Increasing the Efficiency of Foundry Production by Changing the Technology of Pretreatment with Quartzite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation of the Problem
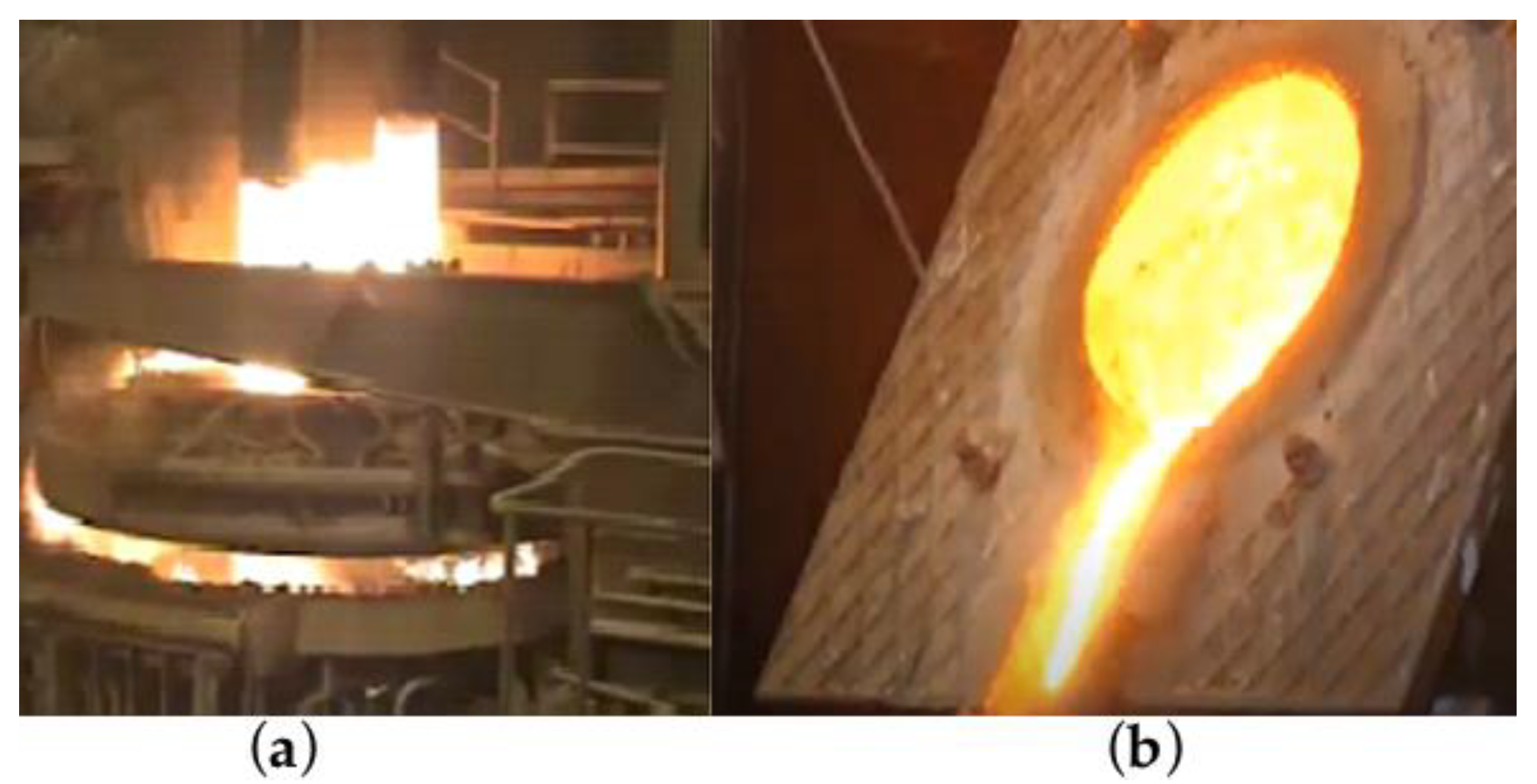
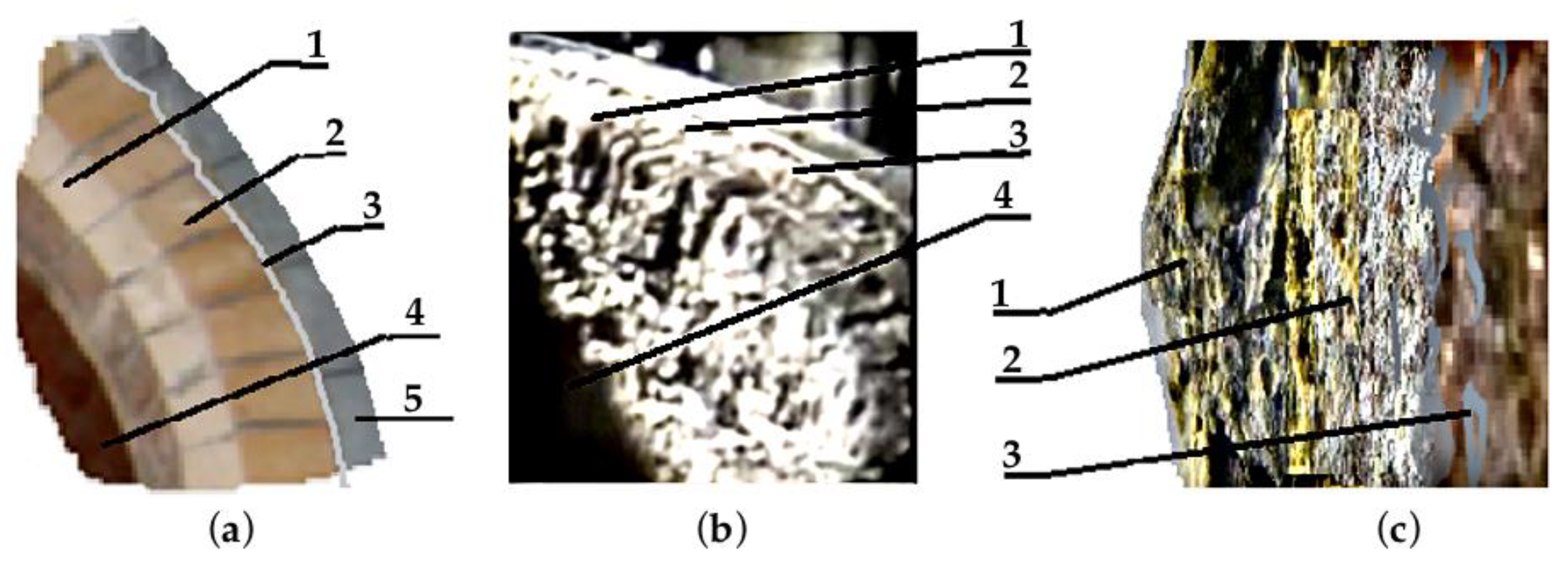
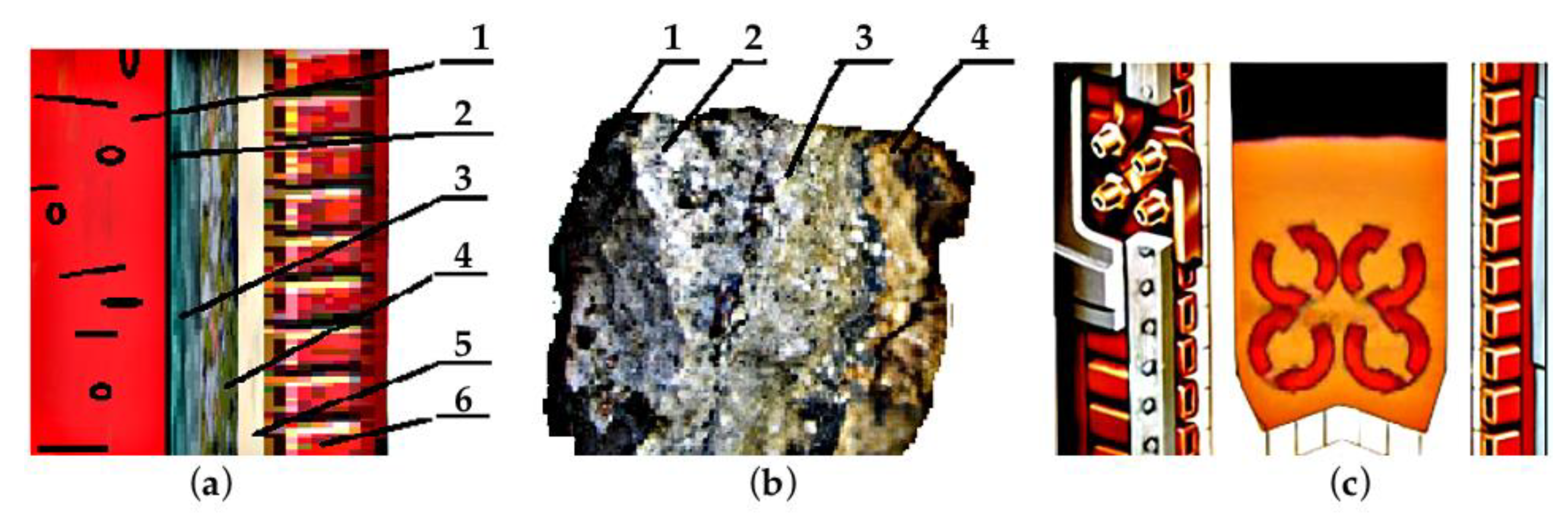
2.2. Description of the Field Experiment
- Remaining on grid No. 2, including—8–14;
- Remaining on the grid No. 3.2, no more—5;
- Passage through grid No. 05, including—46–51;
- Passage through grid No. 01—27–32.
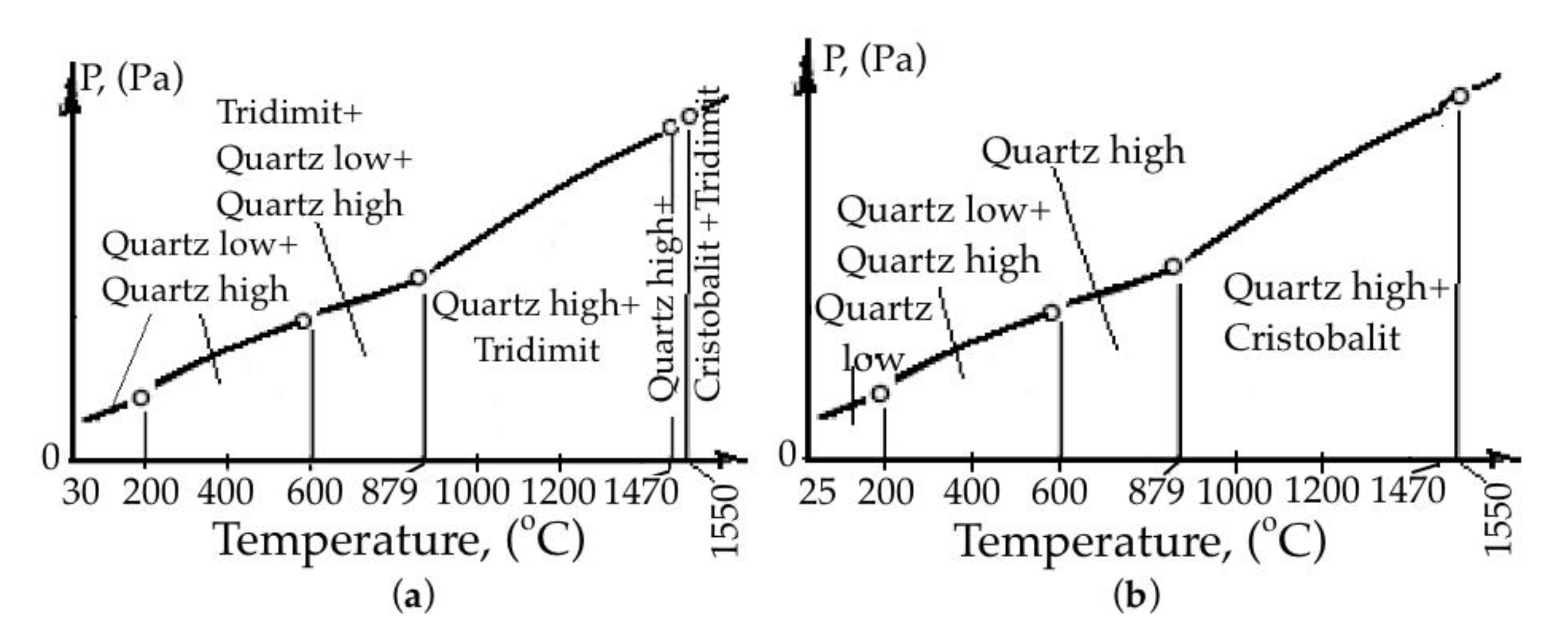
- We heated a portion of raw quartzite to a temperature of 800 °C, allowed exposure for 1 h and cooling to room temperature, and then subjected it to subsequent heating with exposure at each point to remove the card and determine the parameters of the crystal lattice, at 200, 400, 600, 870, 1000, 1200, 1470, 1550 °C;
- We heated the next portion of raw quartzite to a temperature of 200 °C, allowed exposure for 1 h and cooling to room temperature, and then determined its chemical composition and subjected it to subsequent heating with exposure to remove the card and determine the parameters of the crystal lattice, at 200, 400, 600, 870, 1000, 1200, 1470, 1550 °C.
- 200 °C—approximate value of the free moisture removal temperature;
- 400 °C—the appearance of cells in the structure of quartzite with a lower molecular weight (M = 59.2 g/mol), established in [53];
- 600 °C—an intense phase transformation occurs with the release of heat, which was established in [54];
- 1025 °C—furnace drain temperature;
- 1470–1550 °C—melting mode;
- 1550–1570 °C—carrying out the operation of alloying and modification.
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kukartsev, V.V.; Antamoshkin, O.A. Combined Method of Decision-Making on the Reproduction of Fixed Production Assets. Probl. Mech. Eng. Autom. 2011, 2, 56–60. [Google Scholar]
- Adetunji, O.; Ojo, S.S.; Oyetunji, A.; Itua, N.; Adetunji, O.; Ojo, S.S.; Oyetunji, A.; Itua, N. Melting Time Prediction Model for Induction Furnace Melting Using Specific Thermal Consumption from Material Charge Approach. J. Miner. Mater. Charact. Eng. 2020, 9, 61–74. [Google Scholar] [CrossRef]
- Vatalis, K.I.; Charalambides, G.; Benetis, N.P. Market of High Purity Quartz Innovative Applications. Procedia Econ. Financ. 2015, 24, 734–742. [Google Scholar] [CrossRef] [Green Version]
- Nagurney, A.B.; Caddick, M.J.; Law, R.D.; Ross, N.L.; Kruckenberg, S.C. Crystallographically Controlled Void Space at Grain Boundaries in the Harkless Quartzite. J. Struct. Geol. 2021, 143, 104235. [Google Scholar] [CrossRef]
- Benmore, C.J. A Review of High-Energy X-ray Diffraction from Glasses and Liquids. Int. Sch. Res. Netw. 2012, 2012, 852905. [Google Scholar] [CrossRef]
- Cartwright, J.H.E.; García-Ruiz, J.M.; Piro, O.; Sainz-Díaz, C.I.; Tuval, I. Chiral Symmetry Breaking during Crystallization: An Advection-Mediated Nonlinear Autocatalytic Process. Phys. Rev. Lett. 2004, 93, 035502. [Google Scholar] [CrossRef] [Green Version]
- Zavertkin, A.S. Development of the Composition of the Lining of Induction Furnaces from Pervouralsk Quartzite and the Practice of Its Application. Proc. Kola Sci. Cent. Russ. Acad. Sci. 2015, 44, 532–534. [Google Scholar]
- Grinchuk, P.S. Mathematical Modeling of Thermal Modes of Operation of Electric Resistance Furnaces. Eng. Phys. J. 2010, 83, 28–37. [Google Scholar]
- Sassa, V.S. Lining of Induction Melting Furnaces and Mixers; Energoatomizdat: Moscow, Russia, 1983. [Google Scholar]
- Kostyukova, A.P. Institutional Method as the Basis for Monitoring the Induction Melting Furnace. Adv. Mod. Sci. 2017, 4, 19–23. [Google Scholar]
- Kukartsev, V.A. Application of Pervouralsky Quartzite in Acid Lining for Induction Iron-Smelting Furnaces. Liteyshchik Russ. 2012, 2, 35–37. [Google Scholar]
- Kihara, K. An X-ray Study of the Temperature Dependence of the Quartz Structure. Eur. J. Miner. 1990, 2, 63–78. [Google Scholar] [CrossRef]
- Skamnitskaya, L.; Bubnova, T. Mineralogi-Technological Evaluation of Girvas Occurrence Vein Quartz (Karelia, Russia). Int. Multidiscip. Sci. Geoconf. Surv. Geol. Min. Ecol. Manag. SGEM 2019, 19, 877–882. [Google Scholar] [CrossRef]
- Mazukabzov, A.M.; Fedorov, A.M.; Nepomnyashchikh, A.I. Structure of the Bural-Sardyk Superquartzite Deposit (Eastern Sayan). Geodyn. Tectonophys. 2020, 11, 244–261. [Google Scholar] [CrossRef]
- Shakirov, K.M.; Sokolov, G.S.; Nelyub, V.A. Research of Transversal Properties of Winding Basalt Plastics Based on Basalt Fiber with Experimental Lubricants. J. Phys. Conf. Ser. 2021, 1990, 012044. [Google Scholar] [CrossRef]
- Orlov, M.A.; Nelyub, V.A.; Kalinnikov, A.N.; Borodulin, A.S. Modeling Basalt Fibers Wetting Processes Used in the Basalt Rebar Production. J. Phys. Conf. Ser. 2021, 1990, 012042. [Google Scholar] [CrossRef]
- Ananiev, Y.S.; Ananyeva, L.G.; Dolgov, I.V.; Korobeinikov, A.F.; Korovkin, M.V. Search, Evaluation and Enrichment of Quartz Raw Materials for High Technologies. Bull. Tomsk Polytech. Univ. Georesour. Eng. 2001, 304, 123–130. [Google Scholar]
- Sokolov, Y.D.; Baranov, A.N. Silicon Is the Main Element in the Development of Solar Energy. Actual Probl. Humanit. Nat. Sci. 2016, 1, 21–22. [Google Scholar]
- Naumova, O.V.; Chesnokov, B.P.; Mavzovin, V.M.; Sheshukova, M.D.; Tronin, B.A. Obtaining Silicon for Solar Cells from Mineral Raw Materials. Tech. Regul. Transp. Constr. 2020, 4, 320–332. [Google Scholar]
- Morton, O. Solar Energy: A New Day Dawning? Nature 2006, 443, 19–23. [Google Scholar] [CrossRef]
- Nelyub, V.A. The Effect of Copper and Zinc Coatings on the Properties of Carbon Fibers and Composites Based on Them. Polym. Sci. Ser. D 2021, 14, 260–264. [Google Scholar] [CrossRef]
- Nelyub, V.A.; Fedorov, S.Y.; Malysheva, G.V. The Study of the Structure and Properties of Elementary Carbon Fibers with Metal Coatings. Inorg. Mater. Appl. Res. 2021, 12, 1037–1041. [Google Scholar] [CrossRef]
- Nelyub, V.; Fedorov, S.; Klimovich, Y. Investigation of Properties of Elementary Carbon Fibers with Different Technologies for Preparing Their Surfaces before Metallization. J. Phys. Conf. Ser. 2021, 1990, 012078. [Google Scholar] [CrossRef]
- Ignatova, A.M.; Shekhireva, A.M. Comparative Petrography of Natural Materials and Synthetic Mineral Alloys of Stone Casting. Bull. Perm Univ. Geol. 2011, 4, 20–31. [Google Scholar]
- Lapin, V.V. Petrography of Metallurgical and Fuel Slags; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1956. [Google Scholar]
- Zosin, A.P.; Priymak, T.I.; Koshkina, L.B.; Masloboev, V.A. Synthesis and Application of Adsorption-Active Materials Based on Magnesian-Ferrous Slags of Non-Ferrous Metallurgy for the Purification of Process Wastewater from Non-Ferrous Metal Cations. Ecol. Ind. Prod. 2007, 1, 70–74. [Google Scholar]
- Platonov, B.P.; Akimenko, A.D.; Bagutskaya, S.M. Induction Furnaces for Cast Iron Melting; Mashinostroyeniye: Moscow, Russia, 1976. [Google Scholar]
- Satr, P. New Refractory Technology for Melting Cast Iron Alloys and Alloy Steel in Induction Furnaces. Liteyshchik Russ. 2019, 1, 11–15. [Google Scholar]
- Hanagiri, S.; Matsui, T.; Shimpo, A.; Aso, S.; Inuzuka, T.; Matsuda, T.; Sakaki, S.; Nakagawa, H. Recent Improvement of Recycling Technology for Refractories. Shinnittetsu Giho 2008, 388, 93–98. [Google Scholar]
- Kukartsev, V.A.; Kukartsev, V.V.; Kukartsev, A.V. Effect of the Temperature Treatment of Quartzite on the Lining Resistance of Commercial-Frequency Induction Crucible Furnaces. Refract. Ind. Ceram. 2018, 59, 252–256. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Ringdalen, E. Changes in Quartz During Heating and the Possible Effects on Si Production. JOM 2015, 67, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Pryanishnikov, V.P. Silica System; Stroyizdat: Leningrad, Russia, 1971; Volume 238. [Google Scholar]
- Novakovic, R.; Radic, S.M.; Ristic, M.M. Kinetics and Mechanism of Quartz-Tridymite Transformation. Nterceram Interceram 1986, 35, 29–30. [Google Scholar]
- Breneman, R.C.; Halloran, J.W. Kinetics of Cristobalite Formation in Sintered Silica. J. Am. Ceram. Soc. 2014, 97, 2272–2278. [Google Scholar] [CrossRef] [Green Version]
- Shchiptsov, V.V.; Perepelitsyn, V.A.; Grishenkov, E.E.; Enenko, V.P.; Zavertkin, A.S. Pervoural’skii and Karel’skii Quartzites for the Lining of Crucible-Type Induction Furnaces. Refract. Ind. Ceram. 2003, 44, 67–74. [Google Scholar] [CrossRef]
- Nikiforova, E.M.; Eromasov, R.G.; Simonova, N.S.; Vasilyeva, M.N.; Taskin, V.Y. Phase Transformations in the Siliceous Rocks-Mineralizer System. Mod. Probl. Sci. Educ. 2012, 1, 218. [Google Scholar]
- Li, W.; Xu, C.; Xie, A.; Chen, K.; Yang, Y.; Liu, L.; Zhu, S. Microstructure Study of Phase Transformation of Quartz in Potassium Silicate Glass at 900 °C and 1000 °C. Crystals 2021, 11, 1481. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Tynchenko, V.V.; Sergienko, R.B.; Bashmur, K.A.; Lysyannikov, A.V. The Technology of Using Liquid Glass Mixture Waste for Reducing the Harmful Environmental Impact. Materials 2022, 15, 1220. [Google Scholar] [CrossRef] [PubMed]
- Nelyub, V.A.; Fedorov, S.Y.; Malysheva, G.V.; Berlin, A.A. Properties of Carbon Fibers after Applying Metal Coatings on Them by Magnetron Sputtering Technology. Fibre Chem. 2021, 53, 252–257. [Google Scholar] [CrossRef]
- Wahl, F.M.; Grim, R.E.; Graf, R.B. Phase Transformations in Silica as Examined by Continuous X-ray Diffraction|American Mineralogist|GeoScienceWorld. Am. Miner. J. Earth Planet. Mater. 1961, 46, 196–208. [Google Scholar]
- Benarieb, I.; Duyunova, V.A.; Oglodkov, M.S.; Puchkov, Y.A.; Pakhomkin, S.I. Quench Factor Analysis for Predicting Precipitation Hardening of Sheets from Aluminum Alloy V-1341 of the Al–Mg–Si System. Met. Sci. Heat Treat. 2022, 63, 583–589. [Google Scholar] [CrossRef]
- Voronin, A.S.; Fadeev, Y.V.; Makeev, M.O.; Mikhalev, P.A.; Osipkov, A.S.; Provatorov, A.S.; Ryzhenko, D.S.; Yurkov, G.Y.; Simunin, M.M.; Karpova, D.V.; et al. Low Cost Embedded Copper Mesh Based on Cracked Template for Highly Durability Transparent EMI Shielding Films. Materials 2022, 15, 1449. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Cherepanov, A.I.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Popov, A.M.; Sergienko, R.B.; Tynchenko, S.V. X-ray Diffraction Phase Analysis of Changes in the Lattice of Pervouralsk Quartzite upon Heating. Minerals 2022, 12, 233. [Google Scholar] [CrossRef]
- Zavertkin, A.S. Effect of Quartzite Heat Treatment on Induction Furnace Lining Failure Mechanism. Refract. Ind. Ceram. 2019, 60, 67–70. [Google Scholar] [CrossRef]
- He, J.; Jusnes, K.F.; Tangstad, M. Phase Transformation in Quartz at Elevated Temperatures. Asp. Min. Miner. Sci. 2021, 6, 691–699. [Google Scholar] [CrossRef]
- Skamnitskaya, L.S.; Svetova, E.N.; Shanina, S.N. The Effect of Fluid Inclusions on the Vein Quartz Grade. Obogashchenie Rud 2019, 2019, 20–26. [Google Scholar] [CrossRef]
- Zavertkin, A.S. Development of a Lining Made from Karelian Quartzites for Crucible Induction Furnaces. Refract. Ind. Ceram. 2010, 50, 400–405. [Google Scholar] [CrossRef]
- Rajan, H.; Uchida, H.; Bryan, D.L.; Swaminathan, R.; Downs, R.T.; Hall-Wallace, M. Building the American Mineralogist Crystal Structure Database: A Recipe for Construction of a Small Internet Database. Geoinform. Data Knowl. Geol. Soc. Am. Spec. Pap. 2006, 397, 73–80. [Google Scholar] [CrossRef]
- Kosenko, N.F.; Smirnova, M.A. Mechanostimulated Polymorphic Transitions of Quartz. Refract. Tech. Ceram. 2012, 7, 7–13. [Google Scholar]
- Druzhevsky, M.A.; Subdue, B. Lining of Induction Melting Furnaces with Materials Based on Quartzite. Liteynoye Proizv. 2010, 4, 33–38. [Google Scholar]
- Riposan, I.; Chisamera, M.; Stan, S. Enhanced Quality in Electric Melt Grey Cast Irons. ISIJ Int. 2013, 53, 1683–1695. [Google Scholar] [CrossRef] [Green Version]
- Kukartsev, V.A.; Abkaryan, A.K.; Temnykh, V.I.; Kukartsev, V.V.; Tynchenko, V.S.; Kukartsev, A.V. The Changes of Pervouralsky Quartzite Crystal Lattice under Heating from up 25 to 600 °C. Novyye Ogneup. 2021, 1, 34–39. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Trunova, A.I.; Kukartsev, A.V. Thermal Analysis of Quartzite Used to Line a Crucible-Equipped Industrial-Frequency Induction Furnace. Refract. Ind. Ceram. 2014, 55, 220–222. [Google Scholar] [CrossRef]

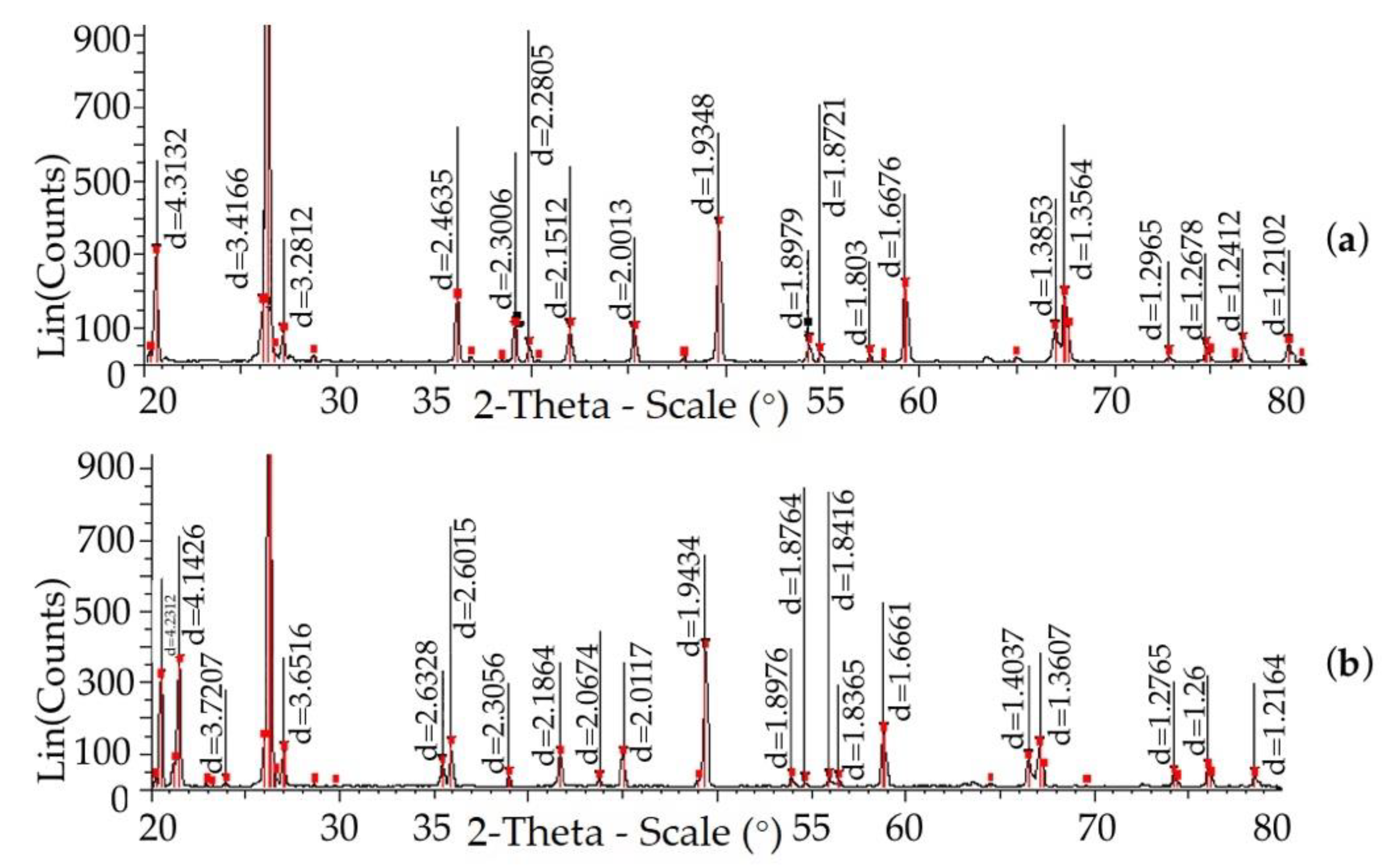
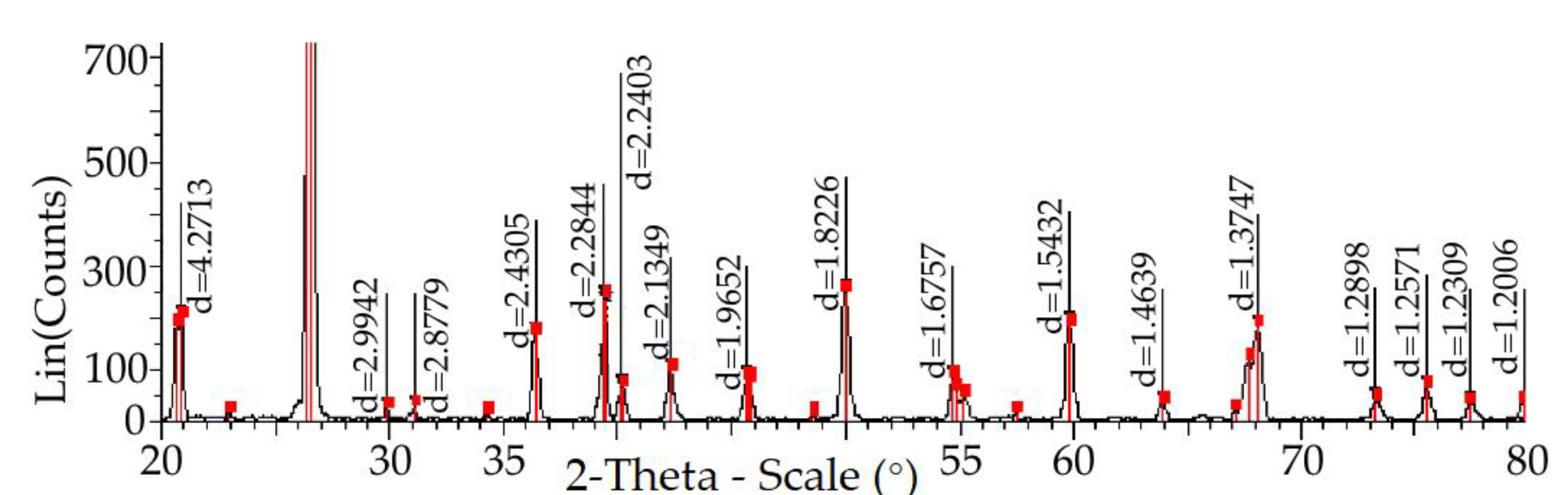
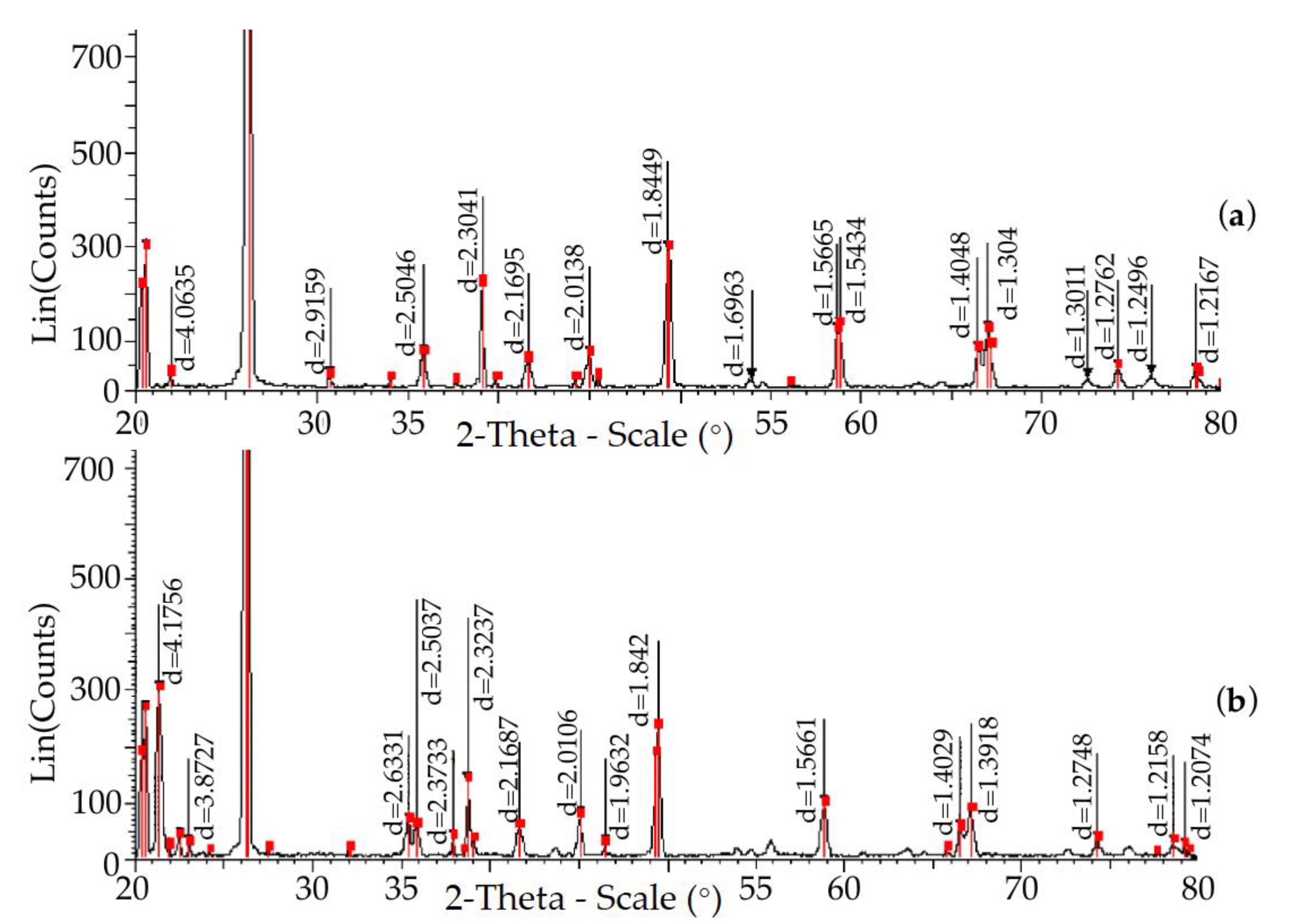
| Field | Content, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | TiO2 | Fe2O3 | P2O5 | MnO | CaO | Na2O | K2O | |
| Bobrovskoe | 97 | 1.1 | 0.6 | 0.05 | 0.06 | 0.6 | 0.01 | - | 0.16 | - | 0.021 |
| Pervouralskoe | 99 | 0.5 | 0.01 | 0.02 | 0.09 | 0.15 | 0.015 | 0.1 | 0.2 | 0.06 | 0.06 |
| Chemical Composition of PKMVI-1 Quartzite | Content, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | TiO2 | Fe2O3 | P2O5 | MnO | CaO | Na2O | K2O | |
| According to the TS | 97.5 | 1.1 | - | - | - | 0.6 | - | - | - | - | - |
| Spectrometer dried at 200 °C | 96.74 | 0.862 | 0.097 | 0.027 | 0.275 | 1.02 | 0.015 | 0.038 | 0.098 | 0.12 | 0.584 |
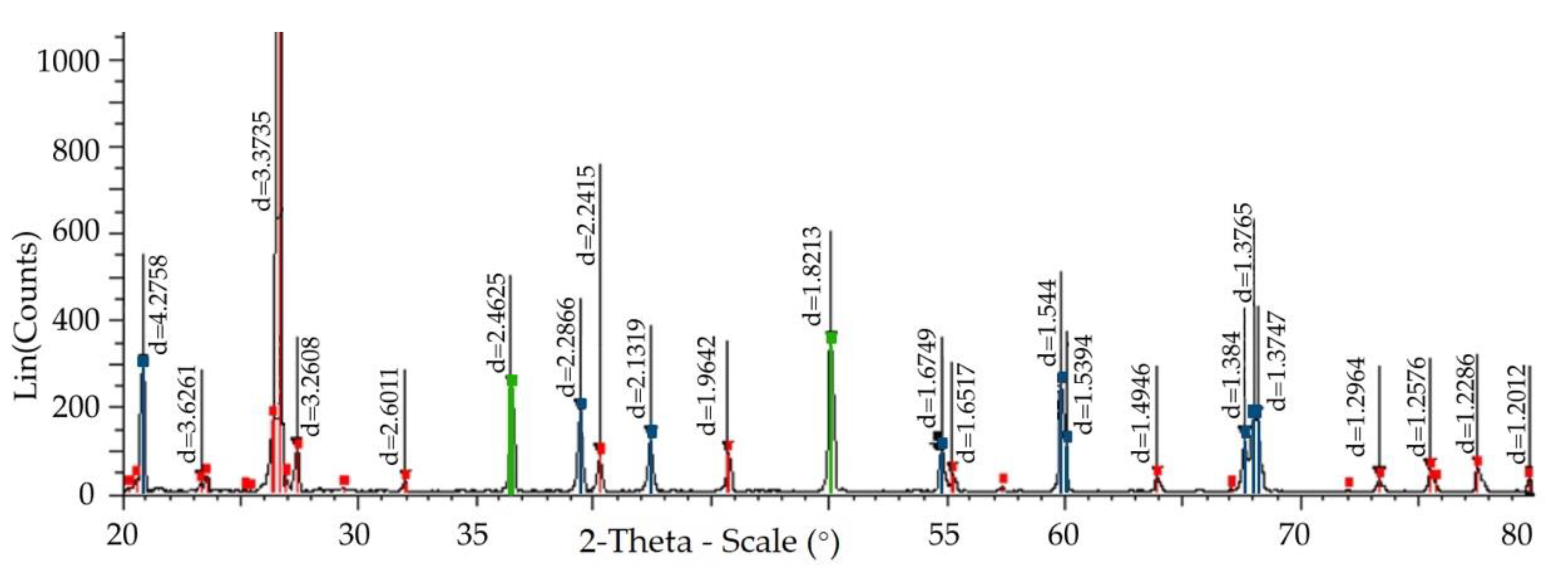
| Indicators | Patent: 00-012-0708 SiO2 Silicon Oxid Quartz Hexagonal P3221 (154) | Patent: 01-071-0911 SiO2 Silicon Oxid Quartz Hexagonal P6222 (180) | Patent: 00-005-0490 SiO2 Silicon Oxid Quartz Hexagonal P3121 (152) | Patent: 01-083-2187 SiO2 Silicon Oxid Quartz Hexagonal P3221 (152) | Patent: 01-070-7344 SiO2 Silicon Oxid Quartz Hexagonal P3221 (154) | Patent: 02-002-0278 SiO2 Silicon Oxid Cristobalite Cubic Fd-3m (227) | Patent: 01-011-0695 SiO2 Silicon Oxid Tetragonal P41212 (92) | Patent: 01-085-0621 SiO2 Silicon Oxid Cristobalite Cubic P213 (198) | Patent: 01-071-0032 SiO2 Silicon Oxid Tridymite Monoclinic Cc (9) | Patent: 00-018-1170 SiO2 Silicon Oxid Tridymite Monoclinic Cc (9) |
|---|---|---|---|---|---|---|---|---|---|---|
| a, (Å) | 4.994 | 5 | 4.913 | 4.965 | 4.915 | 7.12 | 4.971 | 7.16 | 18.494 | 18.504 |
| b, (Å) | 5.438 | 5.49 | 5.405 | 5.424 | 5.406 | - | - | - | 4.991 | 5.006 |
| Mol. weight, (g/mol) | 60.08 | 52.87 | 60.08 | 60.08 | 60.08 | 60.08 | 60.08 | 60.08 | 60.08 | 60.08 |
| Volume, (Å3) | 117.45 | 118.86 | 112.98 | 115.79 | 113.09 | 360.94 | 170.95 | 367.06 | 2110.15 | 2215.08 |
| Dx, (g/cm3) | 2.548 | 2.216 | 2.649 | 2.58 | 2.647 | 2.211 | 2.335 | 2.174 | 2.270 | 2.254 |
| c, (Å) | - | - | - | - | - | - | - | - | 26.832 | 23.845 |
| Temperature, (°C) | The Content of Elementary Cells, %, 1/2 Stage of the Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 00-012-0708 | 01-071-0911 | 01-083-2187 | 01-070-7344 | 01-071-0032 | 00-018-1170 | 01-085-0621 | 00-005-0490 | 01-011-0695 | 02-002-0278 | |
| 25 (initial) | 58 | 42 | ||||||||
| 800/200 | 10/0 | 90/0 | 0/67 | 0/23 | 0/10 | |||||
| 30/25 | 0/6 | 65/69 | 35/10 | 0/15 | ||||||
| 200 | 0/4 | 86/74 | 14/15 | 0/7 | ||||||
| 400 | 0/8 | 100/92 | ||||||||
| 600 | 61/0 | 0/68 | 20/32 | 19/0 | ||||||
| 870 | 24/0 | 0/87 | 76/0 | 0/13 | ||||||
| 1000 | 20/0 | 0/89 | 80/0 | 0/11 | ||||||
| 1200 | 19/0 | 0/89 | 81/0 | 0/11 | ||||||
| 1470 | 18/0 | 0/89 | 82/0 | 0/11 | ||||||
| 1550 | 18/0 | 0/79 | 73/0 | 9/0 | 0/9 | 0/12 | ||||
| Parameter Gratings | Temperature, °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 Raw | 800 | 30 Dry | 200 | 400 | 600 | 870 | 1000 | 1200 | 1470 | 1550 | |
| davg, Å (change, %) | 2.676 | 3.012 | 2.757 | 2.901 | 2.946 | 3.008 | 3.055 | 3.105 | 3.126 | 3.156 | 3.216 |
| - | 12.56 | 3.04 | 8.42 | 10.09 | 12.39 | 14.14 | 16.02 | 16.83 | 17.93 | 20.16 | |
| Vavg, Å3 (change, %) | 115.4 | 129.85 | 114.83 | 115.41 | 115.79 | 647.47 | 1653.02 | 1722.83 | 1742.69 | 1767.17 | 1606.96 |
| - | 12.52 | −0.49 | 0.0 | 0.34 | 561.06 | 1432.42 | 1492.92 | 1510.13 | 1531.34 | 1392.51 | |
| Davg, g/cm3 (change, %) | 2.59 | 2.249 | 2.601 | 2.592 | 2.585 | 2.502 | 2.269 | 2.266 | 2.265 | 2.265 | 2.258 |
| - | −13.17 | +0.42 | +0.08 | −0.19 | −3.4 | −12.41 | −12.51 | −12.54 | −12.54 | −12.81 | |
| Mavg, g/mol (change, %) | 60.08 | 51.01 | 60.08 | 60.08 | 60.08 | 60.08 | 58.38 | 58.63 | 58.7 | 58.79 | 58.79 |
| - | - | - | - | - | - | −2.83 | −2.41 | −2.3 | −2.15 | −2.15 | |
| Parameter Gratings | Temperature, (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 Raw | 200 | 25 Dry | 200 | 400 | 600 | 870 | 1000 | 1200 | 1470 | 1550 | |
| davg, Å (change, %) | 2.676 | 2.908 | 2.814 | 2.834 | 2.892 | 2.791 | 2.928 | 2.98 | 2.992 | 3.038 | 3.262 |
| - | 8.67 | 5.16 | 5.9 | 8.08 | 4.31 | 9.41 | 11.34 | 11.79 | 13.54 | 21.99 | |
| Vavg, Å3 (change, %) | 115.4 | 116.35 | 119.1 | 116.55 | 115.97 | 117.47 | 125.86 | 124.06 | 124.19 | 124.04 | 143.65 |
| - | 0.82 | 3.2 | 0.99 | 0.49 | 1.79 | 9.06 | 7.5 | 7.62 | 7.49 | 24.48 | |
| Davg, g/cm3 (change, %) | 2.590 | 2.552 | 2.597 | 2.552 | 2.558 | 2.333 | 2.292 | 2.291 | 2.229 | 2.229 | 2.227 |
| - | −1.5 | +0.27 | −1.5 | −1.24 | −9.93 | −11.51 | −11.54 | −13.93 | −13.93 | −14.01 | |
| Mavg, g/mol (change, %) | 60.08 | 60.08 | 60.08 | 60.08 | 59.54 | 55.16 | 53.91 | 53.66 | 53.67 | 53.66 | 54.41 |
| - | - | - | - | −0.9 | −8.19 | −10.27 | −10.59 | −10.67 | −10.69 | −9.44 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukartsev, V.A.; Cherepanov, A.I.; Kukartsev, V.V.; Tynchenko, V.S.; Kurashkin, S.O.; Sergienko, R.B.; Tynchenko, V.V.; Bashmur, K.A. Increasing the Efficiency of Foundry Production by Changing the Technology of Pretreatment with Quartzite. Metals 2022, 12, 1266. https://doi.org/10.3390/met12081266
Kukartsev VA, Cherepanov AI, Kukartsev VV, Tynchenko VS, Kurashkin SO, Sergienko RB, Tynchenko VV, Bashmur KA. Increasing the Efficiency of Foundry Production by Changing the Technology of Pretreatment with Quartzite. Metals. 2022; 12(8):1266. https://doi.org/10.3390/met12081266
Chicago/Turabian StyleKukartsev, Viktor Alekseevich, Aleksandr Ivanovich Cherepanov, Vladislav Viktorovich Kukartsev, Vadim Sergeevich Tynchenko, Sergei Olegovich Kurashkin, Roman Borisovich Sergienko, Valeriya Valerievna Tynchenko, and Kirill Aleksandrovich Bashmur. 2022. "Increasing the Efficiency of Foundry Production by Changing the Technology of Pretreatment with Quartzite" Metals 12, no. 8: 1266. https://doi.org/10.3390/met12081266
APA StyleKukartsev, V. A., Cherepanov, A. I., Kukartsev, V. V., Tynchenko, V. S., Kurashkin, S. O., Sergienko, R. B., Tynchenko, V. V., & Bashmur, K. A. (2022). Increasing the Efficiency of Foundry Production by Changing the Technology of Pretreatment with Quartzite. Metals, 12(8), 1266. https://doi.org/10.3390/met12081266







