Associations of Bone Mineral Density with RANKL and Osteoprotegerin in Arab Postmenopausal Women: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Biochemical Analysis
2.3. Data Analysis
3. Results
3.1. Clinical Characteristics of Study Groups
3.2. Bivariate Correlations of RANKL, OPG, and the RANKL/OPG Ratio with Other Parameters
3.3. Stepwise Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, N.B.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr. Pract. 2010, 16, 1016. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.-i.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Lacey, D.; Timms, E.; Tan, H.-L.; Kelley, M.; Dunstan, C.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Khosla, S. Minireview: The opg/rankl/rank system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Kostenuik, P.J. Osteoprotegerin A Physiological and Pharmacological Inhibitor of Bone Resorption. Curr. Pharm. Des. 2001, 7, 613–635. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9, S1. [Google Scholar] [CrossRef]
- Aubin, J.E.; Bonnelye, E. Osteoprotegerin and its ligand: A new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporos. Int. 2000, 11, 905–913. [Google Scholar] [CrossRef]
- Wright, H.; McCarthy, H.S.; Middleton, J.; Marshall, M.J. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr. Rev. Musculoskelet. Med. 2009, 2, 56–64. [Google Scholar] [CrossRef]
- Tyrovola, J.B.; Spyropoulos, M.N.; Makou, M.; Perrea, D. Root resorption and the OPG/RANKL/RANK system: A mini review. J. Oral Sci. 2008, 50, 367–376. [Google Scholar] [CrossRef]
- Martin, T.J.; Sims, N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 2005, 11, 76–81. [Google Scholar] [CrossRef]
- Weitzmann, M.N. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica 2013, 2013, 125705. [Google Scholar] [CrossRef]
- Caetano-Lopes, J.; Canhao, H.; Fonseca, J. Osteoimmunology—The hidden immune regulation of bone. Autoimmun. Rev. 2009, 8, 250–255. [Google Scholar]
- Khosla, S.; Arrighi, H.; Melton, L., III; Atkinson, E.; O’fallon, W.; Dunstan, C.; Riggs, B. Correlates of osteoprotegerin levels in women and men. Osteoporos. Int. 2002, 13, 394–399. [Google Scholar] [CrossRef]
- Clowes, J.A.; Riggs, B.L.; Khosla, S. The role of the immune system in the pathophysiology of osteoporosis. Immunol. Rev. 2005, 208, 207–227. [Google Scholar] [CrossRef]
- Clarke, B.L.; Khosla, S. Female reproductive system and bone. Arch. Biochem. Biophys. 2010, 503, 118–128. [Google Scholar] [CrossRef]
- Kostenuik, P.J. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr. Opin. Pharmacol. 2005, 5, 618–625. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef]
- Buckley, K.A.; Fraser, W.D. Receptor activator for nuclear factor kappaB ligand and osteoprotegerin: Regulators of bone physiology and immune responses/potential therapeutic agents and biochemical markers. Ann. Clin. Biochem. 2002, 39, 551–556. [Google Scholar] [CrossRef]
- Rogers, A.; Saleh, G.; Hannon, R.; Greenfield, D.; Eastell, R. Circulating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 4470–4475. [Google Scholar] [CrossRef]
- Kudlacek, S.; Schneider, B.; Woloszczuk, W.; Pietschmann, P.; Willvonseder, R.F.; Austrian Study Group on Normative Values of Bone Metabolism. Serum levels of osteoprotegerin increase with age in a healthy adult population. Bone 2003, 32, 681–686. [Google Scholar] [CrossRef]
- Terpos, E.; Szydlo, R.; Apperley, J.F.; Hatjiharissi, E.; Politou, M.; Meletis, J.; Viniou, N.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Soluble receptor activator of nuclear factor κB ligand–osteoprotegerin ratio predicts survival in multiple myeloma: Proposal for a novel prognostic index. Blood 2003, 102, 1064–1069. [Google Scholar] [CrossRef]
- Alvarez, L.; Peris, P.; Guanabens, N.; Vidal, S.; Ros, I.; Pons, F.; Filella, X.; Monegal, A.; Muñoz-Gomez, J.; Ballesta, A. Serum osteoprotegerin and its ligand in Paget’s disease of bone: Relationship to disease activity and effect of treatment with bisphosphonates. Arthritis Rheum. 2003, 48, 824–828. [Google Scholar] [CrossRef]
- Ziolkowska, M.; Kurowska, M.; Radzikowska, A.; Luszczykiewicz, G.; Wiland, P.; Dziewczopolski, W.; Filipowicz-Sosnowska, A.; Pazdur, J.; Szechinski, J.; Kowalczewski, J. High levels of osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in serum of rheumatoid arthritis patients and their normalization after anti–tumor necrosis factor α treatment. Arthritis Rheum. 2002, 46, 1744–1753. [Google Scholar] [CrossRef]
- Zamani, M.; Zamani, V.; Heidari, B.; Parsian, H.; Esmaeilnejad-Ganji, S.M. Prevalence of osteoporosis with the World Health Organization diagnostic criteria in the Eastern Mediterranean Region: A systematic review and meta-analysis. Arch. Osteoporos. 2018, 13, 129. [Google Scholar] [CrossRef]
- Wani, K.; Yakout, S.M.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alokail, M.S.; Sheshah, E.; Aljohani, N.J.; Al-Saleh, Y.; Reginster, J.-Y. Metabolic syndrome in Arab adults with low bone mineral density. Nutrients 2019, 11, 1405. [Google Scholar] [CrossRef]
- Al-Saleh, Y.; Al-Daghri, N.M.; Sabico, S.; Alessa, T.; Al Emadi, S.; Alawadi, F.; Al Qasaabi, S.; Alfutaisi, A.; Al Izzi, M.; Mukhaimer, J.; et al. Diagnosis and management of osteoporosis in postmenopausal women in Gulf Cooperation Council (GCC) countries: Consensus statement of the GCC countries’ osteoporosis societies under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Arch. Osteoporos. 2020, 15, 109. [Google Scholar] [CrossRef]
- Yano, K.; Tsuda, E.; Washida, N.; Kobayashi, F.; Goto, M.; Harada, A.; Ikeda, K.; Higashio, K.; Yamada, Y. Immunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibitory factor: Increased serum concentrations in postmenopausal women with osteoporosis. J. Bone Miner. Res. 1999, 14, 518–527. [Google Scholar] [CrossRef]
- Catalano, A.; Loddo, S.; Bellone, F.; Pecora, C.; Lasco, A.; Morabito, N. Pulsed electromagnetic fields modulate bone metabolism via RANKL/OPG and Wnt/β-catenin pathways in women with postmenopausal osteoporosis: A pilot study. Bone 2018, 116, 42–46. [Google Scholar] [CrossRef]
- Fahrleitner-Pammer, A.; Dobnig, H.; Piswanger-Soelkner, C.; Bonelli, C.; Dimai, H.-P.; Leb, G.; Obermayer-Pietsch, B. Osteoprotegerin serum levels in women: Correlation with age, bone mass, bone turnover and fracture status. Wien. Klin. Wochenschr. 2003, 115, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Lein, M.; Hösslin, K.v.; Grosse, A.; Roth, S.; Possinger, K.; Lüftner, D. Osteoprotegerin and receptor activator of nuclear factor-κB ligand (RANKL) in the serum of healthy adults. Int. J. Biol. Markers 2002, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Eghbali-Fatourechi, G.; Khosla, S.; Sanyal, A.; Boyle, W.J.; Lacey, D.L.; Riggs, B.L. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Investig. 2003, 111, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Browner, W.S.; Lui, L.-Y.; Cummings, S.R. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 631–637. [Google Scholar] [CrossRef]
- Mezquita-Raya, P.; de la Higuera, M.; García, D.F.; Alonso, G.; Ruiz-Requena, M.E.; de Dios Luna, J.; Escobar-Jimenez, F.; Munoz-Torres, M. The contribution of serum osteoprotegerin to bone mass and vertebral fractures in postmenopausal women. Osteoporos. Int. 2005, 16, 1368–1374. [Google Scholar] [CrossRef]
- Stern, A.; Laughlin, G.A.; Bergstrom, J.; Barrett-Connor, E. The sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor κB legend with bone mineral density in older adults: The Rancho Bernardo study. Eur. J. Endocrinol. 2007, 156, 555–562. [Google Scholar] [CrossRef]
- Nabipour, I.; Larijani, B.; Vahdat, K.; Assadi, M.; Jafari, S.M.; Ahmadi, E.; Movahed, A.; Moradhaseli, F.; Sanjdideh, Z.; Obeidi, N. Relationships among serum receptor of nuclear factor-κB ligand, osteoprotegerin, high-sensitivity C-reactive protein, and bone mineraldensity in postmenopausal women: Osteoimmunity versus osteoinflammatory. Menopause 2009, 16, 950–955. [Google Scholar] [CrossRef]
- Chiba, Y.; Onouchi, T.; Ikeda, T.; Adachi, J.; Tamura, Y.; Horiuchi, T. Implications of measuring soluble receptor activators of nuclear factor-κB ligand and osteoprotegerin in bone metabolism of elderly women. Gerontology 2009, 55, 275–280. [Google Scholar] [CrossRef]
- Indridason, O.S.; Franzson, L.; Sigurdsson, G. Serum osteoprotegerin and its relationship with bone mineral density and markers of bone turnover. Osteoporos. Int. 2005, 16, 417–423. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Ning, G.; Zhao, Y.; Chen, Y.; Zhang, Z.; Sun, L.; Xu, M.-Y.; Chen, J. Relationships between the changes of serum levels of OPG and RANKL with age, menopause, bone biochemical markers and bone mineral density in Chinese women aged 20–75. Calcif. Tissue Int. 2005, 76, 1–6. [Google Scholar] [CrossRef]
- Ulivieri, F.; Piodi, L.; Marchelli, D.; Corradini, C.; Verdoia, C.; Gherardi, P.G. Osteoprotegerin: A valid new marker of bone turnover in post-menopausal osteoporosis? J. Orthop. Traumatol. 2005, 6, 88–90. [Google Scholar] [CrossRef]
- O’neill, T.; Cooper, C.; Cannata, J.; Lopez, J.D.; Hoszowski, K.; Johnell, O.; Lorenc, R.; Nilsson, B.; Raspe, H.; Stewart, O. Reproducibility of a questionnaire on risk factors for osteoporosis in a multicentre prevalence survey: The European Vertebral Osteoporosis Study. Int. J. Epidemiol. 1994, 23, 559–565. [Google Scholar] [CrossRef]
- Bord, S.; Ireland, D.; Beavan, S.; Compston, J. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003, 32, 136–141. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Spelsberg, T.C.; Riggs, B.L. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 1999, 140, 4367–4370. [Google Scholar] [CrossRef]
- Saika, M.; Inoue, D.; Kido, S.; Matsumoto, T. 17β-Estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-α. Endocrinology 2001, 142, 2205–2212. [Google Scholar] [CrossRef][Green Version]
- Khosla, S.; Atkinson, E.J.; Dunstan, C.R.; O’fallon, W. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J. Clin. Endocrinol. Metab. 2002, 87, 1550–1554. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Hicok, K.C.; Chen, D.; Khosla, S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. Eur. J. Endocrinol. 2002, 147, 269–273. [Google Scholar] [CrossRef]
- Ucer, S.; Iyer, S.; Kim, H.N.; Han, L.; Rutlen, C.; Allison, K.; Thostenson, J.D.; de Cabo, R.; Jilka, R.L.; O’Brien, C. The effects of aging and sex steroid deficiency on the murine skeleton are independent and mechanistically distinct. J. Bone Miner. Res. 2017, 32, 560–574. [Google Scholar] [CrossRef]
- Martin, A.; Yu, J.; Xiong, J.; Khalid, A.B.; Katzenellenbogen, B.; Kim, S.H.; Katzenellenbogen, J.A.; Malaivijitnond, S.; Gabet, Y.; Krum, S.A. Estrogens and androgens inhibit association of RANKL with the pre-osteoblast membrane through post-translational mechanisms. J. Cell. Physiol. 2017, 232, 3798–3807. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Schoppet, M. Osteoprotegerin: A link between osteoporosis and arterial calcification? Lancet 2001, 358, 257–259. [Google Scholar] [CrossRef]
- Knudsen, S.; Foss, C.; Poulsen, P.; Andersen, N.; Mogensen, C.; Rasmussen, L. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur. J. Endocrinol. 2003, 149, 39–42. [Google Scholar] [CrossRef]
- Al-Musharaf, S.; Fouda, M.A.; Turkestani, I.Z.; Al-Ajlan, A.; Sabico, S.; Alnaami, A.M.; Wani, K.; Hussain, S.D.; Alraqebah, B.; Al-Serehi, A.; et al. Vitamin D Deficiency Prevalence and Predictors in Early Pregnancy among Arab Women. Nutrients 2018, 10, 489. [Google Scholar] [CrossRef]
| Parameters | Normal | Osteopenia | Osteoporosis | p-Value |
|---|---|---|---|---|
| n | 169 | 282 | 169 | |
| Age (year) | 56.1 ± 6.1 | 57.7 ± 6.9 | 58.7 ± 7.5 A | 0.003 |
| Age of menarche | 13.1 ± 1.4 | 13.4 ± 1.5 | 13.5 ± 1.7 | 0.08 |
| Age at first pregnancy | 19.3 ± 3.7 | 18.6 ± 3.3 | 19.4 ± 4.0 | 0.04 |
| Years since menopause | 6.2 ± 4.9 | 8.5 ± 6.0 A | 10.1 ± 7.9 AB | <0.001 |
| BMI (kg/m2) | 34.0 ± 5.7 | 33.4 ± 5.5 | 30.3 ± 6.2 A | <0.001 |
| WHR | 0.92 ± 0.11 | 0.92 ± 0.09 | 0.92 ± 0.09 | 0.85 |
| BMD (spine) | 1.16 ± 0.08 | 0.97 ± 0.9 A | 0.83 ± 0.08 AB | <0.001 |
| BMD (femoral neck left) | 1.04 ± 0.09 | 0.90 ± 0.8 A | 0.80 ± 0.13 AB | <0.001 |
| T-score (spine) | −0.03 ± 0.7 | −1.68 ± 0.5 A | −3.04 ± 0.6 AB | <0.001 |
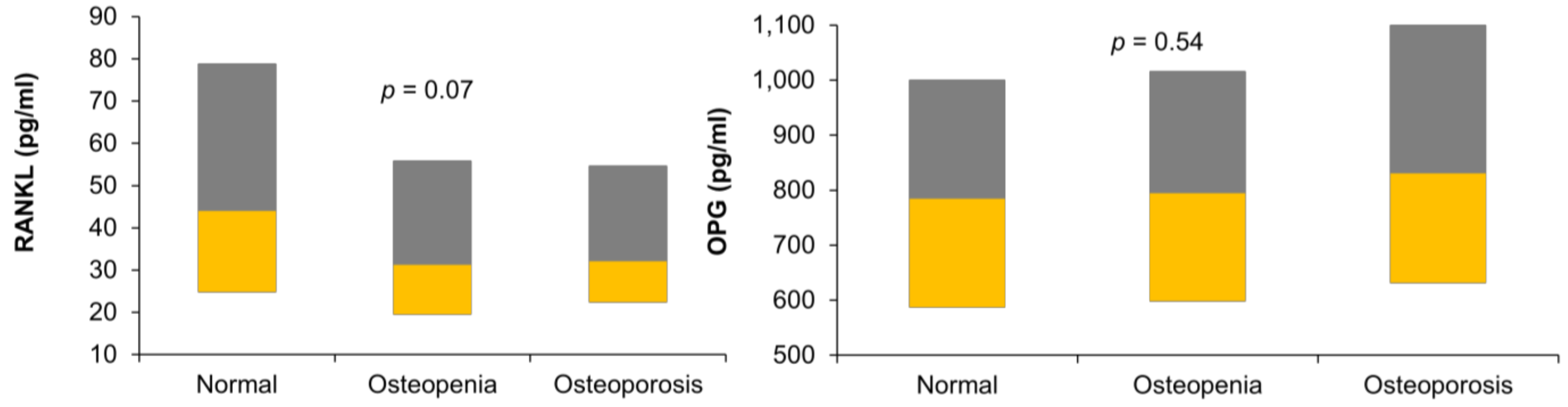
| RANKL (pg/mL) | 44 (24.8–78.9) | 31.2 (19.4–55.9) | 32.1 (22.4–54.7) | 0.07 |
| OPG (pg/mL) | 784.1 (587–1001) | 794.4 (597.8–1016) | 830.5 (631.3–1102) | 0.54 |
| RANKL/OPG | 0.04 (0.03–0.09) | 0.04 (0.02–0.06) | 0.04 (0.02–0.05) | 0.48 |
| Osteocalcin (ng/mL) | 11.7 (3.6–16.7) | 8.5 (2.8–13.7) | 10.0 (3.8–13.0) | 0.10 |
| NTx (nmol) | 57.8 (45–73.4) | 54.3 (41.4–69.2) | 58.4 (40.9–77.2) | 0.91 |
| Testosterone (ng/mL) | 0.8 (0.5–1.4) | 0.6 (0.3–1.0) | 0.7 (0.3–1.0) | 0.09 |
| Estradiol (pg/mL) | 69.3 (39.7–183.1) | 63.7 (34.2–195.7) | 59.6 (34.2–205.3) | 0.93 |
| Parameters | All | Normal | Osteopenia | Osteoporosis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RANKL | OPG | RANKL/OPG | RANKL | OPG | RANKL | OPG | RANKL/OPG | RANKL | OPG | RANKL/OPG | |
| Age (year) | 0.20 ** | 0.29 * | |||||||||
| Age at first pregnancy | −0.34 ** | ||||||||||
| BMI (kg/m2) | 0.34 * | ||||||||||
| WHR | −0.38 * | ||||||||||
| T-score AP spine | |||||||||||
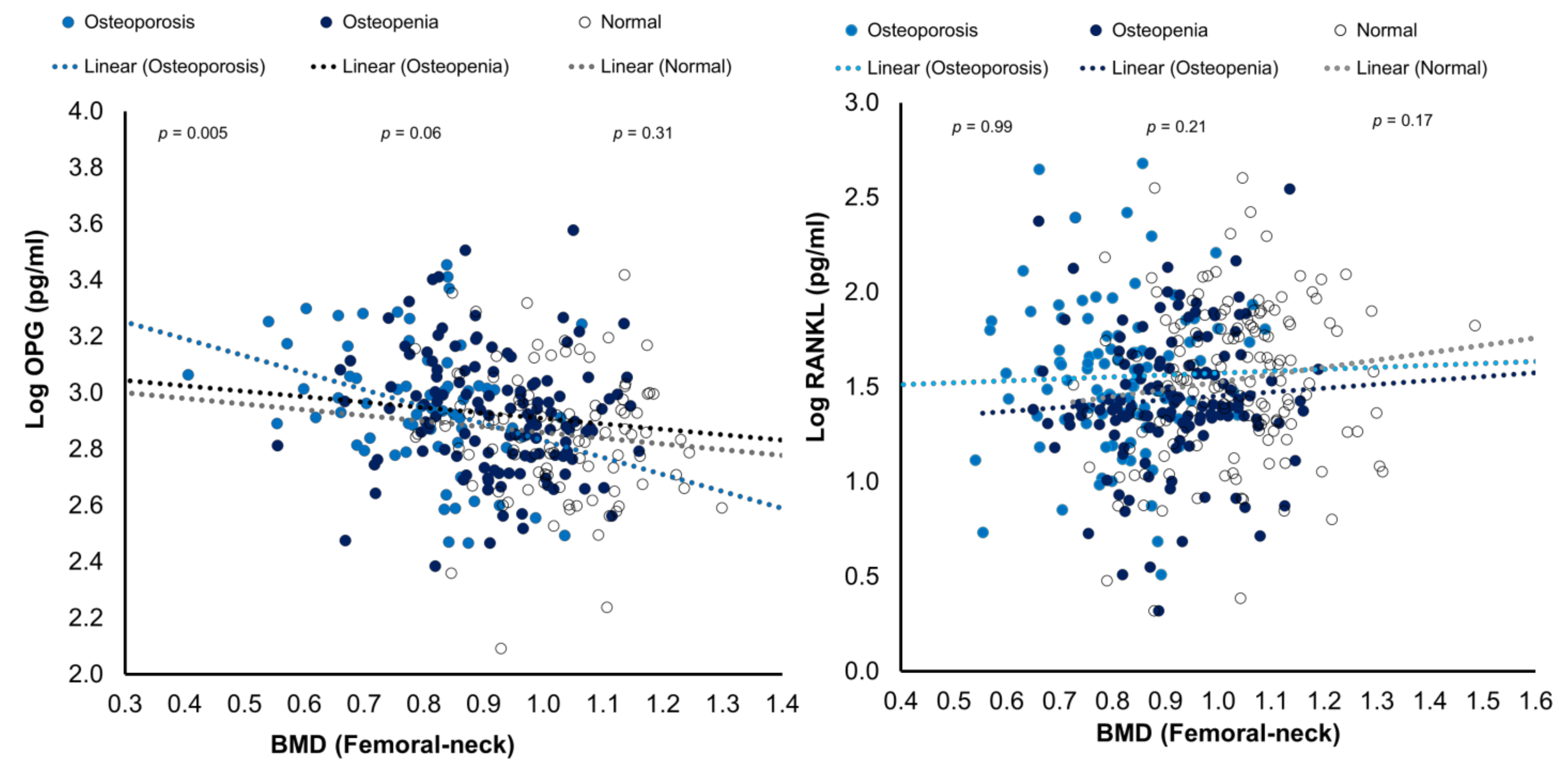
| femoral neck left BMD | −0.27 * | 0.37 ** | 0.36 * | −0.56 ** | |||||||
| NTx (nmol) | −0.37 * | −0.41 * | |||||||||
| Testosterone (ng/mL) | 0.19 * | 0.58 ** | |||||||||
| Estradiol (pg/mL) | 0.21 * | 0.55 ** | |||||||||
| Parameters | All Participants | |
|---|---|---|
| β (SE) | p-Value | |
| T-score (spine) | ||
| RANKL | 0.05 (0.55) | 0.93 |
| OPG | −0.96 (0.81) | 0.23 |
| RANKL/OPG | −0.58 (2.30) | 0.81 |
| BMD (spine) | ||
| RANKL | 0.12 (0.10) | 0.38 |
| OPG | −0.14 (0.2) | 0.39 |
| RANKL/OPG | −0.60 (0.4) | 0.1 |
| BMD (femoral neck) | ||
| RANKL | 0.16 (0.10) | 0.09 |
| OPG | −0.32 (0.12) | 0.009 |
| RANKL/OPG | −0.25 (0.50) | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, O.E.; Wani, K.; Ansari, M.G.A.; Alnaami, A.M.; Aljohani, N.; Abdi, S.; Hussain, S.D.; Al-Daghri, N.M.; Alokail, M.S. Associations of Bone Mineral Density with RANKL and Osteoprotegerin in Arab Postmenopausal Women: A Cross-Sectional Study. Medicina 2022, 58, 976. https://doi.org/10.3390/medicina58080976
Amer OE, Wani K, Ansari MGA, Alnaami AM, Aljohani N, Abdi S, Hussain SD, Al-Daghri NM, Alokail MS. Associations of Bone Mineral Density with RANKL and Osteoprotegerin in Arab Postmenopausal Women: A Cross-Sectional Study. Medicina. 2022; 58(8):976. https://doi.org/10.3390/medicina58080976
Chicago/Turabian StyleAmer, Osama E., Kaiser Wani, Mohammed G. A. Ansari, Abdullah M. Alnaami, Naji Aljohani, Saba Abdi, Syed D. Hussain, Nasser M. Al-Daghri, and Majed S. Alokail. 2022. "Associations of Bone Mineral Density with RANKL and Osteoprotegerin in Arab Postmenopausal Women: A Cross-Sectional Study" Medicina 58, no. 8: 976. https://doi.org/10.3390/medicina58080976
APA StyleAmer, O. E., Wani, K., Ansari, M. G. A., Alnaami, A. M., Aljohani, N., Abdi, S., Hussain, S. D., Al-Daghri, N. M., & Alokail, M. S. (2022). Associations of Bone Mineral Density with RANKL and Osteoprotegerin in Arab Postmenopausal Women: A Cross-Sectional Study. Medicina, 58(8), 976. https://doi.org/10.3390/medicina58080976







