Selective Supercritical CO2 Extraction and Biocatalytic Valorization of Cucurbita pepo L. Industrial Residuals
Abstract
:1. Introduction
2. Results and Discussion
2.1. sc-CO2 Extraction Rationale
Cucurbita pepo L. Seed Waste Extraction
2.2. Extract Characterization
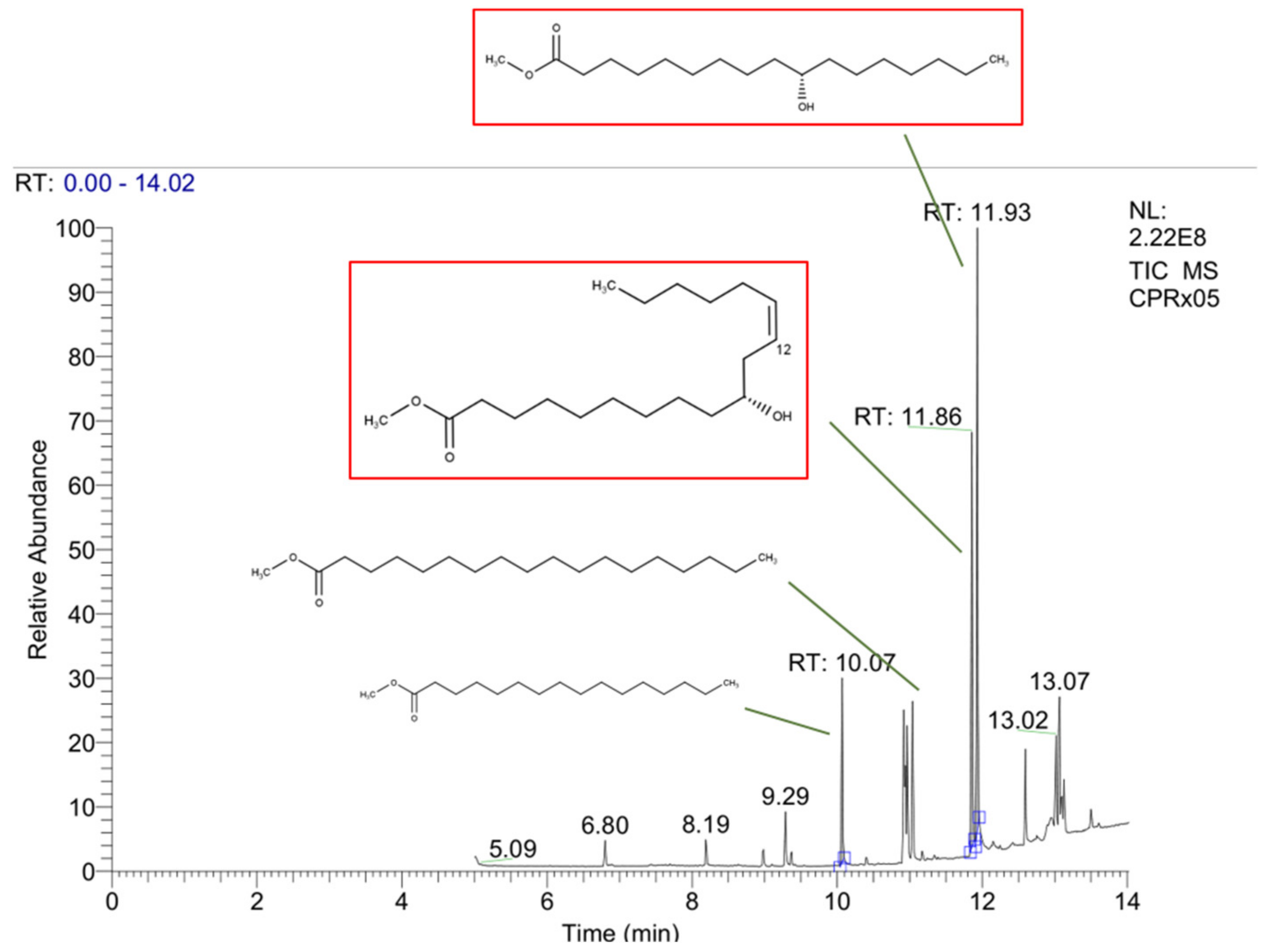
2.2.1. Cucurbita pepo L. Acid Fraction Compositions
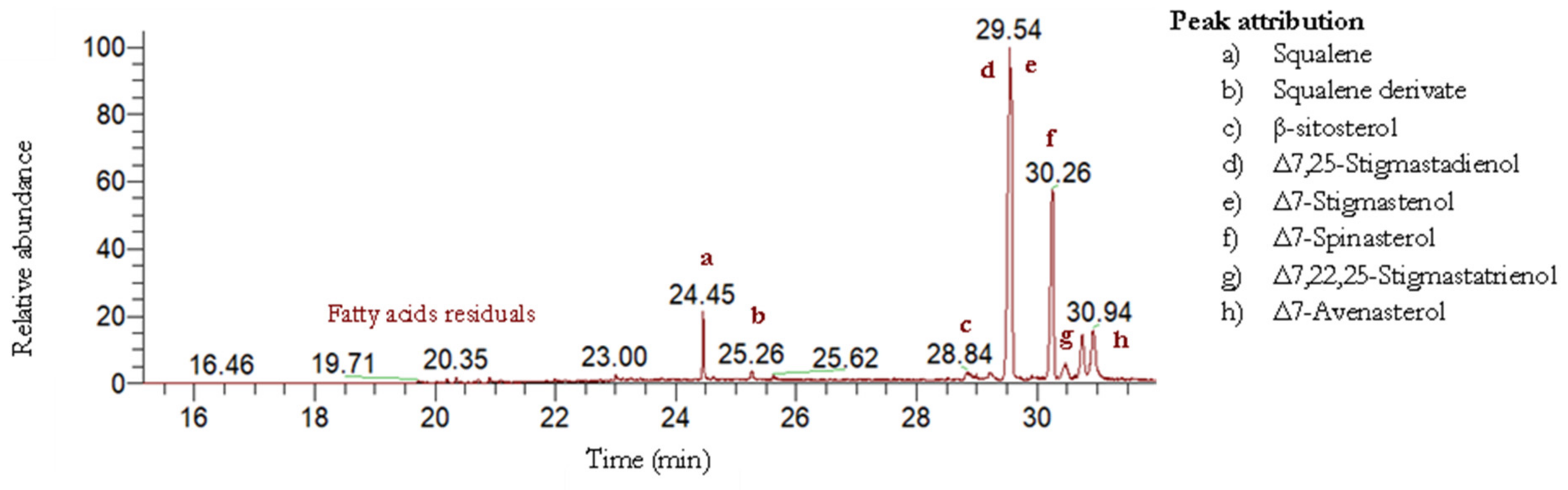
2.2.2. Cucurbita pepo L. Unsaponifiable Fraction Compositions
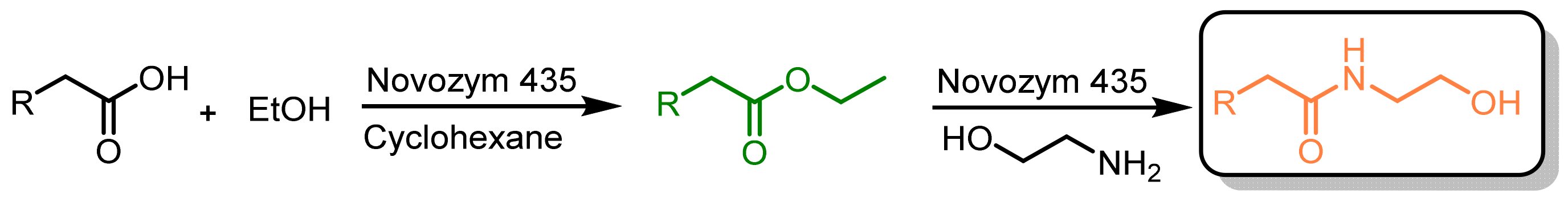
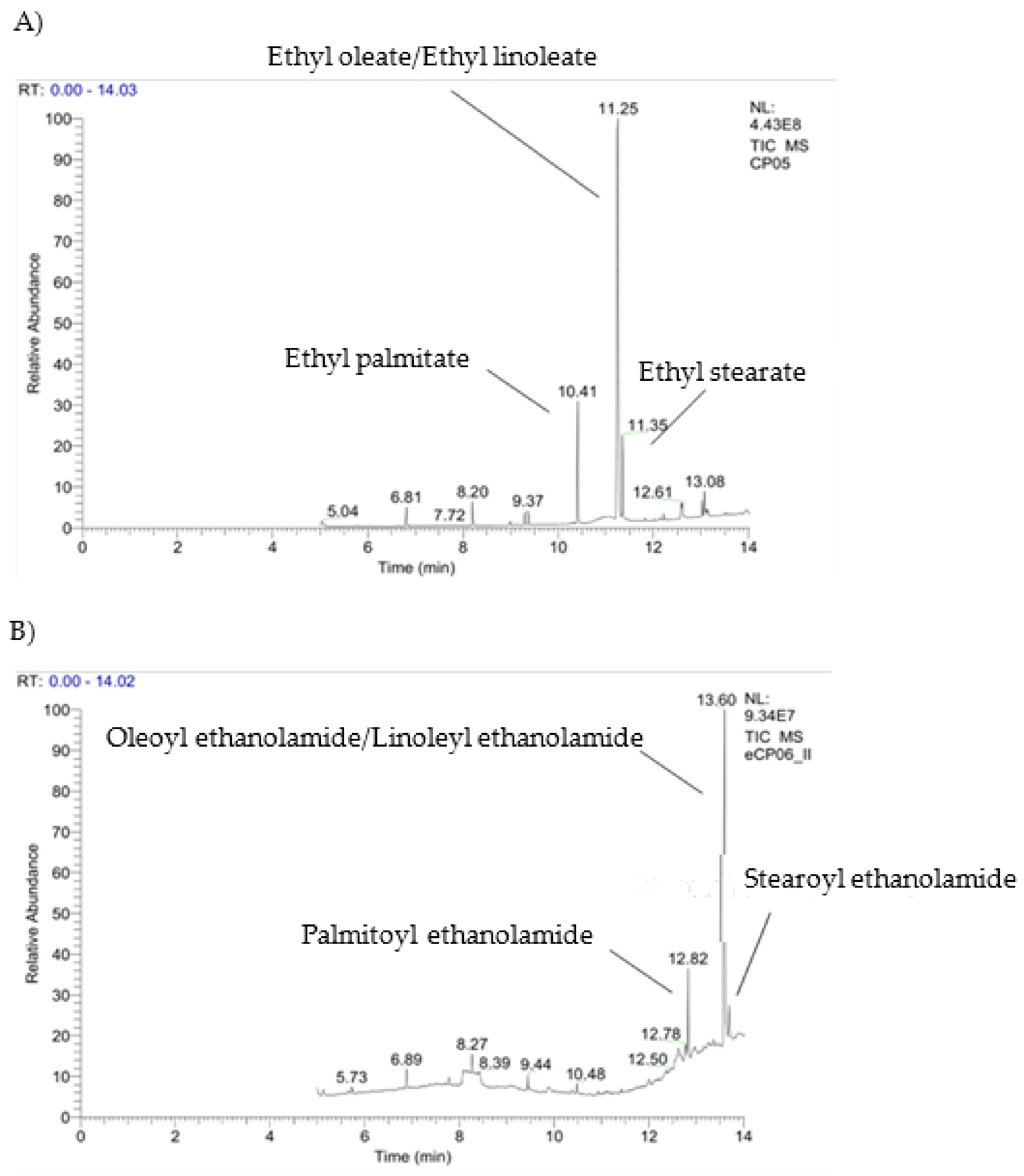
2.3. Enzymatic Manipulation of Cucurbita pepo L. Extracts
3. Materials and Methods
3.1. Materials
3.2. Cucurbita pepo L. Residual Extractions
3.2.1. n-Hexane Residual Extraction
3.2.2. Supercritical CO2: Single-Step Extraction
3.2.3. Supercritical CO2: Sequential Extraction
3.3. Purification and Separation of Neutral and Acidic Fractions
3.4. GC-MS Characterization
3.5. Production and Purification of Recombinant Oleate Hydratase OhyA2
3.6. Biotransformations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Vickers, A.; Zollman, C. ABC of complementary medicine: Herbal medicine. BMJ 1999, 319, 1050–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patwardhan, B.; Mashelkar, R.A. Traditional medicine-inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov. Today 2009, 14, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Beghyn, T.; Deprez-Poulain, R.; Willand, N.; Folleas, B.; Deprez, B. Natural compounds: Leads or ideas? Bioinspired molecules for drug discovery. Chem. Biol. Drug Des. 2008, 72, 3–15. [Google Scholar] [CrossRef]
- Waterman, P.G. Phytochemical Dictionary. A Handbook of Bioactive Compounds from Plants. Biochem. Syst. Ecol. 1993, 21, 849. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC-Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value-added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Hrabovski, N.; Sinadinović-Fišer, S.; Nikolovski, B.; Sovilj, M.; Borota, O. Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO2. Eur. J. Lipid Sci. Technol. 2012, 114, 1204–1211. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Medjakovic, S.; Hobiger, S.; Ardjomand-Woelkart, K.; Bucar, F.; Jungbauer, A. Pumpkin seed extract: Cell growth inhibition of hyperplastic and cancer cells, independent of steroid hormone receptors. Fitoterapia 2016, 110, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Vahlensieck, W.; Theurer, C.; Pfitzer, E.; Patz, B.; Banik, N.; Engelmann, U. Effects of pumpkin seed in men with lower urinary tract symptoms due to benign prostatic hyperplasia in the one-year, randomized, placebo-controlled GRANU study. Urol. Int. 2015, 94, 286–295. [Google Scholar] [CrossRef]
- Montesano, D.; Blasi, F.; Simonetti, M.S.; Santini, A.; Cossignani, L. Chemical and nutritional characterization of seed oil from Cucurbita maxima L. (Var. Berrettina) pumpkin. Foods 2018, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Heim, S.; Seibt, S.; Stier, H.; Moré, M.I. Uromedic® Pumpkin Seed Derived Δ7-Sterols, Extract and Oil Inhibit 5α-Reductases and Bind to Androgen Receptor. Pharmacol. Pharm. 2018, 9, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Ivanovic, J.; Ristic, M.; Skala, D. Supercritical CO2 extraction of Helichrysum italicum: Influence of CO2 density and moisture content of plant material. J. Supercrit. Fluids 2011, 57, 129–136. [Google Scholar] [CrossRef]
- Machmudah, S.; Kondo, M.; Sasaki, M.; Goto, M.; Munemasa, J.; Yamagata, M. Pressure effect in supercritical CO2 extraction of plant seeds. J. Supercrit. Fluids 2008, 44, 301–307. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Herrero, M.; Castro-Puyana, M.; Ibáñez, E. Supercritical fluid extraction. RSC Green Chem. 2013, 1, 196–230. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, J.P.; Pryde, E.H. Supercritical CO2 extraction of lipid-bearing materials and characterization of the products. J. Am. Oil Chem. Soc. 1984, 61, 223–228. [Google Scholar] [CrossRef]
- Im, D.-S. GPR119 and GPR55 as Receptors for Fatty Acid Ethanolamides, Oleoylethanolamide and Palmitoylethanolamide. Int. J. Mol. Sci. 2021, 22, 1034. [Google Scholar] [CrossRef] [PubMed]
- Laleh, P.; Yaser, K.; Alireza, O. Oleoylethanolamide: A novel pharmaceutical agent in the management of obesity—An updated review. J. Cell. Physiol. 2019, 234, 7893–7902. [Google Scholar] [CrossRef] [PubMed]
- Engleder, M.; Pichler, H. On the current role of hydratases in biocatalysis. Appl. Microbiol. Biotechnol. 2018, 102, 5841–5858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagedoorn, P.L.; Hollmann, F.; Hanefeld, U. Novel oleate hydratases and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2021, 105, 6159–6172. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; De Simeis, D.; Marzorati, S.; Valentino, M. Oleate hydratase from lactobacillus rhamnosus atcc 53103: A fadh2-dependent enzyme with remarkable industrial potential. Catalysts 2021, 11, 1051. [Google Scholar] [CrossRef]
- Castagna, A.; De Simeis, D.; Ferrandi, E.E.; Marzorati, S.; Monti, D.; Serra, S.; Valentino, M. Recombinant oleate hydratase from lactobacillus rhamnosus atcc 53103: Enzyme expression and design of a reliable experimental procedure for the stereoselective hydration of oleic acid. Catalysts 2020, 10, 1122. [Google Scholar] [CrossRef]
- Bonina-Noseworthy, J.; Loy, J.B.; Curran-Celentano, J.; Sideman, R.; Kopsell, D.A. Carotenoid concentration and composition in winter squash: Variability associated with different cultigens, harvest maturities, and storage times. HortScience 2016, 51, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. A simple model for super critical fluid extraction of bio oils from biomass. Energy Convers. Manag. 2011, 52, 652–657. [Google Scholar] [CrossRef]
- El-Aziz, A.B.A.; El-Kalek, H.H.A. Antimicrobial proteins and oil seeds from pumpkin (Cucurbita moschata). Nat. Sci. 2011, 9, 105–119. [Google Scholar]
- Shi, X.; Wu, H.; Shi, J.; Xue, S.J.; Wang, D.; Wang, W.; Cheng, A.; Gong, Z.; Chen, X.; Wang, C. Effect of modifier on the composition and antioxidant activity of carotenoid extracts from pumpkin (Cucurbita maxima) by supercritical CO2. LWT Food Sci. Technol. 2013, 51, 433–440. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.H.; Lee, S.M.; Lee, J.; Park, J.B. Adding value to plant oils and fatty acids: Biological transformation of fatty acids into ω-hydroxycarboxylic, α,ω-dicarboxylic, and ω-aminocarboxylic acids. J. Biotechnol. 2015, 216, 158–166. [Google Scholar] [CrossRef]
- Patel, S. Pumpkin (Cucurbita sp.) seeds as nutraceutic: A review on status quo and scopes. Med. J. Nutr. Metab. 2013, 6, 183–189. [Google Scholar] [CrossRef]
- Schinas, P.; Karavalakis, G.; Davaris, C.; Anastopoulos, G.; Karonis, D.; Zannikos, F.; Stournas, S.; Lois, E. Pumpkin (Cucurbita pepo L.) seed oil as an alternative feedstock for the production of biodiesel in Greece. Biomass Bioenergy 2009, 33, 44–49. [Google Scholar] [CrossRef]
- Srbinoska, M.; Hrabovski, N.; Rafajlovska, V.; Sinadinović-Fišer, S. Characterization of the seed and seed extracts of the pumpkins Cucurbita maxima D. and Cucurbita pepo L. from Macedonia. Maced. J. Chem. Chem. Eng. 2012, 31, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Grzybek, M.; Kukula-Koch, W.; Strachecka, A.; Jaworska, A.; Phiri, A.M.; Paleolog, J.; Tomczuk, K. Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts—In Vitro and In Vivo studies. Int. J. Mol. Sci. 2016, 17, 1456. [Google Scholar] [CrossRef]
- Younis, Y.M.H.; Ghirmay, S.; Al-Shihry, S.S. African Cucurbita pepo L.: Properties of seed and variability in fatty acid composition of seed oil. Phytochemistry 2000, 54, 71–75. [Google Scholar] [CrossRef]
- Younis, Y.M.H.; Ghirmay, S. Characteristics and chemical composition of the oil of Cucurbita pepo seeds. Bull. Chem. Soc. Ethiop. 1998, 12, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Petkova, Z.Y.; Antova, G.A. Changes in the composition of pumpkin seeds (Cucurbita moschata) during development and maturation. Grasas y Aceites 2015, 66, e058. [Google Scholar] [CrossRef] [Green Version]
- Akomolafe, S.F.; Oboh, G.; Oyeleye, S.I.; Molehin, O.R.; Ogunsuyi, O.B. Phenolic composition and inhibitory ability of methanolic extract from pumpkin (Cucurbita pepo L.) seeds on Fe-induced thiobarbituric acid reactive species in albino rat’s testicular tissue in-vitro. J. Appl. Pharm. Sci. 2016, 6, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Durante, M.; Lenucci, M.S.; D’Amico, L.; Piro, G.; Mita, G. Effect of drying and co-matrix addition on the yield and quality of supercritical CO2 extracted pumpkin (Cucurbita moschata Duch.) oil. Food Chem. 2014, 148, 314–320. [Google Scholar] [CrossRef]
- Whitten, K.M.; Makriyannis, A.; Vadivel, S.K. Enzymatic synthesis of N-acylethanolamines: Direct method for the aminolysis of esters. Tetrahedron Lett. 2012, 53, 5753–5755. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Jiang, C.; Wang, X.; Wang, X.; Jin, Q.; Wang, X. Eco-Friendly Production of Fatty Amides Using 1-Monoacylglycerols as Acyl Donors. ACS Sustain. Chem. Eng. 2020, 8, 9589–9596. [Google Scholar] [CrossRef]
- Lima, R.N.; dos Anjos, C.S.; Orozco, E.V.M.; Porto, A.L.M. Versatility of Candida antarctica lipase in the amide bond formation applied in organic synthesis and biotechnological processes. Mol. Catal. 2019, 466, 75–105. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wang, T. Synthesis of oleoylethanolamide using lipase. J. Agric. Food Chem. 2012, 60, 451–457. [Google Scholar] [CrossRef]
- Tufvesson, P.; Annerling, A.; Hatti-Kaul, R.; Adlercreutz, D. Solvent-free enzymatic synthesis of fatty alkanolamides. Biotechnol. Bioeng. 2007, 97, 447–453. [Google Scholar] [CrossRef]
- Couturier, L.; Taupin, D.; Yvergnaux, F. Lipase-catalyzed chemoselective aminolysis of various aminoalcohols with fatty acids. J. Mol. Catal. B Enzym. 2009, 56, 29–33. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Ma, Y.; Jin, Q.; Wang, X. Lipozyme 435-catalyzed synthesis of eicosapentaenoyl ethanolamide in a solvent-free system. J. Mol. Catal. B Enzym. 2015, 122, 233–239. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Jin, Q.; Wang, X.; Hu, J.; Wang, X. Preparation of arachidonoyl ethanolamide by enzymatic amidation of arachidonic acid purified from microbial oil. Process Biochem. 2018, 66, 120–125. [Google Scholar] [CrossRef]
- Quintana, P.G.; García Liñares, G.; Chanquia, S.N.; Gorojod, R.M.; Kotler, M.L.; Baldessari, A. Improved Enzymatic Procedure for the Synthesis of Anandamide and N -Fatty Acylalkanolamine Analogues: A Combination Strategy to Antitumor Activity. Eur. J. Org. Chem. 2016, 2016, 518–528. [Google Scholar] [CrossRef]
- Gunawan, E.R.; Suhendra, D.; NuansaWindari, B.; Kurniawati, L. Enzymatic synthesis of palmitoylethanolamide from ketapang kernel oil. J. Phys. Conf. Ser. 2019, 1321, 022034. [Google Scholar] [CrossRef]
- Song, J.-W.; Jeon, E.-Y.; Song, D.-H.; Jang, H.-Y.; Bornscheuer, U.T.; Oh, D.-K.; Park, J.-B. Multistep Enzymatic Synthesis of Long-Chain α,ω-Dicarboxylic and ω-Hydroxycarboxylic Acids from Renewable Fatty Acids and Plant Oils. Angew. Chem. Int. Ed. 2013, 52, 2534–2537. [Google Scholar] [CrossRef]
- Song, J.-W.; Lee, J.-H.; Bornscheuer, U.T.; Park, J.-B. Microbial Synthesis of Medium-Chain α,ω-Dicarboxylic Acids and ω-Aminocarboxylic Acids from Renewable Long-Chain Fatty Acids. Adv. Synth. Catal. 2014, 356, 1782–1788. [Google Scholar] [CrossRef]
- Brenna, E.; Colombo, D.; Di Lecce, G.; Gatti, F.G.; Ghezzi, M.C.; Tentori, F.; Tessaro, D.; Viola, M. Conversion of Oleic Acid into Azelaic and Pelargonic Acid by a Chemo-Enzymatic Route. Molecules 2020, 25, 1882. [Google Scholar] [CrossRef]
- Kang, W.-R.; Seo, M.-J.; Shin, K.-C.; Park, J.-B.; Oh, D.-K. Comparison of Biochemical Properties of the Original and Newly Identified Oleate Hydratases from Stenotrophomonas maltophilia. Appl. Environ. Microbiol. 2017, 83, e03351-16. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Kishino, S.; Hirata, A.; Park, S.B.; Kitamura, N.; Ogawa, J. Characterization of the linoleic acid δ9 hydratase catalyzing the first step of polyunsaturated fatty acid saturation metabolism in Lactobacillus plantarum AKU 1009a. J. Biosci. Bioeng. 2015, 119, 636–641. [Google Scholar] [CrossRef] [Green Version]
- Engleder, M.; Pavkov-Keller, T.; Emmerstorfer, A.; Hromic, A.; Schrempf, S.; Steinkellner, G.; Wriessnegger, T.; Leitner, E.; Strohmeier, G.A.; Kaluzna, I.; et al. Structure-Based Mechanism of Oleate Hydratase from Elizabethkingia meningoseptica. ChemBioChem 2015, 16, 1730–1734. [Google Scholar] [CrossRef] [Green Version]
- Allow, C. Determination of Saponification Value and Unsaponifiable value. Eur. Pharm. 5.0 1998, 129–130. [Google Scholar]
- Priestap, H.A.; Quirke, J.M.E.; Houle, P.; Bennett, B.C. Fatty acid composition of fruits of two forms of Serenoa repens. Chem. Nat. Compd. 2011, 47, 511–514. [Google Scholar] [CrossRef]
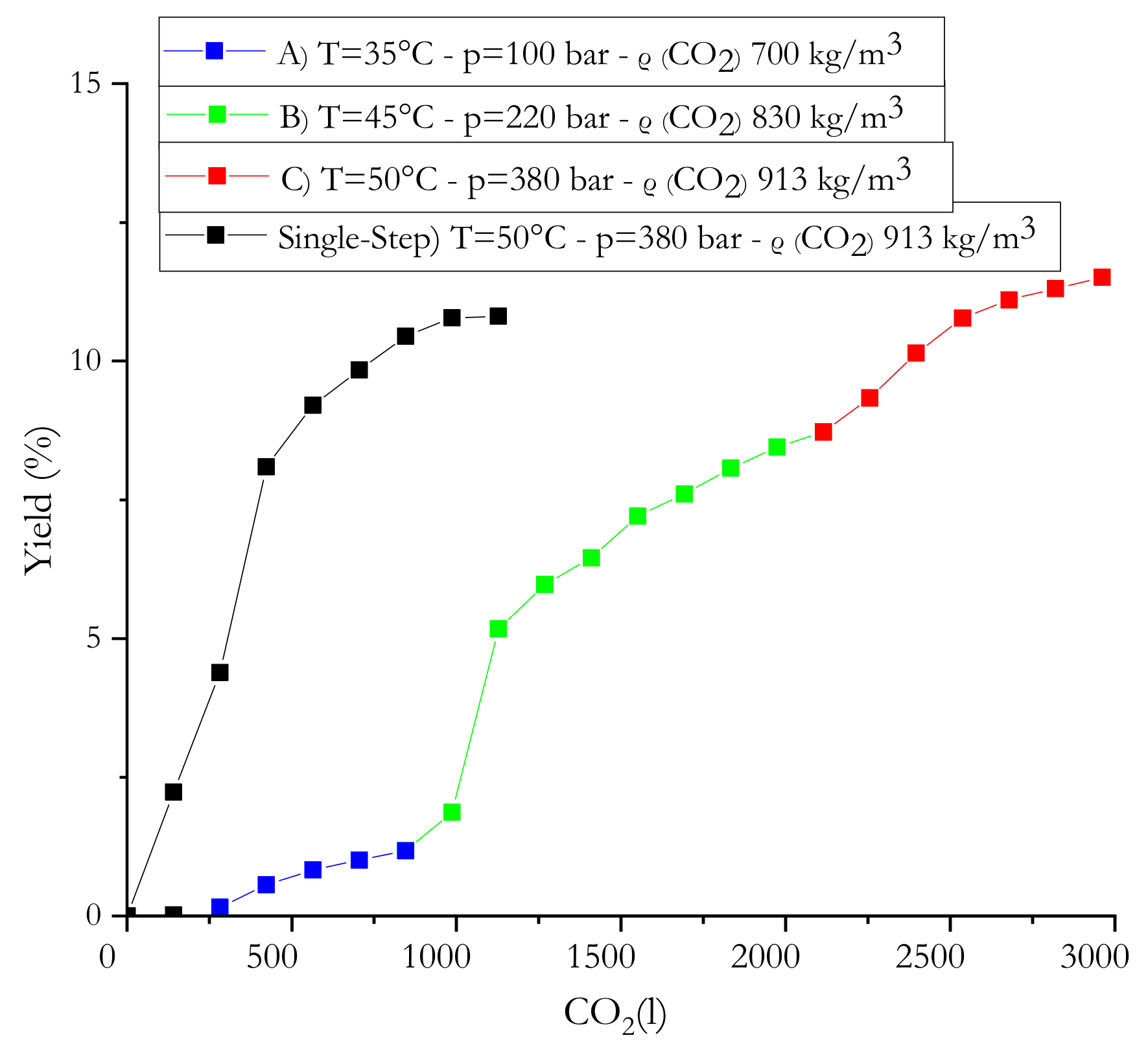
| Entry | Extraction Conditions | Extraction Yield (%) | Free Fatty Acids (%) | Neutral Components (%) |
|---|---|---|---|---|
| 1 | Single step T = 50 °C; p = 380 bar dCO2 913 kg/m3 | 10.8 | 14.5 | Saponifiable 84.4 Unsaponifiable 1.1 |
| 2 | (A) T = 35 °C; p = 100 bar dCO2 700 kg/m3 | 1.2 | 71.0 | Saponifiable 28.5 Unsaponifiable 0.5 |
| 3 | (B) T = 45 °C; p = 220 bar dCO2 830 kg/m3 | 7.5 | 23.6 | Saponifiable 72.1 Unsaponifiable 4.1 |
| 4 | (C) T = 50 °C; p = 380 bar dCO2 913 kg/m3 | 2.8 | 4.2 | Saponifiable 95.0 Unsaponifiable 0.8 |
| A + B + C | 11.5 | |||
| 5 | n-Hexane | 10.3 | 15.3 | Saponifiable 83.6 Unsaponifiable 1.1 |
| Fatty Acids | Cp_sc-CO2/Single-Step Relative Abundance (%) * | Cp_n-Hexane Relative Abundance (%) * | Cp Oils from Literature Relative Abundance (%) [15,33,35,36,37,38,39,40,41,42,43,44] ** |
|---|---|---|---|
| Palmitic acid (16.0) | 11 ± 1 | 12.1 ± 0.5 | 8–15 |
| Linoleic acid (18.2) | 34 ± 1 | 33.6 ± 3 | 25–35 |
| Oleic acid (18.1) | 45 ± 2 | 44.7 ± 1 | 35–50 |
| Stearic acid (18.0) | 8.0 ± 0.4 | 8.4 ± 1 | 5–15 |
| Arachidic acid (20.0) | 0.7 ± 0.2 | 1.2 ± 0.1 | 0.5–1 |
| Saturated fatty acids | 19 ± 1 | 20 ± 2 | 15–25 |
| Monounsaturated fatty acids | 45 ± 1 | 45 ± 3 | 35–45 |
| Polyunsaturated fatty acids | 33 ± 2 | 34 ± 1 | 25–35 |
| Sterols | Main Fragmentation Ions, m/z | ||||
|---|---|---|---|---|---|
| [M]+ | [M-15]+ | [M-90]+ | [M-105]+ | Sterol Abundance (%) | |
| β-Sitosterol | 486 | 471 | 396 | 381 | 1.6 ± 0.3 |
| Δ7,25-Stigmastadienol | 484 | 469 | 394 | 379 | 31 ± 2 |
| Δ7-Stigmastenol | 486 | 471 | 396 | 381 | 39 ± 2 |
| Δ7-Spinasterol | 484 | 469 | 394 | 379 | 27 ± 3 |
| Δ7,22,25-Stigmastatrienol | 482 | 467 | 392 | 377 | 4.1 ± 0.6 |
| Δ7-Avenasterol | 484 | 469 | 394 | 379 | 7 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massironi, A.; Di Fonzo, A.; Bassanini, I.; Ferrandi, E.E.; Marzorati, S.; Monti, D.; Verotta, L. Selective Supercritical CO2 Extraction and Biocatalytic Valorization of Cucurbita pepo L. Industrial Residuals. Molecules 2022, 27, 4783. https://doi.org/10.3390/molecules27154783
Massironi A, Di Fonzo A, Bassanini I, Ferrandi EE, Marzorati S, Monti D, Verotta L. Selective Supercritical CO2 Extraction and Biocatalytic Valorization of Cucurbita pepo L. Industrial Residuals. Molecules. 2022; 27(15):4783. https://doi.org/10.3390/molecules27154783
Chicago/Turabian StyleMassironi, Alessio, Alessandro Di Fonzo, Ivan Bassanini, Erica Elisa Ferrandi, Stefania Marzorati, Daniela Monti, and Luisella Verotta. 2022. "Selective Supercritical CO2 Extraction and Biocatalytic Valorization of Cucurbita pepo L. Industrial Residuals" Molecules 27, no. 15: 4783. https://doi.org/10.3390/molecules27154783
APA StyleMassironi, A., Di Fonzo, A., Bassanini, I., Ferrandi, E. E., Marzorati, S., Monti, D., & Verotta, L. (2022). Selective Supercritical CO2 Extraction and Biocatalytic Valorization of Cucurbita pepo L. Industrial Residuals. Molecules, 27(15), 4783. https://doi.org/10.3390/molecules27154783











