The Biotransformation of Lupine Seeds by Lactic Acid Bacteria and Penicillium camemberti into a Plant-Based Camembert Alternative, and Its Physicochemical Changes during 7 Weeks of Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Products Preparation, Fermentation, Ripening, Microbial Analyses, and Acidity Determination
2.3. Determination of Bioactive Compounds
2.4. Determination of Antioxidant Activity
2.5. Textural Analyses
2.6. Statistical Analysis
3. Results and Discussion
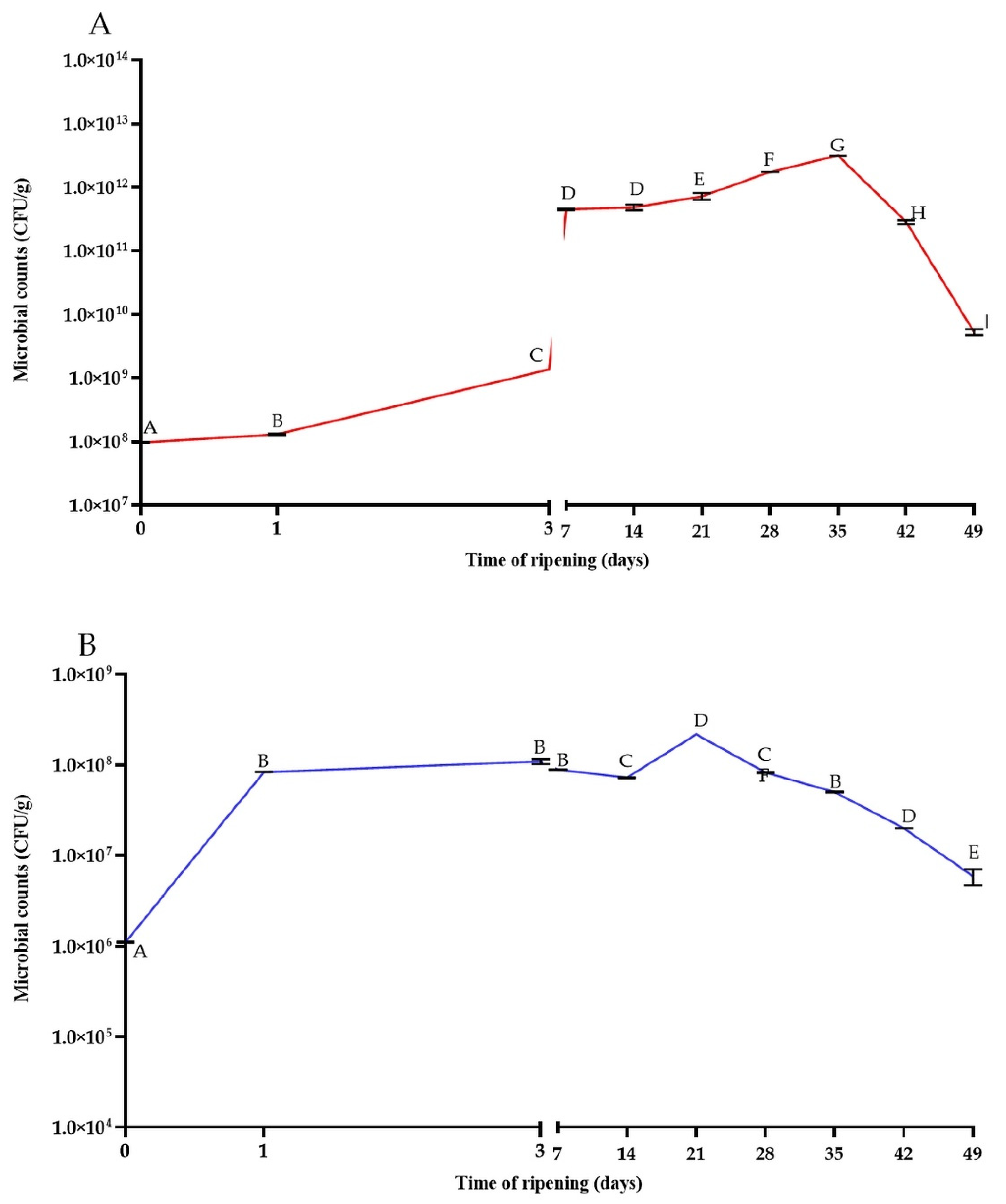
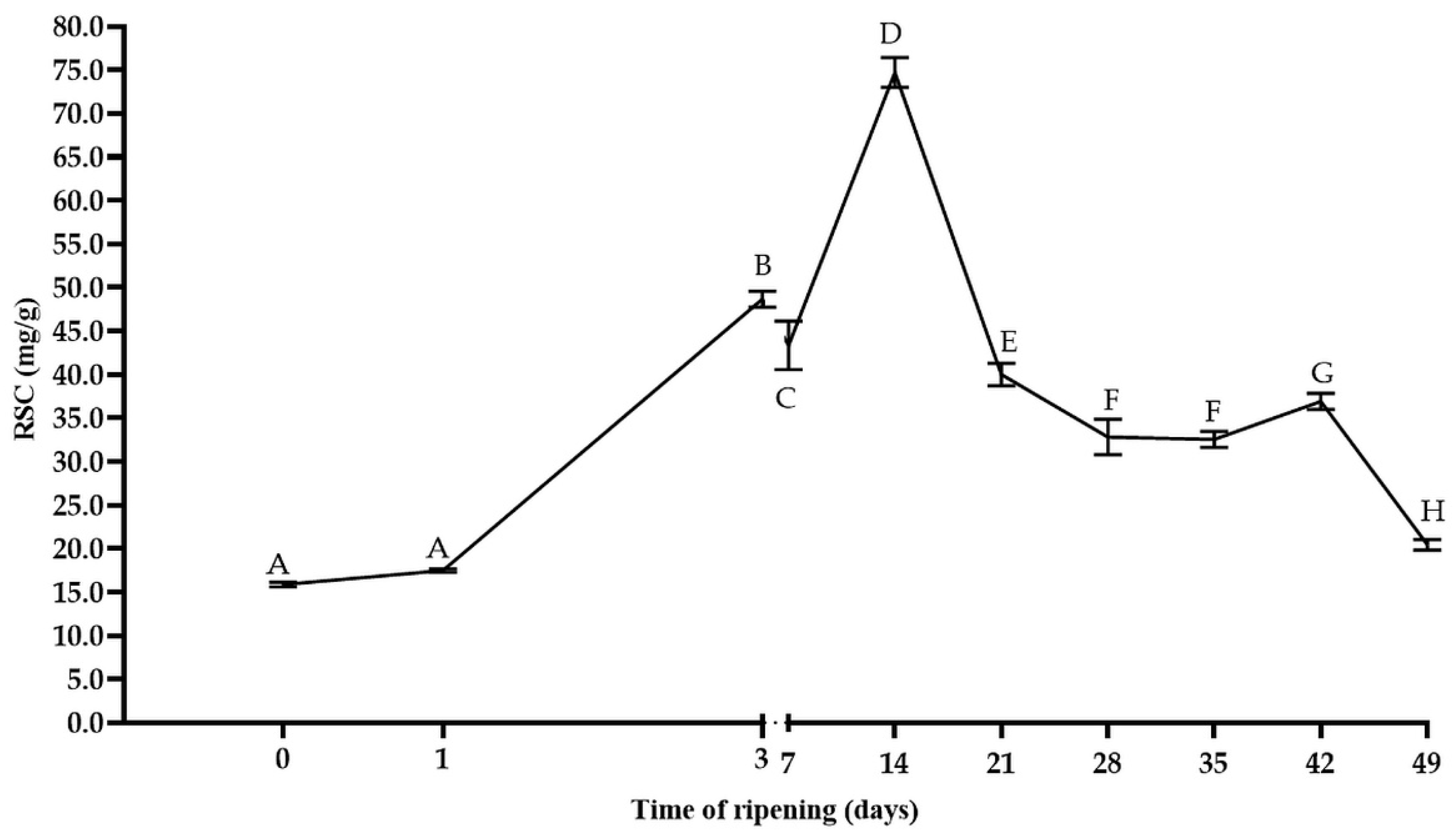
3.1. The Changes in Microbial Population, Acidity, and Reducing Sugars Content during the Ripening
3.2. The Changes in Total Polyphenolics Content, Total Flavonoids Content, Total Free Amino Acids Level, and Antioxidant Activity
3.3. The Textural Changes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; McClements, D.J. The science of plant-based foods: Approaches to create nutritious and sustainable plant-based cheese analogs. Trends Food Sci. Technol. 2021, 118, 207–229. [Google Scholar] [CrossRef]
- Pua, A.; Tang, V.C.Y.; Goh, R.M.V.; Sun, J.; Lassabliere, B.; Liu, S.Q. Ingredients, Processing, and Fermentation: Addressing the Organoleptic Boundaries of Plant-Based Dairy Analogues. Foods 2022, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L. Comparative Study of Chemical Composition and Texture Profile Analysis between Camembert Cheese and Chinese Sufu. Biotechnol. Front. 2012, 1, 1–8. [Google Scholar]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Yano, H. Development of “New” Bread and Cheese. Processes 2020, 8, 1–23. [Google Scholar]
- Boukind, S.; Bouaouina, J.; Bouras, H.; Benhamou, A.A.; Ablouh, E.-H.; Kassab, Z.; Khouloud, M.; El Achaby, M.; Sehaqui, H. Powerful cellulose phosphorylation by fertilizer-grade phosphate enables excellent methylene blue paper sorbent. Int. J. Biol. Macromol. 2022, 219, 949–963. [Google Scholar] [CrossRef]
- Kamath, R.; Basak, S.; Gokhale, J. Recent trends in the development of healthy and functional cheese analogues-a review. LWT 2021, 155, 112991. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, D.; Amin, A. Development of a functional fermented peanut-based cheese analog using probiotic bacteria. BioTechnologia 2018, 99, 435–441. [Google Scholar] [CrossRef]
- Mohamed, A.; Shalaby, S.; Gafour, W.A. Quality Characteristics and Acceptability of an Analogue Processed Spreadable Cheese Made with Carrot Paste (Daucus carota L.). Int. J. Dairy Sci. 2016, 11, 91–99. [Google Scholar] [CrossRef]
- Awad; Salama, W.M.; Farahat, A.M. Effect of lupine as cheese base substitution on technological and nutritional properties of processed cheese analogue. Acta Sci. Pol. Technol. Aliment. 2014, 13, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Villaluenga, C.; Zieliński, H.; Frias, J.; Piskula, M.K.; Kozłowska, H.; Vidal-Valverde, C. Antioxidant capacity and polyphenolic content of high-protein lupin products. Food Chem. 2009, 112, 84–88. [Google Scholar] [CrossRef]
- Abdel–Salam, A.F.; Ali, J.B.; Zayan, A.F. Effect of lupine powder on rheological, chemical and microbiological properties of yoghurt. J. Food Dairy Sci. 2015, 6, 531–546. [Google Scholar] [CrossRef]
- Al-Saedi, N.; Agarwal, M.; Ma, W.; Islam, S.; Ren, Y. Proteomic Characterisation of Lupin (Lupinus angustifolius) Milk as Influenced by Extraction Techniques, Seed Coat and Cultivars. Molecules 2020, 25, 1782. [Google Scholar] [CrossRef]
- Bartkiene, E.; Sakiene, V.; Bartkevics, V.; Juodeikiene, G.; Lele, V.; Wiacek, C.; Braun, P.G. Modulation of the nutritional value of lupine wholemeal and protein isolates using submerged and solid-state fermentation with Pediococcus pentosaceus strains. Int. J. Food Sci. Technol. 2018, 53, 1896–1905. [Google Scholar] [CrossRef]
- Xia, Y.; Yuan, R.; Weng, S.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; Liu, W.; Ai, L. Proteolysis, lipolysis, texture and sensory properties of cheese ripened by Monascus fumeus. Food Res. Int. 2020, 137, 109657. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H. Effect of emulsifying salts on the physicochemical properties of processed cheese made from Mozzarella. J. Dairy Sci. 2012, 95, 4823–4830. [Google Scholar] [CrossRef]
- Zhang, X.; Esmail, G.A.; Alzeer, A.F.; Arasu, M.V.; Vijayaraghavan, P.; Choi, K.C.; Al-Dhabi, N.A. Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saudi J. Biol. Sci. 2020, 27, 3505–3513. [Google Scholar] [CrossRef]
- Leclercq-Perlat, M.-N.; Corrieu, G.; Spinnler, H.-E. Controlled production of camembert-type cheeses: Part III role of the ripening microflora on free fatty acid concentrations. J. Dairy Res. 2007, 74, 218–225. [Google Scholar] [CrossRef]
- Mei, J.; Guo, Q.; Wu, Y.; Li, Y.; Yu, H. Study of proteolysis, lipolysis, and volatile compounds of a Camembert-type cheese manufactured using a freeze-dried Tibetan kefir co-culture during ripening. Food Sci. Biotechnol. 2015, 24, 393–402. [Google Scholar] [CrossRef]
- Oyeyinka, A.T.; Odukoya, J.O.; Adebayo, Y.S. Nutritional composition and consumer acceptability of cheese analog from soy and cashew nut milk. J. Food Process. Preserv. 2019, 43, e14285. [Google Scholar] [CrossRef]
- Eudier, H.; Ben-Harb, S.; Lorand, J.; Duthil, F.; Negahban, M.; Saiter, J.; Chan-Huot, M. Properties Of An Analogue Cheese Obtained From Raw Peanut. Peanut Sci. 2020, 47, 81–88. [Google Scholar] [CrossRef]
- Mohamed, A.G.; Shalaby, S.M. Texture, Chemical Properties and Sensory Evaluation of a Spreadable Processed Cheese Analogue Made with Apricot Pulp (Prunus armeniaca L.). Int. J. Dairy Sci. 2016, 11, 61–68. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Analytical nutritional characteristics of seed proteins in six wild Lupinus species from Southern Spain. Food Chem. 2009, 117, 466–469. [Google Scholar] [CrossRef]
- Torres, A.; Frias, J.; Vidal-Valverde, C. Changes in chemical composition of lupin seeds (Lupinus angustifolius) after selective α-galactoside extraction. J. Sci. Food Agric. 2005, 85, 2468–2474. [Google Scholar] [CrossRef]
- Oomah, B.D.; Tiger, N.; Olson, M.; Balasubramanian, P. Phenolics and Antioxidative Activities in Narrow-Leafed Lupins (Lupinus angustifolius L.). Mater. Veg. 2006, 61, 86–92. [Google Scholar] [CrossRef]
- Wäsche, A.; Müller, K.; Knauf, U. New processing of lupin protein isolates and functional properties. Food/Nahrung 2001, 45, 393–395. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E. Production and Characterization of Yogurt-Like Fermented Beverage Based on Camelina (Camelina sativa L.) Seed Press Cake. Appl. Sci. 2022, 12, 1085. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Śmietana, N.; Paradowska, D.; Drozłowska, E. Black Cumin (Nigella sativa L.) Seed Press Cake as a Novel Material for the Development of New Non-Dairy Beverage Fermented with Kefir Grains. Microorganisms 2022, 10, 300. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseed oil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-Like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef]
- Rodriguez-Martin, N.M.; La Paz, S.M.-D.; Toscano, R.; Grao-Cruces, E.; Villanueva, A.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. Hemp (Cannabis sativa L.) Protein Hydrolysates Promote Anti-Inflammatory Response in Primary Human Monocytes. Biomolecules 2020, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kwiatkowski, P.; Bartkowiak, A.; Gefrom, A.; Sienkiewicz, M. The Effect of Fermentation with Kefir Grains on the Physicochemical and Antioxidant Properties of Beverages from Blue Lupin (Lupinus angustifolius L.) Seeds. Molecules 2020, 25, 5791. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, C.; Hernández-Sánchez, H.; Dávila-Ortiz, G. Production of a yogurt-like product from Lupinus campestris seeds. J. Sci. Food Agric. 2003, 83, 515–522. [Google Scholar] [CrossRef]
- Zaouadi, K.N.; Ziane-Zafour, A.A.H.; Ouazib, A.M.; Arab, A.Y.; Hacini, K.; Aslan, S.S. Formulation and Optimization by Experimental Design of Dairy Dessert Based on Lupinus albus L. and Stevia rebaudiana Extracts. Asian J. Dairy Food Res. 2019, 38, 281–287. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Lee, J.-S.; Bae, I. Quality Characteristics, Changes in Physiochemical Properties and Functional Properties of Camembert Cheese Containing Red Ginseng Powder. Korean J. Food Sci. Anim. Resour. 2018, 38, 64–77. [Google Scholar] [CrossRef]
- Chen, J.M.; Al, K.F.; Craven, L.J.; Seney, S.; Coons, M.; McCormick, H.; Reid, G.; O’Connor, C.; Burton, J.P. Nutritional, Microbial, and Allergenic Changes during the Fermentation of Cashew ‘Cheese’ Product Using a Quinoa-Based Rejuvelac Starter Culture. Nutrients 2020, 12, 648. [Google Scholar] [CrossRef]
- Bartkiene, E.; Schleining, G.; Juodeikiene, G.; Vidmantiene, D.; Krungleviciute, V.; Rekstyte, T.; Basinskiene, L.; Stankevicius, M.; Akuneca, I.; Ragazinskiene, O.; et al. The influence of lactic acid fermentation on biogenic amines and volatile compounds formation in flaxseed and the effect of flaxseed sourdough on the quality of wheat bread. LWT-Food Sci. Technol. 2014, 56, 445–450. [Google Scholar] [CrossRef]
- Trojanowska, K.; Markiewicz, M.; Kolanowska, A.; Czaczyk, K.; Mueller, A.; Gulewicz, K. Ekstrakt Łubinowy Surowiec w Biosyntezie Paszowego Drodze Mikrobioiogicznej. Biotechnologia 1991, 3, 131–136. [Google Scholar]
- Chen, X.; Gu, Z.; Peng, Y.; Quek, S.Y. What happens to commercial camembert cheese under packaging? Unveiling biochemical changes by untargeted and targeted metabolomic approaches. Food Chem. 2022, 383, 132437. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.; Vogel, R.F.; Toelstede, S. Fermentation performance of lactic acid bacteria in different lupin substrates-influence and degradation ability of antinutritives and secondary plant metabolites. J. Appl. Microbiol. 2015, 119, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Egounlety, M.; Aworh, O. Effect of soaking, dehulling, cooking and fermentation with Rhizopus oligosporus on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (Glycine max Merr.), cowpea (Vigna unguiculata L. Walp) and groundbean (Macrotyloma geocarpa Harms). J. Food Eng. 2003, 56, 249–254. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C.G. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Sciarria, T.P.; Scarafoni, A.; Squillace, P.; Adani, F.; Scaglia, B. Polyphenol bioactivity evolution during the spontaneous fermentation of vegetal by-products. Food Chem. 2022, 374, 131791. [Google Scholar] [CrossRef]
- Ruiz-López, M.A.; Barrientos-Ramírez, L.; García-López, P.M.; Valdés-Miramontes, E.H.; Zamora-Natera, J.F.; Rodríguez-Macias, R.; Salcedo-Pérez, E.; Bañuelos-Pineda, J.; Vargas-Radillo, J.J. Nutritional and Bioactive Compounds in Mexican Lupin Beans Species: A Mini-Review. Nutrients 2019, 11, 1785. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Shah, N.P.; Wang, M.; Lui, W.Y.; Corke, H. Fermentation alters antioxidant capacity and polyphenol distribution in selected edible legumes. Int. J. Food Sci. Technol. 2016, 51, 875–884. [Google Scholar] [CrossRef]
- Zaworska, A.; Kasprowicz-Potocka, M.; Frankiewicz, A.; Zdunczyk, Z.; Juskiewicz, J. Effects of fermentation of narrow-leafed lupine (L. angustifolius) seeds on their chemical composition and physiological parameters in rats. J. Anim. Feed Sci. 2016, 25, 326–334. [Google Scholar] [CrossRef]
- Kasprowicz-Potocka, M.; Zaworska, A.; Frankiewicz, A.; Nowak, W.; Gulewicz, P.; Zduńczyk, Z.; Juśkiewicz, J. The Nutritional Value and Physiological Properties of Diets with Raw and Candida Utilis-Fermented Lupin Seeds in Rats. Food Technol Bio-Technol 2015, 53, 286–297. [Google Scholar]
- Xia, X.; Tobin, J.T.; Sharma, P.; Fenelon, M.; McSweeney, P.L.; Sheehan, J.J. Application of a cascade membrane filtration process to standardise serum protein depleted cheese milk for cheddar cheese manufacture. Int. Dairy J. 2020, 110, 104796. [Google Scholar] [CrossRef]
- Ropars, J.; Didiot, E.; de la Vega, R.C.R.; Bennetot, B.; Coton, M.; Poirier, E.; Coton, E.; Snirc, A.; Le Prieur, S.; Giraud, T. Domestication of the Emblematic White Cheese-Making Fungus Penicillium camemberti and Its Diversification into Two Varieties. Curr. Biol. 2020, 30, 4441–4453.e4. [Google Scholar] [CrossRef]
- Barac, M.; Vucic, T.; Zilic, S.; Pesic, M.; Sokovic, M.; Petrovic, J.; Kostic, A.; Ignjatovic, I.S.; Milincic, D. The Effect of In Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses. Foods 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Antioxidant activities and characterisation of polysaccharides isolated from the seeds of Lupinus angustifolius. Ind. Crop. Prod. 2015, 74, 950–956. [Google Scholar] [CrossRef]
- Sánchez-Gamboa, C.; Hicks-Pérez, L.; Gutiérrez-Méndez, N.; Heredia, N.; García, S.; Nevárez-Moorillón, G.V. Microbiological Changes during Ripening of Chihuahua Cheese Manufactured with Raw Milk and Its Seasonal Variations. Foods 2018, 7, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, B.D.; Baptista, D.P.; Cavalheiro, F.G.; Negrão, F.; Eberlin, M.N.; Gigante, M.L. Peptide profile of Camembert-type cheese: Effect of heat treatment and adjunct culture Lactobacillus rhamnosus GG. Food Res. Int. 2019, 123, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.; Dagastine, R.R.; Kentish, S.E.; Gras, S.L. Microstructure and Composition of Full Fat Cheddar Cheese Made with Ultrafiltered Milk Retentate. Foods 2013, 2, 310–331. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.; Gammoh, S.; Ereifej, K.; Johargy, A.; Alli, I. A review of phenolic compounds in oil-bearing plants: Distribution, identification and occurrence of phenolic compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef]


| Time of Ripening (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | 21 | 28 | 35 | 42 | 48 |
| TPC (mg GAE/g) | |||||||||
| 0.52 ± 0.06 A | 0.48 ± 0.05 A | 0.63 ± 0.11 A | 2.15 ± 0.08 AB | 2.32 ± 0.17 B | 2.75 ± 0.27 BC | 2.48 ± 0.26 B | 3.46 ± 0.00 C | 3.00 ± 0.26 C | 2.70 ± 0.30 BC |
| TFC (mg QE/g) | |||||||||
| 10.59 ± 0.34 A | 10.23 ± 0.46 A | 16.03 ± 0.44 BCD | 16.55 ± 1.13 BD | 16.62 ± 0.25 BD | 15.74 ± 0.13 BCD | 15.37 ± 0.44 C | 15.08 ± 0.46 C | 15.89 ± 0.13 BCD | 15.37 ± 0.76 C |
| TFAA (mg Gly/g) | |||||||||
| 8.32 ± 0.68 A | 16.68 ± 0.72 A | 45.02 ± 1.20 B | 237.34 ± 2.72 C | 262.21 ± 7.55 D | 276.05 ± 2.30 E | 323.50 ± 2.43 F | 365.50 ± 3.45 G | 245.33 ± 13.49 C | 358.29 ± 4.03 G |
| DPPH (%) | |||||||||
| 23.62 ± 5.22 A | 34.32 ± 7.34 B | 26.71 ± 2.72 A | 46.06 ± 2.08 CD | 46.19 ± 2.95 CD | 51.51 ± 1.08 D | 35.83 ± 1.61 B | 58.01 ± 2.05 E | 47.24 ± 1.77 CD | 42.72 ± 1.75 C |
| ABTS% | |||||||||
| 0.46 ± 2.64 A | 1.98 ± 4.12 A | 2.80 ± 1.19 A | 12.57 ± 3.53 B | 17.20 ± 4.43 BC | 20.05 ± 2.14 C | 16.03 ± 2.37 BC | 19.08 ± 2.12 C | 16.23 ± 3.93 BC | 13.89 ± 1.88 B |
| FRAP (mg AAC/g) | |||||||||
| 0.20 ± 0.01 A | 0.19 ± 0.01 A | 0.22 ± 0.03 A | 0.59 ± 0.02 A | 0.63 ± 0.04 AB | 0.74 ± 0.06 B | 0.67 ± 0.06 B | 0.91 ± 0.00 C | 0.80 ± 0.06 C | 0.73 ± 0.07 BC |
| RP (700 nm) | |||||||||
| 0.173 ± 0.003 A | 0.187 ± 0.003 AB | 0.214 ± 0.004 B | 0.324 ± 0.007 C | 0.363 ± 0.001 DE | 0.446 ± 0.002 G | 0.307 ± 0.058 C | 0.265 ± 0.021 F | 0.376 ± 0.004 D | 0.340 ± 0.004 CE |
| Time of Ripening (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | 21 | 28 | 35 | 42 | 49 |
| Hardness (N) | |||||||||
| 2.02 ± 0.23 A | 5.74 ± 0.98 B | 6.14 ± 0.95 C | 15.18 ± 0.21 D | 9.79 ± 0.58 E | 11.09 ± 1.28 F | 8.03 ± 1.49 G | 10.79 ± 1.49 H | 10.03 ± 1.19 H | 8.99 ± 1.35 G |
| Springiness (N) | |||||||||
| 0.95 ± 0.03 A | 0.67 ± 0.16 B | 0.98 ± 0.15 A | 1.99 ± 0.36 C | 1.06 ± 0.24 D | 1.57 ± 0.72 E | 2.85 ± 0.23 F | 1.65 ± 0.81 G | 2.11 ± 0.93 H | 1.01 ± 0.87 I |
| Gumminess (N) | |||||||||
| 1.03 ± 0.03 A | 0.81 ± 0.23 A | 1.11 ± 0.14 A | 3.36 ± 0.28 B | 2.46 ± 0.22 BC | 3.12 ± 0.50 BC | 2.18 ± 0.36 C | 2.64 ± 0.75 BC | 2.89 ± 1.20 BC | 2.26 ± 1.20 C |
| Chewiness (N) | |||||||||
| 0.98 ± 0.03 AB | 0.53 ± 0.23 A | 1.09 ± 0.14 AB | 7.73 ± 1.28 D | 2.59 ± 0.22 ABC | 5.30 ± 0.50 C | 6.19 ± 0.36 C | 4.78 ± 0.75 BC | 6.59 ± 1.20 D | 2.41 ± 0.20 ABC |
| Cohesiveness (N) | |||||||||
| 0.47 ± 0.03 A | 0.13 ± 0.16 B | 0.16 ± 0.20 B | 0.19 ± 0.91 C | 0.24 ± 0.60 D | 0.26 ± 0.21 E | 0.24 ± 0.95 F | 0.22 ± 0.97 F | 0.26 ± 0.66 D | 0.22 ± 0.77 G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewcz, Ł.; Śmietana, N.; Lichwiarska, E.; Mazurkiewicz-Zapałowicz, K.; Gefrom, A.; Drozłowska, E. The Biotransformation of Lupine Seeds by Lactic Acid Bacteria and Penicillium camemberti into a Plant-Based Camembert Alternative, and Its Physicochemical Changes during 7 Weeks of Ripening. Fermentation 2022, 8, 447. https://doi.org/10.3390/fermentation8090447
Łopusiewcz Ł, Śmietana N, Lichwiarska E, Mazurkiewicz-Zapałowicz K, Gefrom A, Drozłowska E. The Biotransformation of Lupine Seeds by Lactic Acid Bacteria and Penicillium camemberti into a Plant-Based Camembert Alternative, and Its Physicochemical Changes during 7 Weeks of Ripening. Fermentation. 2022; 8(9):447. https://doi.org/10.3390/fermentation8090447
Chicago/Turabian StyleŁopusiewcz, Łukasz, Natalia Śmietana, Elżbieta Lichwiarska, Kinga Mazurkiewicz-Zapałowicz, Annett Gefrom, and Emilia Drozłowska. 2022. "The Biotransformation of Lupine Seeds by Lactic Acid Bacteria and Penicillium camemberti into a Plant-Based Camembert Alternative, and Its Physicochemical Changes during 7 Weeks of Ripening" Fermentation 8, no. 9: 447. https://doi.org/10.3390/fermentation8090447
APA StyleŁopusiewcz, Ł., Śmietana, N., Lichwiarska, E., Mazurkiewicz-Zapałowicz, K., Gefrom, A., & Drozłowska, E. (2022). The Biotransformation of Lupine Seeds by Lactic Acid Bacteria and Penicillium camemberti into a Plant-Based Camembert Alternative, and Its Physicochemical Changes during 7 Weeks of Ripening. Fermentation, 8(9), 447. https://doi.org/10.3390/fermentation8090447








