Polymeric Nanorepellent Systems Containing Geraniol and Icaridin Aimed at Repelling Aedes aegypti
Abstract
:1. Introduction
2. Result and Discussion
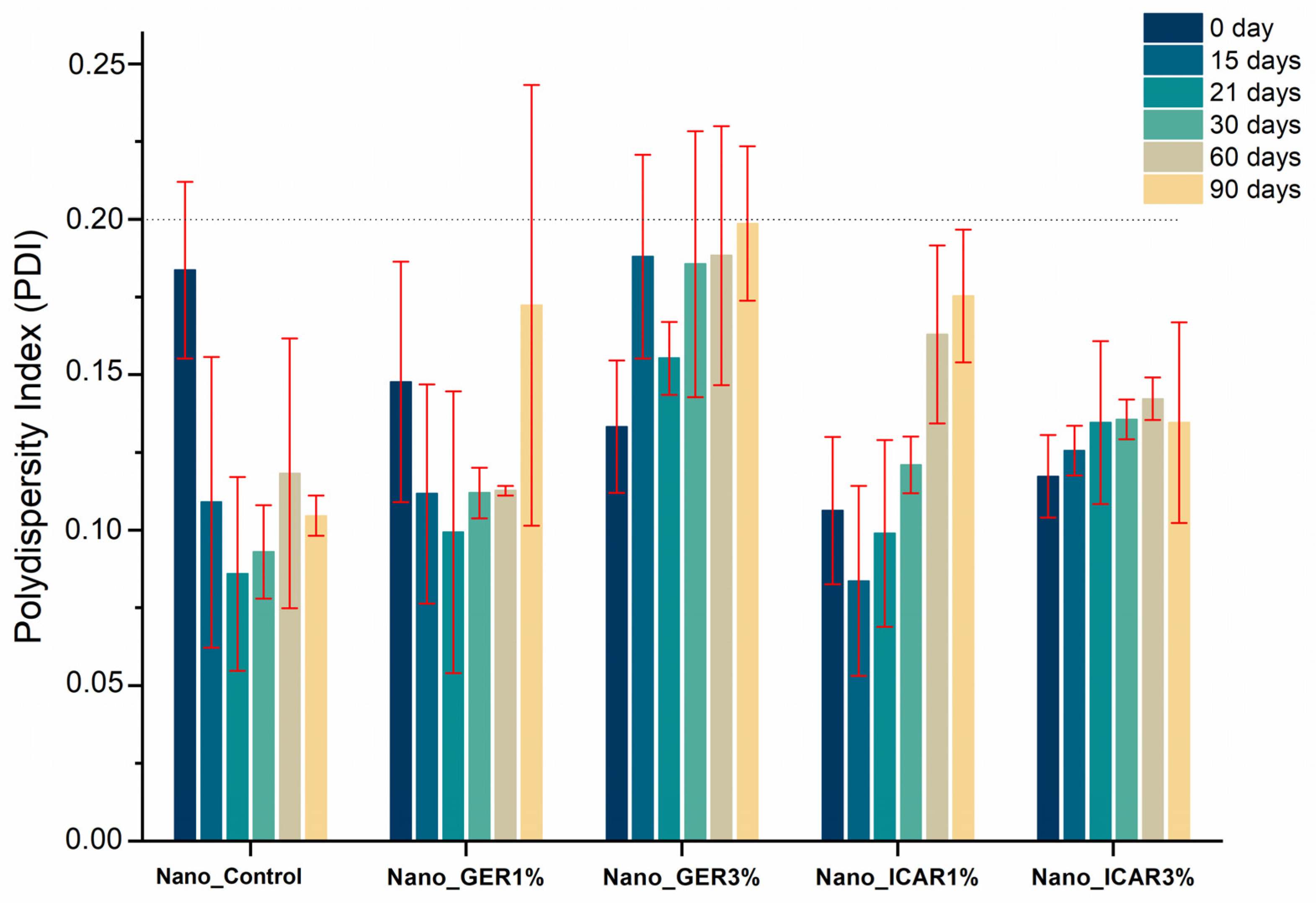
2.1. Characterization of Nanocapsules
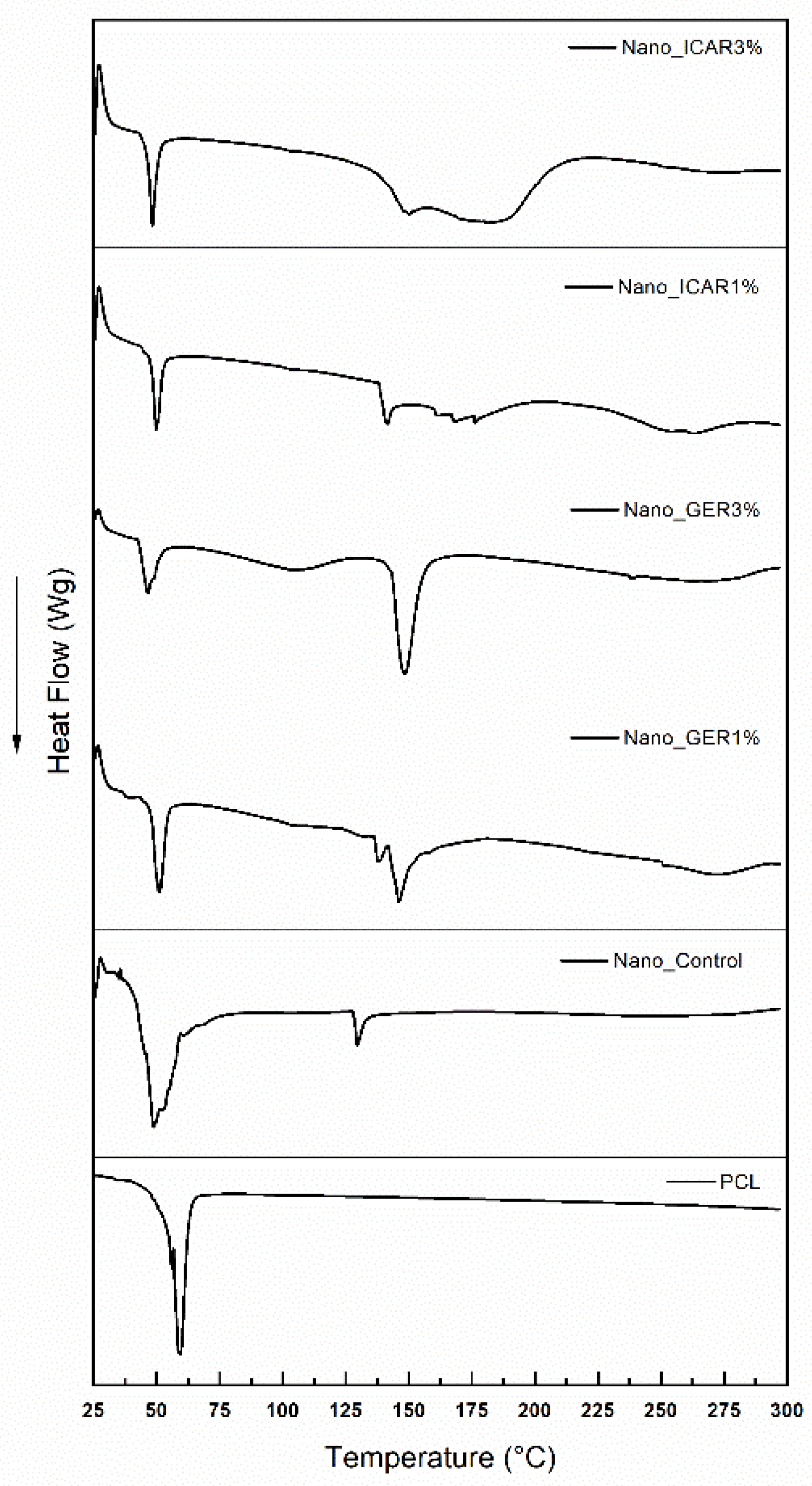
2.2. Differential Scanning Calorimetry (DSC)
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Encapsulation Efficiency
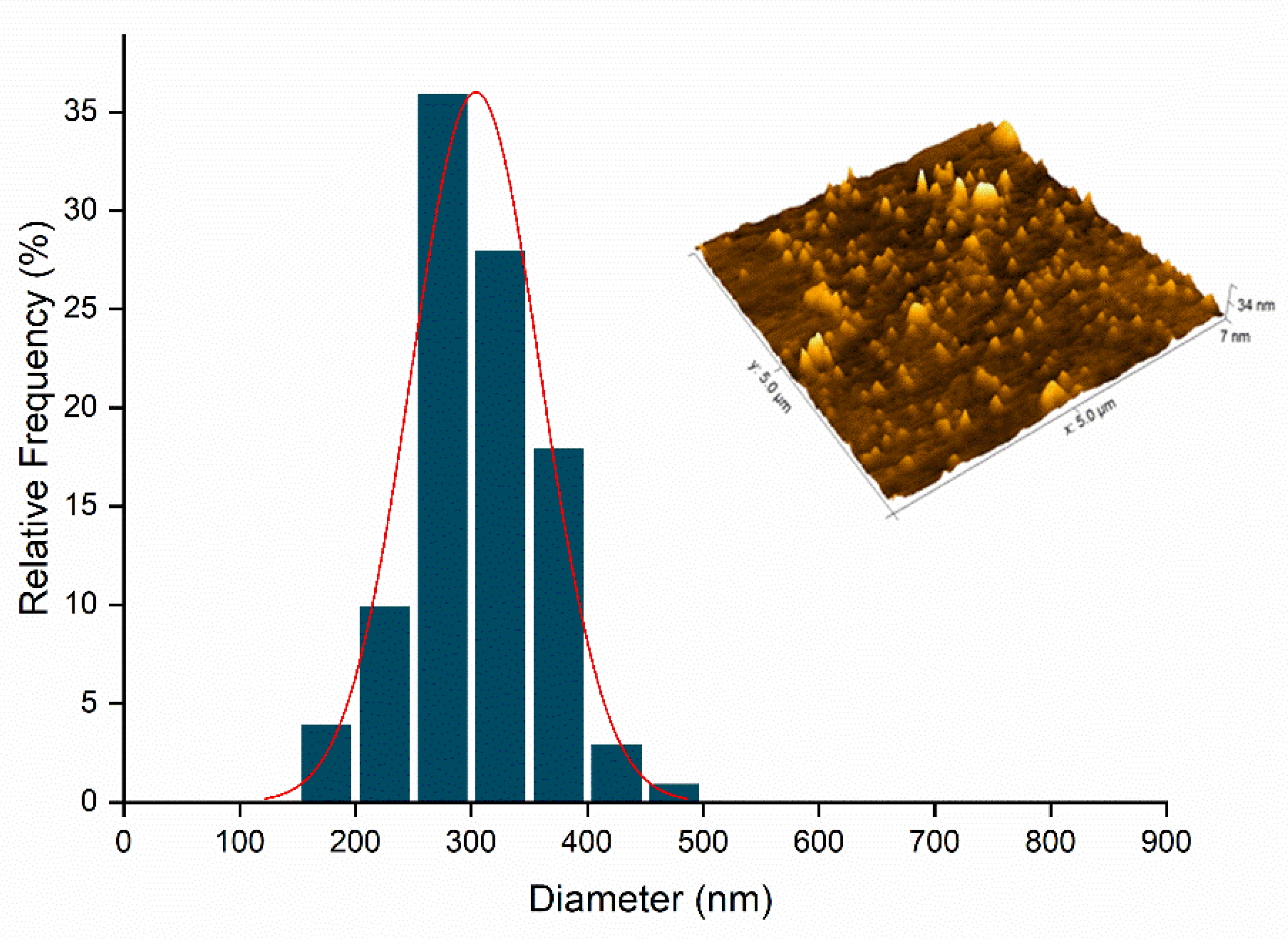
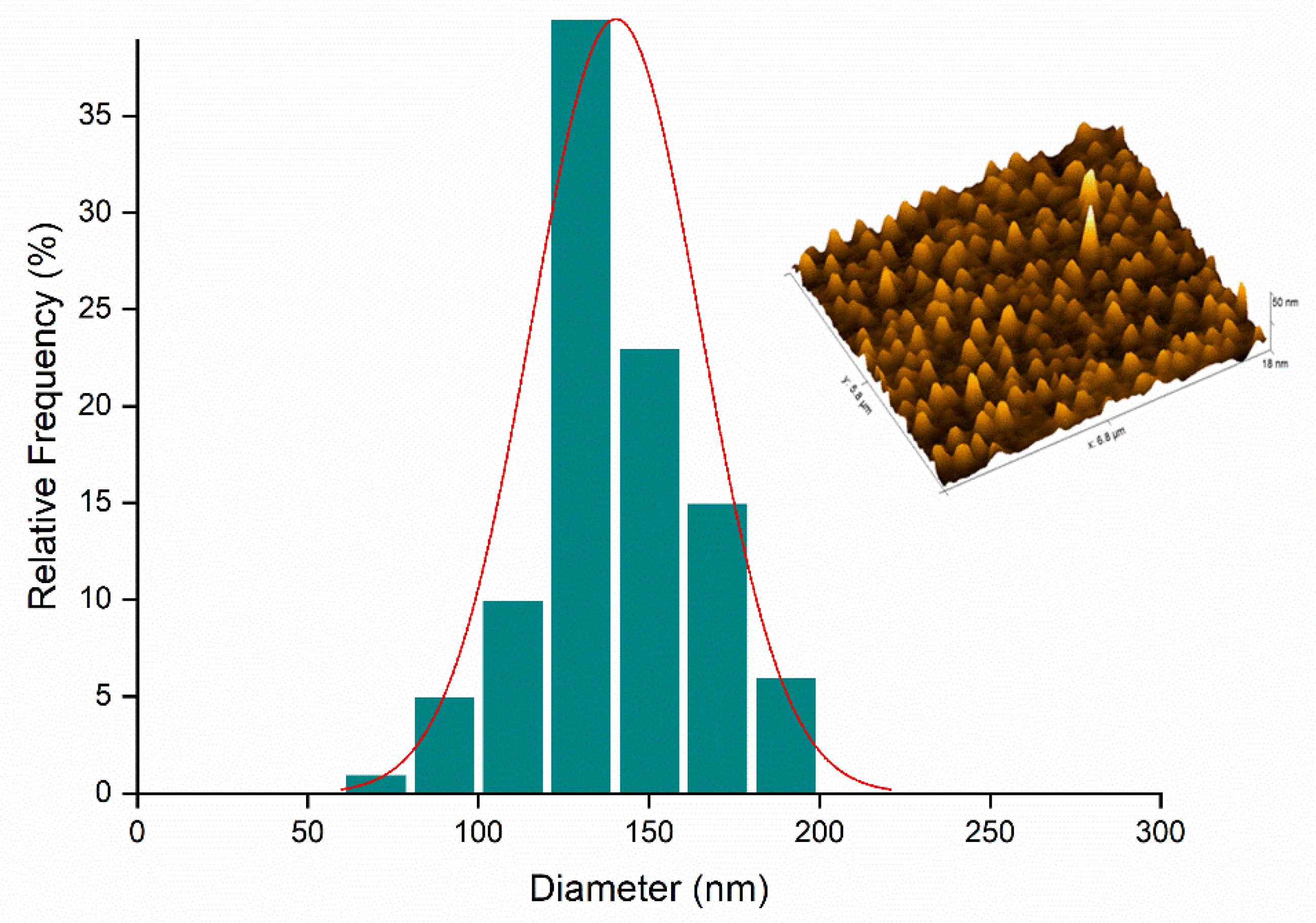
2.5. Atomic Force Microscopy (AFM)
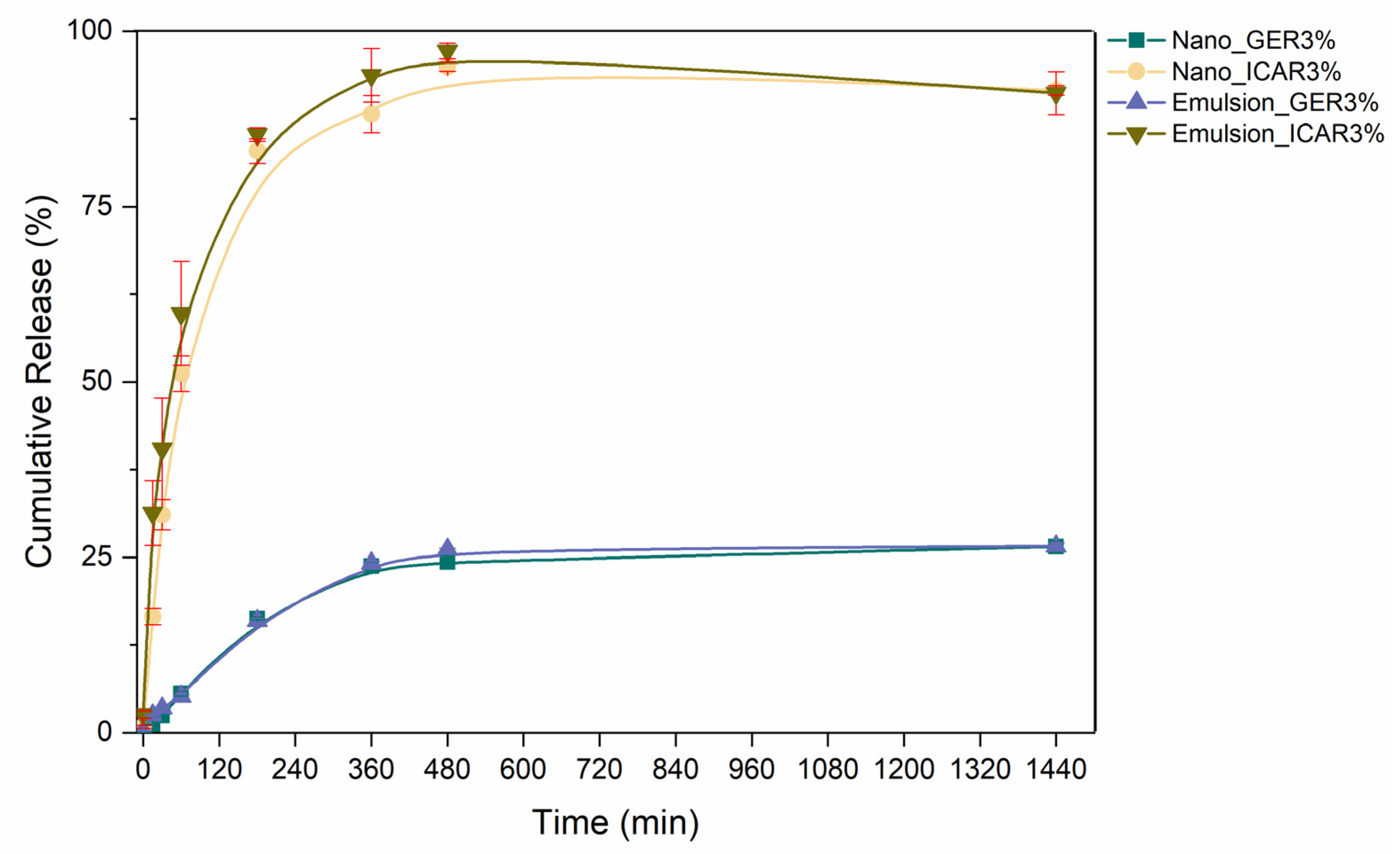
2.6. In Vitro Release
2.7. Evaluation of Cytotoxicity
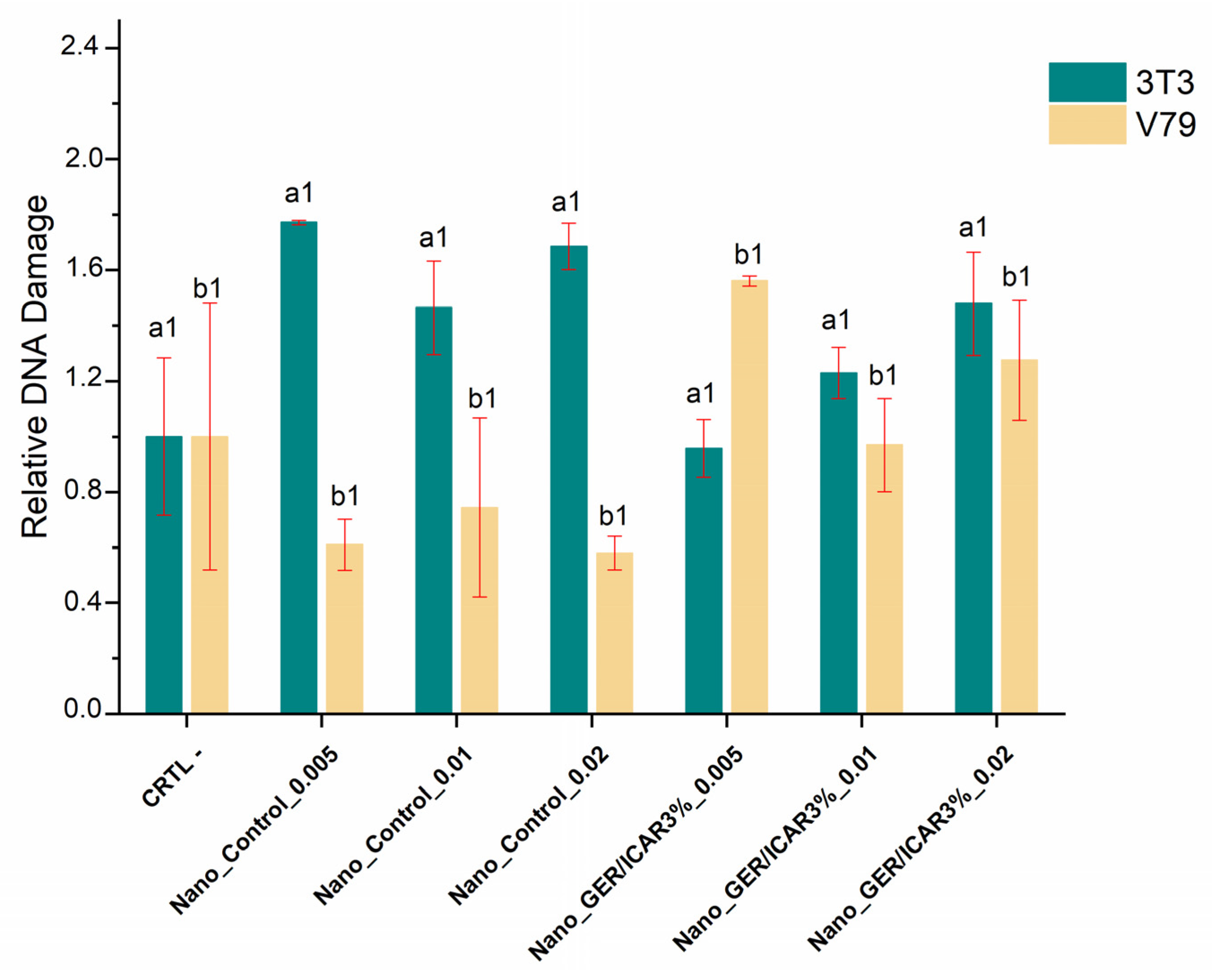
2.8. Evaluation of Genotoxicity
3. Methods and Materials
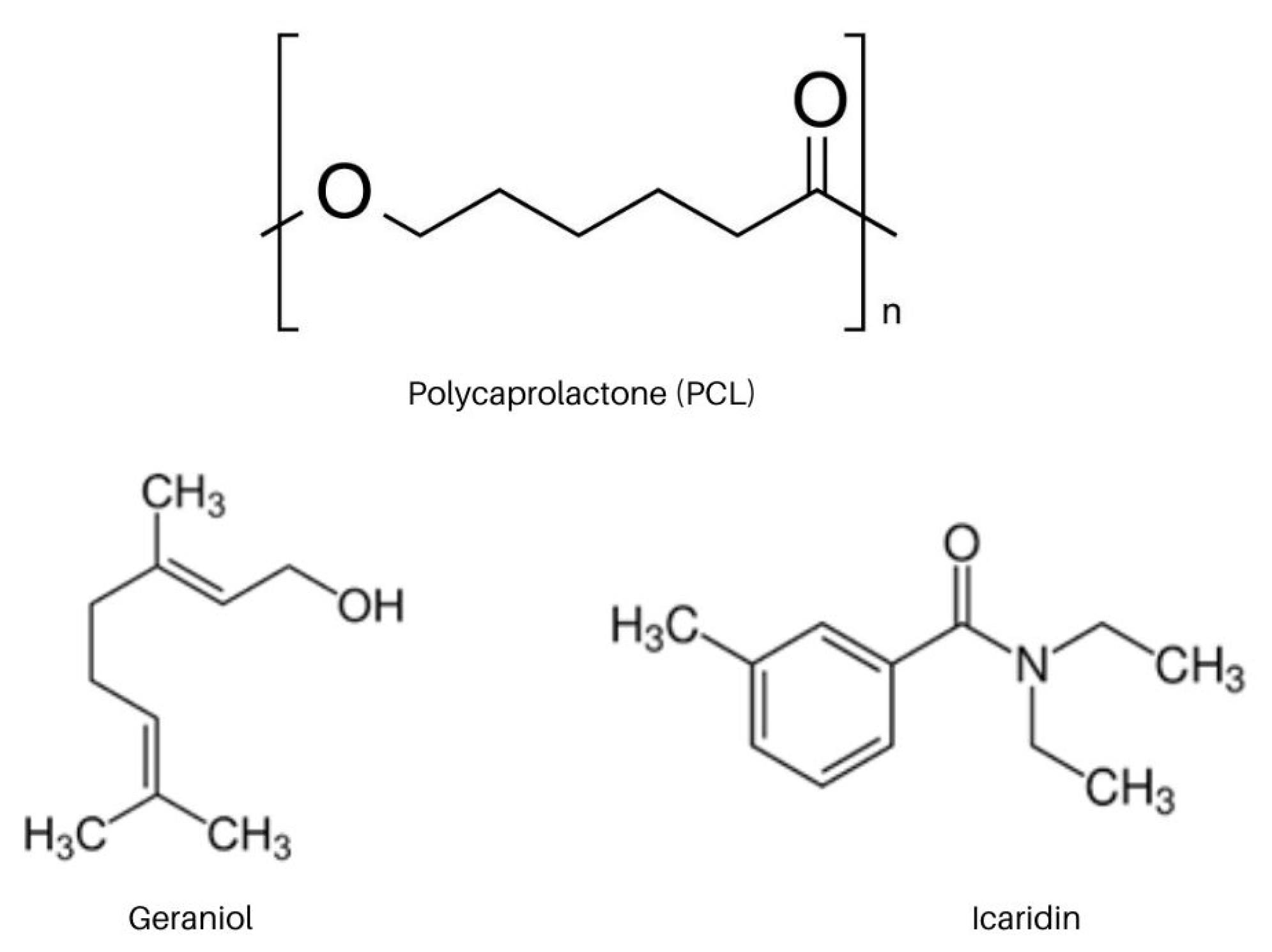
3.1. Materials
3.2. Preparation of Nanocapsules Containing Repellents
3.3. Characterization of Nanocapsules
3.3.1. Particle Size, Polydispersity Index (PDI), and Nanoparticle Tracking Analysis (NTA)
3.3.2. Differential Scanning Calorimetry (DSC)
3.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.4. Encapsulation Efficiency
3.3.5. Atomic Force Electron Microscopy (AFM)
3.4. In Vitro Release Assay
3.5. Cytotoxicity and Genotoxicity Assay of Nanocapsules
3.5.1. Mitochondrial Activity Assay—MTT (3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide)
3.5.2. Genotoxicity Evaluation
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasil. Ministerio da Saude. Secretaria de vigilância em saúde. Monitoramento dos casos de arboviroses urbanas causados por vírus transmitidos pelo mosquito Aedes (dengue, chikungunya e zika), semanas epidemiológicas 1 a 39, 2021. Bol. Epidemiológico 2021, 52, 1–10. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletinsepidemiologicos/edicoes/2021/boletim_epidemiologico_svs_44-2.pdf (accessed on 10 April 2022).
- Teich, V.; Arinelli, R.; Fahham, L. Aedes aegypti e sociedade: O impacto econômico das arboviroses no Brasil. J. Bras. Econ. Saúde 2017, 9, 267–276. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dengue and Severe Dengue. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 8 April 2022).
- Zara, A.L.D.S.A.; Santos, S.M.D.; Fernandes-Oliveira, E.S.; Carvalho, R.G.; Coelho, G.E. Estratégias de controle do Aedes aegypti: Uma revisão. Epidemiol. Serv. Saúde. 2016, 25, 1–2. [Google Scholar]
- Qiu, H.; Jun, H.W.; McCall, J.W. Pharmacokinetics, formulation, and safety of insect repellent N,N-diethyl-3-methylbenzamide (deet): A review. J. Am. Mosq. Control. Assoc. 1998, 14, 12–27. [Google Scholar] [PubMed]
- Swale, D.R.; Bloomquist, J.R. Is DEET a dangerous neurotoxicant? Pest. Manag. Sci. 2019, 75, 2068–2070. [Google Scholar] [CrossRef]
- Anvisa Não vê Restrições no uso de Repelentes por Gestantes Agência Nacional de VIGILÂNCIA SANITÁRIA—Anvisa. 2021. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2016/anvisa-nao-ve-restricoes-no-uso-de-repelentes-por-gestantes (accessed on 14 February 2022).
- Koren, G.; Matsui, D.; Bailey, B. DEET-based insect repellents: Safety implications for children and pregnant and lactating women. CMAJ 2003, 169, 209–212. [Google Scholar]
- Singh, S.P.; Azua, A.; Chaudhary, A.; Khan, S.; Willett, K.L.; Gardinali, P.R. Occurrence and distribution of steroids, hormones and selected pharmaceuticals in South Florida coastal environments. Ecotoxicology 2010, 19, 338–350. [Google Scholar] [CrossRef]
- Sangha, G.K. Toxicology and safety evaluation of the new insect repellent Picaridin (Saltidin). In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010; pp. 2219–2230. [Google Scholar]
- Naucke, T.J.; Kröpke, R.; Benner, G.; Schulz, J.; Wittern, K.P.; Rose, A.; Kröckel, U.; Grünewald, H.W. Field evaluation of the efficacy of proprietary repellent formulations with IR3535® and Picaridin against Aedes aegypti. Parasitol. Res. 2007, 101, 169–177. [Google Scholar] [CrossRef]
- Charlton, N.P.; Murphy, L.T.; Parker Cote, J.L.; Vakkalanka, J.P. The toxicity of picaridin containing insect repellent reported to the National Poison Data System. Clin. Toxicol. 2016, 54, 655–658. [Google Scholar] [CrossRef]
- Silva, B.O.D.; Olivatti, T.O.F.; Kanda, R.G.; Marco, G.N.; Santaella, F.J.; Madeira, N.G.; Haddad, J.V.; Miot, H.A. Efficacy of the main repellents available in the Brazilian market against Aedes aegypti bites under concentrations applied to pediatric populations. Rev. Soc. Bras. Med. Trop. 2018, 51, 256–257. [Google Scholar] [CrossRef]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological properties of geraniol—A review. Planta. Med. 2019, 85, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Tabari, M.A.; Youssefi, M.R.; Esfandiari, A.; Benelli, G. Toxicity of β-citronellol, geraniol and linalool from Pelargonium roseum essential oil against the West Nile and filariasis vector Culex pipiens (Diptera: Culicidae). Res. Vet. Sci. 2017, 114, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, D.M.; Menezes, D.B.; Andrade, L.R.; Lima, F.D.C.; Hollanda, L.; Zielinska, A.; Sanchez-Lopez, E.; Souto, E.B.; Severino, P. Silver nanocapsules obtained from Brazilian pepper extracts with synergistic antimicrobial effect: Production, characterization, hydrogel formulation, cell viability, and in vitro efficacy. Pharm. Dev. Technol. 2021, 26, 539–548. [Google Scholar] [CrossRef]
- Deletre, E.; Martin, T.; Duménil, C.; Chandre, F. Insecticide resistance modifies mosquito response to DEET and natural repellents. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revay, E.E.; Junnila, A.; Xue, R.D.; Kline, D.L.; Bernier, U.R.; Kravchenko, V.D.; Qualls, W.A.; Ghattas, N.; Müller, G.C. Evaluation of commercial products for personal protection against mosquitoes. Acta Tropica. 2013, 125, 226–230. [Google Scholar] [CrossRef]
- Werdin, G.J.O.; Jesser, E.N.; Yeguerman, C.A.; Ferrero, A.A.; Fernández, B.B. Polymer nanocapsules containing essential oils: New options for mosquito control. Environ. Sci. Pollut. Res. Int. 2017, 24, 17006–17015. [Google Scholar] [CrossRef]
- Marques, C.S.F.; Machado Júnior, J.B.; Andrade, L.R.M.; Andrade, L.N.; Santos, A.L.S.D.; Cruz, M.D.S.P.E.; Chaud, M.; Fricks, A.T.; Severino, P. Use of pharmaceutical nanotechnology for the treatment of leishmaniasis. Rev. Soc. Bras. Med. Trop. 2019, 52. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Stolf-Moreira, R.; Martinez, C.B.R.; Grillo, R.; de Jesus, M.B.; Fraceto, L.F. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS ONE 2015, 10, e0132971. [Google Scholar]
- Zorzi, G.; Schuh, R.; Campos, A.; Carvalho, E.; Rott, M.; Teixeira, H.F. Biomateriais para formulações de base nanotecnológica visando terapia gênica ocular. Quim Nova 2017, 40, 74–84. Available online: http://quimicanova.sbq.org.br/audiencia_pdf.asp?aid2=6507&nomeArquivo=RV20160024.pdf (accessed on 27 February 2022). [CrossRef]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nanocapsules for drug delivery: An updated review of the last decade. Recent Pat. Drug Deliv. Formul. 2018, 12, 252–266. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanocapsulesnanocapsules: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Kelidari, H.R.; Moemenbellah-Fard, M.D.; Morteza-Semnani, K.; Amoozegar, F.; Shahriari-Namadi, M.; Saeedi, M.; Osanloo, M. Solid-lipid nanocapsules (SLN)s containing Zataria multiflora essential oil with no-cytotoxicity and potent repellent activity against Anopheles stephensi. J. Parasit. Dis. 2021, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Bezerra, J.A.; Campelo, P.H.; Machado, M.B.; Trovati, G.; Dos Santos, A.L.; da Fonseca Filho, H.D.; et al. Encapsulation of Piper aduncum and Piper hispidinervum essential oils in gelatin nanocapsules: A possible sustainable control tool of Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. J. Sci. Food Agric. 2019, 99, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, D.C.; Rogerio, C.B.; de Oliveira, J.L.; Campos, E.V.R.; de Araújo, D.R.; Pampana, L.C.; Duarte, M.J.; Valadares, G.F.; Fraceto, L.F. Development of a mosquito repellent formulation based on nanostructured lipid carriers. Front. Pharmacol. 2021, 12, 760682. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Martín-Banderas, L.; Gonçalves, L.M.D.; Fernández-Arévalo, M.; Almeida, A.J. Comparative study of chitosan- and PEG-coated lipid and PLGA nanocapsules as oral delivery systems for cannabinoids. J. Nanopart. Res. 2015, 17, 61. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.R.; Fleming, I.M.; Gedik, C.M. In vitro repair of oxidative and ultraviolet-induced DNA damage in supercoiled nucleoid DNA by human cell extract. Biochim. Biophys. Acta 1994, 1219, 724–727. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh, D.F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanocapsules and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.C.; Fraceto, L.F.; De Lima, R. Nanocapsules based on chitosan as carriers for the combined herbicides Imazapic and imazapyr. Sci. Rep. 2016, 6, 19768. [Google Scholar] [CrossRef]
- Kim, A.; Ng, W.B.; Bernt, W.; Cho, N.J. Validation of size estimation of nanoparticle tracking analysis on polydisperse macromolecule assembly. Sci. Rep. 2019, 9, 2639. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Li, Y.J.; Huang, C.C.; Lai, J.Y. Poly(ε-caprolactone) nanocapsule carriers with sustained drug release: Single dose for long-term glaucoma treatment. Nanoscale 2017, 9, 11754–11764. [Google Scholar] [CrossRef]
- Zanetti, M.; Mazon, L.R.; de Meneses, A.C.; Silva, L.L.; de Araújo, P.H.H.; Fiori, M.A.; de Oliveira, D. Encapsulation of geranyl cinnamate in polycaprolactone nanocapsules. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 198–207. [Google Scholar] [CrossRef]
- Aguiar, U.N.; de Lima, S.G.; Rocha, M.S.; de Freitas, R.M.; Oliveira, T.M.; Silva, R.M. Preparação e caracterização do complexo de inclusão do óleo essencial de Croton zehntneri com β-ciclodextrina. Quím Nova 2014, 37, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Songkro, S.; Hayook, N.; Jaisawang, J.; Maneenuan, D.; Chuchome, T.; Kaewnopparat, N. Investigation of inclusion complexes of citronella oil, citronellal and citronellol with β-cyclodextrin for mosquito repellent. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 339–355. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Zha, L.; Yang, W.; Wang, C.; Jiang, X.; Fu, S. Preparation, characterization and application of pyrene-loaded methoxy poly(ethylene glycol)?poly(lactic acid) copolymer nanocapsules. Colloid Polym. Sci. 2004, 282, 1323–1328. [Google Scholar] [CrossRef]
- Mathurin, J.; Pancani, E.; Deniset-Besseau, A.; Kjoller, K.; Prater, C.B.; Gref, R.; Dazzi, A. How to unravel the chemical structure and component localization of individual drug-loaded polymeric nanocapsules by using tapping AFM-IR. Analyst 2018, 143, 5940–5949. [Google Scholar] [CrossRef] [PubMed]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Rudnik, E. Biodegradability testing of compostable polymer materials under laboratory conditions. In Compostable Polymer Materials; Elsevier: Boston, MA, USA, 2019; p. 245. [Google Scholar]
- Pourtalebi, J.L.; Ghazali, M.; Ashrafi, H.; Azadi, A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanocapsules. Heliyon 2020, 6, e03451. [Google Scholar] [CrossRef] [Green Version]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Rochín-Wong, S.; Rosas-Durazo, A.; Zavala-Rivera, P.; Maldonado, A.; Martínez-Barbosa, M.E.; Vélaz, I.; Tánori, J. Drug release properties of diflunisal from layer-by-layer self-assembled κ-carrageenan/chitosan nanocapsules: Effect of deposited layers. Polymers 2018, 10, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz, T.B.; Santos, G.F.; Ventura, S.C.; Hiruma-Lima, C.A.; Gaivão, I.O.M.; Maistro, E.L. Cytotoxic and genotoxic potential of geraniol in peripheral blood mononuclear cells and human hepatoma cell line (HepG2). Genet. Mol. Res. 2017, 16, gmr16039777. [Google Scholar] [CrossRef] [PubMed]
- Udroiu, I.; Sgura, A. Cytogenetic tests for animal production: State of the art and perspectives. Anim Genet. 2017, 48, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Elespuru, R.; Pfuhler, S.; Aardema, M.J.; Chen, T.; Doak, S.H.; Doherty, A.; Farabaugh, C.S.; Kenny, J.; Manjanatha, M.; Mahadevan, B.; et al. Genotoxicity assessment of nanomaterials: Recommendations on best practices, assays, and methods. Toxicol. Sci. 2018, 164, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.P.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Gómez, Y. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanocapsules and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Collins, A.; Dusinská, M.; Franklin, M.; Somorovská, M.; Petrovská, H.; Duthie, S.; Fillion, L.; Panayiotidis, M.; Rašlová, K.; Vaughan, N.; et al. Comet assay in human biomonitoring studies: Reliability, validation, and applications. Environ. Mol. Mutagen. 1997, 30, 139–146. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F.; et al. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]






| Mathematical Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | Higuchi | Korsmeyer–Peppas | Hixson–Crowell | First Order | |||||
| R2 | k | R2 | k | n | R2 | k | R2 | k | |
| Nano_ICAR3% | 0.891 | 8.730 | 0.941 | 16.259 | 0.269 | 0.964 | 0.0051 | 0.930 | 0.0187 |
| Nano_GER3% | 0.907 | 4.556 | 0.822 | 1.4308 | 0.436 | 0.999 | 0.0036 | 0.998 | 0.0114 |
| Geraniol | Icaridin | |
|---|---|---|
| Limit of Detection (μg/mL) | 0.39 ± 0.02 | 2.35 ± 0.86 |
| Limit of Quantification (μg/mL) | 1.30 ± 0.32 | 7.83 ± 1.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade, L.R.M.; Guilger-Casagrande, M.; Germano-Costa, T.; de Lima, R. Polymeric Nanorepellent Systems Containing Geraniol and Icaridin Aimed at Repelling Aedes aegypti. Int. J. Mol. Sci. 2022, 23, 8317. https://doi.org/10.3390/ijms23158317
de Andrade LRM, Guilger-Casagrande M, Germano-Costa T, de Lima R. Polymeric Nanorepellent Systems Containing Geraniol and Icaridin Aimed at Repelling Aedes aegypti. International Journal of Molecular Sciences. 2022; 23(15):8317. https://doi.org/10.3390/ijms23158317
Chicago/Turabian Stylede Andrade, Lucas Rannier Melo, Mariana Guilger-Casagrande, Tais Germano-Costa, and Renata de Lima. 2022. "Polymeric Nanorepellent Systems Containing Geraniol and Icaridin Aimed at Repelling Aedes aegypti" International Journal of Molecular Sciences 23, no. 15: 8317. https://doi.org/10.3390/ijms23158317
APA Stylede Andrade, L. R. M., Guilger-Casagrande, M., Germano-Costa, T., & de Lima, R. (2022). Polymeric Nanorepellent Systems Containing Geraniol and Icaridin Aimed at Repelling Aedes aegypti. International Journal of Molecular Sciences, 23(15), 8317. https://doi.org/10.3390/ijms23158317







