Microbial Evaluation of Ozone Water Combined with Ultrasound Cleaning on Crayfish (Procambarus clarkii)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment of Crayfish
2.3. Microbial Count
2.4. DNA Extraction and Amplification
2.5. Examination of Chromaticity
2.6. E-Nose Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results
3.1. Microbial Count
3.2. Bacterial Diversity
3.2.1. Alpha Diversity
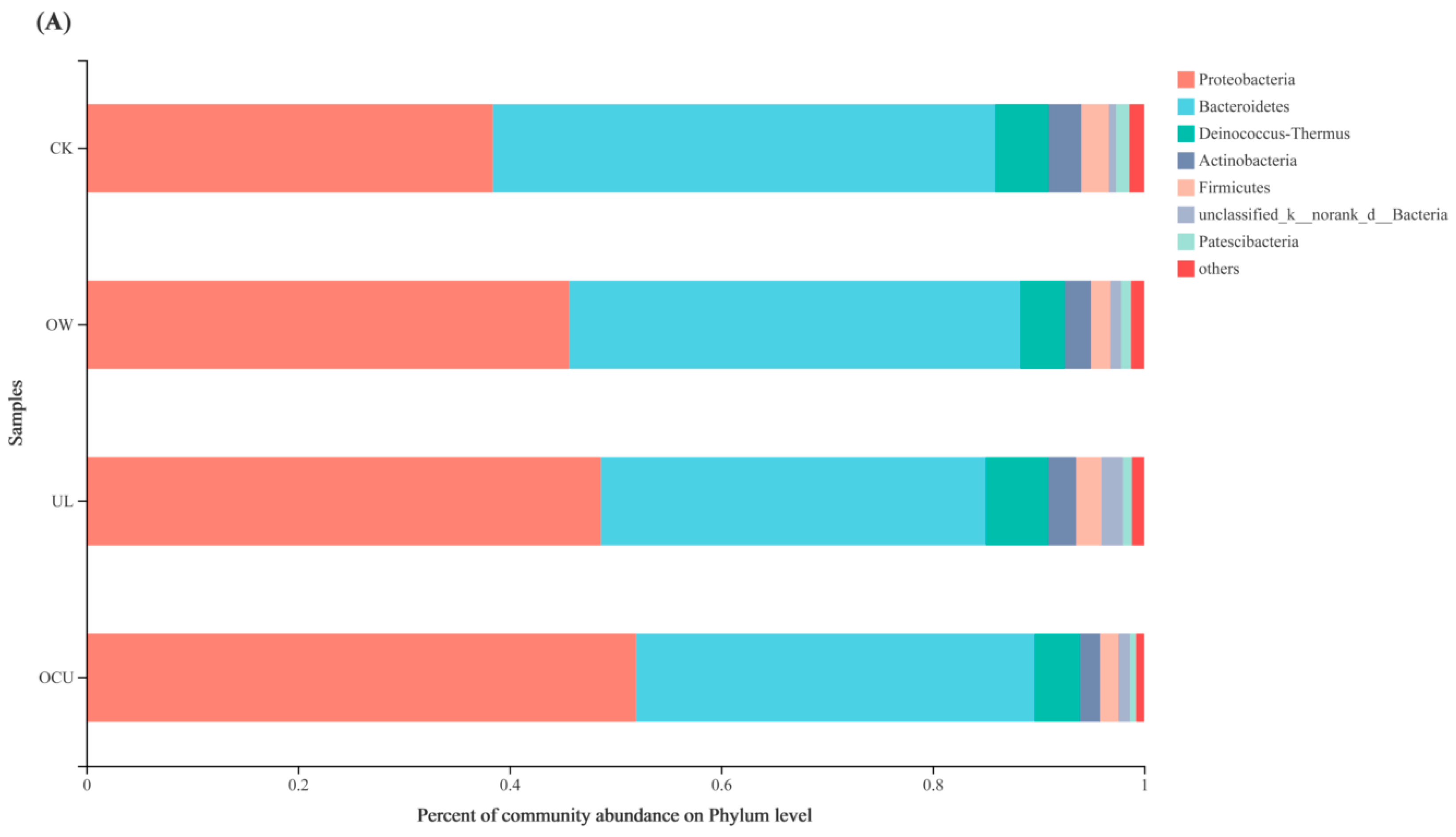
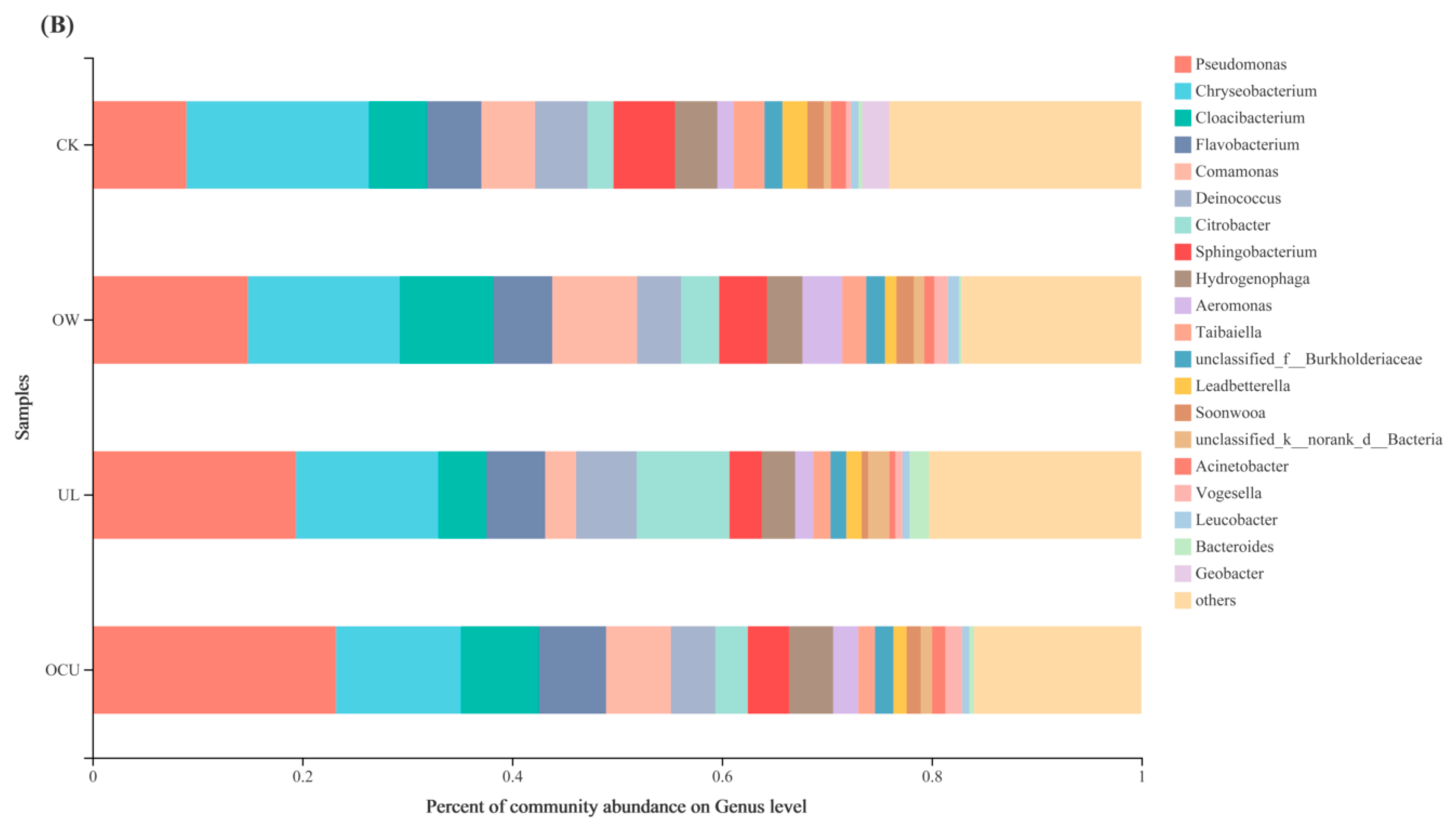
3.2.2. Bacterial Community Composition
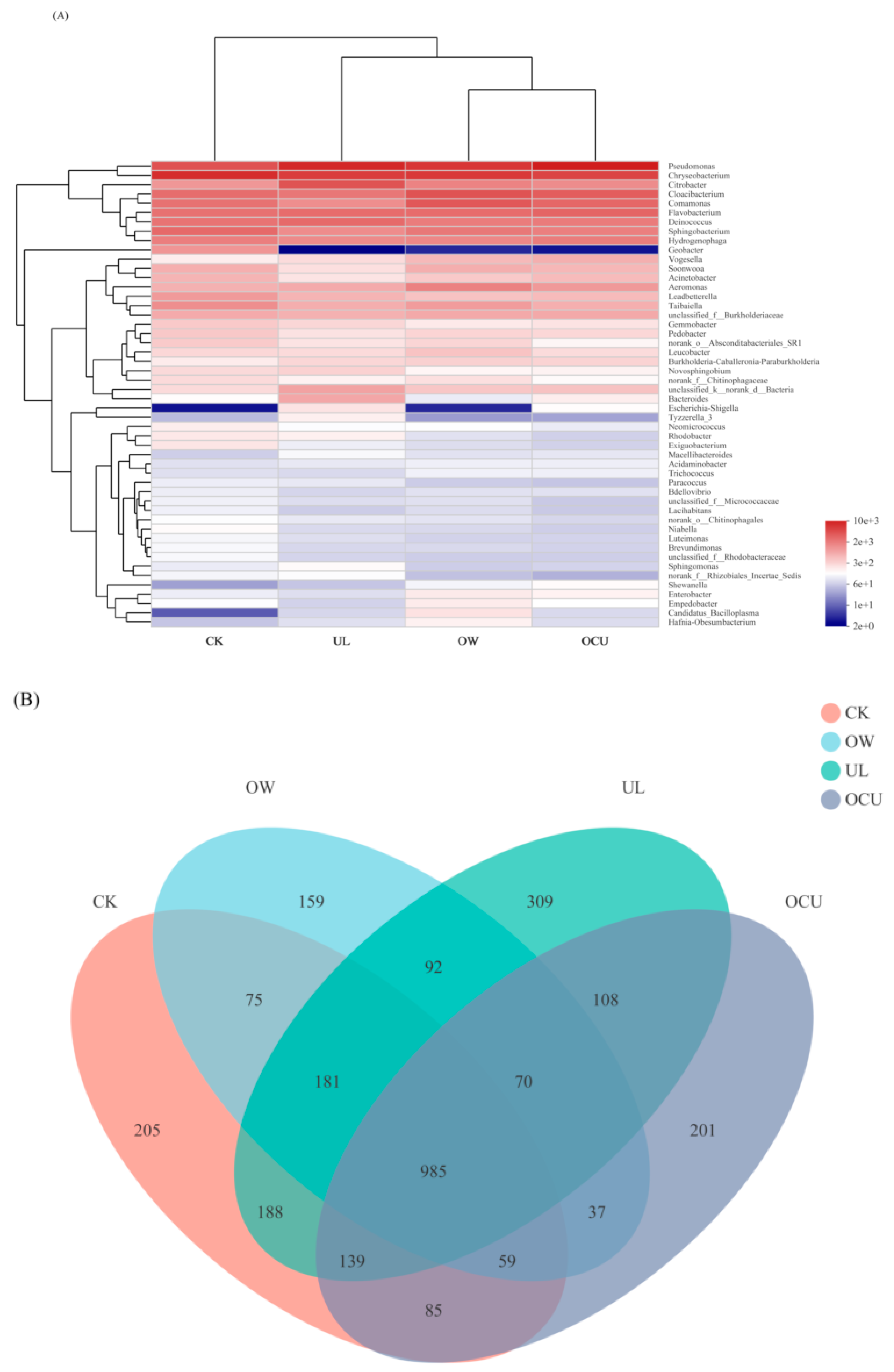
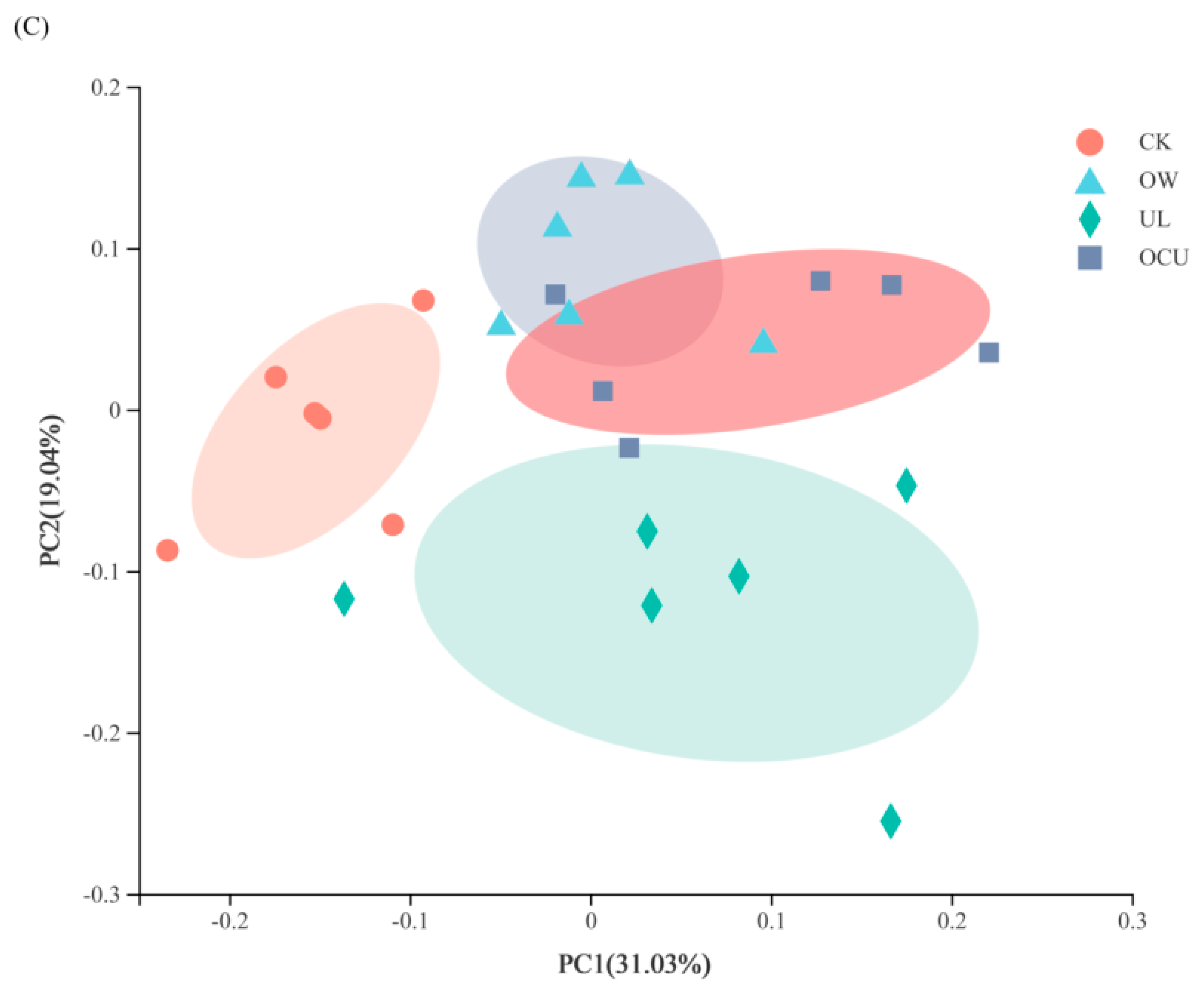
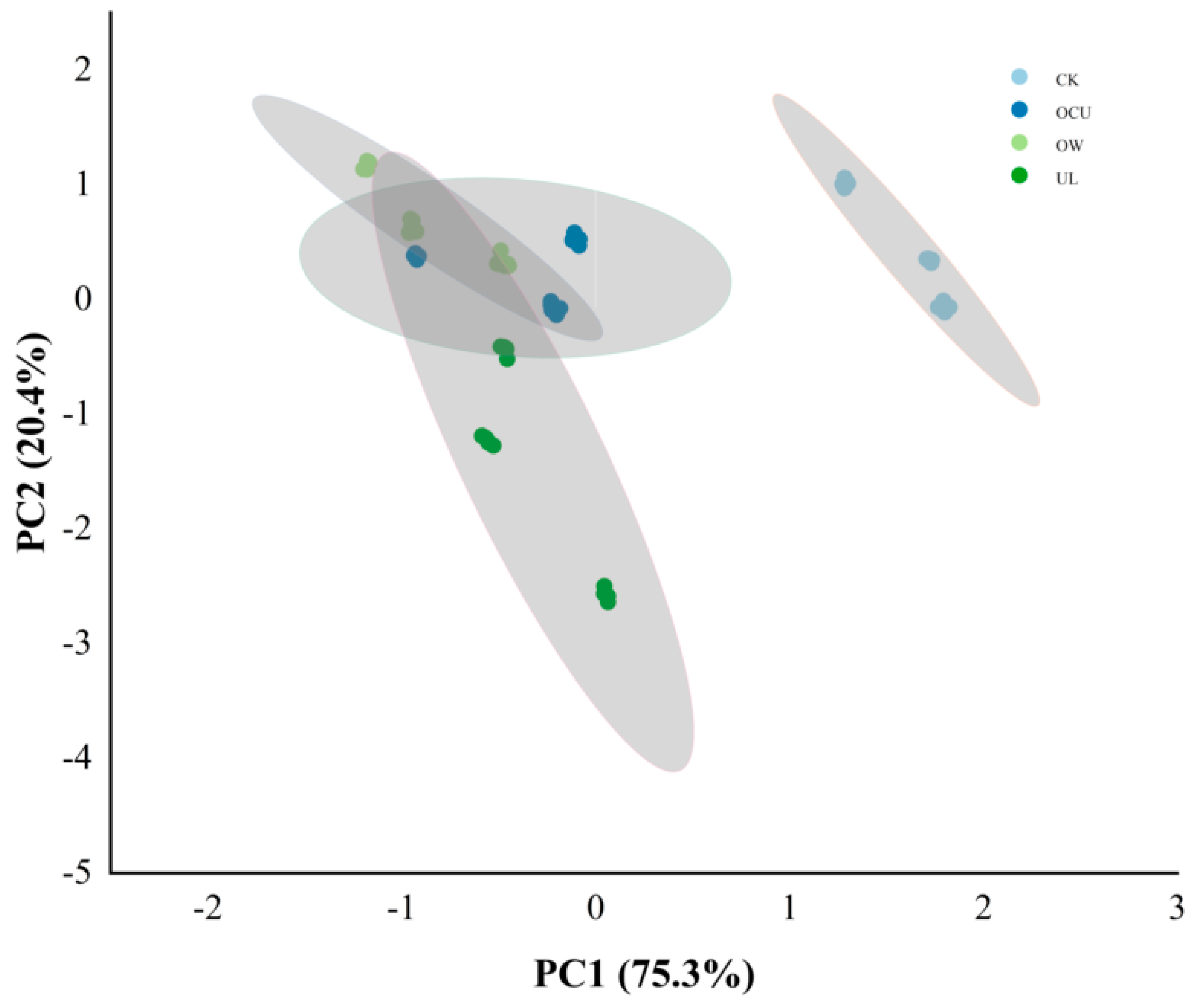
3.2.3. Sample Difference
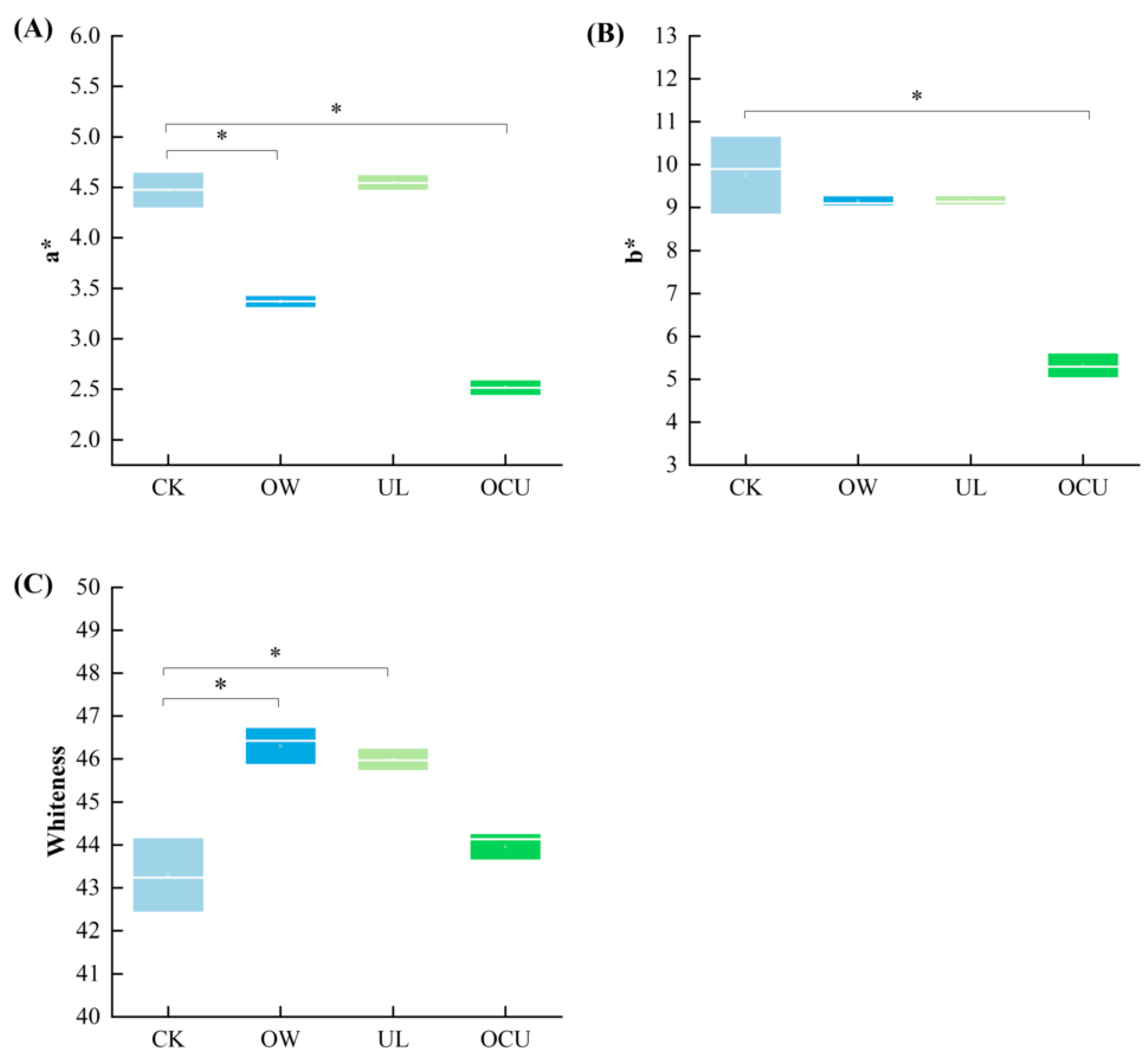
3.3. Chromaticity
3.4. E-Nose
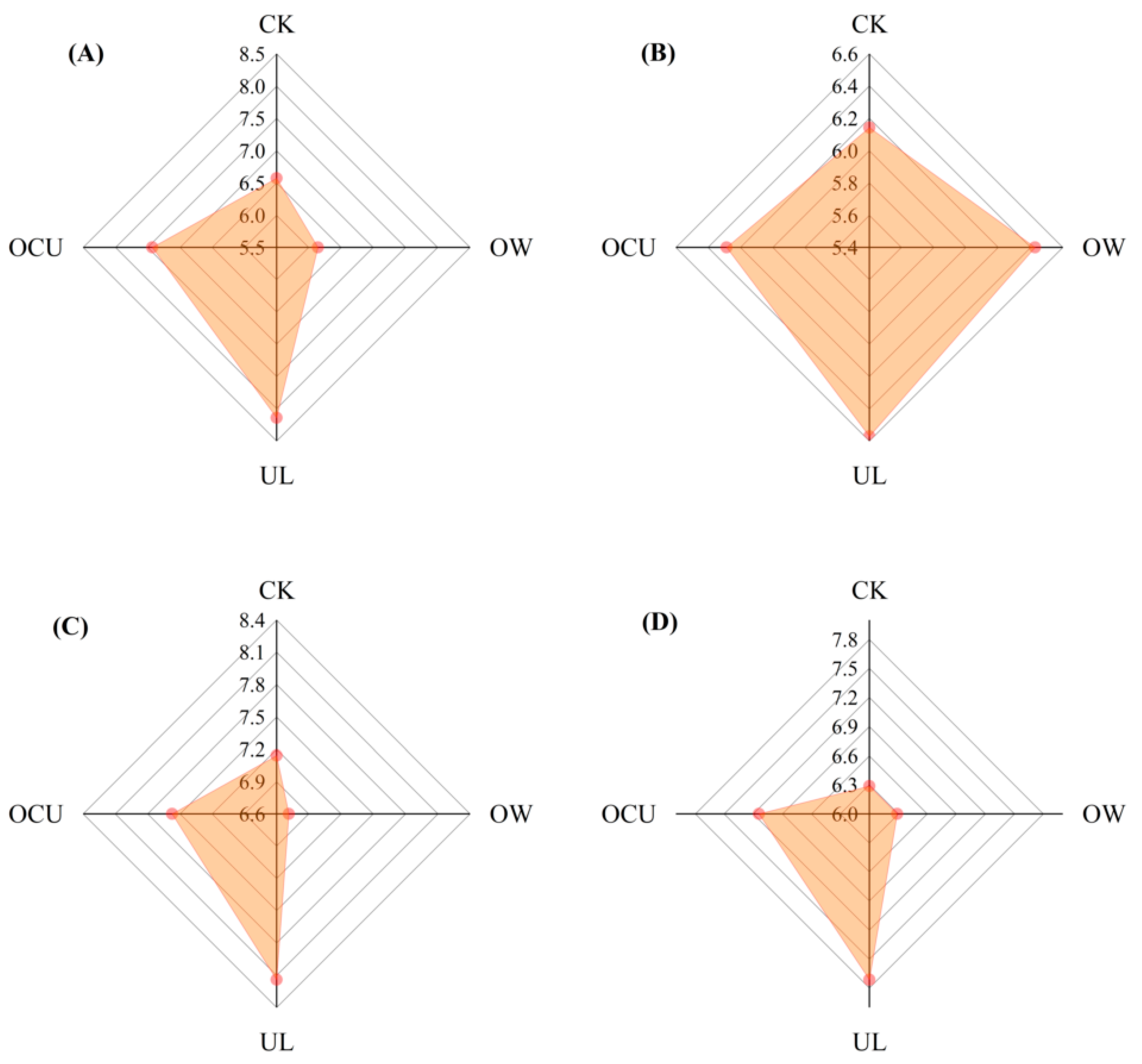
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Guo, M.; Jin, T.Z.; Yang, R.; Antenucci, R.; Mills, B.; Cassidy, J.; Scullen, O.J.; Sites, J.E.; Rajkowski, K.T.; Sommers, C.H. Inactivation of natural microflora and inoculated Listeria innocua on whole raw shrimp by ozonated water, antimicrobial coatings, and cryogenic freezing. Food Control 2013, 34, 24–30. [Google Scholar] [CrossRef]
- Herbert, B. Emergency animal diseases bulletin: Crayfish plague. Aust. Vet. J. 2014, 92, N8–N9. [Google Scholar] [PubMed]
- Zhao, Y.M.; de Alba, M.; Sun, D.W.; Tiwari, B. Principles and recent applications of novel non-thermal processing technologies for the fish industry—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, Y.; Xing, L.; Zhang, W. Influences of ultrasonic-assisted frying on the flavor characteristics of fried meatballs. Innov. Food Sci. Emerg. Technol. 2020, 62, 102365. [Google Scholar] [CrossRef]
- Ercan, S.Ş.; Soysal, Ç. Use of ultrasound in food preservation. Nat. Sci. 2013, 05, 5–13. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Sun, P.; Ma, Y.; Cai, L.; Li, J.R. Effect of ultrasonic thawing on the water-holding capacity, physicochemical properties and structure of frozen tuna (Thunnus tonggol) myofibrillar proteins. J. Sci. Food Agric. 2019, 99, 5083–5091. [Google Scholar] [CrossRef]
- Mason, T.J. Ultrasonic cleaning: An historical perspective. Ultrason. Sonochem. 2016, 29, 519–523. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Vetrimurugan, R.; Hooi, B. Study of ultrasonic parameters on removal of contamination from slider surface by using various cleaning chemistry. Int. J. Chem. Environ. Eng. 2012, 3, 392–396. [Google Scholar]
- Chemat, F.; Zille, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.; Ashokkumar, M.; Kentish, S. The Fundamentals of Power Ultrasound—A Review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]
- Spiteri, D.; Chot-Plassot, C.; Sclear, J.; Karatzas, K.A.; Scerri, C.; Valdramidis, V.P. Ultrasound processing of liquid system(s) and its antimicrobial mechanism of action. Lett. Appl. Microbiol. 2017, 65, 313–318. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M.; Stasiak, D. Effect of ultrasound on survival of gram-negative bacteria on chicken skin surface. Bull. Vet. Inst. Pulawy 2011, 55, 207–210. [Google Scholar]
- da Silva, S.; Luvielmo, M.; Geyer, M.; Pra, I. Potencialidades do uso do ozônio no processamento de alimentos Potential use of ozone in the food processing. Semin. Ciênc. Agrárias 2011, 32, 659–682. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Wang, W.; Sun, Y.; Zhang, J.; Fatima, M.; Jia, X.; Qiu, H.J. Efficient inactivation of African swine fever virus by ozonized water. Vet. Microbiol. 2020, 247, 108796. [Google Scholar] [CrossRef]
- Fonseca, P.M.M.; De Sa, P.L.; Miyakawa, W.; Damiao, A.J.; Melo, L.; Zangaro, R.A.; Fernandes, A.B.; De Lima, C.J. Analysis of Damage on the Streptococcus mutans Immersed in Ozonated Water: Preliminary Study for Application as Mouth Rinse. Ozone-Sci. Eng. 2019, 41, 242–249. [Google Scholar] [CrossRef]
- Rodrigues, A.A.Z.; Queiroz, M.; Neves, A.A.; Oliveira, A.F.; Prates, L.H.F.; Freitas, J.F.; Heleno, F.F.; Faroni, L.R.D. Use of ozone and detergent for removal of pesticides and improving storage quality of tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Doan, H. Disinfection of recycled red-meat-processing wastewater by ozone. J. Chem. Technol. Biotechnol. 2005, 80, 828–833. [Google Scholar] [CrossRef]
- Crowe, K.M.; Skonberg, D.; Bushway, A.; Baxter, S. Application of ozone sprays as a strategy to improve the microbial safety and quality of salmon fillets. Food Control 2012, 25, 464–468. [Google Scholar] [CrossRef]
- Jurado-Alameda, E.; García-Román, M.; Altmajer-Vaz, D.; Jiménez-Pérez, J.L. Assessment of the use of ozone for cleaning fatty soils in the food industry. J. Food Eng. 2012, 110, 44–52. [Google Scholar] [CrossRef]
- Tran, N.; Drogui, P.; Brar, S.K. Sonochemical techniques to degrade pharmaceutical organic pollutants. Environ. Chem. Lett. 2015, 13, 251–268. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z. Mechanism and reaction kinetic of hybrid ozonation-ultrasonication treatment for intensified degradation of emerging organic contaminants in water: A critical review. Chem. Eng. Process.-Process Intensif. 2020, 154, 108047. [Google Scholar] [CrossRef]
- Duan, S.; Zhou, X.; Xiao, H.; Miao, J.; Zhao, L. Characterization of Bacterial Microbiota in Tilapia Fillets Under Different Storage Temperatures. J. Food Sci. 2019, 84, 1487–1493. [Google Scholar] [CrossRef]
- Bott, T.R.; Tianqing, L. Ultrasound enhancement of biocide efficiency. Ultrason. Sonochem. 2004, 11, 323–326. [Google Scholar] [CrossRef]
- Al-Hashimi, A.M.; Mason, T.J.; Joyce, E.M. Combined Effect of Ultrasound and Ozone on Bacteria in Water. Environ. Sci. Technol. 2015, 49, 11697–11702. [Google Scholar] [CrossRef]
- Aday, M.S.; Caner, C. Individual and combined effects of ultrasound, ozone and chlorine dioxide on strawberry storage life. LWT-Food Sci. Technol. 2014, 57, 344–351. [Google Scholar] [CrossRef]
- Yang, S.S.; Guo, W.Q.; Chen, Y.D.; Wu, Q.L.; Luo, H.C.; Peng, S.M.; Zheng, H.S.; Feng, X.C.; Zhou, X.; Ren, N.Q. Economical evaluation of sludge reduction and characterization of effluent organic matter in an alternating aeration activated sludge system combining ozone/ultrasound pretreatment. Bioresour. Technol. 2015, 177, 194–203. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, M.; Zhang, K.F.; Wang, N.; Wang, K.K.; Wang, H.B.; Meng, S.J.; Mu, R.M. Effect of ultrasound irradiation combined with ozone pretreatment on the anaerobic digestion for the biosludge exposed to trace-level levofloxacin: Degradation, microbial community and ARGs analysis. J. Environ. Manag. 2020, 262, 9. [Google Scholar] [CrossRef]
- Li, P.; Peng, Y.; Mei, J.; Xie, J. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage. LWT 2020, 118, 108831. [Google Scholar] [CrossRef]
- Chen, X.J.; Tang, R.; Wang, Y.L.; Yuan, S.J.; Wang, W.; Ali, I.M.; Hu, Z.H. Effect of ultrasonic and ozone pretreatment on the fate of enteric indicator bacteria and antibiotic resistance genes, and anaerobic digestion of dairy wastewater. Bioresour. Technol. 2021, 320, 9. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça Silva, A.M.; Gonçalves, A.A. Effect of aqueous ozone on microbial and physicochemical quality of Nile tilapia processing. J. Food Process. Preserv. 2017, 41, e13298. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Investigation of quality attributes of ice-stored Pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT-Food Sci. Technol. 2014, 57, 538–547. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.; Jiao, C.; Qiao, Y.; Wu, W.; Li, X.; Wang, J.; Ding, A.; Liao, L.; Xiong, G. Effect of Ultrasound Combined with Ozone Water Pretreatment on the Bacterial Communities and the Physicochemical Properties of Red Swamp Crayfish Meat (Procambarus clarkii). Food Bioprocess Technol. 2020, 13, 1778–1790. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Lira Santos, T.C. Improving quality and shelf-life of whole chilled Pacific white shrimp (Litopenaeus vannamei) by ozone technology combined with modified atmosphere packaging. LWT 2019, 99, 568–575. [Google Scholar] [CrossRef]
- Cravero, F.; Englezos, V.; Rantsiou, K.; Torchio, F.; Giacosa, S.; Segade, S.R.; Gerbi, V.; Rolle, L.; Cocolin, L. Ozone treatments of post harvested wine grapes: Impact on fermentative yeasts and wine chemical properties. Food Res. Int. 2016, 87, 134–141. [Google Scholar] [CrossRef]
- Guzzon, R.; Carafa, I.; Tuohy, K.; Cervantes, G.; Vernetti, L.; Barmaz, A.; Larcher, R.; Franciosi, E. Exploring the microbiota of the red-brown defect in smear-ripened cheese by 454-pyrosequencing and its prevention using different cleaning systems. Food Microbiol. 2017, 62, 160–168. [Google Scholar] [CrossRef]
- Botta, C.; Ferrocino, I.; Pessione, A.; Cocolin, L.; Rantsiou, K. Spatiotemporal Distribution of the Environmental Microbiota in Food Processing Plants as Impacted by Cleaning and Sanitizing Procedures: The Case of Slaughterhouses and Gaseous Ozone. Appl. Environ. Microbiol. 2020, 86, e01861-20. [Google Scholar] [CrossRef]
- Moore, G.; Griffith, C.; Peters, A. Bactericidal properties of ozone and its potential application as a terminal disinfectant. J. Food Prot. 2000, 63, 1100–1106. [Google Scholar] [CrossRef]
- Gertzou, I.N.; Drosos, P.E.; Karabagias, I.K.; Riganakos, K.A. Combined effect of ozonation and packaging on shelf life extension of fresh chicken legs during storage under refrigeration. J. Food Sci. Technol.-Mysore 2016, 53, 4270–4277. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Q.; Zhang, T.; Chen, J.; Wang, S.; Hao, J.; Lin, Y.; Li, A. Association of the microbiota dysbiosis in the hepatopancreas of farmed crayfish (Procambarus clarkii) with disease outbreaks. Aquaculture 2021, 536, 736492. [Google Scholar] [CrossRef]
- Zeng, S.; Khoruamkid, S.; Kongpakdee, W.; Wei, D.; Yu, L.; Wang, H.; Deng, Z.; Weng, S.; Huang, Z.; He, J.; et al. Dissimilarity of microbial diversity of pond water, shrimp intestine and sediment in Aquamimicry system. AMB Express 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Wei, D.; Deng, X.; Weng, S.; Yan, Q.; He, J. Intestinal bacterial signatures of white feces syndrome in shrimp. Appl. Microbiol. Biotechnol. 2018, 102, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.; Soares, M.C.; Silva, S.M.; Banha, F.; Gama, M.; Ribeiro, L.; Anastácio, P.; Cardoso, S.C. Environment and host-related factors modulate gut and carapace bacterial diversity of the invasive red swamp crayfish (Procambarus clarkii). Hydrobiologia 2021, 848, 4045–4057. [Google Scholar] [CrossRef]
- Orlić, K.; Šver, L.; Burić, L.; Kazazić, S.; Grbin, D.; Maguire, I.; Pavić, D.; Hrašćan, R.; Vladušić, T.; Hudina, S.; et al. Cuticle-associated bacteria can inhibit crayfish pathogen Aphanomyces astaci: Opening the perspective of biocontrol in astaciculture. Aquaculture 2021, 533, 736112. [Google Scholar] [CrossRef]
- Bassey, A.P.; Chen, Y.; Zhu, Z.; Odeyemi, O.A.; Frimpong, E.B.; Ye, K.; Li, C.; Zhou, G. Assessment of quality characteristics and bacterial community of modified atmosphere packaged chilled pork loins using 16S rRNA amplicon sequencing analysis. Food Res. Int. 2021, 145, 110412. [Google Scholar] [CrossRef]
- Jiang, L.; Li, M.; Tang, J.; Zhao, X.; Zhang, J.; Zhu, H.; Yu, X.; Li, Y.; Feng, T.; Zhang, X. Effect of Different Disinfectants on Bacterial Aerosol Diversity in Poultry Houses. Front. Microbiol. 2018, 9, 2113. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wang, J. Pretreatment of antibiotic fermentation residues by combined ultrasound and alkali for enhancing biohydrogen production. J. Clean Prod. 2020, 268, 122190. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: Energy conversion and microbial community. Energy Convers. Manag. 2020, 225, 113436. [Google Scholar] [CrossRef]
- Lin, Y.; Li, D.; Zeng, S.; He, M. Changes of microbial composition during wastewater reclamation and distribution systems revealed by high-throughput sequencing analyses. Front. Environ. Sci. Eng. 2016, 10, 539–547. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, Q.; Zeng, W.; Liu, G.; Shao, H. The effect of antibiotic cocktails on host immune status is dynamic and does not always correspond to changes in gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 4995–5009. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Tao, D.; Wang, S.; Li, C.; Zheng, F.; Wu, Z. Reduction of Escherichia coli O157:H7, Listeria monocytogenes, and Naturally Present Microbe Counts on Lettuce using an Acid Mixture of Acetic and Lactic Acid. Microorganisms 2019, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.M.M.; Palacios, D.A.B.; de Sa, P.L.; Miyakawa, W.; Damiao, A.J.; Fernandes, A.B.; de Lima, C.J. Preliminary Study: Comparative Analysis of the Effects of Ozone and Ultrasound onStreptococcus Mutans. Ozone Sci. Eng. 2021, 43, 263–275. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Sunoj, S.; Manikantan, M.R.; Kothakota, A.; Hebbar, K.B. Application and Kinetics of Ozone in Food Preservation. Ozone Sci. Eng. 2016, 39, 115–126. [Google Scholar] [CrossRef]
- Walker, N.; Burke, F.J.T.; Palenik, C.J. Comparison of ultrasonic cleaning schemes: A pilot study. Prim. Dent. Care J. Fac. Gen. Dent. Pract. 2006, 13, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Esua, O.J.; Cheng, J.H.; Sun, D.W. Novel technique for treating grass carp (Ctenopharyngodon idella) by combining plasma functionalized liquids and Ultrasound: Effects on bacterial inactivation and quality attributes. Ultrason. Sonochem. 2021, 76, 14. [Google Scholar] [CrossRef]
- Taiye Mustapha, A.; Zhou, C.; Wahia, H.; Amanor-Atiemoh, R.; Otu, P.; Qudus, A.; Abiola Fakayode, O.; Ma, H. Sonozonation: Enhancing the antimicrobial efficiency of aqueous ozone washing techniques on cherry tomato. Ultrason. Sonochem. 2020, 64, 105059. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S.V. Ozone as a novel emerging technology for the dissipation of pesticide residues in foods–a review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Tachikawa, M.; Yamanaka, K.; Nakamuro, K. Studies on the Disinfection and Removal of Biofilms by Ozone Water Using an Artificial Microbial Biofilm System. Ozone Sci. Eng. 2009, 31, 3–9. [Google Scholar] [CrossRef]
- Topić Popović, N.; Sauerborn Klobučar, R.; Maguire, I.; Strunjak-Perović, I.; Kazazić, S.; Barišić, J.; Jadan, M.; Klobučar, G.; Čož-Rakovac, R. High-throughput discrimination of bacteria isolated fromAstacus astacusandA. leptodactylus. Knowl. Manag. Aquat. Ecosyst. 2014, 413, 4–16. [Google Scholar] [CrossRef][Green Version]
- Zhang, C. Effect of low power ultrasound on enhancing water-holding capacity of tilapia fillets. Food Sci. Technol. 2019, 44, 161–166. [Google Scholar] [CrossRef]
- Zhao, D.; Hu, J.; Chen, W. Analysis of the relationship between microorganisms and flavour development in dry-cured grass carp by high-throughput sequencing, volatile flavour analysis and metabolomics. Food Chem. 2022, 368, 130889. [Google Scholar] [CrossRef] [PubMed]
- Wenzheng, S.; Ying, L. Recent advances on deodorization technology of fishy odors. Food Ferment. Ind. 2021, 47, 282–287. [Google Scholar] [CrossRef]

| Samples | Shannon | Simpson | Ace | Chao |
|---|---|---|---|---|
| CK | 4.46 ± 0.22 a | 0.03 ± 0.01 a | 1230.25 ± 133.13 a | 1235.84 ± 141.03 a |
| OW | 4.09 ± 0.10 ab | 0.05 ± 0.01 ab | 996.00 ± 126.95 bc | 980.03 ± 121.72 bc |
| UL | 4.20 ± 0.34 ab | 0.06 ± 0.03 ab | 1190.31 ± 128.74 ab | 1173.43 ± 123.24 ab |
| OCU | 3.91 ± 0.26 b | 0.08 ± 0.03 b | 943.25 ± 91.79 c | 930.18 ± 90.89 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Tan, H.; Shen, L.; Wei, L.; Xiong, G.; Wang, L.; Wu, W.; Qiao, Y. Microbial Evaluation of Ozone Water Combined with Ultrasound Cleaning on Crayfish (Procambarus clarkii). Foods 2022, 11, 2314. https://doi.org/10.3390/foods11152314
Ling Y, Tan H, Shen L, Wei L, Xiong G, Wang L, Wu W, Qiao Y. Microbial Evaluation of Ozone Water Combined with Ultrasound Cleaning on Crayfish (Procambarus clarkii). Foods. 2022; 11(15):2314. https://doi.org/10.3390/foods11152314
Chicago/Turabian StyleLing, Yuzhao, Hongyuan Tan, Lingwei Shen, Lingyun Wei, Guangquan Xiong, Lan Wang, Wenjin Wu, and Yu Qiao. 2022. "Microbial Evaluation of Ozone Water Combined with Ultrasound Cleaning on Crayfish (Procambarus clarkii)" Foods 11, no. 15: 2314. https://doi.org/10.3390/foods11152314
APA StyleLing, Y., Tan, H., Shen, L., Wei, L., Xiong, G., Wang, L., Wu, W., & Qiao, Y. (2022). Microbial Evaluation of Ozone Water Combined with Ultrasound Cleaning on Crayfish (Procambarus clarkii). Foods, 11(15), 2314. https://doi.org/10.3390/foods11152314





