1. Introduction
Over the years, consumers’ demand for disposable personal care products (PCPs) has increased and is forecasted to be a strong market demand for the next five years [
1]. The hygiene and sanitary products that can be rapidly discarded include wet wipes, sanitary napkins, tampons, incontinence products, panties, diapers, and cosmetic pads. Nonwoven textiles are used to manufacture these products due to their excellent absorption characteristics, higher tensile strength, and good durability [
1,
2]. A recent report about the nonwoven textile market released in 2021 by two leading global nonwoven associations estimates that the global market increased by 6.2% from 2010 to 2020. It is estimated that European production of nonwoven textiles for hygiene products and personal care wipes recently reached over 1 million tonnes [
3]. This sector changed the materials used for product development because most were produced using synthetic sources that generate unique characteristics such as breathability, comfort, antileakage, protection, barrier, and antimicrobial properties. However, the costs of the biodegradable materials commonly used and the prices of eco-friendly products are still higher than their synthetic counterparts [
1]. Therefore, the sector demands research and investment to improve technologies and develop new biodegradable products.
Biodegradation is the biologically catalyzed mechanism that breaks down materials into simple substances and includes biodeterioration as a combined result of degradative factors such as moisture, oxygen, UV light, or microbial activity [
4,
5]. In the case of disposable PCPs, this phenomenon has scarcely been studied, and most works focus on the occurrence and persistence of these products during wastewater treatment processes and in aquatic environments [
6,
7]. Because there is no established safe disposal route, the disposal of these products containing a broad spectrum of chemical components could lead to various environmental issues [
2]. Wet wipes have been recently identified as a potential source of microplastic pollution. Moreover, incomplete removal of chemical compounds from pharmaceutical and personal care products makes these products emerging contaminants of concern [
8,
9].
This study focused on the soil biodeterioration of disposable hygiene and sanitary products that contain different percentages of cellulose as raw material and compared the results with the fate of plant- and animal-based materials in the same environmental conditions (availability of oxygen and water, temperature, pH). Biodeterioration of materials after 40 days in direct contact with the ground was direct evaluated based on changes in the material morphology observed using stereomicroscope and scanning electron microscopy (SEM) images and by weight loss (%). These results were correlated with analysis of soil microbial community (total counts and cellulolytic activity) from two different types of soils (soil #1 from Ceatalchioi and soil #2 from Bididia-Mineri).
2. Materials and Methods
2.1. Materials and Sampling
Plant-based woven textiles made from cotton and linen, animal materials containing wool and leather, and disposable hygiene and sanitary products (sanitary pads, make-up removed disks, antimicrobial/sanitary wet wipes) were collected from the market (Bucharest-Romania) and were assigned based on manufacturer label. The plant and animal-based fabrics were washed twice with water and dried before testing, then cut in the square shape of dimensions of 5 × 5 cm2 and buried in the soil. Sanitary pads were square-cut 5 × 5 cm2 in the absorption area; similar size cutting with wet wipes and make-up removed disks with a 5.5 cm/piece diameter. All studied samples were evaluated in triplicate and buried in separate beakers.
2.2. Soil Burial Test—Samples in Direct Contact with Soil
The soil was collected from lakeside area Ceatalchioi (soil #1) and forest Bididia-Mineri (soil #2) from Tulcea—Romania, and had a particle size of 0.5–1 cm, 50–55% content of dry substances, and pH in the range of 4.0 to 7.5. The biodegradation was done by burying the samples in the soil according to the ISO 11721-1:2001 [
10] and ISO 11721-2:2003 [
11] at different times. Still, the results were analyzed after 40 days of exposure in direct contact with soil. The samples (3 repetitions/each) were buried at approximately 5 cm depth in separate beakers of 1000 mL so that different materials may not mix. The beakers containing the buried samples were then placed at room temperature in a dark place and 27 °C. After seven days of incubation, soils from all beakers were moisturized with the same amount of distilled water. Soils with samples were put back for incubation, but one piece of each sample was kept to analyze the biodeterioration. The removed samples were first rinsed in sterilized saline solution for approximately 10 min. In ethanol/water solution 70:30 (
v/v) for 10 min, the soil particle was removed under stereomicroscope Olympus SZX7 before drying at room temperature for one day in a desiccator, and further experiments were done.
The biodeterioration of different samples in soil was evaluated based on weight loss (%) and visual/SEM analysis. The percentage of mass reduction due to the biodeterioration process, expressed as weight loss (%), was calculated by the below formula: where Mbefore and Mafter were the weight of the sample (g/cm2) before and after biodegradation and drying, respectively. First, to calculate the weight loss (%) for wipes, quantify the antimicrobial substances in the sample. The samples with an area of 25 cm2 were dry at room temperature for one day in a desiccator. To quantify the amount of antimicrobial/pure substances from a wet wipe sample, the weight of 24 hours dry sample was subtracted from the weight of the wet sample. The weight loss of dry wipes samples before and after biodeterioration was considered.
For some samples, the reduction in mass percentage (%) was not calculated because small size soil particles attached by fibers from woven materials or entered into the absorption area from sanitary pads could not be removed.
Visually/SEM index was used to evaluate the biodeterioration level based on visually and microscopic pictures, as follow: ++++ very high, +++ high, ++ medium + low—no biodeterioration.
2.3. Scanning Microscope Analysis
The samples were analyzed on a Quanta 200 (FEI) Scanning Electron Microscope (SEM), Low Vacuum Mode, GSED detector (Gaseous Secondary Electron Detector), and various filament voltage values (10–15 kV). The samples were visualized without coating, mounted on metal stubs, in an average area of 1 cm2, at 1000× and 2000× magnification levels. The analyses were carried out before soil exposure, on samples sets that served as control, and after 40 days of soil biodeterioration.
2.4. Microbiological Analysis and Cellulolytic Activity
Microbial community from soil narrow the samples were analyzed by harvesting 1 g of the soil before and after biodegradation. The solution was decimally diluted (10
−1 to 10
−8) and aliquots plated on the appropriate culture media for total counts (NTG) using Tryptone Soya Agar TSA medium (Oxoid) for bacteria and Czapek Dox medium (Oxoid) for fungal growth. After incubation at 25–30 °C for up to 10 days, the colony-forming units (CFU) were counted. The cellulase activity from saline solutions was assayed in carboxymethyl cellulose (CMC) solution by the 3, 5-dinitrosalicylic (DNS) method [
12].
Total counts and cellulolytic activity in 1 g of the soil before biodegradation were considered 100%. The counts and enzyme activity increased (%) were calculated based on data obtained before and after 40 days of biodegradation from the soil nearby the sample.
2.5. Fungal Strain Isolation and Identification Based on PCR
Fungal strains isolated from saline solutions obtained from different samples were identified based on cultural-morphological characteristics under stereomicroscope Olympus SZX7. Genomic DNA from fungal cultures was extracted using a Quick-DNA Miniprep Plus kit (Zymo Research, Irvine, CA, USA), according to the manufacturer’s instructions. High-coverage PCR primers targeting ITS1 and ITS2 regions were used: forward primer ITS1 5′-TCCGTAGGTGAACCTGCGG-3′ and reverse primer ITS 4 5′-TCCTCCGCTTATTGATATGC-3′ [
13]. The PCR reaction was performed using Multigene (Labnet International, Edison, NJ, USA) instrument, with a final volume of 20 μL, among them 2 μL of template DNA, 0.2 μL of FIREPol DNA polymerase 5 U/μL (Solis BioDyne, Tartu, Estonia), 2 μL FIREPol 10× Buffer, 2 μL of MgCl
2 25 mM, 0.2 μL dNTP Mix (200 μM/each), 0.5 μL of forwarding primer and 0.5 μL of reverse primer and nuclease-free water up to 20 μL. The cycling parameters were: initial denaturation of 5 min at 95°, followed by 30 cycles of 30 s at 95 °C, 30 s at 56 °C, 60 s at 72 °C, and final extension of 10 min at 72 °C and amplicons (290 bp) were visualized by gel electrophoresis.
2.6. Statistical Analysis
All the samples and parameters investigated were evaluated in triplicate. The results were expressed as the mean ± standard deviation (SD). The variance analysis was performed with ANOVA at 95% significance using SAS statistical software package (SAS Institute Inc., Cary, NC, USA).
3. Results
Plant-based woven fabrics quickly biodegrade in soil, but the process is not uniform primarily because of the non-homogeneity of the textile fibers. Structural deformation produced by damage to individual fibers on the surface and inside the material is evident by naked eyes and microscopic viewing before and after 40 days of soil biodegradation for both fabrics (
Figure 1). Fibers are highly degraded, and samples showed significant final weight loss due to the microbe activity, with a maximum of 43.12% for linen samples in soil #1 (
Table S1—Supplementary Files). Animal-based materials are less biodegradable than plant-based materials, and the process is not uniform and is related to these materials’ structures. Wool fibers with tightly twisted fibers were analyzed only based on the visual/SEM images and showed more resistance to soil microbial biodegradation than plant-based fibers, but they were more degraded than leather structure (
Figure 2). The slow biodeterioration effect for leather, observed only on the material’s surface, is linked to leather structure (as observed in SEM images) and influenced by the higher mass per unit area of leather (2.561 ± 0.04) compared to wool (1.959 ± 0.10). The loss in leather mass was observed, especially for microbial degradation in soil #1 (8.984%) compared to soil #2 (6.093%), while naked eyes and SEM analysis confirmed the results. For cellulose-rich and animal-based materials, the biodegradation was influenced by the soil composition and is higher in soil from Ceatalchioi based on weight loss (%) and visual/SEM index.
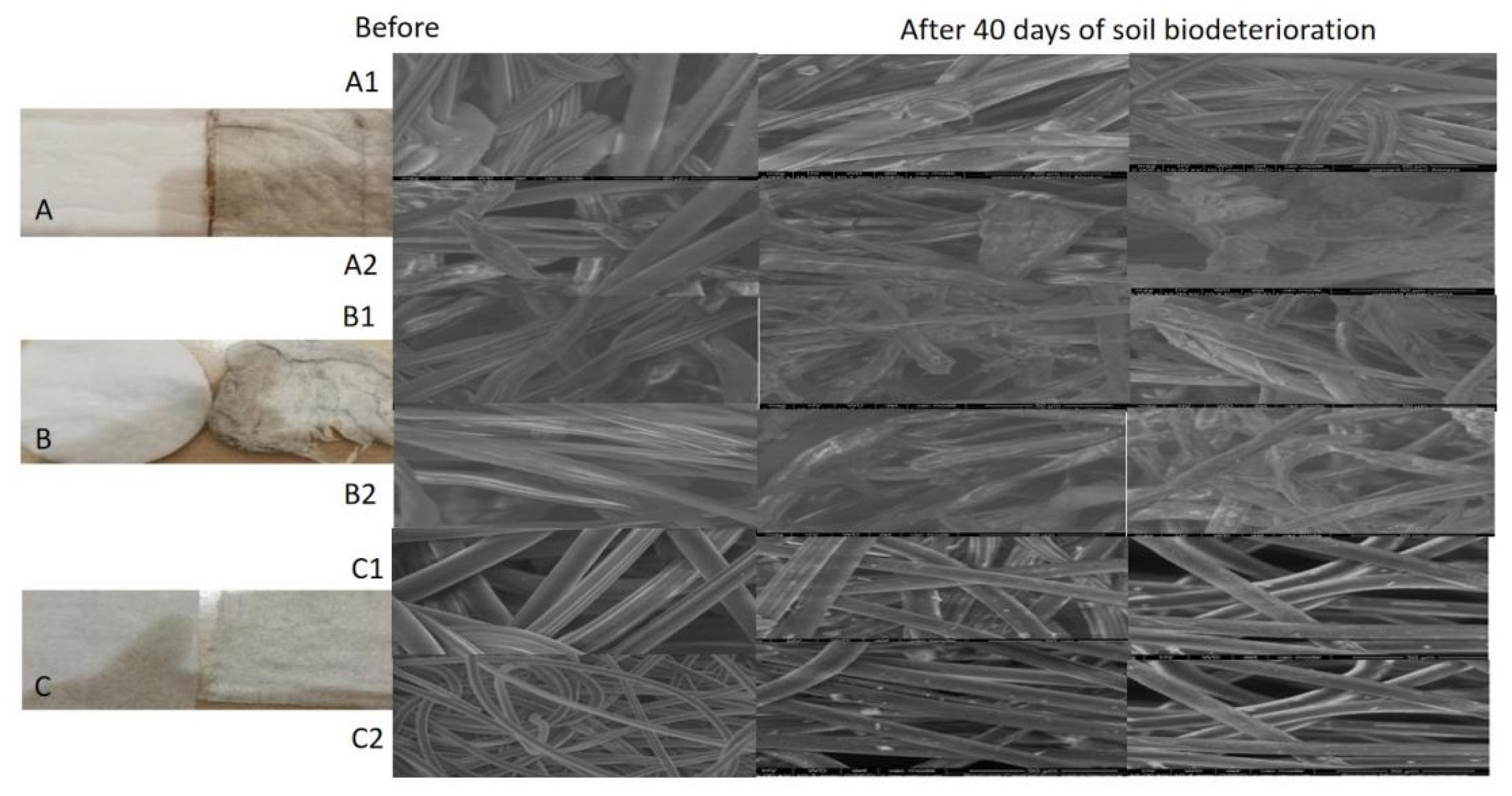
Soil biodegradation processes for different brands of disposable PCPs: sanitary pads, cosmetic pads, and chemical impregnated wet wipes, were analyzed and compared with the biodegradation of natural fiber materials. Nonwoven cellulose fibers are the base material in many sanitary and hygiene products, including wet wipes (made up of over 50% cellulosic fibers), sanitary pads, and cosmetic pads [
14]. In our experiments, disposable PCPs containing cellulosic natural biopolymers were partially biodegradable in soil environments, but less biodegradable than plant-based materials (
Figure 3). Based on analysis of SEM images, the structural deformation produced by the damage to individual fibers is less noticeable in various brands of sanitary pad samples, while wet wipes (antimicrobial and sanitary) were attacked by a low number of microbes and were resistant to soil biodegradation with almost no damage to the structure of individual fibers. Cosmetic pads made from cotton fibers demonstrated higher soil biodeterioration than other tested products based on microscopic evaluation. Visually, the process is not uniform and areas from the external part of the pad are more affected. SEM images support this observation, showing individual fibers attacked by many microbes in both soils. For PCPs, the soil biodeterioration process seems to be less affected by the soil or the brand type.
For all disposable PCPs samples, the weight loss results are not directly correlated with visual and microscopic analysis (
Table S1—Supplementary Files). The mass reduction due to biodegradability was calculated for wet wipes and cosmetic pads. In the case of cosmetic pads, the weight loss (%) calculation was influenced by incomplete removal of soil particles, and visual/SEM pictures have proved the presence of numerous damaged fibers. All wet wipes samples lost weight, with a maximum of 24.332% for antibacterial wipes in soil #1. Still, these seem to be generated by the chemical substance leak into soil rather than microbes’ effect on the fiber structure. At the end of the experiment, antimicrobial and sanitary wipes were very dry. Their weight was slightly higher than the weight of samples dried for 24 h in the laboratory desiccator. For sanitary pads buried in soil for 40 days, SEM images did not show significant changes in their structure for either brand or soil. The biodegradation of hygiene and sanitary disposable PCPs is related to their composition and structure and less influenced by the ground or brand type.
The effect on microbial community in the biodeterioration process of the disposable PCPs was evaluated by counting the total number of microbes (NTG) and cellulolytic activity changes in the soil near the samples and comparing the results with data obtained for linen material in soil #1. These results are shown in
Table S2 (Supplementary Files), alongside samples used for fungal isolation. There is a significant increase in NTG (CFU/g) and cellulolytic activity in soil #1 with a buried linen sample, but the results obtained in soils where cosmetic pads underwent microbial degradation were lower than data obtained for linen biodegradation in soil #1. Moreover, there is a correlation between weight loss (%) and cellulolytic activity increase for cosmetic pads. The biodegradation level is slightly higher in soil #2 (the weight loss is 27.537% for brand A, and enzymatic activity increased to 345.5%). In the case of wet wipes, results from SEM images and antimicrobial/sanitary chemical leak were confirmed by total counts that significantly decreased to 10
4 CFU/g soil, while cellulolytic activity slowly increased up to 133% for antibacterial wet wipes in soil #1. The total counts increased in soils with buried sanitary napkins, but cellulolytic activity decreased.
Finally, eight fungal strains were isolated from the surface of different samples washed with saline solution and then plated on Czapek medium. These strains were identified based on cultural-morphological characteristics observed under a microscope and using genetic analysis (PCR) with high-coverage PCR primers targeting ITS1 and ITS2 regions from Ascomycetes.
4. Discussion
The majority of disposable personal care products and included chemicals enter the environment via direct discharge into soil or waters or indirect discharge when wastewater or treated sludge is used on agricultural farms [
15]. This phenomenon is scarcely studied because there is no proper mechanism for disposing of these products, which could become biological hazards and create alarming pollution to the environment. Therefore, pharmaceuticals and PCPs are considered emerging pollutants that can lead to several environmental issues through persistence, bioaccumulation, and ecotoxicity [
7,
9]. Some problems related to improper disposal of these products cannot be minimized, especially for products that cannot be decomposed within a few days or months through the action of microorganisms and environmental factors (temperature, moisture, and sunlight).
The degradation rate of cellulose and cellulosic textile substrates mostly depends on microorganisms used, especially bacteria and fungi responsible for enzymatic degradation of cellulose. The primary function of these enzymes is to decrease the degree of polymerization, producing damage to the structure of the fibers and causing them to lose their strength. Bacterial degradation of cellulose fabrics begins from the surface towards the inside. At the same time, fungi penetrate the outermost layer of the thread—which is named the cuticle—then enter the inner part of the fiber where they grow. The degradation rate depends on different parameters, including cellulose crystallinity and the presence of non-cellulosic substances [
16]. The biodegradation of cellulose and cellulosic fabrics has been extensively studied [
3,
4]. In our experiments, both cellulose-based woven materials were high biodegradable after 40 days of being buried in direct contact with soil, and the process is not uniform. Both materials were severely attacked by microorganisms, as can be easily observed in SEM images, with a higher rate of biodeterioration and weight loss (%) for the linen sample in soil #1. Arshad et al. (2014) concluded that biodegradation of fibers containing cellulose proceed in similar way, with a faster effect linked to the structure of linen materials because fibers are not tightly twisted in linen fabric compared with cotton [
4]. However, in that study, the biodegradation of linen material was rapid, and in a few weeks, cotton and linen fabrics were tough to separate from the soil. The quicker biodegradation rate compared to our results might be explained by differences in the chemical and biological composition of soils used in the experiments. Our results have shown different rates of microbial degradation in lakeside soil from Ceatalchoi compared to forest soil from the Bibidia-Mineri area. The data from the direct evaluation were correlated with an increase in soil microbial activity, both in number (~10
9 CFU/g soil) and cellulolytic activity (588% for linen sample in soil #1). Higher enzymatic biodegradation of different types of cotton fabrics in the compost environment compared to laboratory conditions has been confirmed [
17]. Animal-based materials (wool, leather) were less soil biodegraded comparable to cellulosic material (cotton, linen, and cosmetic pads), but SEM images showed microbes attached to the wool fiber, trying to penetrate the structure of fiber and on the surface of leather. The presence of wool grease, a hydrophobic substance covering the wool fiber, provides an explanation for their resistance to microbial attack, at least at the beginning of the process [
4]. The changes in the wool and leather samples were not significant, but both materials started to degenerate in direct contact with soil at the end of the experiments.
Prior to this study, little was known about the biodegradation of disposable hygiene and sanitary products. Moreover, information about the composition of disposable materials is scarce; most personal care, hygiene, and cleaning product labeling fails to identify the design of the product. Preliminary studies have shown selective biodegradation of sanitary materials. For example, a cotton swab lasted 15 days, and towels had a degradation time of at least 30 days in an anaerobic sewage sludge digester [
18]. Higher yields require processes of physicochemical degradation and, in the second stage, the use of selected strains. In the case of products containing highly biodegradable components, a two-step process uses mushroom stalks (
Pleurotus ostreatus) to degrade diapers and sanitary pads over three months [
19].
Our research detected the highest biodeterioration for cotton cosmetic pads, based on changes in the samples, by both naked eyes and SEM images. These results were consistent with indirect evaluation based on the number of microbes and cellulolytic activity of the soil near the samples. Despite highly degraded cellulose fibers and many microbes attached to the fiber surface, cosmetic pads seem to be less affected after 40 days buried in direct contact with the ground than linen samples. Cosmetic pads used for cleaning the skin, especially for peeling applications, contain layers of cotton and micro staple fibers. The rough surface of the pad features abrasive particles in a continuous plastic coating, applied to the pad in the liquid state and trapped there. The addition of synthetic fibers in the composition of cosmetic pads can give the cotton pads superior softness, cleaning, and make-up removal abilities [
20]. The design and structure of both types of cotton pads used in our experiments might explain the partial soil biodeterioration of these products, which are labeled as 100% cotton pads but are not as biodegradable. Therefore, in the case of cosmetic pads, the eco-friendly designation should not depend only on the presence of high-quality cotton used in the manufacturing process. Recently studies about cosmetic products containing microplastics have proved that these are a contaminant of increasing concern in the marine environment [
21,
22]. In the experiment with cosmetic pads, the weight loss (%) might not be considered a suitable parameter for biodeterioration evaluation because the samples were difficult to separate from soil particles. A more reliable parameter for evaluating the process is the increase in number of cells (approximately 10
8 CFU/g soil) and cellulolytic activity (207–343.5%) in soil near these samples after 40 days of biodegradation, compared to data obtained at the beginning of the experiment.
Wet wipes—such as baby wipes, facial wipes, moist flushable wipes, and medical and disinfectant wipes—blend viscose fibers, wood pulp, and cotton, and contain cellulosic natural biopolymers. Long viscose fibers shape the load-carrying structure providing the wet strength, while pulp fibers are responsible for liquid absorption [
23]. The main nonwoven fibers which make up over 50% of the raw materials in wet wipes are cellulose and polylactic acid, both of which are considered biodegradable; however, recent findings question the biodegradability of polylactic acid in the marine environment [
24]. The presence of chemical substances with antimicrobial properties or other chemicals used in sanitary napkins, as well as their structure, made these products resistant to microbial degradation. At the end of the experiment, the wet wipes were very dry because the antimicrobial/sanitary liquid leaked into the soil. This hypothesis is supported by the SEM images which prove that the number of soil microbes around these fibers is deficient, and there is almost no damage to the structure of the individual fiber. Moreover, total cell counts decreased to 10
4 CFU/g soil, while cellulolytic activity slowly increased (up to 133% for antibacterial wet wipe in soil #1) in all wet wipe samples.
Sanitary pads have a conventional layer design with a top sheet surface with polyethylene/polypropylene nonwoven fabric, the cellulosic core with polyacrylate polymer foam, and an impermeable back sheet with polyethylene adhesive film and a small amount of perfume in the scented version [
25]. In the United States, the Food and Drug Administration (FDA) regulates sanitary pads as class I medical devices, while in Canada and the European Union, they are considered consumer products [
25]. Most of the studies on sanitary pads to date have focused on the human health risk assessment for these products, including the effect of higher phthalate content that became a worldwide public concern [
26]. In our experiment, the absorption area of sanitary pads were less affected by soil microbes and only affected at the top sheet surface. Based on visual observation, these products dried and shrank, while the SEM images proved that only the nonwoven fibers from the surface had some damage. The total cell counts and cellulolytic activity in soil were not significantly changed. Biodegradable sanitary napkins made from natural fibers, such as jute, banana, cotton, and bamboo, are alternatives to developing hazard-free pure products [
27,
28].
In the case of disposable hygiene and sanitary PCPs, biodegradability is dependent on the total amount of cellulosic fibers, the presence of antimicrobial/hygienic substances, and the product’s structure. Cotton fibers from cosmetic pads containing 100% cotton are clearly more damaged by microbial attacks compared to fibers from products labeled “wet cotton wipes” that should contain at least 50% cotton fibers. Products with complex multi-layer designs and cellulosic cores, such as sanitary pads, were minimally damaged. The biodegradation process is minimally affected by soil microbial community activity and brand type, as suggested by the similarities between the biodegradation processes of the cosmetic pads.
Assimilation of xenobiotics is a critical issue, especially when biodegradation of medical products (gauze, rival buffers), many of which may contain significant amounts of antimicrobial substances, is needed. Compared to bacterial strains (
Actinomycetes), fungal species have a high capacity to degrade cellulosic materials and have the potential to assimilate compounds with xenobiotic potential [
29,
30,
31]. Thus, the inhibitory effect of some chemicals, such as alcohol, rivanol, or iodine, is partially eliminated by using mushroom species [
32]. The microbial load of waste on the biodegradation of hygiene or sanitary products is another critical detail [
33]. It is assumed that interactions may occur that limit the efficiency of the microbial degradation process. Therefore, the selection of competitive, high-yielding strains is a significant aspect. This study isolated eight fungal strains identified based on cultural-morphological characteristics and genetic analysis with high-coverage PCR primers targeting ITS1 and ITS2 regions from
Ascomycetes.
Consumers’ increased environmental awareness has led to changes in the hygiene and sanitary industrial sector, and the market is now providing disposable, partial biodegradable products at a higher price. The current trend in producing disposable sanitary and personal care products is to use plant products and by-products as raw materials. In the last decade, changes have been made in related industrial sectors in correlation with environmental issues, including the elimination of plastics or other components which take a long time to assimilate into the environment. This trend aligns with the current economic context regarding the efficient management of natural resources and improving safety in personal and medical products [
34].
Further studies are required to reveal crucial insights about the potential hazards of different substances in disposable products, i.e., microplastics or chemicals, and how their presence affects biodegradability. As a result of the COVID-19 pandemic, rising sanitation requirements for personal hygiene are boosting the demand for antimicrobial wet wipes, and there are reports and evidence of environmental contamination from these products around the world [
35,
36,
37,
38,
39,
40]. These products are an anthropogenic source of microplastic contamination, both after disposal and when used as cleaning agents [
8,
41]. Lee et al. (2021) suggested that humans are exposed unknowingly to these microplastics and if only 1% of all wet wipes are improperly disposed of, millions of microplastic fibers will be released into the aquatic environment or remain in soil [
42]. Moreover, further studies are needed to find more efficient waste management options, as these products have been an issue for wastewater plant operators for a long time.