Abstract
Adropin is a novel 76-amino acid-peptide that is expressed in different tissues and cells including the liver, pancreas, heart and vascular tissues, kidney, milk, serum, plasma and many parts of the brain. Adropin, encoded by the Enho gene, plays a crucial role in energy homeostasis. The literature review indicates that adropin alleviates the degree of insulin resistance by reducing endogenous hepatic glucose production. Adropin improves glucose metabolism by enhancing glucose utilization in mice, including the sensitization of insulin signaling pathways such as Akt phosphorylation and the activation of the glucose transporter 4 receptor. Several studies have also demonstrated that adropin improves cardiac function, cardiac efficiency and coronary blood flow in mice. Adropin can also reduce the levels of serum triglycerides, total cholesterol and low-density lipoprotein cholesterol. In contrast, it increases the level of high-density lipoprotein cholesterol, often referred to as the beneficial cholesterol. Adropin inhibits inflammation by reducing the tissue level of pro-inflammatory cytokines such as tumor necrosis factor alpha and interleukin-6. The protective effect of adropin on the vascular endothelium is through an increase in the expression of endothelial nitric oxide synthase. This article provides an overview of the existing literature about the role of adropin in different pathological conditions.
1. Introduction
Peptides are short linear chains of amino acids that are often stabilized by disulfide bonds. Peptides can be synthesized and used for different purposes and the sequence can also be modulated using various chemical and biological techniques [1,2,3,4,5,6,7,8]. There are therapeutic peptides, which are used to fight certain diseases such as cancer and diabetes mellitus and its complications [9,10,11].
Therapeutic peptides have several important advantages when compared to proteins and antibodies. They are smaller in size, easy to synthesize and have the ability to penetrate cell membranes. They also have high activity, specificity and affinity. An added benefit of using peptides as a therapeutic agent is that they do not accumulate in specific organs such as the kidney or liver, which can help to minimize their toxic side effects [12]. Therapeutic peptides show great potential in the treatment of many diseases [9,10,11,13]. One of these promising peptides is adropin. Adropin is a short peptide that consists of 76 amino acids. It was discovered by Kumar and colleagues in 2008. They demonstrated its role in glucose and lipid metabolism and energy homeostasis [14]. This review examines adropin peptide from different aspects, starting from the discovery and its biochemical structure to tissue localization. It also outlines the results of human and animal studies on the effects of adropin on physiological and biochemical parameters.
1.1. Discovery of Adropin
Adropin was discovered by Kumar et al. in 2008 [14], while studying the hypothalamic regulation of liver metabolism, and through conducting a microarray analysis of total gene expression in the liver of C57BL/6J mice that were deficient in melanocortin-3 receptor (Mc3r −/−). Kumar and his team distinguished a novel transcript in the liver. This transcript encoded a short and highly conserved amino acid sequence (76 aa), which was downregulated in the obese mice. Further investigation revealed that the coding gene was associated with energy homeostasis and lipid metabolism, so it was named the Energy Homeostasis Associated (Enho) gene. The name “adropin” was derived from the initials of Latin names “aduro”, which means “to set fire to”, and “pinquis”, which means “fats” [14].
Adropin level has been shown to be related to nutrient intake. For example, the investigation of Kumar et al. showed that lean C57BL/6J mice that were fed a high-fat diet (HFD) expressed higher adropin in the liver, compared to the control group, while fasting lean C57BL/6J mice showed a diminished adropin level. Interestingly, another experiment in which diet-induced obesity (DIO) mice were used demonstrated that the level of liver Enho gene expression was reduced, compared to that of lean mice, explaining that the long-term intake of a high-fat diet disrupts the normal activity of adropin. This finding linked the Enho gene to metabolic disorders, such as obesity [14]. Further investigations showed that the blood level of adropin was markedly reduced in obese individuals when compared to those of normal subjects [15,16].
It has been reported that adropin is a secreted, as well as membrane-bound peptide. Previous reports show that adropin is secreted by HEK293 cells and C57BL/6J mice [14]. Other reports have indeed shown that adropin is secreted by brain tissue [16], while other investigators showed that the liver is a strong producer of adropin [17]. In contrast to these reports, there are reports that show that adropin is secreted by many types of tissues in the human body [18].
In contrast, a study that was conducted by Wong et al. showed that adropin was a membrane-bound peptide [19]. Initially, a bioinformatic analysis was used to make a prediction of the transmembrane topology of adropin. It was suggested that amino acids 1–9 of the N-terminal are cytoplasmic, while amino acids 9–30 are transmembrane-bound, and amino acids 30–76 are localized outside of the surface of the plasma membrane. Afterward, an immunohistochemical analysis showed that anti-adropin antibody co-localized with pan-cadherin antibody (membrane marker) using HeLa cells that were transfected with plasmid-expressing adropin. Moreover, it was demonstrated that adropin is expressed on the plasma membrane of C57BL/6N mice brain tissue and HEK293 cells. Despite all of these initial observations regarding the nature and location of adropin, most reports now point to the fact that adropin is indeed a secreted protein.
1.2. Enho Gene and Adropin Peptide Characteristics
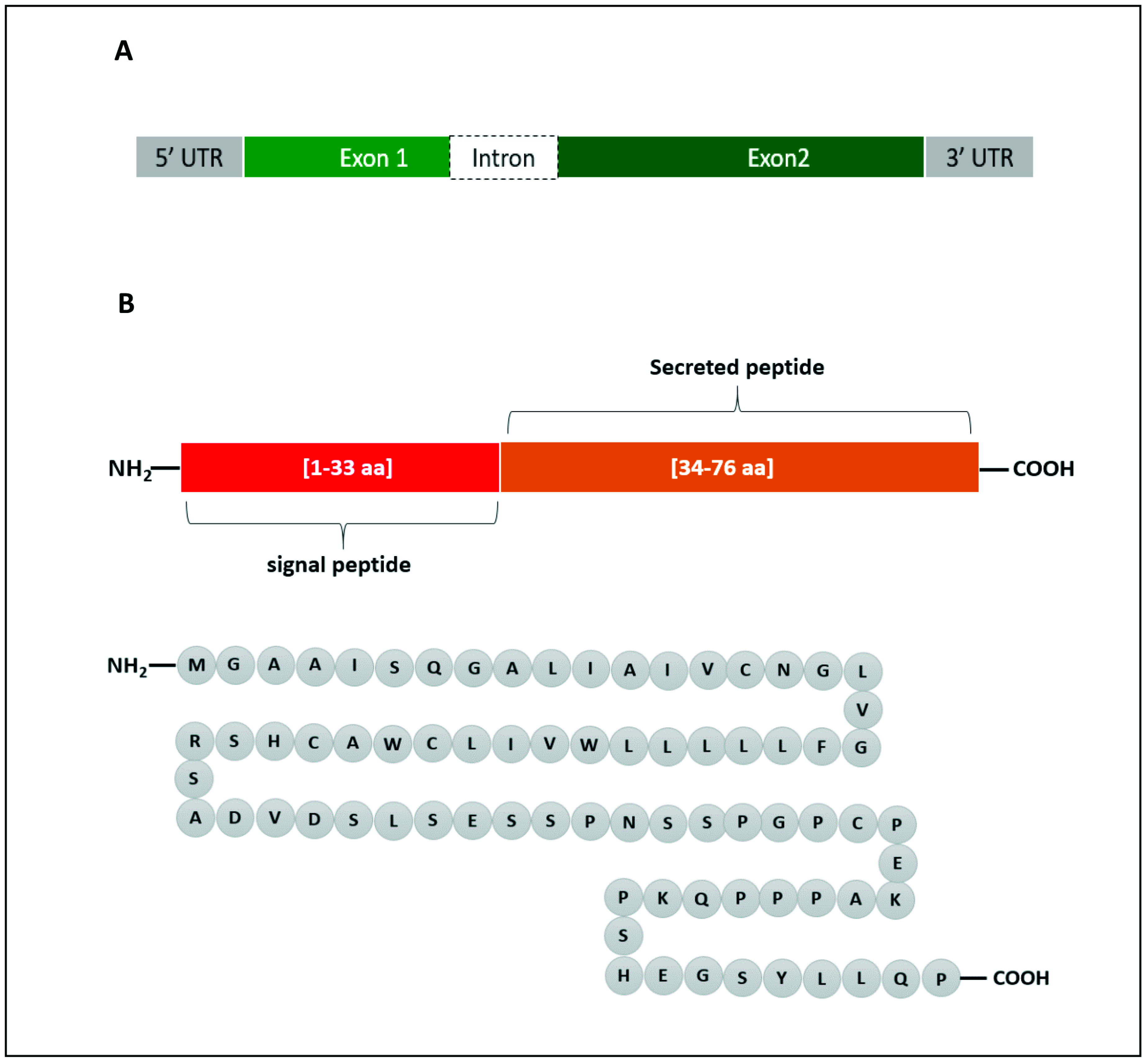
In human, the Enho gene is in chromosome number 9 (9p13.3), position 34,521,043–34,522,990 and it has a size of 1948 bp. It is composed of two exons and one intron (Figure 1A). Adropin, which is encoded by the Enho gene, is 76 amino acids in length (MGAAISQGALIAIVCNGLVGFLLLLLWVILCWACHSRSADVDSLSESSPNSSPGPCPEKAPPPQKPSHEGSYLLQP) and has a molecular weight of 4.499 KDa (Figure 1B). The amino acid sequence is 100% conserved in human, mouse and rat. Adropin1−33 (amino acids 1–33) is a secretory signal peptide [14], and adropin34−76 (amino acids 34–76) is biologically active when it is administered to rats [20].
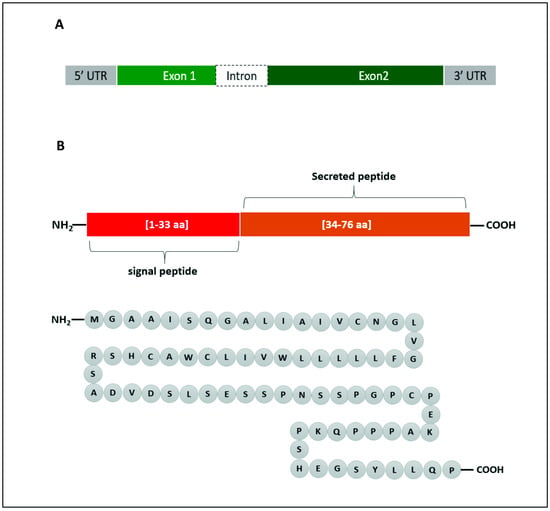
Figure 1.
Graphical representation of Enho gene (A) and the encoded adropin (B). The gene consists of 2 exons and 1 intron. Adropin has a secretory sequence and a bioactive sequence. The full peptide is made up of 76 amino acids (aa).
The literature has not stated a specific receptor upon which adropin exerts its biological activities. In the brain, Stein et al. showed that adropin acts on the orphan G protein-coupled receptor (GPR19) to inhibit water deprivation in rats [21], while in the liver, Kumar et al. demonstrated that Enho gene expression is regulated by Liver X receptor (LXR) [14]. LXR is a nuclear receptor, which also acts as a lipid and glucose sensor [22].
It was demonstrated that adropin stimulated angiogenesis, proliferation and migration of the human umbilical vein and coronary artery endothelial cells. It was also proposed that this novel peptide may exert its activities on vascular endothelial growth factor receptor-2 (VEGFR2) in endothelial cells [23].
2. Tissue Expression of Adropin
The early discovery of adropin demonstrated the expression of the peptide in the liver [14] and brain [19]; thereafter, the tissue distribution of adropin has been shown in various tissues and cells [18]. In the liver, the expression was detected in sinusoidal cells, while in the central nervous system (CNS), immunoreactivity of the peptide was present in the vascular area, pia matter, neuroglial cells, Purkinje cells, granular layer and neurons of the central nervous system of rat [24].
In the pancreas, adropin was detected in acinar cells [24] and in the capillaries of the islets of Langerhans [25] using immunohistochemical technique. Adropin immunoreactivity was observed in the capillaries of the renal glomeruli, peritubular interstitial and peritubular regions [26].
In the circulatory system, Lovren et al. showed that adropin was expressed in endothelial cells—specifically, in cultured human umbilical vein and coronary artery endothelial cells (ECs) [23]. Moreover, this study suggested a vascular effect for adropin through endothelial cells by enhancing capillary-like tube formation and exhibiting greater cell proliferation and migration. Furthermore, a histological analysis displayed the localization of adropin in the endocardium, myocardium and epicardium of rat heart [24]. Several other studies [27,28,29,30,31,32,33] have indeed linked endothelial function to adropin, thus confirming the probable presence in endothelial cells.
Furthermore, mRNA expression of the Enho gene has been detected in the lung tissue of C57BL/6J mice [34]. Interestingly, the same study reported genetic variations in the Enho gene after analyzing myeloperoxidase (MPO) and anti-neutrophil cytoplasm autoantibody (ANCA) in patients with vasculitis and healthy individuals [34].
It was demonstrated that biological fluids such as milk, serum, plasma and cheese whey milk-derived fluid of cow diary contain numerous amino acids and proteins including adropin, nesfatin-1, apelin-12, ghrelin and salusin peptides [35]. Additionally, human serum adropin was measured and correlated well with distinct diseases in several studies, especially coronary artery disease [35,36,37,38,39].
3. Adropin and Energy Homeostasis
The biological process of regulating energy inflow (food intake) and energy expenditure through biosynthetic reactions is identified as energy homeostasis. To maintain energy homeostasis, the amount of energy intake must be at equilibrium with the amount of energy that is expended. As the body intakes nutritional molecules such as carbohydrates, lipids and proteins, which are considered fuel, the required energy is utilized by the body and the excess is stored in a form of adipose tissue and held constant over a period of time [40].
The gastrointestinal tract (GI tract), pancreas and liver are known to provide hormonal signals to specific nuclei of the central nervous system that regulate the energy that is consumed and the energy that is utilized. These hormones include ghrelin; gastric leptin; secretin; glucagon-like peptide 1, secreted by the stomach and intestines; and insulin and glucagon, released from the endocrine pancreas. Adipose tissue is also a major source of energy-related signals—specifically, signals that reflect lipogenesis, storage and lipolysis. These signals send feedback to the region of the central nervous system that controls energy balance [41].
The brain, specifically the medial and lateral nuclei of the hypothalamus, plays a major role in energy homeostasis, with the help of hormonal, as well as nutritional signals. Food intake is controlled by hunger and regulated by the hypothalamus. There are several models related to food intake and energy homeostasis. The lipostatic model says that adipose tissue produces signals such as leptin, which is proportionate to the fat mass and acts on the hypothalamus to decrease food intake and increase energy output, while the GI tract-peptide model, hypothesizes that when the food enters the gastrointestinal tract, the release of cholecystokinin and glucagon hormone are induced. These hormones have receptors in the hypothalamus, which produce the feeling of fullness. Another model of energy balancing and food intake involves glucose utilization in brain neurons [40].
During feeding and fasting cycles, carbohydrates and fatty acids are the two primary substrates in oxidative metabolism to keep energy balanced. Several studies have reported the role of the hormonal peptide, adropin, in regulating substrate oxidation preferences. Initially, when adropin was discovered, its biological role was linked to glucose homeostasis and lipid metabolism [14]. Kumar et al., however, showed that liver Enho expression is regulated by the leptin and melanocortin receptor, as obese leptin knockout mice (Lepob/Lepob) and melanocortin 3 receptor knockout mice (Mc3r−/−) exhibited downregulation in Enho expression [14]. Melanocortin 3 receptor (Mc3r) is a member of the melanocortin receptors family, highly expressed in brain, as well as other tissues such as liver. The Mc3 receptor is involved in energy homeostasis, and the absence of the Mc3r gene causes increased adiposity in tissues [42]. Leptin is encoded by the ob gene and is secreted into the blood circulation by adipose cells. It also acts on hypothalamic receptors to inhibit feeding and initiates increased thermogenesis. It is an important regulator of energy homeostasis [43]. Another significant finding that was reported by Kumar et al., while investigating how nutritional status affects liver Enho mRNA expression, was that a high-fat diet increased Enho mRNA expression in lean C57BL/6J mice. However, introducing a high-fat diet to diet-induced obesity (DIO) C57BL/6J mice in a period of 3 months was associated with reduced liver Enho mRNA expression, compared to lean controls. This suggests that a chronic obese state caused metabolic disorder, which consequently disturbed adropin expression.
Additionally, adropin metabolism is not only associated with lipid, but also with carbohydrate metabolism and energy status. It is worth mentioning that adropin-overexpressing transgenic C57BL/6J mice that were fed a high-fat diet showed improvement in glucose homeostasis, as well as delayed development of obesity. Normally, a high-fat diet leads to obesity and disruption in glucose homeostasis [14,44].
In another study, elevated fasting glucose level in DIO mice was controlled and regulated by administrating an intraperitoneal injection of adropin. It was found that endogenous hepatic glucose production was reduced in adropin-treated obese mice [16,18,45,46,47,48].
4. The Role of Adropin in Health and Disease
The levels of adropin in blood circulation have been proposed to direct the metabolic state in skeletal muscle by influencing fuel selection preference towards glucose oxidation in the fed state [20]. Studies have shown that adropin regulates the expression of hepatic lipogenic genes and the PPARγ receptor (peroxisome proliferator-activated receptor gamma), the major regulator of lipogenesis [14]. Moreover, adropin regulates angiogenesis, increases blood flow, boosts capillary density and has a protective role for endothelial cells [23]. Apparently, the tissue level of adropin varies in several physiological and biological conditions such as multiple sclerosis [49], COVID-19 [50], gestational diabetes [51] obstructive sleep apnea [52], rheumatoid arthritis [53], coronary artery ectasia [54], acute mesenteric ischemia [55] and diabetic nephropathy [56].
4.1. Diabetes Mellitus
When adropin was first discovered, most of the attention was given to its role in lipid and carbohydrate metabolism and insulin resistance. Interestingly, some studies show that adropin deficiency plays a role in the development and progression of chronic diseases, such as diabetes mellitus. Zang et al. reported that serum concentrations of adropin were significantly decreased in Chinese type 2 diabetic patients, compared to control subjects [57]. Other studies confirmed this finding by reporting the downregulation in circulating adropin in adults with type 2 diabetes mellitus [58], liver disease [59] and children with type 1 diabetes mellitus [60]. In contrast, it was elucidated that higher insulin resistance and higher fasting plasma glucose positively correlated with serum adropin levels in patients with type 2 diabetes mellitus [61].
Additionally, low adropin levels have been shown to correlate with a risk of developing diabetic complications such as diabetic retinopathy [62], diabetic nephropathy [63] and gestational diabetes mellitus [64].
As it was reported that serum adropin levels vary between diabetic and normal subjects, several investigations tried to understand the mechanisms underlying these variations. For instance, it was demonstrated that hyperglycemia was associated with increased adropin expression, as well as the signal transducer and activator of transcription 3 (STAT3) activation in the liver of streptozotocin-induced diabetic rats. The mechanism underlying the elevation of adropin levels and Enho gene expression in the diabetic rats was suggested to be through STAT3 activation [65].
During the discovery of adropin, its physiological role was linked to glucose homeostasis. It is thus important to understand the potential role of adropin in controlling hyperglycemia and its effect on insulin-sensitive tissues.
In skeletal muscle, a study that was performed by Gao et al. showed that adropin played a crucial role in modulating glucose utilization in DIO mice with insulin resistance [20]. Adropin was able to promote glucose oxidation and diminish fatty acid oxidation in skeletal muscle, and that led to an increase in glucose uptake and enhanced mitochondrial function. The metabolic actions for enhancing mitochondrial function were mediated by suppressing the activity of peroxisome proliferator-activated receptor gamma coactivator-1a (PGC-1a), a transcription co-activator that regulates the expression of the genes that are involved in fatty acid oxidation. Moreover, adropin promoted skeletal muscle sensitization to insulin signaling actions by increasing insulin-induced Akt phosphorylation and the cell surface expression of glucose transporter 4 (GLUT4) [20].
In contrast, a study utilizing insulin-resistant hepatocytes showed that adropin could reduce glucose production in the liver. Adropin treatment downregulated the transcription of hepatic gluconeogenesis genes by inhibiting the binding site of transcription factors forkhead box protein O1(FoxO1) and cAMP-response element binding protein (CREB), along with their co-activators, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) and CREB regulated transcription coactivator 2 (CRTC2), respectively, to the promoter of gluconeogenesis genes [47,66]. FoxO1-PGC1α and CREB-CRTC2 complex promoter binding is required to activate the transcription of genes that are involved in hepatic glucose production [67,68].
Interestingly, this effect of adropin was not observed in insulin-sensitive hepatocytes [66]. This research team earlier reported that a downregulation in adropin expression led to systemic insulin resistance in mice that were introduced to a high-fat diet for a long period [16].
Furthermore, it was reported that adropin was associated with incretins in obese males with type 2 diabetes receiving a 3-month treatment of liraglutide [69]. There was an increase in plasma adropin levels in those subjects. Liraglutide is an anti-diabetic agent, a specific glucagon-like peptide-1 receptor (GLP-1) agonist, also known as incretin mimetic molecule. Incretins are endogenous peptide hormones that are secreted by the GI tract to stimulate insulin secretion from pancreatic β-cells after meals [70,71,72,73].
It is worth mentioning that irisin, which is a peptide hormone involved in glucose homeostasis [5,74], has a similar effect on incretins, specifically GLP-1. Both adropin and irisin can enhance glucose-stimulated insulin secretion [75]. In addition, the peptide apelin increased plasma GLP-1 levels in rats that were intraperitoneally injected with apelin-13 [76]. However, the role of adropin in inducing incretin secretion and augmenting incretin effect remains unclear and needs more study to elucidate the mechanism by which it regulates these GI tract hormones Table 1.

Table 1.
Effect of adropin on metabolic parameters.
4.2. Obesity
Obesity is a major health problem worldwide [44,81]. Studies performed on humans and animal models suggest that adropin may play a role in lipid metabolism and obesity.
C57BL/6J mice that were fed a high-fat diet exhibited a rapid increase in Enho gene expression, while fasting reduced the expression of this gene, when compared to the control mice. However, liver Enho gene expression declined when a high-fat diet was introduced to the mice for a longer period of time, suggesting a regulatory role of the Enho gene in nutrition, but the expression of adropin is diet-dependent [14]. Moreover, Kumar et al. generated an adropin knockout mice, which exhibited increased adiposity [82].
In humans, adropin level was negatively correlated with body mass index (BMI) [83] in diabetic patients, as overweight and obese patients had considerably reduced levels of adropin, compared to lean patients [57]. Moreover, when adropin levels were measured in plasma samples that were obtained from healthy subjects, it was found that the peptide levels correlated negatively with BMI and aging [84]. This observation was supported by studying patients who underwent bariatric surgery and monitoring serum adropin levels before and after surgery [85]. Serum adropin levels were higher 6 months after bariatric surgery than at baseline, leading to the conclusion that, in some patients, body mass reduction may restore the impaired production of adropin. Another study was conducted to investigate the role of adropin in children with obesity or metabolic syndrome [86]. The results of this study showed that—there was no significant difference between the plasma level of adropin in obese children, and those individuals with normal weight Table 1.
4.3. Cardiovascular Diseases
There is overwhelming evidence that cardiovascular diseases are common in patients suffering from diabetes mellitus [77,87,88,89,90,91,92]. Several studies and reports indicate the involvement of adropin in the functioning of the cardiovascular system. As mentioned previously, the immunoreactivity of adropin has been detected in many tissues, including the three layers of the heart [24].
High cardiac fatty acid oxidation rates and impaired cardiac insulin signaling are associated with decreased cardiac efficiency and various cardiac diseases [93]. Altamimi et al. investigated the effect of adropin on cardiac energy metabolism, insulin signaling and cardiac efficiency [94]. C57Bl/6 mice were injected with a secretable form of adropin (450 nmol/kg, i.p.) three times over 24 h, then they were fasted, and the hearts were isolated and perfused. Altamimi et al. demonstrated that adropin administration improved cardiac function, cardiac efficiency and coronary flow, compared to the untreated mice. Moreover, by measuring glucose and palmitate contribution in catabolic pathways for ATP production, the important role of adropin on the preference of cardiac glucose oxidation and the inhibition of cardiac fatty acid oxidation were reported. In cardiomyocytes, adropin regulates cell bioenergetics through GPR19 activation. The receptor activation leads to stimulation of p44/42 phosphorylation and, consequently, the downregulation of pyruvate dehydrogenase kinase 4 (PDK4) and pyruvate dehydrogenase (PDH) phosphorylation [95,96,97,98].
Hyperlipidemia is a risk factor that is associated with cardiovascular diseases [99]. Akcilar et al. demonstrated the role of a low dose of adropin in reducing hyperlipidemia in rats that were fed a high-fat diet. A reduction in the levels of serum triglycerides, total cholesterol and low-density lipoprotein cholesterol (LDL-C), as well as an increment in the level of high-density lipoprotein cholesterol (HDL-C) were reported [100]. Additionally, adropin administration reduced the mRNA expression of pro-inflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). This suggests that adropin may have an anti-inflammatory role in the liver and possibly in other organs, including the heart.
Interestingly, a recent meta-analysis found an association between serum adropin and coronary artery disease (CAD) [101]. The study stated that the serum adropin level in patients with CAD was lower than in healthy individuals, indicating that the decrease in adropin concentration might play an important role in the development of CAD. Another cardiovascular disease, which has been correlated with adropin, is atrial fibrillation. Atrial fibrillation is a condition of abnormal heart rhythm [102,103]. Decreased serum adropin concentrations were found in atrial fibrillation patients compared with healthy controls. Patients with chronic atrial fibrillation had a significantly reduced serum adropin concentration compared with control patients. Hence, adropin deficiency may contribute to the development and progression of atrial fibrillation [104] Table 1.
4.4. Inflammation
Researchers and scientists have also investigated the role of adropin in inflammation and related diseases such as atherosclerosis. Atherosclerosis is a chronic inflammatory disease in response to injury of the arterial wall and the formation of plaque [80]. Vascular inflammation stimulates the expression of certain adhesion molecules, such as intercellular adhesion molecule 1, vascular cell adhesion molecule 1 in endothelial cells. These adhesion molecules stimulate monocyte adhesion to endothelial cells and monocyte infiltration into the subendothelial space, causing an accumulation of macrophage foam cells. Chronic inflammation of the cardiovascular system is a common feature of chronic diseases including diabetes mellitus, hyperlipidemia and nosocomial conditions [105,106,107]. Hormones such as adropin and plant-based antioxidants have been used to mitigate the adverse effects of these vascular lesions. Adropin is expressed in human endothelial cells [23], and it has been shown earlier that adropin inhibits tumor necrosis factor α (TNFα). Sato et al. investigated the relationship between TNFα, monocyte adhesion, human endothelial cells, atherosclerosis and adropin. The experiment involved incubating human endothelial cells with adropin and TNFα and assessing the expression of the adhesion molecules that are involved in atherosclerosis. The results showed that an incubation of adropin alone had no significant effect on the mRNA expression of these adhesion molecules, which are usually stimulated by TNFα. However, when adropin and TNFα were both incubated with human endothelial cells, adropin suppressed the TNFα-induced mRNA expression of adhesion molecules, suggesting a role for adropin in the anti-atherosclerosis process by inhibiting endothelial cells’ adhesion molecules via the suppression of TNFα [108].
In the case of obesity, the infiltration of macrophages into adipose tissues causes chronic inflammation. Adipocytes secrete cytokines such as TNFα and MCP-1 that attract macrophages and regulatory T cells, leading to fat inflammation. Adropin regulates the expression of PPAR-γ by activating the AKT pathway, thus inhibiting the differentiation of 3T3-L1 preadipocytes into mature adipocytes and consequently reducing fat accumulation and fat inflammation [109].
In another study, lower adropin plasma levels and increased inflammation markers such as TNFα and interleukin-6 (IL-6) were reported in male patients with moderate and severe obstructive sleep apnea, compared to healthy individuals [110].
Furthermore, in order to investigate how adropin could affect hepatocyte inflammation and injury in nonalcoholic steatohepatitis (NASH), immunohistochemistry using the inflammation markers F4/80, CD45 and MCP-1, and a gene expression analysis for TNFα and IL-6 genes, were performed using liver tissues from adropin knockout C57BL/6J mice and the control wild-type, which were fed a methionine-choline deficient diet [111]. Methionine-choline deficient diet is the classic dietary model for studying NASH, and usually, rodents consuming this diet develop steatohepatitis, necroinflammation, and fibrosis, similar to human NASH [78]. The pathohistological analysis showed a higher signal of F4/80, CD45 and MCP1 and a substantial induction of genes TNFα and IL-6 in adropin knockout mice. These results indicate the presence of elevated inflammatory responses in adropin knockout mice, when compared to that of the wild-type mice [111] (Table 1).
4.5. Cell Proliferation and Differentiation
Far beyond the classical action, adropin can stimulate cell proliferation and differentiation. Lovren et al. showed that adropin has the ability to induce the proliferation and capillary-like tube formation of endothelial cells that stimulate angiogenesis [23]. Adropin upregulated endothelial NO synthase expression through VEGFR2 2-PI3K-Akt and VEGFR2-extracellular signal-regulated kinase pathways to reduce inflammation.
Furthermore, in rat primary preadipocytes and 3T3-L1 cells, preadipocyte proliferation was increased by adropin treatment, while the differentiation of those preadipocytes into mature adipocytes was reduced [79]. The suppression of adipogenic markers and lipid accumulation demonstrate the important role of adropin in the fight against obesity [109]. The same research team reported a similar effect of adropin on primary brown preadipocytes that were isolated from the interscapular region in rat [112].
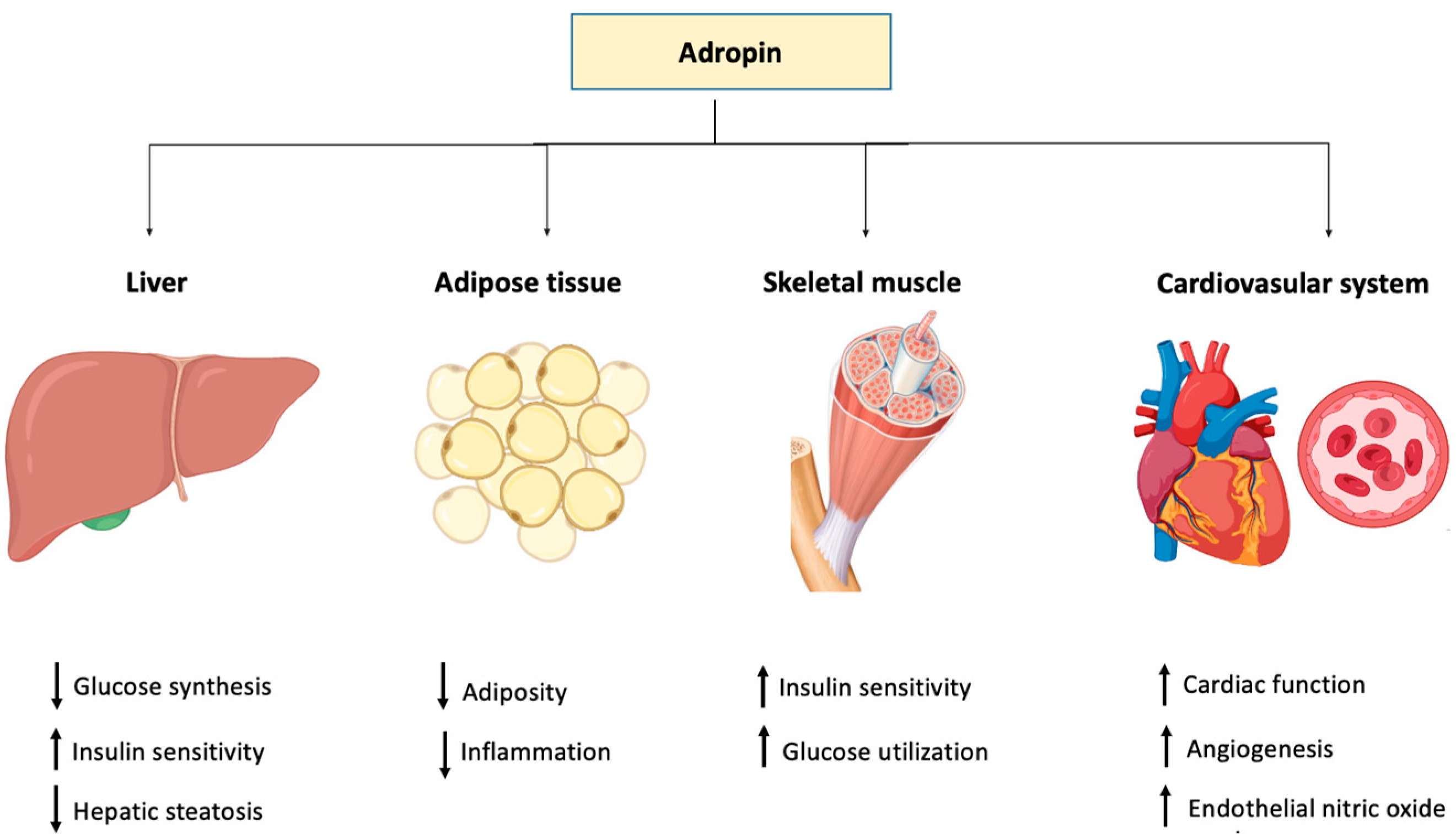
On the other hand, it was reported that adropin downregulated the proliferation and migration of human aortic smooth muscle cells (HASMCs) in vitro, providing evidence that the peptide is also protective against atherosclerosis [108] (Table 1). The role of adropin in different tissues and organ systems is depicted in Figure 2.
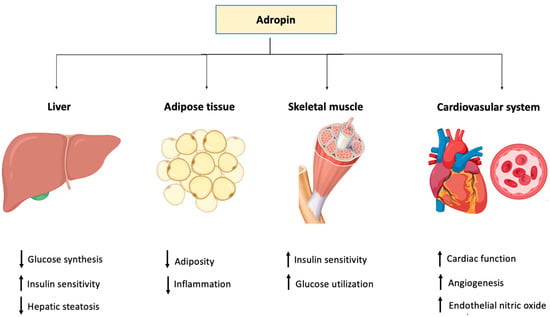
Figure 2.
Graphical representation of the function of adropin in different body tissues.
5. Characteristics of Adropin Knockout Mice
Knockout mice are usually used to study what happens in an organism when a particular gene is absent. Studying knockout mice can provide information about how the knocked-out gene normally functions, including the gene’s biochemical, developmental, physical and behavioral roles.
It was shown that adropin knockout C57BL/6J mice exhibited remarkable insulin resistance, dyslipidemia, failure in the suppression of endogenous glucose production in a hyperinsulinemic condition and increased adiposity in liver [82]. These knockouts were generated by targeting the open reading frame of the Enho gene—specifically in exon 2—and carried out Cre-mediated deletion using Cre-LoxP recombination technology [82].
In another study, adropin knockout C57BL/6J mice were used to investigate the pathogenicity of fatty pancreas [58]. Interestingly, these mice developed fatty pancreas and showed a significant decrease in the amount of regulatory T cells; regulatory T cells are involved in controlling the inflammatory state [113]. Other pathological conditions included an increased severity of obesity-related impaired glucose homeostasis, lipid metabolism disorder and reduced endothelial nitric oxide synthase phosphorylation, as reported in earlier studies. Nitric oxide synthase is an important molecule regulating endothelial function including blood flow and vascular integrity [114,115].
Chen et al., as well as Gao et al., used clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) technology and Enho single-guide RNA, the latest tool in genome editing, to generate adropin knockout C57BL/6J mice [34,58]. They reported a higher susceptibility to developing myeloperoxidase and anti-neutrophil cytoplasm autoantibody (MPO-ANCA)-associated lung injury in adropin knockout C57BL/6J mice [34].
6. Conclusions
In summary, adropin, a 76-amino acid peptide, is membrane-bound and secreted by cells. It is present in the endothelial cells of the capillaries of the brain, liver and kidney. Adropin is involved in several biological activities, and it is regulated by nutrients including lipids and carbohydrates. The administration of adropin can enhance the oxidation of glucose, with a concomitant reduction in fatty acid oxidation in skeletal muscle cells. Adropin has a protective effect in type 2 diabetes mellitus, as it can reduce insulin resistance and prevent the development of obesity by enhancing lipid catabolism. It promotes insulin signaling pathways through Akt phosphorylation and the cell surface expression of GLUT4. Additionally, adropin modulates lipid metabolism by regulating the expression of hepatic lipogenic genes and the PPARγ receptor, the major regulator of lipogenesis. Therefore, adropin-based treatments could emerge as a new line of therapy against glucose and lipid metabolism-related diseases.
It has also been shown that adropin improves coronary blood flow and cardiac function. Adropin promotes cardiac glucose oxidation and the inhibition of cardiac fatty acid oxidation, enhancing cardiac energy metabolism and cardiac efficiency. Moreover, the anti-inflammatory effect of adropin can suppress TNFα expression in atherosclerosis.
Studies showed that Enho gene expression, adropin serum/plasma levels and/or protein expression level in tissue can fluctuate, according to the type of illness. These conditions include but are not limited to diabetes mellitus, obesity, cardiovascular diseases and inflammation. Thus, adropin could also be used as a diagnostic biomarker to detect these clinical conditions, especially metabolic and cardiovascular diseases. However, more research needs to be carried out to understand its mechanism of action in specific tissues and to discover if it acts on a specific receptor, other than GPR19.
Author Contributions
Conceptualization, I.I.A. and E.A.; methodology, I.I.A. and E.A.; formal analysis, I.I.A., C.D., J.S., E.A.; resources, I.I.A., C.D., J.S., E.A.; data curation, I.I.A., E.A.; writing—original draft preparation, I.I.A.; writing—review and editing, I.I.A., C.D., J.S., E.A.; supervision, E.A.; project administration, E.A.; funding acquisition, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
The project was supported by United Arab Emirates University Grant#: G00003451, and Zayed Foundation for Health Sciences, United Arab Emirates University, Al Ain, UAE, Grant#: G00003417.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayashi, M.A.F.; Ducancel, F.; Konno, K. Natural peptides with potential applications in drug development, diagnosis, and/or biotechnology. Int. J. Pept. 2014, 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Elabadlah, H.; Hameed, R.; D’Souza, C.; Mohsin, S.; Adeghate, E.A. Exogenous Ghrelin Increases Plasma Insulin Level in Diabetic Rats. Biomolecules 2020, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Lotfy, M.; D’Souza, C.; Alseiari, S.M.; Alsaadi, A.A.; Qahtan, S.A. Hypocretin/orexin modulates body weight and the metabolism of glucose and insulin. Diabetes/Metab. Res. Rev. 2020, 36, e3229. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Fernandez-Cabezudo, M.; Hameed, R.; El-Hasasna, H.; El Wasila, M.; Abbas, T.; Al-Ramadi, B. Orexin-1 receptor co-localizes with pancreatic hormones in islet cells and modulates the outcome of streptozotocin-induced diabetes mellitus. PLoS ONE 2010, 5, e8587. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.; Baniyas, M.; Adeghate, E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef]
- Lotfy, M.; Singh, J.; Rashed, H.; Tariq, S.; Zilahi, E.; Adeghate, E. Mechanism of the beneficial and protective effects of exenatide in diabetic rats. J. Endocrinol. 2014, 220, 291–304. [Google Scholar] [CrossRef]
- Adeghate, E. Visfatin: Structure, function and relation to diabetes mellitus and other dysfunctions. Curr. Med. Chem. 2008, 15, 1851–1862. [Google Scholar] [CrossRef]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharm. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Adeghate, E.; Mohsin, S.; Adi, F.; Ahmed, F.; Yahya, A.; Kalász, H.; Tekes, K.; Adeghate, E.A. An update of SGLT1 and SGLT2 inhibitors in early phase diabetes-type 2 clinical trials. Expert Opin. Investig. Drugs 2019, 28, 811–820. [Google Scholar] [CrossRef]
- Howarth, F.C.; Jacobson, M.; Shafiullah, M.; Adeghate, E. Effects of insulin treatment on heart rhythm, body temperature and physical activity in streptozotocin-induced diabetic rat. Clin. Exp. Pharmacol. Physiol. 2006, 33, 327–331. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-based Drugs. Chem. Biol. Drug Design. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of Adropin as a Secreted Factor Linking Dietary Macronutrient Intake with Energy Homeostasis and Lipid Metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Kolahdouz-Mohammadi, R.; Aydin, S.; Yosaee, S.; Clark, C.; Abdollahi, S. Circulating levels of adropin and overweight/obesity: A systematic review and meta-analysis of observational studies. Hormones 2022, 21, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Xie, B.; Manning, J.R.; Zhang, M.; Stoner, M.W.; Huckestein, B.R.; Edmunds, L.R.; Zhang, X.; Dedousis, N.L.; O’Doherty, R.M.; et al. Adropin reduces blood glucose levels in mice by limiting hepatic glucose production. Physiol. Rep. 2019, 7, e14043. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Stoner, M.W.; Zhang, M.; Xie, B.; Manning, J.R.; Guimaraes, D.; Shiva, S.; Jurczak, M.J.; Scott, I. Adropin regulates pyruvate dehydrogenase in cardiac cells via a novel GPCR-MAPK-PDK4 signaling pathway. Redox Biol. 2018, 18, 25–32. [Google Scholar] [CrossRef]
- Butler, A.A.; Havel, P.J. Adropin and insulin resistance: Integration of endocrine, circadian, and stress signals regulating glucose metabolism. Obesity 2021, 29, 1799–1801. [Google Scholar] [CrossRef]
- Wong, C.M.; Wang, Y.; Lee, J.T.; Huang, Z.; Wu, D.; Xu, A.; Lam, K.S. Adropin is a brain membrane-bound protein regulating physical activity via the NB-3/notch signaling pathway in mice. J. Biol. Chem. 2014, 289, 25976–25986. [Google Scholar] [CrossRef]
- Gao, S.; McMillan, R.P.; Zhu, Q.; Lopaschuk, G.D.; Hulver, M.W.; Butler, A.A. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 2015, 4, 310–324. [Google Scholar] [CrossRef]
- Stein, L.M.; Yosten, G.L.C.; Samson, W.K. Adropin acts in brain to inhibit water drinking: Potential interaction with the orphan G protein-coupled receptor, GPR19. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R476–R480. [Google Scholar] [CrossRef] [PubMed]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRS AND FXR: The Yin and Yang of Cholesterol and Fat Metabolism. Annu. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin is a novel regulator of endothelial function. Circulation 2010, 11 (Suppl. S1), 185–192. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Kocaman, N.; Citil, C.; Kendir, Y. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol. Cell. Biochem. 2013, 380, 73–81. [Google Scholar] [CrossRef]
- Aydin, S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef]
- Kuloglu, T.; Aydin, S. Immunohistochemical expressions of adropin and inducible nitric oxide synthase in renal tissues of rats with streptozotocin-induced experimental diabetes. Biotech. Histochem. 2014, 89, 104–110. [Google Scholar] [CrossRef]
- Akkaya, H.; Güntürk, E.E.; Akkaya, F.; Karabıyık, U.; Güntürk, İ.; Yılmaz, S. Assessment of the Relatıonshıp between the Adropın Levels and the Coronary Collateral Cırculatıon in Patıents wıth Chronıc Coronary Syndrome. Avaliação da Relação entre Níveis de Adropina e Circulação Colateral Coronária em Pacientes com Síndrome Coronariana Crônica. Arq. Bras. De Cardiol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Dodd, W.S.; Patel, D.; Lucke-Wold, B.; Hosaka, K.; Chalouhi, N.; Hoh, B.L. Adropin decreases endothelial monolayer permeability after cell-free hemoglobin exposure and reduces MCP-1-induced macrophage transmigration. Biochem. Biophys. Res. Commun. 2021, 582, 105–110. [Google Scholar] [CrossRef]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Males, I.; Borovac, J.A.; Martinovic, D.; Vilovic, M. Role of Adropin in Cardiometabolic Disorders: From Pathophysiological Mechanisms to Therapeutic Target. Biomedicines 2021, 9, 1407. [Google Scholar] [CrossRef]
- Li, B.; Li, N.; Guo, S.; Zhang, M.; Li, J.; Zhai, N.; Wang, H.; Zhang, Y. The changing features of serum adropin, copeptin, neprilysin and chitotriosidase which are associated with vascular endothelial function in type 2 diabetic retinopathy patients. J. Diabetes Its Complicat. 2020, 34, 107686. [Google Scholar] [CrossRef]
- Celikhisar, H.; Ilkhan, G.D. Alterations in Serum Adropin, Adiponectin, and Proinflammatory Cytokine Levels in OSAS. Can. Respir. J. 2020, 2020, 2571283. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, Y.; Zou, F.; Xu, T.; Pan, P.; Hu, C.; Su, X. Serum adropin level is associated with endothelial dysfunction in patients with obstructive sleep apnea and hypopnea syndrome. Sleep Breath. 2021, 25, 117–123. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Yuan, X.; Xiong, C.; Chen, L. Adropin reduces hypoxia/reoxygenation-induced myocardial injury via the reperfusion injury salvage kinase pathway. Exp. Ther. Med. 2019, 18, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Fang, J.; Chen, F.; Wang, C.; Chen, S.; Zhang, S.; Lv, X.; Zhang, J.; He, Q.; Weng, S.; et al. Enho Mutations Causing Low Adropin: A Possible Pathomechanism of MPO-ANCA Associated Lung Injury. EBioMedicine 2016, 9, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. Presence of adropin, nesfatin-1, apelin-12, ghrelins and salusins peptides in the milk, cheese whey and plasma of dairy cows. Peptides 2013, 43, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; You, T.; Chan, S.P.; Chen, J.C.; Xu, W.T. Adropin is associated with hyperhomocysteine and coronary atherosclerosis. Exp. Ther. Med. 2016, 11, 1065–1070. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Ren, Y.G.; Liu, J. Low Serum Adropin Levels are Associated with Coronary Slow Flow Phenomenon. Acta Cardiol. Sin. 2018, 34, 307–312. [Google Scholar] [CrossRef]
- Celik, H.T.; Bilen, M.; Kazancı, F.; Yildirim, M.E.; İncebay, İ.B.; Erdamar, H. Serum adropin as a predictive biomarker of erectile dysfunction in coronary artery disease patients. Cent. Eur. J. Urol. 2019, 72, 302–306. [Google Scholar] [CrossRef]
- Yang, M.; Pei, Q.; Zhang, J.; Weng, H.; Jing, F.; Yi, Q. Association between adropin and coronary artery lesions in children with Kawasaki disease. Eur. J. Pediatrics 2021, 180, 2253–2259. [Google Scholar] [CrossRef]
- Woods, S.C.; Seeley, R.J.; Porte, D.; Schwartz, M.W. Signals that regulate food intake and energy homeostasis. Science 1998, 280, 1378–1383. [Google Scholar] [CrossRef]
- Powley, T.L.; Keessey, R.E. Body energy homeostasis. Appetite 2008, 51, 442–445. [Google Scholar] [CrossRef]
- Renquist, B.J.; Lippert, R.; Sebag, J.A.; Ellacott, K.L.J.; Cone, R.D. Physiological roles of the melanocortin MC 3 receptor. Eur. J. Pharmacol. 2010, 660, 13–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jéquier, E. Leptin Signaling, Adiposity, and Energy Balance. Ann. N. Y. Acad. Sci. 2006, 967, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, S.A.; Cohen, A.; Saeed, Z.; Ojha, S.; Singh, J.; Adeghate, E. Obesity: Molecular Mechanisms, Epidemiology, Complications and Pharmacotherapy. In Cellular and Biochemical Mechanisms of Obesity; Springer: Cham, Switzerland, 2021; pp. 249–266. [Google Scholar]
- Gao, S.; Ghoshal, S.; Zhang, L.; Stevens, J.R.; McCommis, K.S.; Finck, B.N.; Lopaschuk, G.D.; Butler, A.A. The peptide hormone adropin regulates signal transduction pathways controlling hepatic glucose metabolism in a mouse model of diet-induced obesity. J. Biol. Chem. 2019, 294, 13366–13377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Q.; Huang, Z.; Jiang, Q. Adropin inhibited tilapia hepatic glucose output and triglyceride accumulation via AMPK activation. J. Endocrinol. 2020, 246, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, S.; Shen, T.; Yang, W.; Chen, Q.; Zhang, P.; You, Y.; Sun, X.; Xu, H.; Tang, Y.; et al. Adropin regulates hepatic glucose production via PP2A/AMPK pathway in insulin-resistant hepatocytes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 10056–10072. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghoshal, S.; Stevens, J.R.; McCommis, K.S.; Gao, S.; Castro-Sepulveda, M.; Mizgier, M.L.; Girardet, C.; Kumar, K.G.; Galgani, J.E.; et al. Hepatocyte expression of the micropeptide adropin regulates the liver fasting response and is enhanced by caloric restriction. J. Biol. Chem. 2020, 295, 13753–13768. [Google Scholar] [CrossRef]
- Demirdöğen, F.; Akdağ, T.; Gündüz, Z.B.; Odabaş, F.Ö. Investigation of serum adropin levels and its relationship with hypothalamic atrophy in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 66, 103948. [Google Scholar] [CrossRef]
- Aydın, P.; Uzunçakmak, S.K.; Tör, İ.H.; Bilen, A.; Özden, A. Comparison of Serum Adropin Levels in Patients with Diabetes Mellitus, COVID-19, and COVID-19 with Diabetes Mellitus. Eurasian J. Med. 2022, 54, 197–201. [Google Scholar] [CrossRef]
- Ruszała, M.; Pilszyk, A.; Niebrzydowska, M.; Kimber-Trojnar, Ż.; Trojnar, M.; Leszczyńska-Gorzelak, B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus 2.0. Int. J. Mol. Sci. 2022, 23, 4364. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, Y. Soluble Vascular Adhesion Protein-1 Level Correlates With Adropin and Inflammatory Biomarkers in Patients With Obstructive Sleep Apnea. Ear Nose Throat J. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Simac, P.; Perkovic, D.; Bozic, I.; Bilopavlovic, N.; Martinovic, D.; Bozic, J. Serum Adropin Levels in Patients with Rheumatoid Arthritis. Life 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.A.; Kuyumcu, M.S. Serum irisin and adropin levels may be predictors for coronary artery ectasia. Clin. Exp. Hypertens. 2022, 44, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kurt, E.; Tekin, E.; Kurt, N.; Bayramoglu, A. The role of adropin, HIF-1α and apelin biomarkers in the diagnosis of acute mesentaric ischemia. Am. J. Emerg. Med. 2022, 51, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Es-Haghi, A.; Al-Abyadh, T.; Mehrad-Majd, H. The Clinical Value of Serum Adropin Level in Early Detection of Diabetic Nephropathy. Kidney Blood Press. Res. 2021, 46, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Jiang, F.; Cheng, X.; Xu, H.; Hu, X. Serum adropin levels are decreased in chinese type 2 diabetic patients and negatively correlated with body mass index. Endocr. J. 2018, 65, 685–691. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, K.; Liu, Q.C.; Guo, Z.; Zhang, S.; Chen, X.R.; Lin, J.H.; Wen, J.P.; Zhao, C.F.; Lin, X.H.; et al. Adropin deficiency worsens HFD-induced metabolic defects. Cell Death Dis. 2017, 8, e3008. [Google Scholar] [CrossRef]
- Li, N.; Xie, G.; Zhou, B.; Qu, A.; Meng, H.; Liu, J.; Wang, G. Serum Adropin as a Potential Biomarker for Predicting the Development of Type 2 Diabetes Mellitus in Individuals With Metabolic Dysfunction-Associated Fatty Liver Disease. Front. Physiol. 2021, 12, 696163. [Google Scholar] [CrossRef]
- Polkowska, A.; Pasierowska, I.E.; Pasławska, M.; Pawluczuk, E.; Bossowski, A. Assessment of Serum Concentrations of Adropin, Afamin, and Neudesin in Children with Type 1 Diabetes. BioMed Res. Int. 2019, 2019, 6128410. [Google Scholar] [CrossRef]
- Hosseini, A.; Shanaki, M.; Emamgholipour, S.; Nakhjavani, M.; Razi, F.; Golmohammadi, T. Elevated serum levels of adropin in patients with type 2 diabetes mellitus and its association with insulin resistance. J. Biol. Today’s World 2016, 5, 44–49. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Hu, W.; Liu, Y.; Lin, D.; Duan, H.; Liu, F. The association of serum and vitreous adropin concentrations with diabetic retinopathy. Ann. Clin. Biochem. Int. J. Lab. Med. 2019, 56, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, L. Association of Serum Adropin Concentrations with Diabetic Nephropathy. Mediat. Inflamm. 2016, 2016, 6038261. [Google Scholar] [CrossRef] [PubMed]
- Beigi, A.; Shirzad, N.; Nikpour, F.; Nasli Esfahani, E.; Emamgholipour, S.; Bandarian, F. Association between serum adropin levels and gestational diabetes mellitus; a case–control study. Gynecol. Endocrinol. 2015, 31, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.Y.; Cheng, K.C.; Li, Y.; Cheng, J.T.; Tsai, C.C. Promotion of adropin expression by hyperglycemia is associated with STAT3 activation in diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2269–2277. [Google Scholar] [CrossRef]
- Ozkan, A.; Aslan, M.A.; Sinen, O.; Munzuroglu, M.; Derin, N.; Parlak, H.; Bulbul, M.; Agar, A. Effects of adropin on learning and memory in rats tested in the Morris water maze. Hippocampus 2022, 32, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1a interaction. Nature 2003, 423, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J.; Suzuki, S.; Segars, J.H.; Kino, T. CRTC2 is a coactivator of GR and couples GR and CREB in the regulation of hepatic gluconeogenesis. Mol. Endocrinol. 2016, 30, 104–117. [Google Scholar] [CrossRef]
- Kurir, T.T.; Miličević, T.; Novak, A.; Vilović, M.; Božić, J. Adropin—potential link in cardiovascular protection for obese male type 2 diabetes mellitus patients treated with liraglutide. Acta Clin. Croat. 2020, 59, 344–350. [Google Scholar] [CrossRef]
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Sanlioglu, S. Incretins: Their physiology and application in the treatment of diabetes mellitus. Diabetes/Metab. Res. Rev. 2014, 30, 354–371. [Google Scholar] [CrossRef]
- Lotfy, M.; Singh, J.; Kalász, H.; Tekes, K.; Adeghate, E. Medicinal Chemistry and Applications of Incretins and DPP-4 Inhibitors in the Treatment of Type 2 Diabetes Mellitus. Open Med. Chem. J. 2011, 5, 82–92. [Google Scholar] [CrossRef]
- Adeghate, J.O.; D’Souza, C.; Kántor, O.; Tariq, S.; Souid, A.K.; Adeghate, E. Early (5-Day) Onset of Diabetes Mellitus Causes Degeneration of Photoreceptor Cells, Overexpression of Incretins, and Increased Cellular Bioenergetics in Rat Retina. Cells 2021, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Singh, J.; Rashed, H.; Tariq, S.; Zilahi, E.; Adeghate, E. The effect of glucagon-like peptide-1 in the management of diabetes mellitus: Cellular and molecular mechanisms. Cell Tissue Res. 2014, 358, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Ison, J.; Voor, M.J.; Tyagi, N. Exercise-Linked Skeletal Irisin Ameliorates Diabetes-Associated Osteoporosis by Inhibiting the Oxidative Damage-Dependent miR-150-FNDC5/Pyroptosis Axis. Diabetes 2022, db210573. [Google Scholar] [CrossRef] [PubMed]
- Marrano, N.; Biondi, G.; Borrelli, A.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F.; Natalicchio, A. Irisin and incretin hormones: Similarities, differences, and implications in type 2 diabetes and obesity. Biomolecules 2021, 11, 286. [Google Scholar] [CrossRef]
- Wattez, J.S.; Ravallec, R.; Cudennec, B.; Knauf, C.; Dhulster, P.; Valet, P.; Breton, C.; Vieau, D.; Lesage, J. Apelin stimulates both cholecystokinin and glucagon-like peptide 1 secretions in vitro and in vivo in rodents. Peptides 2013, 48, 134–136. [Google Scholar] [CrossRef]
- Howarth, F.C.; Jacobson, M.; Shafiullah, M.; Adeghate, E. Long-term effects of type 2 diabetes mellitus on heart rhythm in the Goto-Kakizaki rat. Exp. Physiol. 2008, 93, 362–369. [Google Scholar] [CrossRef]
- Marcolin, É.; Forgiarini, L.F.; Tieppo, J.; Dias, A.S.; de Freitas, L.A.R.; Marroni, N.P. Methionine- and choline-deficient diet induces hepatic changes characteristic of non-alcoholic steatohepatitis. Arq. De Gastroenterol. 2011, 48, 72–79. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Wojciechowicz, T.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Effects of adropin on proliferation and differentiation of 3T3-L1 cells and rat primary preadipocytes. Mol. Cell. Endocrinol. 2019, 496, 110532. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Hart, D.A. Obesity, the Obesity Epidemic, and Metabolic Dysfunction: The Conundrum Presented by the Disconnect between Evolution and Modern Societies. J. Biomed. Sci. Eng. 2021, 14, 203–211. [Google Scholar] [CrossRef]
- Ganesh-Kumar, K.; Zhang, J.; Gao, S.; Rossi, J.; McGuinness, O.P.; Halem, H.H.; Culler, M.D.; Mynatt, R.L.; Butler, A.A. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity 2012, 20, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Erman, H.; Ozdemir, A.; Sitar, M.E.; Cetin, S.I.; Boyuk, B. Role of serum adropin measurement in the assessment of insulin resistance in obesity. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2021, 69, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Tam, C.S.; Stanhope, K.L.; Wolfe, B.M.; Ali, M.R.; O’Keeffe, M.; St-Onge, M.P.; Ravussin, E.; Havel, P.J. Low Circulating Adropin Concentrations with Obesity and Aging Correlate with Risk Factors for Metabolic Disease and Increase after Gastric Bypass Surgery in Humans. J. Clin. Endocrinol. Metab. 2012, 97, 3783–3791. [Google Scholar] [CrossRef]
- Glück, M.; Glück, J.; Wiewióra, M.; Rogala, B.; Piecuch, J. Serum Irisin, Adropin, and Preptin in Obese Patients 6 Months After Bariatric Surgery. Obes. Surg. 2019, 29, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Kocaoglu, C.; Buyukinan, M.; Erdem, S.S.; Ozel, A. Are obesity and metabolic syndrome associated with plasma adropin levels in children? J. Pediatric Endocrinol. Metab. 2015, 28, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.A.; Kalász, H.; Al Jaberi, S.; Adeghate, J.; Tekes, K. Tackling type 2 diabetes-associated cardiovascular and renal comorbidities: A key challenge for drug development. Expert Opin. Investig. Drugs 2021, 30, 85–93. [Google Scholar] [CrossRef]
- Singh, R.M.; Waqar, T.; Howarth, F.C.; Adeghate, E.; Bidasee, K.; Singh, J. Hyperglycemia-induced cardiac contractile dysfunction in the diabetic heart. Heart Fail. Rev. 2018, 23, 37–54. [Google Scholar] [CrossRef]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef]
- Adeghate, E.; Singh, J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail. Rev. 2014, 19, 15–23. [Google Scholar] [CrossRef]
- Adeghate, E.; Schattner, P.; Dunn, E. An update on the etiology and epidemiology of diabetes mellitus. Ann. N. Y. Acad. Sci. 2006, 1084, 1–29. [Google Scholar] [CrossRef]
- Adeghate, E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: A short review. Mol. Cell. Biochem. 2004, 261, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, N.; Mori, J.; Lopaschuk, G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014, 171, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, T.R.; Gao, S.; Karwi, Q.G.; Fukushima, A.; Rawat, S.; Wagg, C.S.; Zhang, L.; Lopaschuk, G.D. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metab. Clin. Exp. 2019, 98, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Xie, B.; Zhang, M.; Stoner, M.W.; Manning, J.R.; Huckestein, B.R.; Edmunds, L.R.; Mullett, S.J.; McTiernan, C.F.; Wendell, S.G.; et al. Adropin treatment restores cardiac glucose oxidation in pre-diabetic obese mice. J. Mol. Cell. Cardiol. 2019, 129, 174–178. [Google Scholar] [CrossRef]
- Arkadievich, O.D. Metabolic markers of myocardium insulin resistance in dogs with heart failure. Open Vet. J. 2021, 10, 363–370. [Google Scholar] [CrossRef]
- Thapa, D.; Xie, B.; Mushala, B.; Zhang, M.; Manning, J.R.; Bugga, P.; Stoner, M.W.; Jurczak, M.J.; Scott, I. Diet-induced obese mice are resistant to improvements in cardiac function resulting from short-term adropin treatment. Curr. Res. Physiol. 2022, 5, 55–62. [Google Scholar] [CrossRef]
- Mushala, B.A.S.; Scott, I. Adropin: A hepatokine modulator of vascular function and cardiac fuel metabolism. Am. J. Physiol. Circ. Physiol. 2021, 320, H238–H244. [Google Scholar] [CrossRef]
- Poss, J.; Custodis, F.; Werner, C.; Weingartner, O.; Bohm, M.; Laufs, U. Cardiovascular Disease and Dyslipidemia: Beyond LDL. Curr. Pharm. Des. 2011, 17, 861–870. [Google Scholar] [CrossRef]
- Akcılar, R.; Emel Koçak, F.; Şimşek, H.; Akcılar, A.; Bayat, Z.; Ece, E.; Kökdaşgil, H. The effect of adropin on lipid and glucose metabolism in rats with hyperlipidemia. Iran. J. Basic Med. Sci. 2016, 19, 245–251. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, M.; Chen, L.; Yin, F.; Zhu, X.; Gou, J.; Zeng, W.; Lv, Z. Association between serum adropin level and coronary artery disease: A systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 2002, 415, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Jacobson, M.; Adeghate, E.; Oulhaj, A.; Shafiullah, M.; Qureshi, A.; Howarth, F.C. Effects of obesity and diabesity on heart rhythm in the Zucker rat. Clin. Exp. Pharmacol. Physiol. 2021, 48, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xue, Y.; Shang, F.; Ni, S.; Liu, X.; Fan, B.; Wang, H. Association of serum adropin with the presence of atrial fibrillation and atrial remodeling. J. Clin. Lab. Anal. 2019, 33, e22672. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid type-2 (CB2) receptors in rats. Chem. Biol. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Al-Taee, H.; Azimullah, S.; Meeran, M.; Alaraj Almheiri, M.K.; Al Jasmi, R.A.; Tariq, S.; Ab Khan, M.; Adeghate, E.; Ojha, S. β-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharmacol. 2019, 858, 172467. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, E.; Kumaraswamy, S.M.; Kader, K.H.; Venkataraman, B.; Ojha, S.; Adeghate, E.; Rajesh, M. Genipin attenuates cisplatin-induced nephrotoxicity by counteracting oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2017, 93, 1083–1097. [Google Scholar] [CrossRef]
- Sato, K.; Yamashita, T.; Shirai, R.; Shibata, K.; Okano, T.; Yamaguchi, M.; Mori, Y.; Hirano, T.; Watanabe, T. Adropin contributes to anti-atherosclerosis by suppressing monocyte-endothelial cell adhesion and smooth muscle cell proliferation. Int. J. Mol. Sci. 2018, 19, 1293. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as A Fat-Burning Hormone with Multiple Functions-Review of a Decade of Research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef]
- Bozic, J.; Borovac, J.A.; Galic, T.; Kurir, T.T.; Supe-Domic, D.; Dogas, Z. Adropin and inflammation biomarker levels in male patients with obstructive sleep apnea: A link with glucose metabolism and sleep parameters. J. Clin. Sleep Med. 2018, 14, 1109–1118. [Google Scholar] [CrossRef]
- Chen, X.; Xue, H.; Fang, W.; Chen, K.; Chen, S.; Yang, W.; Shen, T.; Chen, X.; Zhang, P.; Ling, W. Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol. 2019, 21, 101068. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Wojciechowicz, T.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin stimulates proliferation but suppresses differentiation in rat primary brown preadipocytes. Arch. Biochem. Biophys. 2020, 692, 108536. [Google Scholar] [CrossRef] [PubMed]
- Kondělková, K.; Vokurková, D.; Krejsek, J.; Borská, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med. (Hradec Králové)/Univ. Carol. Fac. Med. Hradec Králové 2010, 53, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; al-Ramadi, B.; Saleh, A.M.; Vijayarasathy, C.; Ponery, A.S.; Arfat, K.; Howarth, F.C.; El-Sharkawy, T. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell. Mol. Life Sci. 2003, 60, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Emerald, B.S.; Mohsin, S.; D’Souza, C.; John, A.; El-Hasasna, H.; Ojha, S.; Raza, H.; Al-Ramadi, B.; Adeghate, E. Diabetes Mellitus Alters the Immuno-Expression of Neuronal Nitric Oxide Synthase in the Rat Pancreas. Int. J. Mol. Sci. 2022, 23, 4974. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).