Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield
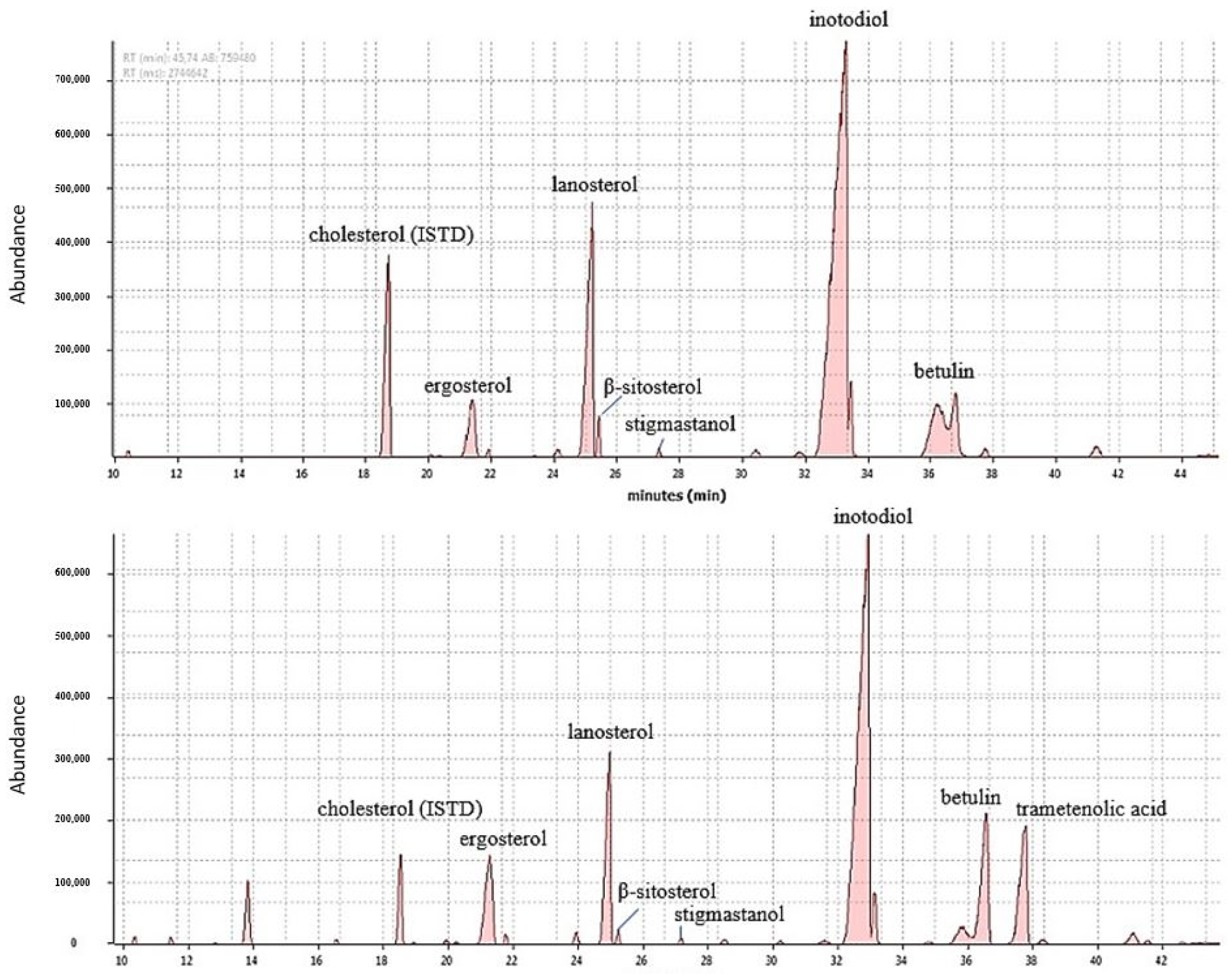
2.2. Identification of Triterpenoids in SFE and Folch Extracts by Mass Spectra of GC-MS
2.3. Quantification of Triterpenoids in SFE and Folch Extracts
2.4. Recovery of Triterpenoids with SFE
3. Materials and Methods
3.1. Chemicals
3.2. Sample Material
3.3. SFE Apparatus and Procedure
3.4. Folch Extraction
3.5. Saponification of the Extracts
3.6. Gas Chromatographic Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balandaykin, M.E.; Zmitrovich, I.V. Review on Chaga Medicinal Mushroom, Inonotus obliquus (Higher Basidiomycetes): Realm of Medicinal Applications and Approaches on Estimating its Resource Potential. Int. J. Med. Mushrooms 2015, 17, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Saar, M. Fungi in khanty folk medicine. J. Ethnopharmacol. 1991, 31, 175–179. [Google Scholar] [CrossRef]
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.D.; Yu, L.; Wang, P.; Kou, P.; Li, J.; Wang, L.T.; Wang, W.; Yao, L.P.; Zhao, X.H.; Fu, Y.J. Inotodiol inhibits cells migration and invasion and induces apoptosis via p53-dependent pathway in HeLa cells. Phytomedicine 2019, 60, 152957. [Google Scholar] [CrossRef]
- Nomura, M.; Takahashi, T.; Uesugi, A.; Tanaka, R.; Kobayashi, S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res. 2008, 28, 2691–2696. [Google Scholar]
- Zhong, X.H.; Wang, L.B.; Sun, D.Z. Effects of inotodiol extracts from inonotus obliquus on proliferation cycle and apoptotic gene of human lung adenocarcinoma cell line A549. Chin. J. Integr. Med. 2011, 17, 218–223. [Google Scholar] [CrossRef]
- Nakata, T.; Yamada, T.; Taji, S.; Ohishi, H.; Wada, S.I.; Tokuda, H.; Sakuma, K.; Tanaka, R. Structure determination of inonotsuoxides A and B and in vivo anti-tumor promoting activity of inotodiol from the sclerotia of Inonotus obliquus. Bioorganic Med. Chem. 2007, 15, 257–264. [Google Scholar] [CrossRef]
- Zhao, F.; Mai, Q.; Ma, J.; Xu, M.; Wang, X.; Cui, T.; Qiu, F.; Han, G. Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia 2015, 101, 34–40. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, W. Deciphering the antitumoral potential of the bioactive metabolites from medicinal mushroom Inonotus obliquus. J. Ethnopharmacol. 2021, 265, 113321. [Google Scholar] [CrossRef]
- Jalali, H.T.; Petronilho, S.; Villaverde, J.J.; Coimbra, M.A.; Domingues, M.R.M.; Ebrahimian, Z.J.; Silvestre, A.J.D.; Rocha, S.M. Assessment of the sesquiterpenic profile of Ferula gummosa oleo-gum-resin (galbanum) from Iran. Contributes to its valuation as a potential source of sesquiterpenic compounds. Ind. Crops Prod. 2013, 44, 185–191. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Domingues, R.M.A.; Oliveira, C.S.D.; Villaverde, J.J.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Secondary metabolites from Eucalyptus grandis wood cultivated in Portugal, Brazil and South Africa. Ind. Crops Prod. 2017, 95, 357–364. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Uddin, M.S.; Sarker, M.Z.I.; Ferdosh, S.; Akanda, M.J.H.; Easmin, M.S.; Bt Shamsudin, S.H.; Yunus, K. Bin Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: A review. J. Sci. Food Agric. 2015, 95, 1385–1394. [Google Scholar] [CrossRef]
- Huang, L.; Zhong, T.; Chen, T.; Ye, Z.; Chen, G. Identification of β-sitosterol, stigmasterol and ergosterin in A. roxburghii using supercritical fluid extraction followed by liquid chromatography/atmospheric pressure chemical ionization ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3024–3032. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Aldars-García, L.; Palanisamy, M.; Jiverdeanu, R.M.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Sterol enriched fractions obtained from Agaricus bisporus fruiting bodies and by-products by compressed fluid technologies (PLE and SFE). Innov. Food Sci. Emerg. Technol. 2013, 18, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.M.A.; Oliveira, E.L.G.; Freire, C.S.R.; Couto, R.M.; Simões, P.C.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of Eucalyptus globulus bark-A promising approach for triterpenoid production. Int. J. Mol. Sci. 2012, 13, 7648–7662. [Google Scholar] [CrossRef] [Green Version]
- Abu-Reidah, I.M.; Critch, A.L.; Manful, C.F.; Rajakaruna, A.; Vidal, N.P.; Pham, T.H.; Cheema, M.; Thomas, R. Effects of ph and temperature on water under pressurized conditions in the extraction of nutraceuticals from chaga (Inonotus obliquus) mushroom. Antioxidants 2021, 10, 1322. [Google Scholar] [CrossRef]
- Fink, J.K. Terpene resins. In Reactive Polymers Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 303–315. [Google Scholar]
- Cole, B.J.W.; Bentley, M.D.; Hua, Y.; Bu, L. Triterpenoid Constituents in The Outer Bark of Betula Alleghaniensis (Yellow Birch). J. Wood Chem. Technol. 1991, 11, 209–223. [Google Scholar] [CrossRef]
- Vikström, F.; Holmbom, B.; Hamunen, A. Sterols and Triterpenyl alcohols in common pulpwoods and black liquor soaps. Holz Als Roh-Und Werkst. 2005, 63, 303–308. [Google Scholar] [CrossRef]
- Kahlos, K.; Hiltunen, R. Gas Chromatographic mass spectrometric study of some sterols and lupines from Inonotus obliquus. Acta Pharm. Fenn. 1987, 96, 80–85. [Google Scholar]
- Kahlos, K.; Hiltunen, R. Gas chromatographic spectrometry of some lanostanes from Inonotus obliquus. Acta Pharm. Fenn. 1988, 97, 45–49. [Google Scholar]
- Goad, J.L.; Akihisa, T. Analysis of Sterols; Chapman and Hall: New York, NY, USA, 1997. [Google Scholar]
- Honda, M.; Tint, G.S.; Honda, A.; Batta, A.K.; Chen, T.S.; Shefer, S.; Salen, G. Measurement of 3 beta-hydroxysteroid delta 7-reductase activity in cultured skin fibroblasts utilizing ergosterol as a substrate: A new method for the diagnosis of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 1996, 37, 2433–2438. [Google Scholar] [CrossRef]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Knights, B.A. Identification of Plant Sterols Using Combined GLC/Mass Spectrometry. J. Chromatogr. Sci. 1967, 5, 273–282. [Google Scholar] [CrossRef]
- Modugno, F.; Ribechini, E.; Colombini, M.P. Chemical study of triterpenoid resinous materials in archaeological findings by means of direct exposure electron ionisation mass spectrometry and gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1787–1800. [Google Scholar] [CrossRef]
- Prokes, L.; Hlozek, M. Identification of Some Adhesives and Wood Pyrolysis Products of Archaeological Origin by Direct Inlet Mass Spectrometry. Chem. Anal. 2007, 52, 700–713. [Google Scholar]
- Ahmad, F.B.H.; Ghaffari, M.; Basri, M.; Rahman, M.B.A. Spectroscopic Data of 3-O-Acetyl-betulinic Acid: An Antitumor Reagent. Asian J. Chem. 2010, 22, 3186–3192. [Google Scholar]
- Wang, L.X.; Lu, Z.M.; Geng, Y.; Zhang, X.M.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Stimulated production of steroids in Inonotus obliquus by host factors from birch. J. Biosci. Bioeng. 2014, 118, 728–731. [Google Scholar] [CrossRef]
- Armbruster, M.; Mönckedieck, M.; Scherließ, R.; Daniels, R.; Wahl, M.A. Birch bark dry extract by supercritical fluid technology: Extract characterisation and use for stabilisation of semisolid systems. Appl. Sci. 2017, 7, 292. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Liu, T.; Xiang, X.; Gu, Q. Sterol composition in field-grown and cultured mycelia of Inonotus obliquus. Yao Xue Xue Bao Acta Pharm. Sin. 2007, 42, 750–756. [Google Scholar]
- Kim, J.; Yang, S.C.; Hwang, A.Y.; Cho, H.; Hwang, K.T. Composition of Triterpenoids in Inonotus obliquus and Their Anti-Proliferative Activity on Cancer Cell Lines. Molecules 2020, 25, 4066. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- RStudio. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 14 December 2021).

| Extraction Limiting Factor | Temperature (K) | Temperature (°C) | Pressure (bar) | CO2 Density (kgm−3) | Hildebrand Solubility Parameter * (MPa1/2) | Solvent/ Sample (g/g) | Extraction Yield # (%) | Total Triterpenoids (mg/100 g Chaga) |
|---|---|---|---|---|---|---|---|---|
| 50 mL | 314 | 40 | 281 | 898.45 | 15.95 | 22.22 ± 0.16 | 0.06 ± 0.02 a | 184.85 ± 6.05 a |
| 50 mL | 324 | 50 | 350 | 899.40 | 15.96 | 22.42 ± 0.06 | 0.11 ± 0.02 b | 197.66 ± 15.08 b |
| 50 mL | 314 | 40 | 350 | 934.90 | 16.59 | 23.17 ± 0.13 | 0.07 ± 0.03 a | 179.66 ± 3.81 a |
| 15 min | 314 | 40 | 281 | 898.45 | 15.95 | 22.21 ± 1.49 | 0.14 ± 0.07 b | 179.60 ± 5.18 a |
| 20 min | 324 | 50 | 350 | 899.40 | 15.96 | 31.83 ± 2.04 | 0.06 ± 0.02 a | 197.21 ± 8.67 b |
| Folch | - | - | - | - | 25.53 | 102.36 ± 0.52 § | 1.21 ± 0.01 c | 352.73 ± 4.04 c |
| Triterpenoid | Reference b | Fragment Ions (m/z) a | ||||||
|---|---|---|---|---|---|---|---|---|
| Lanosterol | 1 | 393 (100) | 69(78) | 498 (52, M+) | 483(40) | 109 (32) | 187 (12) | 227 (11) |
| Inotodiol | 2 | 393 (100) | 69 (98) | 498 (31, M+) | 483 (21) | 109 (38) | 187 (16) | 227 (14) |
| 1 | 297 (85) | 517 (73) | 571 (65) | 427 (57) | 387 (33) | 337 (10) | 586 (7, M+) | |
| Trametenolic acid | 2 | 297 (85) | 517 (31) | 571 (5) | 427 (17) | 387 (5) | 337 (100) | - |
| 1 | 281 (24) | 187 (24) | 213 (17) | 585 (7) | 495 (7) | 405 (3) | 600 (3, M+) | |
| Ergosterol | 2 | 281 (31) | 187 (21) | 213 (9) | 585 (31) | 495 (19) | - | 600 (13, M+) |
| 1 | 363 (100) | 69 (85) | 337 (58) | 468 (56, M+) | 253 (55) | 131 (48) | 378 (28) | |
| Cholesterol | 2 | 363 (100) | 69 (48) | 337 (65) | 468 (19, M+) | - | 131 (20) | 378 (19) |
| 1 | 129 (100) | 229 (44) | 107 (40) | 121 (36) | 368 (25) | 353 (15) | 255 (15) | |
| β-sitosterol | 2 | 129 (100) | - | 107 (29) | - | 368 (71) | 353 (33) | 255 (17) |
| 2 | 129 (100) | 357 (72) | 396 (67) | 73 (51) | 75 (33) | - | - | |
| Stigmastanol | 2 | 69 (100) | 393 (46) | 73 (40) | 75 (25) | - | - | - |
| Extraction Limiting Factor | Temperature (K) | Pressure (bar) | CO2 Density (kgm−3) | Ergosterol (mg/100 g Chaga) | Lanosterol (mg/100 g Chaga) | β-sitosterol (mg/100 g Chaga) | Stigmastanol (mg/100 g Chaga) | Inotodiol (mg/100 g Chaga) | Betulin (mg/100 g Chaga) | Trametenolic Acid (mg/100 g Chaga) |
|---|---|---|---|---|---|---|---|---|---|---|
| 50 mL | 314 | 281 | 898.45 | 16.74 ± 0.7 a | 59.87 ± 5.05 a | 3.47 ± 0.23 a,b | 1.33 ± 0.09 a | 91.34 ± 7.37 a | 12.11 ± 0.82 a | n.d. |
| 50 mL | 324 | 350 | 899.4 | 17.19 ± 0.54 a | 62.65 ± 4.26 a | 3.69 ± 0.31 b | 1.29 ± 0.5 a | 99.63 ± 6.29 b | 13.21 ± 5.48 a | n.d. |
| 50 mL | 314 | 350 | 934.9 | 17.05 ± 0.57 a | 58.83 ± 2.08 a | 3.19 ± 0.23 a | 1.12 ± 0.43 a | 87.69 ± 1.07 a | 11.80 ± 0.84 a | n.d. |
| 15 min | 314 | 281 | 898.45 | 17.27 ± 0.48 a | 61.33 ± 2.49 a | 3.91 ± 0.6 b | 1.23 ± 0.47 a | 87.48 ± 5.83 a | 8.39 ± 5.31 a | n.d. |
| 20 min | 324 | 350 | 899.4 | 18.49 ± 0.58 b | 58.98 ± 4.28 a | 3.57 ± 0.23 a,b | 1.04 ± 0.6 a | 101.08 ± 3.56 b | 14.05 ± 5.37 a | n.d. |
| Folch | - | - | - | 39.86 ± 1.46 c | 80.54 ± 0.98 b | 5.43 ± 0.36 c | 1.90 ± 0.21 b | 138.58 ± 4.42 c | 37.58 ± 3.96 b | 48.84 ± 0.95 |
| Extraction Limiting Factor | Temperature (K) | Pressure (bar) | CO2 Density (kgm−3) | Ergosterol (%) | Lanosterol (%) | β-Sitosterol (%) | Stigmastanol (%) | Inotodiol (%) | Betulin (%) | Total Terpenes (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 50 mL | 314 | 281 | 898.45 | 41.91 ± 1.04 a | 74.35 ± 6.84 a | 63.63 ± 4.02 a.b | 70.31 ± 9.2 a | 66.17 ± 5.37 a.b | 32.39 ± 4.97 a.b | 52.41 ± 1.72 a |
| 50 mL | 324 | 350 | 899.40 | 43.11 ± 2.48 a | 77.72 ± 4.56 a | 67.66 ± 4.61 a | 76.15 ± 8.74 a | 72.16 ± 4.3 b.c | 39.53 ± 11.52 a | 56.04 ± 4.27 b |
| 50 mL | 314 | 350 | 934.90 | 42.74 ± 2.16 a | 73.04 ± 3.35 a | 58.79 ± 7.4 a | 66.34 ± 10.67 a | 63.54 ± 2.18 a | 31.30 ± 2.47 a.b | 50.94 ± 1.08 a |
| 15 min | 314 | 281 | 898.45 | 43.31 ± 2.22 a | 76.11 ± 2.82 a | 71.7 ± 9.79 b | 72.25 ± 5.74 a | 63.47 ± 5.8 a | 26.36 ± 13.37 b | 50.92 ± 1.47 a |
| 20 min | 324 | 350 | 899.40 | 46.33 ± 1.31 b | 73.16 ± 4.69 a | 65.84 ± 7.03 a.b | 70.9 ± 12.1 a | 73.28 ± 4.1 c | 42.90 ± 8.08 a | 55.91 ± 2.46 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, N.; Beltrame, G.; Tarvainen, M.; Suomela, J.-P.; Yang, B. Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus. Molecules 2022, 27, 1880. https://doi.org/10.3390/molecules27061880
Huynh N, Beltrame G, Tarvainen M, Suomela J-P, Yang B. Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus. Molecules. 2022; 27(6):1880. https://doi.org/10.3390/molecules27061880
Chicago/Turabian StyleHuynh, Nghia, Gabriele Beltrame, Marko Tarvainen, Jukka-Pekka Suomela, and Baoru Yang. 2022. "Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus" Molecules 27, no. 6: 1880. https://doi.org/10.3390/molecules27061880
APA StyleHuynh, N., Beltrame, G., Tarvainen, M., Suomela, J.-P., & Yang, B. (2022). Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus. Molecules, 27(6), 1880. https://doi.org/10.3390/molecules27061880







