides by A. terreus can be optimized by modifying the substrate with glucose (2.39%), NH4NO3 (0.957%) and pH (8.79), leading to an increase in total polysaccharides, including β-glucans, to 1.34 g/L [79]. The authors of [80] were able to increase β-glucan to 0.32 g/g mycelium (D.W.) using a medium containing corn starch (4.5 g/L) and yeast extract (0.4 g/L). In addition, the different medium composition was able to affect β-glucan production by A. terreus, which varied from 0.06 to 0.32 g/L.
Even though the use of β-glucan for polygastric animals is just beginning to be explored, initial results have corroborated its potential as a modulator of the ruminal microbiota and the ruminant immune system. Supplementation of yeast β-glucan at 75 mg/kg in calves’ ration increased the apparent digestibility parameters (i.e., dry matter, crude protein, and ether extract) of the feed. Furthermore, an increase in serum immunoglobulins G and M, and a decrease in serum triglycerides, and total cholesterol were observed [16]. Oral intake of Aureobasidium pullulans β-glucan affects cytokine expression in the serum of cows and influences the bacterial microbiota in the intestines of calves [81]. In calves, it was also observed that β-1,3/1,6-D-glucan influenced the percentages of different immune system cells, such as phagocytic granulocytes, monocytes and the percentage of bacteria engulfed by granulocytes [82]. Supplementing 4.7 g/day of β-glucan to beef cattle improved rice straw intake, as well as crude protein digestibility [83]. An increase in matter intake and milk production in postpartum cows was observed with commercial β-glucan intake; furthermore, it also reduced the serum parameters of aspartate and alanine aminotransferase, and glutamyl transpeptidase, indicating a hepatoprotective effect [84].
In sheep, Saadei [17] demonstrated that oral administration of β-glucan affects various aspects of the immune system, including hematologic parameters, such as serum IgG and interferon-gamma. Zabek [10] observed that lambs with diets supplemented with β-glucan have a higher growth rate and muscle tissue development. Furthermore, an increase in blood gamma-globulin, lysozyme activity, respiratory burst activity, and potential killing activity response of T and B-cells were also observed.
In monogastric animals, β-1,3/1,6-glucan molecules will pass through the small intestine and become the substrate for colon microorganisms [85]. Similar studies that support any benefits for the rumen microbiota are still lacking. The authors of [86] fed cattle with barley β-glucan and found low digestibility of the polysaccharide. Indeed, this research group recovered 0.03 to 0.11 g of β-glucan/kg (D.W.) from the feces of mature cattle fed for 42 days. Further studies should be performed to determine whether fungal β-glucan also has the same effect.
3.4. Lovastatin Production
Most methane is produced by methanogenic archaea present in the rumen microbiota [87]. Some archaea that use the HMG-CoA enzyme to produce phospholipids (isoprenyl) associated with membrane formation, have their growth compromised in the presence of lovastatin [88]. Thus, lovastatin has been used as a ruminant microbiota modulator [9]. Due to its potential mitigation effect on ruminant methane production, the presence of lovastatin was evaluated in the fermented substrates analyzed in this work.
Lovastatin production was only detected in A. terreus ATCC 20542 fermentations. From the three substrates evaluated, ST-09 (based on sorghum and pigeon pea) presented the highest lovastatin content (1.10 ± 0.17 mg/g), followed by fermented corn (CT-01) and oat (OT-01) grain, with 0.69 ± 0.03 and 0.29 ± 0.02 mg/mg of lovastatin content, respectively. Notably, in all fermented substrates studied, the β-hydroxy acid form (active) was higher than the lactone form of lovastatin (Table 5).

Table 5.
Lovastatin content in substrates fermented by A. terreus ATCC 20542.
Lovastatin production by A. terreus has been evaluated for several agricultural biomasses, mainly for optimized drug obtention. Drug production is dependent on substrate composition and fermentation conditions. The authors of [89] obtained a lovastatin yield of 70.17 and 63.76 mg/kg (dry matter, D.M.) from oil palm frond fermentation supplemented with soybean meal by A. terreus ATCC 20542 and A. terreus ATCC 74135, respectively. The authors also produced lovastatin in rice straw, achieving a yield of 154.48 ± 22.88 and 157.070 ± 1.92 mg/kg (D.M.) by both fungi. Different substrates interfere in lovastatin production, soybean cake particle (1.0 ± 0.039 mg/g of lovastatin), rice (2.2 ± 0.085 mg/g), corn particle (1.2 ± 0.047 mg/g), wheat bran (2.0 ± 0.038 mg/g) and rice husk (0.6 ± 0.030 mg/g). Lovastatin yield is also affected by the substrate particle size, and nutrient supplementation (glucose and peptone) [90]. Patil [91] achieved different lovastatin yields with A. terreus PM3 in wheat bran (12.5 mg/g), red gram (Cajanas cajan) husk (8.2 mg/g), rice bran (3.1 mg/g), green gram (Phaseolus mungo) straw (9.3 mg/g) and soybean husk (8.3 mg/g). The authors also found that substrate moisture affects the obtention of the molecule, 70% moisture being ideal for lovastatin production. When A. terreus is grown on the substrate containing a high concentration of biodiesel-derived crude glycerol, lovastatin production is inhibited, and only 3.4 g/L (crude glycerol at 50 g/L) was obtained. However, when the same concentration of pure glycerol is used, lovastatin production increased to 18.9 g/L, indicating that the presence of impurities in the carbon source used by the fungi interferes with lovastatin production. The authors were also able to increase lovastatin production by adding methanol or NaCl to the medium containing pure glycerol [92].
Lovastatin production by A. terreus has been evaluated due to its ability to reduce methane production by ruminants. The concentration of 4.14 mg/kg (D.W.) of lovastatin reduced methane production by 32% in goats fed with A. terreus fermented rice straw: also, substrate digestibility was improved by 13% [93]. The authors of [19] also observed methanogenic archaea and a methane reduction in in vitro digestion with A. terreus fermented rice straw fermented for 8 days containing 260.8 mg/kg D.M. An increase in cellulolytic bacteria and in vitro dry matter digestibility were also observed. In an in vitro experiment, Miller [94] demonstrated that lovastatin selectively inhibits by 50% the growth of Methanobrevibacter ZA 10 with 4 µmol/mL of lovastatin, and concentrations ≥ 10 µmol/mL completely inhibited the strain growth and CH4 production. On the other hand, lovastatin did not interfere with the growth of cellulolytic bacteria (e.g., Butyrivibrio fibrisolven, Ruminococcus albus, Ruminococcus flavefacien, Fibrobacter succinogenes, and Selenomonas ruminantium). Recently, it was demonstrated that feed supplemented with less than 20% of fermented oat straw (189 and 284 mg/L of the fermented substrate to obtain lovastatin concentration of 100 and 150 mg/L in the final feed formulation, respectively) was able to decrease methane formation up to 38% without affecting other nutrition parameters. This shows that solid-state fermentation by specific fungi may be an economical and sustainable process to mitigate CH4 production in ruminants [9].
Even if the other fungi do not produce lovastatin, they have the potential to produce other bioactive molecules and need more in-depth studies to explore potential benefits for animal production. For example, F. hepatica produces the antifungal molecule feldin [95]. G. lucidum produces ganoderic acid that has several potential therapeutic activities such as antitumor, anti-inflammatory, anti-viral and hipocholesterolemic [96]. P. pulmonarius produces nematicidal molecules such as S-coriolic acid, linoleic acid and p-anisaldehyde [97]. Therefore, there is great potential for the use of filamentous fungi as a source of metabolites in ruminant feed.
It is well known that factors such as fungal strain, substrate composition, and fermentation conditions directly affect metabolite profile and production. The optimization of the fermentation process to enrich bioactive molecules (nutraceuticals) is key to the establishment of industrial production. Finally, it is noteworthy that the nutritional value of residual biomasses can benefit from a combination of fungi with other organisms [98].
4. Conclusions
In summary, fermentative processes with filamentous fungi were able to add nutraceutical value to animal feed biomasses commonly used in Brazil. Depending on the formulation prepared, G. lucidum and F. hepatica presented the highest growth rates, P. lecomtei showed superior lacase activity, and A. terreus displayed increased levels of total protein, β-glucan, and lovastatin. These results show that commonly used animal feed biomasses can be enriched for nutraceuticals by fungal fermentation with potential benefits in animal nutrient use efficiency, as well as in mitigation of the use of antibiotics and the production of greenhouse gases. Further work will address the effects of the fermentation of feed biomasses by fungi on in vitro digestibility and gas production.
Author Contributions
Conceptualization, F.G.d.S., E.G.d.A. and S.M.; methodology, F.G.d.S., E.G.d.A. and A.A.C.; Data curation, F.G.d.S., E.G.d.A. and A.A.C.; validation, F.G.d.S. and E.G.d.A.; formal analysis, F.G.d.S., E.G.d.A. and T.D.M.; resources, F.G.d.S.; writing—original draft preparation, A.A.C.; writing—review and editing, F.G.d.S., B.F.Q., E.G.d.A. and A.A.C.; supervision, F.G.d.S., E.G.d.A. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to Embrapa Agroenergy and to members of the University of Mato Grosso for the technical support and the Nadia Parachin for the availability of A. terreus strain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucchese-Cheung, T.; de Aguiar, L.K.; de Lima, L.C.; Spers, E.E.; Quevedo-Silva, F.; Alves, F.V.; Giolo de Almeida, R. Brazilian Carbon Neutral Beef as an Innovative Product: Consumption Perspectives Based on Intentions’ Framework. J. Food Prod. Mark. 2021, 27, 384–398. [Google Scholar] [CrossRef]
- Feltran-Barbieri, R.; Féres, J.G. Degraded Pastures in Brazil: Improving Livestock Production and Forest Restoration. R. Soc. Open Sci. 2021, 8, 201854. [Google Scholar] [CrossRef] [PubMed]
- Salton, J.C.; Mercante, F.M.; Tomazi, M.; Zanatta, J.A.; Concenço, G.; Silva, W.M.; Retore, M. Integrated Crop-Livestock System in Tropical Brazil: Toward a Sustainable Production System. Agric. Ecosyst. Environ. 2014, 190, 70–79. [Google Scholar] [CrossRef]
- D’aurea, A.P.; da Silva Cardoso, A.; Guimarães, Y.S.R.; Fernandes, L.B.; Ferreira, L.E.; Reis, R.A. Mitigating Greenhouse Gas Emissions from Beef Cattle Production in Brazil through Animal Management. Sustainability 2021, 13, 7207. [Google Scholar] [CrossRef]
- Nur-Nazratul, F.M.Y.; Rakib, M.R.M.; Zailan, M.Z.; Yaakub, H. Enhancing in Vitro Ruminal Digestibility of Oil Palm Empty Fruit Bunch by Biological Pre-Treatment with Ganoderma Lucidum Fungal Culture. PLoS ONE 2021, 16, e0258065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Y.; Luo, L.; Zhang, H.; Liao, Y.; Gou, C. Enhancement of the Nutritional Value of Fermented Corn Stover as Ruminant Feed Using the Fungi Pleurotus spp. Sci. Rep. 2021, 11, 11961. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lu, N.; Liu, Y.; Zhang, S.; Jiang, H.; Wang, H.; Wang, W.; Li, S. Aspergillus Oryzae and Aspergillus Niger Co-Cultivation Extract Affects in Vitro Degradation, Fermentation Characteristics, and Bacterial Composition in a Diet-Specific Manner. Animals 2021, 11, 1248. [Google Scholar] [CrossRef]
- Lubis, D.; Wina, E.; Haryanto, B.; Suhargiyantatmo, T. Feeding of Aspergillus Oryzae Fermentation Culture (AOFC) to Growing Sheep: 2. Growth Rate and Feed Efficiency. J. Ilmu Ternak Vet. 2002, 7, 214–219. [Google Scholar]
- Ábrego-Gacía, A.; Poggi-Varaldo, H.M.; Mendoza-Vargas, A.; Mercado-Valle, F.G.; Ríos-Leal, E.; Ponce-Noyola, T.; Calva-Calva, G. Effects of Fermented Oat Straw as a Lovastatin Carrier on in Vitro Methane Production and Rumen Microbiota. Front. Energy Res. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Zabek, K.; Milewski, S.; Wójcik, R.; Siwicki, A.K. Effect of β-1,3/1,6-D-Glucan in Diet on Productivity and Humoral and Cellular Defense Mechanisms in Sheep. Acta Vet. Brno 2013, 82, 141–146. [Google Scholar] [CrossRef]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals an Alternative Strategy for the Use of Antimicrobials. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 507–534. [Google Scholar] [CrossRef] [PubMed]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Prospects and Feasibility of Fungal Pretreatment of Agricultural Biomass for Ruminant Feeding. Anim. Feed Sci. Technol. 2020, 268, 114577. [Google Scholar] [CrossRef]
- Kumar, V.P.; Rao, R.G.; Dhali, A.; Thammiaha, V.; Sridhar, M. Evaluation of Exogenous Laccase Enzyme Treated Finger Millet Straw on Body Weight Gain, DM Intake and Nutrient Digestibility in Heifers. Indian J. Anim. Res. 2021, 55, 457–462. [Google Scholar] [CrossRef]
- Lucio, B.S.V.-D.; Domínguez, E.M.H.; García, M.V.; Díaz-Godínez, G.; Mandujano-Gonzalez, V.; Mendoza-Mendoza, B.; Álvarez-Cervantes, J. Exogenous Enzymes as Zootechnical Additives in Animal Feed: A Review. Catalysts 2021, 11, 851. [Google Scholar] [CrossRef]
- Yue, Z.Q.; Xu, Y.Z.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Pei, C.X.; Zhang, Y.L.; et al. Effects of Dietary Laccase Supplementation on Growth Performance, Nutrient Digestion, Rumen Fermentation and Microbiota in Dairy Bulls. Anim. Feed Sci. Technol. 2020, 269, 114645. [Google Scholar] [CrossRef]
- Tao, M.A.; Yan, T.U.; Zhang, N.F.; Guo, J.P.; Deng, K.D.; Yi, Z.H.O.U.; Qiang, Y.U.N.; Diao, Q.Y. Effects of Dietary Yeast β-Glucan on Nutrient Digestibility and Serum Profiles in Pre-Ruminant Holstein Calves. J. Integr. Agric. 2015, 14, 749–757. [Google Scholar] [CrossRef]
- Saadei Khalkhane, A.; Habibian, R. Effect of Dietary B-Glucan Supplementation on Humoral and Cellular Immunologic Factors in Lambs. Glob. Vet. 2013, 11, 38–43. [Google Scholar] [CrossRef]
- Kruger, D.; Werf, M. Van der Benefits of Application of Yeast β-Glucansruminants; Ohly GmbH: Hamburg, Germany, 2018. [Google Scholar]
- Jahromi, M.F.; Liang, J.B.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P.; Ho, Y.W. Lovastatin-Enriched Rice Straw Enhances Biomass Quality and Suppresses Ruminal Methanogenesis. BioMed Res. Int. 2013, 2013, 397934. [Google Scholar] [CrossRef]
- Neto, C.B.S.; Conceição, A.A.; Gomes, T.G.; de Aquino Ribeiro, J.A.; Campanha, R.B.; Barroso, P.A.V.; Machado, A.E.V.; Mendonça, S.; De Siqueira, F.G.; Miller, R.N.G. A Comparison of Physical, Chemical, Biological and Combined Treatments for Detoxification of Free Gossypol in Crushed Whole Cottonseed. Waste Biomass Valoriz. 2021, 12, 3965–3975. [Google Scholar] [CrossRef]
- Guimarães, M.B.; de Siqueira, F.G.; Campanha, R.B.; de Aquino Ribeiro, J.A.; Oliveira Magalhães, P.; Mendonça, S. Evaluation of Bio-Detoxification of Jatropha Curcas Seed Cake and Cottonseed Cake by Basidiomycetes: Nutritional and Antioxidant Effects. Waste Biomass Valoriz. 2022, 13, 1475–1490. [Google Scholar] [CrossRef]
- Su, B.; Chen, X. Current Status and Potential of Moringa Oleifera Leaf as an Alternative Protein Source for Animal Feeds. Front. Vet. Sci. 2020, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heinzkill, M.; Bech, L.; Halkier, T.; Schneider, P.; Anke, T. Characterization of Laccases and Peroxidases from Wood-Rotting Fungi (Family Coprinaceae). Appl. Environ. Microbiol. 1998, 64, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, B.S.; Willson, R. Radical-Cations as Reference Chromogens in Kinetic Studies of Ono-Electron Transfer Reactions: Pulse Radiolysis Studies of 2,2′-Azinobis-(3-Ethylbenzthiazoline-6-Sulphonate). J. Chem. Soc. Perkin Trans. 1982, 2, 805–812. [Google Scholar] [CrossRef]
- Chebaibi, S.; Leriche Grandchamp, M.; Burgé, G.; Clément, T.; Allais, F.; Laziri, F. Improvement of Protein Content and Decrease of Anti-Nutritional Factors in Olive Cake by Solid-State Fermentation: A Way to Valorize This Industrial by-Product in Animal Feed. J. Biosci. Bioeng. 2019, 128, 384–390. [Google Scholar] [CrossRef]
- Krotz, L. Elemental Analysis: N/Protein and CHNS Determination of Insect-Based Food and Animal Feed by Dumas Method; Thermo Fisher Scientific: Milan, Italy, 2019. [Google Scholar]
- Barbarino, E.; Lourenço, S.O. Comparison of CHN Analysis and Hach Acid Digestion to Quantify Total Nitrogen in Marine Organisms. Limnol. Oceanogr. Methods 2009, 7, 751–760. [Google Scholar] [CrossRef]
- Megazyme. Mushroom and Yeast Beta-Glucan Assay Procedure (K-YBGL 02/21); Megazyme: Wicklow, Ireland, 2021; Volume 21. [Google Scholar]
- Wang, J.; Luzum, J.A.; Phelps, M.A.; Kitzmiller, J.P. Liquid Chromatography—Tandem Mass Spectrometry Assay for the Simultaneous Quantification of Simvastatin, Lovastatin, Atorvastatin, and Their Major Metabolites in Human Plasma. J. Chromatogr. B 2015, 983, 18–25. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, F.; Fang, Y.; Zhou, D.; Wang, S.; Wu, D.; Wang, L.; Zhong, R. High-Potency White-Rot Fungal Strains and Duration of Fermentation to Optimize Corn Straw as Ruminant Feed. Bioresour. Technol. 2020, 312, 67–78. [Google Scholar] [CrossRef]
- Sharma, R.; Kocher, G.S.; Rao, S.S.; Oberoi, H.S. Improved Production of Multi-Component Cellulolytic Enzymes Using Sweet Sorghum Bagasse and Thermophilic Aspergillus terreus RWY Through Statistical Process Optimization. Waste Biomass Valoriz. 2020, 11, 3355–3369. [Google Scholar] [CrossRef]
- Tuyen, V.D.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Fungal Strain and Incubation Period Affect Chemical Composition and Nutrient Availability of Wheat Straw for Rumen Fermentation. Bioresour. Technol. 2012, 111, 336–342. [Google Scholar] [CrossRef]
- Shrivastava, B.; Jain, K.K.; Kalra, A.; Kuhad, R.C. Bioprocessing of Wheat Straw into Nutritionally Rich and Digested Cattle Feed. Sci. Rep. 2014, 4, 6360. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The Impact of Mushroom Polysaccharides on Gut Microbiota and Its Beneficial Effects to Host: A Review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Alzaban, M.I.; Albarakaty, F.M.; El-aziz, A.R.M.A.; Alrokban, A.H.; Mahmoud, M.A. Transcriptome Profiling Reveals Differential Gene Expression of Laccase Genes in Aspergillus terreus KC462061 during Biodegradation of Crude Oil. Biology 2022, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.A.; Cabreras, C.E.V.; Torta, L.; Laudicina, A.; Mirabile, G. Ligninolytic Potential of Curvularia Kusanoi L7 Laccases for Animal Production. Cuba. J. Agric. Sci. 2020, 54, 157–167. [Google Scholar]
- Beauchemin, K.A.; Colombatto, D.; Morgavi, D.P.; Yang, W.Z.; Rode, L.M. Mode of Action of Exogenous Cell Wall Degrading Enzymes for Ruminants. Can. J. Anim. Sci. 2003, 84, 22. [Google Scholar] [CrossRef]
- Peláez, R.D.R.; Wischral, D.; Mendes, T.D.; Pacheco, T.F.; Urben, A.F.; Helm, C.V.; Mendonça, S.; Balan, V.; de Siqueira, F.G. Co-Culturing of Micro- and Macro-Fungi for Producing Highly Active Enzyme Cocktail for Producing Biofuels. Bioresour. Technol. Rep. 2021, 16, 100833. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Jokharidze, T.; Kobakhidze, A.; Elisashvili, V. Enhancement of Laccase Production by Cerrena Unicolor through Fungal Interspecies Interaction and Optimum Conditions Determination. Arch. Microbiol. 2021, 203, 3905–3917. [Google Scholar] [CrossRef] [PubMed]
- Lallawmsanga; Leo, V.V.; Passari, A.K.; Muniraj, I.K.; Uthandi, S.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Singh, B.P. Elevated Levels of Laccase Synthesis by Pleurotus Pulmonarius BPSM10 and Its Potential as a Dye Decolorizing Agent. Saudi J. Biol. Sci. 2019, 26, 464–468. [Google Scholar] [CrossRef]
- Kirst Tychanowicz, G.; De Souza, D.F.; Souza, C.G.M.; Kadowaki, M.K.; Peralta, R.M. Copper Improves the Production of Laccase by the White- Rot Fungus Pleurotus Pulmonarius in Solid State Fermentation. Braz. Arch. Biol. Technol. 2006, 49, 699–704. [Google Scholar] [CrossRef]
- De Souza, D.F.; Tychanowicz, G.K.; De Souza, C.G.M.; Peralta, R.M. Co-Production of Ligninolytic Enzymes by Pleurotus Pulmonarius on Wheat Bran Solid State Cultures. J. Basic Microbiol. 2006, 46, 126–134. [Google Scholar] [CrossRef]
- Floudas, D.; Held, B.W.; Riley, R.; Nagy, L.G.; Koehler, G.; Ransdell, A.S.; Younus, H.; Chow, J.; Chiniquy, J.; Lipzen, A.; et al. Evolution of Novel Wood Decay Mechanisms in Agaricales Revealed by the Genome Sequences of Fistulina Hepatica and Cylindrobasidium Torrendii. Fungal Genet. Biol. 2015, 76, 78–92. [Google Scholar] [CrossRef]
- Peláez, F.; Martínez, M.J.; Martínez, A.T. Screening of 68 Species of Basidiomycetes for Enzymes Involved in Lignin Degradation. Mycol. Res. 1995, 99, 37–42. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhang, J.L.; Hu, K.H.; Zhang, W.G. The Relationship between Lignin Peroxidase and Manganese Peroxidase Production Capacities and Cultivation Periods of Mushrooms. Microb. Biotechnol. 2013, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, F.W.M.R.; Baum, S.; Fink, S. Dual Modes of Degradation by Fistulina Hepatica in Xylem Cell Walls of Quercus Robur. Mycol. Res. 2000, 104, 846–852. [Google Scholar] [CrossRef]
- Yuliana, T.; Putri, N.Z.; Komara, D.Z.; Mardawati, E.; Lanti, I.; Rahimah, S. Study of Ganoderma Lucidum in Laccase Production Using Corncob and Paddies Straw Substrates on Submerged Fermentation System. Pakistan J. Biol. Sci. 2020, 23, 1060–1065. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Thangavelu, K.P.; Heese, K. Characterization of Optimized Production, Purification and Application of Laccase from Ganoderma Lucidum. Biochem. Eng. J. 2013, 70, 106–114. [Google Scholar] [CrossRef]
- Rodrigues, E.M.; Karp, S.G.; Malucelli, L.C.; Helm, C.V.; Alvarez, T.M. Evaluation of Laccase Production by Ganoderma Lucidum in Submerged and Solid-State Fermentation Using Different Inducers. J. Basic Microbiol. 2019, 59, 784–791. [Google Scholar] [CrossRef]
- Shaikh, S.; Shaikh, I.; Dixit, P. Production and Characterization of Laccase from Aspergillus terreus Isolated from Saw Mill Soil of Osmanabad. Int. J. Adv. Res. 2020, 8, 626–632. [Google Scholar] [CrossRef]
- Stoffel, F.; de Santana, W.O.; Gregolon, J.G.N.; Kist, T.B.L.; Fontana, R.C.; Camassola, M. Production of Edible Mycoprotein Using Agroindustrial Wastes: Influence on Nutritional, Chemical and Biological Properties. Innov. Food Sci. Emerg. Technol. 2019, 58, 102227. [Google Scholar] [CrossRef]
- Ellamar, J.B.; Valdez, M.T.S.; Inalvez, A.E.; Maniquez, M.C.; Rodriguez, J.M.; Tarozza, D.B. Bioconversion of Lignocellulosic Agricultural By-Products by Microorganisms into High Mycoprotein Feeds. Philipp. J. Vet. Anim. Sci. 2019, 45, 75–86. [Google Scholar]
- Khonkhaeng, B.; Cherdthong, A. Improving Nutritive Value of Purple Field Corn Residue and Rice Straw by Culturing with White-Rot Fungi. J. Fungi 2020, 6, 69. [Google Scholar] [CrossRef]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Variation in the Solubilization of Crude Protein in Wheat Straw by Different White-Rot Fungi. Anim. Feed Sci. Technol. 2018, 242, 135–143. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Mahboubi, A.; Ferreira, J.A.; Lundh, T.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Nutritional Composition of Pure Filamentous Fungal Biomass as a Novel Ingredient for Fish Feed. Fermentation 2021, 7, 152. [Google Scholar] [CrossRef]
- Ekute, B. Nutritional Profile of Two Nigerian Edible Mushrooms: Pleurotus Ostreatus and Pleurotus Pulmonarius. J. Appl. Sci. Environ. Manag. 2018, 22, 1745–1747. [Google Scholar] [CrossRef]
- Oyetayo, V.O.; Ogidi, C.O.; Bayode, S.O.; Enikanselu, F.F. Evaluation of Biological Efficiency, Nutrient Contents and Antioxidant Activity of Pleurotus Pulmonarius Enriched with Zinc and Iron. Indian Phytopathol. 2021, 74, 901–910. [Google Scholar] [CrossRef]
- Inácio, F.D.; Ferreira, R.O.; de Araujo, C.A.V.; Peralta, R.M.; de Souza, C.G.M. Production of Enzymes and Biotransformation of Orange Waste by Oyster Mushroom, Pleurotus pulmonarius (Fr.). Adv. Microbiol. 2015, 5, 52997. [Google Scholar] [CrossRef]
- Akinfemi, A.; Adu, O.A.; Doherty, F. Conversion of Sorghum Stover into Animal Feed with White-Rot Fungi: Pleurotus Ostreatus and Pleurotus Pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef]
- Akinfemi, A.; Ogunwole, O.A. ChemiCal Composition and in Vitro Digestibility of RiCe Straw Treated with Pleurotus Ostreatus, Pleurotus Pulmonarius and Pleurotus Tuber-Regium. Slovak J. Anim. Sci. 2012, 45, 14–20. [Google Scholar]
- Montañez-Valdez, O.D.; Flores, E.O.G.; García, J.A.M.; Chavira, J.S.; Rubio, R.R.; Ortiz, J.J.G.P. Use of Pleurotus Pulmonarius to Change the Nutritional Quality of Wheat Straw. I. Effect on Chemical Composition. Interciencia 2008, 33, 435–438. [Google Scholar]
- Martinez, Y.; Paredes, J.; Avellaneda, M.; Botello, A.; Valdivie, M. Diets with Ganoderma Lucidum Mushroom Powder and Zinc-Bacitracin on Growth Performance, Carcass Traits, Lymphoid Organ Weights and Intestinal Characteristics in Broilers. Brazilian J. Poult. Sci. 2022, 24, 1–12. [Google Scholar] [CrossRef]
- Chen, S.D.; Hsieh, M.C.; Chiou, M.T.; Lai, Y.S.; Cheng, Y.H. Effects of Fermentation Products of Ganoderma Lucidum on Growth Performance and Immunocompetence in Weanling Pigs. Arch. Anim. Nutr. 2008, 62, 22–32. [Google Scholar] [CrossRef]
- Misra, A.K.; Mishra, A.S.; Tripathi, M.K.; Prasad, R.; Vaithiyanathan, S.; Jakhmola, R.C. Optimization of Solid State Fermentation of Mustard (Brassica Campestris) Straw for Production of Animal Feed by White Rot Fungi (Ganoderma lucidum). Asian-Australas. J. Anim. Sci. 2007, 20, 208–213. [Google Scholar] [CrossRef]
- Han, J.R.; An, C.H.; Yuan, J.M. Solid-State Fermentation of Cornmeal with the Basidiomycete Ganoderma Lucidum for Degrading Starch and Upgrading Nutritional Value. J. Appl. Microbiol. 2005, 99, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Jaganmohan, B.; Daas, B.P.; Prasad, S.V. Production of Single Cell Protein (SCP) with Aspergillus terreus Using Solid State Fermentation. Eur. J. Biol. Sci. 2013, 5, 38–43. [Google Scholar] [CrossRef]
- Shahzad, M.A.; Rajoka, M.I. Single Cell Protein Production from Aspergillus terreus and Its Evaluation in Broiler Chick. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 137–141. [Google Scholar] [CrossRef]
- Garg, S.K.; Neelakantan, S. Production of SCP and Cellulase by Aspergillus terreus from Bagasse Substrate. Biotechnol. Bioeng. 1982, 24, 2407–2417. [Google Scholar] [CrossRef]
- Miller, T.F.; Srinivasan, V.R. Producton of Single-cell Protein from Cellulose by Aspergillus terreus. Biotechnol. Bioeng. 1983, 25, 1509–1519. [Google Scholar] [CrossRef]
- Ouzouni, P.K.; Petridis, D.; Koller, W.D.; Riganakos, K.A. Nutritional Value and Metal Content of Wild Edible Mushrooms Collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Cortina-escribano, M.; Pihlava, J.; Miina, J.; Veteli, P.; Linnakoski, R.; Vanhanen, H. Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β -Glucan Content of Ganoderma Lucidum Fruiting Bodies. Molecules 2020, 25, 4732. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of Beta-Glucan Contents in Commercially Cultivated and Wild Growing Mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Hamzah, N.S.A. Keong Screening and Characterization of Endopolysaccharides from Pleurotus Pulmonarius in Submerged Culture Fermentation. J. Sains Nukl. Malays. 2017, 29, 52–59. [Google Scholar]
- Contato, A.G.; Inácio, F.D.; de Araújo, C.A.V.; Brugnari, T.; Maciel, G.M.; Haminiuk, C.W.I.; Bracht, A.; Peralta, R.M.; de Souza, C.G.M. Comparison between the Aqueous Extracts of Mycelium and Basidioma of the Edible Mushroom Pleurotus Pulmonarius: Chemical Composition and Antioxidant Analysis. J. Food Meas. Charact. 2020, 14, 830–837. [Google Scholar] [CrossRef]
- Atoji-Henrique, K.; Henrique, D.S.; Glória, L.S.; Mazaro, S.M.; Casagrande, M. Influence of Substrate Composition on Beta-Glucans Production and Growth of Ganoderma Lucidum. J. Agric. Sci. 2017, 9, 190. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma Lucidum and Pleurotus Ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- Tsubaki, K. Betaglucan-Containing Fat and Oil Compositions and Novel Microorganism Capable of Producing Beta-Glucan [EP1533382A1]. U.S. Patent 7,442,541, 28 October 2008. [Google Scholar]
- Wang, C.; Mao, W.; Chen, Z.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Yan, M.; Liu, X.; Guo, T. Purification, Structural Characterization and Antioxidant Property of an Extracellular Polysaccharide from Aspergillus terreus. Process Biochem. 2013, 48, 1395–1401. [Google Scholar] [CrossRef]
- Costa, C.R.L.d.M.; Menolli, R.A.; Osaku, E.F.; Tramontina, R.; de Melo, R.H.; do Amaral, A.E.; Duarte, P.A.D.; de Carvalho, M.M.; Smiderle, F.R.; Silva, J.L.d.C.; et al. Exopolysaccharides from Aspergillus terreus: Production, Chemical Elucidation and Immunoactivity. Int. J. Biol. Macromol. 2019, 139, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, B.; Saravanan, T. Optimization of B-Glucan Production from Lower Fungi Using Central Composite Design and Its Biological Application. Int. J. Comput. Appl. 2012, 49, 23–28. [Google Scholar] [CrossRef]
- Uchiyama, H.; Iwai, A.; Asada, Y.; Muramatsu, D.; Aoki, S.; Kawata, K.; Kusano, K.; Nagashima, K.; Yasokawa, D.; Okabe, M.; et al. A Small Scale Study on the Effects of Oral Administration of the β-Glucan Produced by Aureobasidium pullulans on Milk Quality and Cytokine Expressions of Holstein Cows, and on Bacterial Flora in the Intestines of Japanese Black Calves. BMC Res. Notes 2012, 5, 189. [Google Scholar] [CrossRef]
- Wójcik, R. The Effect of Leiber Beta-S (1,3-1,6-β-D-Glucan) on the Phagocytic Activity and Oxidative Metabolism of Peripheral Blood Granulocytes and Monocytes in Calves. Acta Vet. Brno 2014, 83, 347–354. [Google Scholar] [CrossRef][Green Version]
- Cherdthong, A.; Seankamsorn, A.; Suriyapha, C.; Chanjula, P.; Wanapat, M. Effect of Beta-Glucan Supplementation on Feed Intake, Digestibility of Nutrients and Ruminal Fermentation in Thai Native Beef Cattle. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1509–1514. [Google Scholar] [CrossRef]
- Wang, L.; Xia, W.H.; Wang, J.H.; Fan, R.F.; Niu, X.D.; Wang, Y.M.; Li, Q.L.; Wang, Z.Y.; Wang, Z.H. Effects of Beta-1,3-Glucan Supplementation on Concentrations of Serum Metabolites in Transition Holstein Cows. Res. Vet. Sci. 2020, 132, 250–256. [Google Scholar] [CrossRef]
- Raa, J. Immune Modulation by Non-Digestible and Non-Absorbable Beta-1,3/1,6-Glucan. Microb. Ecol. Health Dis. 2015, 26, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Grove, A.V.; Kaiser, C.R.; Iversen, N.; Hafla, A.; Robison, B.L.; Bowman, J.G.P. Digestibility of Barley Beta-Glucan in Cattle. Proc. West. Sect. Am. Soc. Anim. Sci. 2006, 57, 367–369. [Google Scholar]
- Lan, W.; Yang, C. Ruminal Methane Production: Associated Microorganisms and the Potential of Applying Hydrogen-Utilizing Bacteria for Mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Demonfort Nkamga, V.; Armstrong, N.; Drancourt, M. In Vitro Susceptibility of Cultured Human Methanogens to Lovastatin. Int. J. Antimicrob. Agents 2017, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.F.; Liang, J.B.; Ho, Y.W.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P. Lovastatin Production by Aspergillus terreus Using Agro-Biomass as Substrate in Solid State Fermentation. J. Biomed. Biotechnol. 2012, 2012, 196264. [Google Scholar] [CrossRef]
- Wei, P.; Xu, Z.; Cen, P. Lovastatin Production by Aspergillus terreus in Solid State and Submerged Fermentations. J. Zhejiang Univ. Sci. A 2007, 8, 1521–1526. [Google Scholar] [CrossRef]
- Patil, R.H.; Krishnan, P.; Maheshwari, V.L. Production of Lovastatin by Wild Strains of Aspergillus terreus. Nat. Prod. Commun. 2011, 6, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.H.A.; Hasan, H.; Montoya, A.; Abbas, A. Lovastatin and (+)-Geodin Production by Aspergillus terreus from Crude Glycerol. Eng. Life Sci. 2015, 15, 220–228. [Google Scholar] [CrossRef]
- Mohd Azlan, P.; Jahromi, M.F.; Ariff, M.O.; Ebrahimi, M.; Candyrine, S.C.L.; Liang, J.B. Aspergillus terreus Treated Rice Straw Suppresses Methane Production and Enhances Feed Digestibility in Goats. Trop. Anim. Health Prod. 2018, 50, 565–571. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Inhibition of Growth of Methane-Producing Bacteria of the Ruminant Forestomach by Hydroxymethylglutaryl~SCoA Reductase Inhibitors. J. Dairy Sci. 2001, 84, 1445–1448. [Google Scholar] [CrossRef]
- Lee, J.; Shi, Y.M.; Grün, P.; Gube, M.; Feldbrügge, M.; Bode, H.; Hennicke, F. Identification of Feldin, an Antifungal Polyyne from the Beefsteak Fungus Fistulina Hepatica. Biomolecules 2020, 10, 1502. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological Production and Application of Ganoderic Acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Degenkolb, T.; Vilcinskas, A. Metabolites from Nematophagous Fungi and Nematicidal Natural Products from Fungi as Alternatives for Biological Control. Part II: Metabolites from Nematophagous Basidiomycetes and Non-Nematophagous Fungi. Appl. Microbiol. Biotechnol. 2016, 100, 3813–3824. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Reis, C.E.; Ogero D’Otaviano, L.; Rajendran, A.; Hu, B. Co-Culture of Filamentous Feed-Grade Fungi and Microalgae as an Alternative to Increase Feeding Value of Ethanol Coproducts. Fermentation 2018, 4, 86. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).