Modifiable and Non-Modifiable Risk Factors for the Development of Non-Hereditary Pancreatic Cancer
Abstract
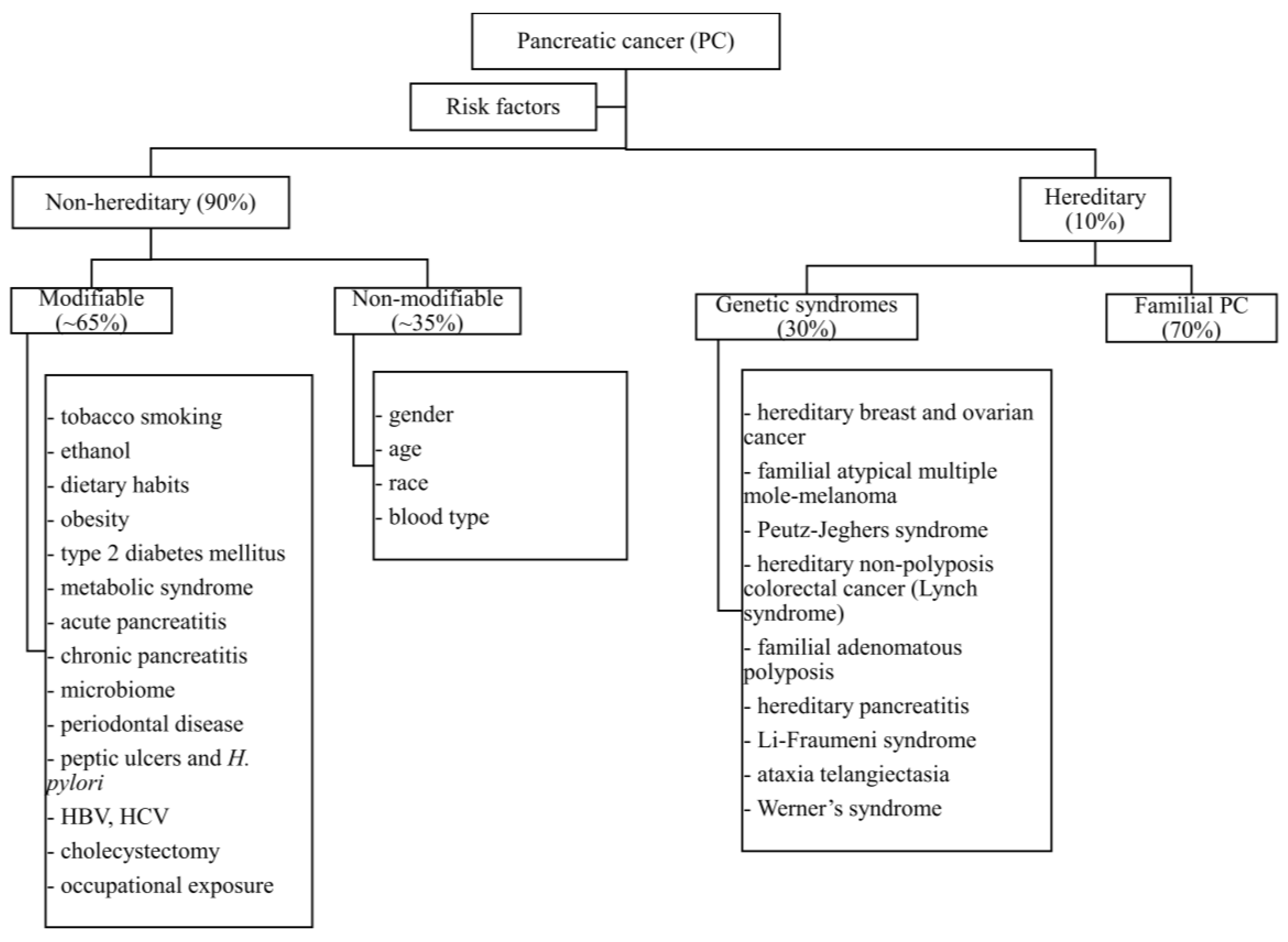
1. Introduction
2. Materials and Methods
3. Modifiable Risk Factors for PC
3.1. Tobacco Smoking
3.2. Ethanol
3.3. Dietary Habits
3.3.1. Red Meat
3.3.2. Carbohydrates
3.3.3. Lipids
3.3.4. Diet Decreasing the Risk of PC
3.4. Obesity
3.5. Type 2 Diabetes Mellitus
3.6. Metabolic Syndrome
3.7. Acute Pancreatitis (AP)
3.8. Chronic Pancreatitis (CP)
3.9. Microbiome
3.10. Periodontal Diseases
3.11. H. pylori Infection and Peptic Ulcer Disease
3.12. HBV and HCV Infection
3.13. Cholecystectomy
3.14. Occupational Exposure
4. Non-Modifiable Risk Factors for Pancreatic Cancer
4.1. Gender
4.2. Age
4.3. Race
4.4. Blood Type
4.5. Non-Hereditary Genetic Alterations
5. Pancreatic Cancer Prevention
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Czaderny, K.; Ciuba, A.; Olasek, P.; Didkowska, J. Cancer in Poland in 2016; National Research Institute of Oncology: Warszawa, Poland, 2018; ISSN 0867-8251. [Google Scholar]
- Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 4 May 2022).
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; Vrieling, A.; Lubin, J.H.; Kraft, P.; Mendelsohn, J.B.; Hartge, P.; Canzian, F.; Steplowski, E.; Arslan, A.A.; Gross, M.; et al. Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Am. J. Epidemiol. 2009, 170, 403–413. [Google Scholar] [CrossRef]
- Koyanagi, Y.N.; Ito, H.; Matsuo, K.; Sugawara, Y.; Hidaka, A.; Sawada, N.; Wada, K.; Nagata, C.; Tamakoshi, A.; Lin, Y.; et al. Smoking and Pancreatic Cancer Incidence: A Pooled Analysis of 10 Population-Based Cohort Studies in Japan. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1370–1378. [Google Scholar] [CrossRef]
- Lugo, A.; Peveri, G.; Bosetti, C.; Bagnardi, V.; Crippa, A.; Orsini, N.; Rota, M.; Gallus, S. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: A comprehensive review and meta-analysis. Eur. J. Cancer 2018, 104, 117–126. [Google Scholar] [CrossRef]
- Iodice, S.; Gandini, S.; Maisonneuve, P.; Lowenfels, A.B. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbecks. Arch. Surg. 2008, 393, 535–545. [Google Scholar] [CrossRef]
- Ben, Q.W.; Liu, J.; Sun, Y.W.; Wang, L.F.; Zou, D.W.; Yuan, Y.Z. Cigarette Smoking and Mortality in Patients with Pancreatic Cancer: A Systematic Review and Meta-analysis. Pancreas 2019, 48, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.; Bueno-de-Mesquita, H.B.; Boshuizen, H.C.; Michaud, D.S.; Severinsen, M.T.; Overvad, K.; Olsen, A.; Tjønneland, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Cigarette smoking, environmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2010, 126, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, C.; Han, Z.; Xu, S.; Li, D.; Meng, X.; Chen, D. Environmental tobacco smoke and pancreatic cancer: A case-control study. Int. J. Clin. Exp. Med. 2015, 8, 16729–16732. [Google Scholar] [PubMed]
- Polakowska, M.; Kaleta, D.; Piotrowski, W.; opór-Mądry, R.; Puch-Walczak, A.; Niklas, A.; Bielecki, W.; Kozakiewicz, K.; Pająk, A.; Tykarski, A.; et al. Tobacco smoking in Poland in the years from 2003 to 2014. Multi-centre National Population Health Examination Survey (WOBASZ). Pol. Arch. Intern. Med. 2017, 127, 91–99. [Google Scholar]
- Weissman, S.; Takakura, K.; Eibl, G.; Pandol, S.J.; Saruta, M. The Diverse Involvement of Cigarette Smoking in Pancreatic Cancer Development and Prognosis. Pancreas 2020, 49, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Morales-Oyarvide, V.; Babic, A.; Clish, C.B.; Kraft, P.; Bao, Y.; Qian, Z.R.; Rubinson, D.A.; Ng, K.; Giovannucci, E.L.; et al. Cigarette Smoking and Pancreatic Cancer Survival. J. Clin. Oncol. 2017, 35, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Alkashash, A.M.; Elsebaie, M.A.; Bikhet, M.H.; Morsi, M.; Paluri, R.K. Predictors of Survival among Early Onset Pancreatic Adenocarcinoma Patients A Tertiary Care Center Experience. Chirurgia 2021, 116, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Piciucchi, M.; Capurso, G.; Valente, R.; Larghi, A.; Archibugi, L.; Signoretti, M.; Stigliano, S.; Zerboni, G.; Barucca, V.; La Torre, M.; et al. Early onset pancreatic cancer: Risk factors, presentation and outcome. Pancreatology 2015, 15, 151–155. [Google Scholar] [CrossRef]
- Hawk, E.T.; Colbert Maresso, K. E-Cigarettes: Unstandardized, Under-Regulated, Understudied, and Unknown Health and Cancer Risks. Cancer Res. 2019, 79, 6079–6083. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Sancho, P.; Cañamero, M.; Martinelli, P.; Madriles, F.; Michl, P.; Gress, T.; de Pascual, R.; Gandia, L.; Guerra, C.; et al. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology 2014, 147, 1119–1133.e4. [Google Scholar] [CrossRef]
- Yan, R.; Chen, X.L.; Xu, Y.M.; Lau, A.T.Y. Epimutational effects of electronic cigarettes. Environ. Sci. Pollut. Res. Int. 2021, 14, 17044–17067. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Mohamadnejad, M.; Pourshams, A.; Poustchi, H.; Islami, F.; Sharafkhah, M.; Mirminachi, B.; Nasseri-Moghaddam, S.; Semnani, S.; Shakeri, R.; et al. Opium Use and Risk of Pancreatic Cancer: A Prospective Cohort Study. Cancer Epidemiol. Biomark. Prev. 2018, 27, 268–273. [Google Scholar] [CrossRef]
- Al-Awwad, N.; Allehdan, S.; Al-Jaberi, T.; Hushki, A.; Albtoush, Y.; Bani-Hani, K.; Tayyem, R.F. Dietary and Lifestyle Factors Associated with Gastric and Pancreatic Cancers: A Case-Control Study. Prev. Nutr. Food Sci. 2021, 26, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Dunn, J.A.; Hoover, R.N.; Schiffman, M.; Lillemoe, K.D.; Schoenberg, J.B.; Brown, L.M.; Greenberg, R.S.; Hayes, R.B.; Swanson, G.M.; et al. Cigarette smoking and pancreas cancer: A case-control study based on direct interviews. J. Natl. Cancer Inst. 1994, 86, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Lea, C.S.; Holly, E.A.; Bracci, P.M. Cigarette smoking and risk of pancreatic cancer: A clinic-based case-control study in the San Francisco Bay Area. Ann. Epidemiol. 2015, 25, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J.; Holly, E.A.; Wang, F.; Bracci, P.M. Cigarette, cigar and pipe smoking, passive smoke exposure, and risk of pancreatic cancer: A population-based study in the San Francisco Bay Area. BMC Cancer 2011, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens-Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Genkinger, J.M.; Spiegelman, D.; Anderson, K.E.; Bergkvist, L.; Bernstein, L.; van den Brandt, P.A.; English, D.R.; Freudenheim, J.L.; Fuchs, C.S.; Giles, G.G.; et al. Alcohol intake and pancreatic cancer risk: A pooled analysis of fourteen cohort studies. Cancer Epidemiol. Biomark. Prev. 2009, 18, 765–776. [Google Scholar] [CrossRef]
- Wang, Y.T.; Gou, Y.W.; Jin, W.W.; Xiao, M.; Fang, H.Y. Association between alcohol intake and the risk of pancreatic cancer: A dose-response meta-analysis of cohort studies. BMC Cancer 2016, 16, 212. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Vrieling, A.; Boffetta, P.; Stolzenberg-Solomon, R.Z.; Lowenfels, A.B.; Jensen, M.K.; Overvad, K.; Olsen, A.; Tjonneland, A.; et al. Ethanol intake and the risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 2009, 20, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Naudin, S.; Li, K.; Jaouen, T.; Assi, N.; Assi, N.; Kyrø, C.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.C.; Rebours, V.; et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2018, 143, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Holmes, M.V.; Guo, Y.; Yang, L.; Bian, Z.; Chen, Y.; Iona, A.; Millwood, I.Y.; Bragg, F.; Chen, J.; et al. Smoking, alcohol, and diet in relation to risk of pancreatic cancer in China: A prospective study of 0.5 million people. Cancer Med. 2018, 7, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Polesel, J.; Bosetti, C.; Serraino, D.; Negri, E.; La Vecchia, C. Population attributable risk for pancreatic cancer in Northern Italy. Pancreas 2015, 44, 216–220. [Google Scholar] [CrossRef]
- Talamini, R.; Polesel, J.; Gallus, S.; Dal Maso, L.; Zucchetto, A.; Negri, E.; Bosetti, C.; Lucenteforte, E.; Boz, G.; Franceschi, S.; et al. Tobacco smoking, alcohol consumption and pancreatic cancer risk: A case-control study in Italy. Eur. J. Cancer 2010, 46, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Brown, L.M.; Hoover, R.N.; Schiffman, M.; Lillemoe, K.D.; Schoenberg, J.B.; Swanson, G.M.; Hayes, R.B.; Greenberg, R.S.; Benichou, J. Alcohol and pancreatic cancer in blacks and whites in the United States. Cancer Res. 1995, 55, 4899–4905. [Google Scholar] [PubMed]
- Key, T.J.; Bradbury, K.B.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br. J. Cancer 2012, 106, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yin, Z.; Pu, Z.; Zhao, Q. Association Between Consumption of Red and Processed Meat and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Castro-Webb, N.; Gerlovin, H.; Bethea, T.N.; Li, S.; Ruiz-Narváez, E.A.; Rosenberg, L.; Palmer, J.R. A Prospective Analysis of Intake of Red and Processed Meat in Relation to Pancreatic Cancer among African American Women. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1775–1783. [Google Scholar] [CrossRef]
- Zheng, J.; Stuff, J.; Tang, H.; Hassan, M.M.; Daniel, C.R.; Li, D. Dietary N-nitroso compounds and risk of pancreatic cancer: Results from a large case-control study. Carcinogenesis 2019, 40, 254–262. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.M.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Cade, J.E.; Burley, V.J.; Norat, T. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: A systematic review and meta-analysis of cohort studies. Ann. Oncol. 2012, 23, 2536–2546. [Google Scholar] [CrossRef]
- Pericleous, M.; Rossi, R.E.; Mandair, D.; Whyand, T.; Caplin, M.E. Nutrition and pancreatic cancer. Anticancer Res. 2014, 34, 9–21. [Google Scholar] [PubMed]
- Gordon-Dseagu, V.L.Z.; Thompson, F.E.; Subar, A.F.; Ruder, E.H.; Thiébaut, A.; Potischman, N.; Stolzenberg-Solomon, R. A Cohort Study of Adolescent and Midlife Diet and Pancreatic Cancer Risk in the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2017, 186, 305–317. [Google Scholar] [CrossRef]
- Liu, S.Z.; Chen, W.Q.; Wang, N.; Yin, M.M.; Sun, X.B.; He, Y.T. Dietary factors and risk of pancreatic cancer: A multi-centre case-control study in China. Asian Pac. J. Cancer Prev. 2014, 15, 7947–7950. [Google Scholar] [CrossRef]
- Salem, A.A.; Mackenzie, G.G. Pancreatic cancer: A critical review of dietary risk. Nutr. Res. 2018, 52, 1–13. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Christakoudi, S.; Pagoni, P.; Ferrari, P.; Cross, A.J.; Tzoulaki, I.; Muller, D.C.; Weiderpass, E.; Freisling, H.; Murphy, N.; Dossus, L.; et al. Weight change in middle adulthood and risk of cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int. J. Cancer 2021, 148, 1637–1651. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int. J. Cancer 2007, 120, 1993–1998. [Google Scholar] [CrossRef]
- Brocco, D.; Florio, R.; De Lellis, L.; Veschi, S.; Grassadonia, A.; Tinari, N.; Cama, A. The Role of Dysfunctional Adipose Tissue in Pancreatic Cancer: A Molecular Perspective. Cancers 2020, 12, 1849. [Google Scholar] [CrossRef]
- Shyam, S.; Greenwood, D.; Mai, C.W.; Tan, S.S.; Mohd Yusof, B.N.; Moy, F.M.; Cade, J. Traditional and Novel Adiposity Indicators and Pancreatic Cancer Risk: Findings from the UK Women’s Cohort Study. Cancers 2021, 13, 1036. [Google Scholar] [CrossRef]
- Jiao, L.; Chen, L.; White, D.L.; Tinker, L.; Chlebowski, R.T.; van Horn, L.V.; Richardson, P.; Lane, D.; Sangi-Haghpeykar, H.; El-Serag, H.B. Low-fat Dietary Pattern and Pancreatic Cancer Risk in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl. Cancer Inst. 2018, 110, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Diabetes and pancreatic cancer. Mol. Carcinog. 2012, 51, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Kartsonaki, C.; Guo, Y.; Bragg, F.; Yang, L.; Bian, Z.; Chen, Y.; Iona, A.; Millwood, I.Y.; Lv, J.; et al. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int. J. Cancer 2017, 140, 1781–1788. [Google Scholar] [CrossRef]
- Sah, R.P.; Nagpal, S.J.; Mukhopadhyay, D.; Chari, S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 423–433. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G. Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress. Cancers 2021, 13, 313. [Google Scholar] [CrossRef]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients with New-Onset Diabetes. Gastroenterology 2018, 155, 730–739.e3. [Google Scholar] [CrossRef]
- Li, D.; Yeung, S.C.; Hassan, M.M.; Konopleva, M.; Abbruzzese, J.L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009, 137, 482–488. [Google Scholar] [CrossRef]
- Bosetti, C.; Rosato, V.; Li, D.; Petersen, G.M.; Bracci, P.M.; Neale, R.E.; Muscat, J.; Anderson, K.; Gallinger, S.; Olson, S.H.; et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2014, 25, 2065–2072. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Coscia, C.; Gómez-Rubio, P.; Fernández, A.; Boenink, R.; Rava, M.; Márquez, M.; Molero, X.; Löhr, M.; Sharp, L.; et al. PanGenEU Study Investigators. Deciphering the complex interplay between pancreatic cancer, diabetes mellitus subtypes and obesity/BMI through causal inference and mediation analyses. Gut 2021, 70, 319–329. [Google Scholar]
- Lee, D.Y.; Yu, J.H.; Park, S.; Han, K.; Kim, N.H.; Yoo, H.J.; Choi, K.M.; Baik, S.H.; Kim, N.H.; Seo, J.A. The influence of diabetes and antidiabetic medications on the risk of pancreatic cancer: A nationwide population-based study in Korea. Sci. Rep. 2018, 8, 9719. [Google Scholar] [CrossRef]
- Drzewoski, J.; Drozdowska, A.; Sliwińska, A. Do we have enough data to confirm the link between antidiabetic drug use and cancer development? Pol. Arch. Med. Wewn. 2011, 121, 81–87. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute. What Is Metabolic Syndrome? Available online: https://www.nhlbi.nih.gov/health/health-topics/topics/ms (accessed on 5 May 2022).
- Rosato, V.; Tavani, A.; Bosetti, C.; Pelucchi, C.; Talamini, R.; Polesel, J.; Serraino, D.; Negri, E.; La Vecchia, C. Metabolic syndrome and pancreatic cancer risk: A case-control study in Italy and meta-analysis. Metabolism 2011, 60, 1372–1378. [Google Scholar] [CrossRef]
- Xia, B.; He, Q.; Pan, Y.; Gao, F.; Liu, A.; Tang, Y.; Chong, C.; Teoh, A.; Li, F.; He, Y.; et al. Metabolic syndrome and risk of pancreatic cancer: A population-based prospective cohort study. Int. J. Cancer 2020, 147, 3384–3393. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Oh, C.M.; Kim, M.H.; Ha, E.; Choi, Y.S.; Ryoo, J.H. Metabolic syndrome, metabolic components, and their relation to the risk of pancreatic cancer. Cancer 2020, 126, 1979–1986. [Google Scholar] [CrossRef]
- Johansen, D.; Stocks, T.; Jonsson, H.; Lindkvist, B.; Björge, T.; Concin, H.; Almquist, M.; Häggström, C.; Engeland, A.; Ulmer, H.; et al. Metabolic factors and the risk of pancreatic cancer: A prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2307–2317. [Google Scholar] [CrossRef]
- Park, J.H.; Han, K.; Hong, J.Y.; Park, Y.S.; Hur, K.Y.; Kang, G.; Park, J.O. Changes in metabolic syndrome status are associated with altered risk of pancreatic cancer: A nationwide cohort study. Gastroenterology 2021, 162, 509–520. [Google Scholar] [CrossRef]
- Sadr-Azodi, O.; Oskarsson, V.; Discacciati, A.; Videhult, P.; Askling, J.; Ekbom, A. Pancreatic Cancer Following Acute Pancreatitis: A Population-based Matched Cohort Study. Am. J. Gastroenterol. 2018, 113, 1711–1719. [Google Scholar] [CrossRef]
- Kirkegård, J.; Cronin-Fenton, D.; Heide-Jørgensen, U.; Mortensen, F.V. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology 2018, 154, 1729–1736. [Google Scholar] [CrossRef]
- Teng, D.; Wu, K.; Sun, Y.; Zhang, M.; Wang, D.; Wu, J.; Yin, T.; Gong, W.; Ding, Y.; Xiao, W.; et al. Significant increased CA199 levels in acute pancreatitis patients predicts the presence of pancreatic cancer. Oncotarget 2018, 9, 12745–12753. [Google Scholar] [CrossRef]
- Cho, J.; Scragg, R.; Petrov, M.S. Postpancreatitis Diabetes Confers Higher Risk for Pancreatic Cancer Than Type 2 Diabetes: Results from a Nationwide Cancer Registry. Diabetes Care 2020, 43, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, A.P.; Bakker, O.J.; Ahmed, A.U.; Hagenaars, J.; van Santvoort, H.C.; Besselink, M.G.; Bollen, T.L.; van Eijck, C.H.; Dutch Pancreatitis Study Group. Risk of Pancreatic Cancer after a Primary Episode of Acute Pancreatitis. Pancreas 2017, 46, 1018–1022. [Google Scholar] [CrossRef]
- Löhr, M.; Klöppel, G.; Maisonneuve, P.; Lowenfels, A.B.; Lüttges, J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: A meta-analysis. Neoplasia 2005, 7, 17–23. [Google Scholar] [CrossRef]
- Kong, X.; Sun, T.; Kong, F.; Du, Y.; Li, Z. Chronic Pancreatitis and Pancreatic Cancer. Gastrointest. Tumors 2014, 1, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Hao, L.; Zeng, X.P.; Xin, L.; Wang, D.; Pan, J.; Bi, Y.W.; Ji, J.T.; Du, T.T.; Lin, J.H.; Zhang, D.; et al. Incidence of and risk factors for pancreatic cancer in chronic pancreatitis: A cohort of 1656 patients. Dig. Liver Dis. 2017, 49, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andrén-Sandberg, A.; Domellöf, L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Dugic, A.; Maisonneuve, P.; Aljic, A.; Berggren, R.; Panic, N.; Valente, R.; Pozzi Mucelli, R.; Waldthaler, A.; Ghorbani, P.; et al. Risk of Developing Pancreatic Cancer in Patients with Chronic Pancreatitis. J. Clin. Med. 2020, 9, 3720. [Google Scholar] [CrossRef] [PubMed]
- Owyang, C.; Wu, G.D. The gut microbiome in health and disease. Gastroenterology 2014, 146, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fuhler, G.M.; Bn, N.; Jose, T.; Bruno, M.J.; Peppelenbosch, M.P.; Konstantinov, S.R. Pancreatic cyst fluid harbors a unique microbiome. Microbiome 2017, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, E.; Meier, R.; Chung, M.; Koestler, D.C.; Chen, T.; Paster, B.J.; Charpentier, K.P.; Kelsey, K.T.; Izard, J.; Michaud, D.S. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol. Biomark. Prev. 2019, 28, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, G.; You, L.; Yang, J.; Feng, M.; Qiu, J.; Zhao, F.; Liu, Y.; Cao, Z.; Zheng, L.; et al. Role of the microbiome in occurrence, development and treatment of pancreatic cancer. Mol. Cancer 2019, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Dave, S. Risk Factors for Periodontitis. J. Int. Acad. Periodontol. 2005, 7, 3–7. [Google Scholar] [PubMed]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Raderer, M.; Wrba, F.; Kornek, G.; Maca, T.; Koller, D.Y.; Weinlaender, G.; Hejna, M.; Scheithauer, W. Association between Helicobacter pylori infection and pancreatic cancer. Oncology 1998, 55, 16–19. [Google Scholar] [CrossRef]
- Mei, Q.X.; Huang, C.L.; Luo, S.Z.; Zhang, X.M.; Zeng, Y.; Lu, Y.Y. Characterization of the duodenal bacterial microbiota in patients with pancreatic head cancer vs. healthy controls. Pancreatology 2018, 18, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Blaser, M.J.; Limburg, P.J.; Perez-Perez, G.; Taylor, P.R.; Virtamo, J.; Albanes, D.; ATBC Study. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J. Natl. Cancer Inst. 2001, 93, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Murphy, G.; Michel, A.; Weinstein, S.J.; Männistö, S.; Albanes, D.; Pawlita, M.; Stolzenberg-Solomon, R.Z. Seropositivity to Helicobacter pylori and risk of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2416–2419. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Y.; Gao, Y. Association between Helicobacter pylori infection and pancreatic cancer development: A meta-analysis. PLoS ONE 2013, 8, e75559. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, J. Helicobacter pylori infection and pancreatic cancer risk: A meta-analysis. J. Cancer Res. Ther. 2016, 12, C229–C232. [Google Scholar] [PubMed]
- Risch, H.A. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J. Natl. Cancer. Inst. 2003, 95, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.E.; Kim, A.S.; Kim, M.R.; Ko, H.J.; Jung, M.K. Does the Use of Proton Pump Inhibitors Increase the Risk of Pancreatic Cancer? A Systematic Review and Meta-Analysis of Epidemiologic Studies. Cancers 2020, 12, 2220. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhou, Q.; Zhou, Y.; Lin, Q.; Zeng, B.; Chen, R.; Li, Z. Gastrectomy and risk of pancreatic cancer: Systematic review and meta-analysis of observational studies. Cancer Causes Control 2012, 23, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.H.; Jiang, F. Hepatitis B virus infection increases the risk of pancreatic cancer: A meta-analysis. Scand. J. Gastroenterol. 2021, 56, 252–258. [Google Scholar] [CrossRef]
- Arafa, A.; Eshak, E.S.; Abdel Rahman, T.A.; Anwar, M.M. Hepatitis C virus infection and risk of pancreatic cancer: A meta-analysis. Cancer Epidemiol. 2020, 65, 101691. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Sakurai, I.; Shimoda, T.; Abe, K.; Okano, T.; Shikata, T. Detection of HBsAg in the Pancreas. Acta Path. Jpn. 1981, 31, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Brechot, C.; Pourcel, C.; Louise, A.; Rain, B.; Tiollais, P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature 1980, 286, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, G.; Nair, A.S.; Narayanan, V.A.; Balakrishnan, V. Acute pancreatitis in viral infections, with possible progression to chronic pancreatitis. Indian J. Gastroenterol. 2008, 27, 162–164. [Google Scholar]
- Wang, C.C.; Tseng, M.H.; Wu, S.W.; Yang, T.W.; Chen, H.Y.; Sung, W.W.; Su, C.C.; Wang, Y.T.; Chen, W.L.; Lai, H.C.; et al. Symptomatic cholelithiasis patients have an increased risk of pancreatic cancer: A population-based study. J. Gastroenterol. Hepatol. 2021, 36, 1187–1196. [Google Scholar] [CrossRef]
- Huang, D.; Lee, J.; Song, N.; Cho, S.; Choe, S.; Shin, A. Gallstones, Cholecystectomy and the Risk of Hepatobiliary and Pancreatic Cancer: A Nationwide Population-based Cohort Study in Korea. J. Cancer Prev. 2020, 25, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Negri, E.; Bosetti, C.; Gomez-Rubio, P.; Consortium, P.; Maisonneuve, P.; Miller, A.B.; Bueno-de-Mesquita, H.B.; Baghurst, P.A.; Zatonski, W. Gallbladder disease, cholecystectomy, and pancreatic cancer risk in the International Pancreatic Cancer Case-Control Consortium (PanC4). Eur. J. Cancer Prev. 2020, 29, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.T.; Silverman, D.T.; Stewart, P.A.; Blair, A.; Swanson, G.M.; Baris, D.; Greenberg, R.S.; Hayes, R.B.; Brown, L.M.; Lillemoe, K.D.; et al. Occupational exposure to pesticides and pancreatic cancer. Am. J. Ind. Med. 2001, 39, 92–99. [Google Scholar] [CrossRef]
- Antwi, S.O.; Eckert, E.C.; Sabaque, C.V.; Leof, E.R.; Hawthorne, K.M.; Bamlet, W.R.; Chaffee, K.G.; Oberg, A.L.; Petersen, G.M. Exposure to environmental chemicals and heavy metals, and risk of pancreatic cancer. Cancer Causes Control 2015, 26, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Porta, M.; Silverman, D.T.; Milne, R.L.; Kogevinas, M.; Rothman, N.; Cantor, K.P.; Jackson, B.P.; Pumarega, J.A.; López, T.; et al. Pancreatic cancer risk and levels of trace elements. Gut 2012, 61, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Jones, R.R.; James, P.; Stolzenberg-Solomon, R.Z. Light at Night and Risk of Pancreatic Cancer in the NIH-AARP Diet and Health Study. Cancer Res. 2021, 81, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Wahi, M.M.; Shah, N.; Schrock, C.E.; Rosemurgy, A.S., 2nd; Goldin, S.B. Reproductive factors and risk of pancreatic cancer in women: A review of the literature. Ann. Epidemiol. 2009, 19, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. The global burden of pancreatic cancer. Arch. Med. Sci. 2020, 16, 820–824. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P. Epidemiology and risk factors for pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Blackford, A.L.; Dal Molin, M.; Wolfgang, C.L.; Goggins, M. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut 2015, 64, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2021, 124, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Blanco, B.A.; Poulson, M.; Kenzik, K.M.; McAneny, D.B.; Tseng, J.F.; Sachs, T.E. The Impact of Residential Segregation on Pancreatic Cancer Diagnosis, Treatment, and Mortality. Ann. Surg. Oncol. 2021, 28, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Hoover, R.N.; Brown, L.M.; Swanson, G.M.; Schiffman, M.; Greenberg, R.S.; Hayes, R.B.; Lillemoe, K.D.; Schoenberg, J.B.; Schwartz, A.G.; et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology 2003, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.R.; Wieben, E.D.; Chaffee, K.G.; Antwi, S.O.; Raskin, L.; Olopade, O.I.; Li, D.; Highsmith, W.E., Jr.; Colon-Otero, G.; Khanna, L.G.; et al. CDKN2A Germline Rare Coding Variants and Risk of Pancreatic Cancer in Minority Populations. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1364–1370. [Google Scholar] [CrossRef]
- Pernick, N.L.; Sarkar, F.H.; Philip, P.A.; Arlauskas, P.; Shields, A.F.; Vaitkevicius, V.K.; Dugan, M.C.; Adsay, N.V. Clinicopathologic analysis of pancreatic adenocarcinoma in African Americans and Caucasians. Pancreas 2003, 26, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, D.S.; Karagas, M.R.; Tosteson, T.D.; Mott, L.A. Racial differences in pancreatic cancer: Comparison of survival and histologic types of pancreatic carcinoma in Asians, blacks, and whites in the United States. Pancreas 2000, 21, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Nio, Y.; Tamura, K.; Song, M.M.; Guo, K.J.; Guo, R.X.; Dong, Y.T. Ki-ras point mutation and p53 expression in human pancreatic cancer: A comparative study among Chinese, Japanese, and Western patients. Cancer Epidemiol. Biomark. Prev. 2000, 9, 279–284. [Google Scholar]
- Wolpin, B.M.; Chan, A.T.; Hartge, P.; Chanock, S.J.; Kraft, P.; Hunter, D.J.; Giovannucci, E.L.; Fuchs, C.S. ABO blood group and the risk of pancreatic cancer. J. Natl. Cancer Inst. 2009, 101, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Amundadottir, L.; Kraft, P.; Stolzenberg-Solomon, R.Z.; Fuchs, C.S.; Petersen, G.M.; Arslan, A.A.; Bueno-de-Mesquita, H.B.; Gross, M.; Helzlsouer, K.; Jacobs, E.J.; et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat. Genet. 2009, 41, 986–990. [Google Scholar] [CrossRef]
- Hofmann, B.T.; Stehr, A.; Dohrmann, T.; Güngör, C.; Herich, L.; Hiller, J.; Harder, S.; Ewald, F.; Gebauer, F.; Tachezy, M.; et al. ABO blood group IgM isoagglutinins interact with tumor-associated O-glycan structures in pancreatic cancer. Clin. Cancer Res. 2014, 20, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.K.; Hwang, J.; Rostgaard, K.; Nyrén, O.; Ullum, H.; Pedersen, O.; Erikstrup, C.; Melbye, M.; Hjalgrim, H.; Pawitan, Y.; et al. ABO blood group and risk of cancer: A register-based cohort study of 1.6 million blood donors. Cancer Epidemiol. 2016, 44, 40–43. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Kraft, P.; Xu, M.; Steplowski, E.; Olsson, M.L.; Arslan, A.A.; Bueno-de-Mesquita, H.B.; Gross, M.; Helzlsouer, K.; Jacobs, E.J.; et al. Variant ABO blood group alleles, secretor status, and risk of pancreatic cancer: Results from the pancreatic cancer cohort consortium. Cancer Epidemiol. Biomark. Prev. 2010, 19, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- El Jellas, K.; Hoem, D.; Hagen, K.G.; Kalvenes, M.B.; Aziz, S.; Steine, S.J.; Immervoll, H.; Johansson, S.; Molven, A. Associations between ABO blood groups and pancreatic ductal adenocarcinoma: Influence on resection status and survival. Cancer Med. 2017, 6, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Lowenfels, A.B. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int. J. Epidemiol. 2015, 44, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xu, J.W.; Cheng, Y.G.; Gao, J.Y.; Hu, S.Y.; Wang, L.; Zhan, H.X. Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer 2017, 141, 231–241. [Google Scholar] [CrossRef]
- Parkin, D.M.; Boyd, L.; Walker, L.C. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 2011, 105 (Suppl. 20), 77–81. [Google Scholar] [CrossRef]
- Vanella, G.; Archibugi, L.; Stigliano, S.; Capurso, G. Alcohol and gastrointestinal cancers. Curr. Opin. Gastroenterol. 2019, 35, 107–113. [Google Scholar] [CrossRef]
- Xu, M.; Jung, X.; Hines, O.J.; Eibl, G.; Chen, Y. Obesity and Pancreatic Cancer: Overview of Epidemiology and Potential Prevention by Weight Loss. Pancreas 2018, 47, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.N.; Crowe, F.L.; Bradbury, K.E.; Travis, R.C.; Key, T.J. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am. J. Clin. Nutr. 2016, 103, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Wu, L.; Zheng, L.Q.; Xu, X.; Ji, C.; Gong, T.T. Consumption of fruit and vegetables reduces risk of pancreatic cancer: Evidence from epidemiological studies. Eur. J. Cancer 2016, 25, 196–205. [Google Scholar] [CrossRef]
- Mossine, V.V.; Mawhinney, T.P.; Giovannucci, E.L. Dried Fruit Intake and Cancer: A Systematic Review of Observational Studies. Adv. Nutr. 2020, 11, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Schacht, S.R.; Olsen, A.; Dragsted, L.O.; Overvad, K.; Tjønneland, A.; Kyrø, C. Whole-Grain Intake and Pancreatic Cancer Risk-The Danish, Diet, Cancer and Health Cohort. J. Nutr. 2021, 151, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, H.; Qin, S.; Wang, M.; Wang, X.; Zhang, X.; Liu, F.; Zhang, S. The association between dietary vitamin A intake and pancreatic cancer risk: A meta-analysis of 11 studies. Biosci. Rep. 2016, 36, e00414. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, W.; Shao, L.; Zhong, D.; Wu, Y.; Cai, J. Association between intake of antioxidants and pancreatic cancer risk: A meta-analysis. Int. J. Food Sci. Nutr. 2016, 67, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.H.; Mao, Q.Q. Vitamin B6, vitamin B12 and methionine and risk of pancreatic cancer: A meta-analysis. Nutr. J. 2020, 19, 111. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Sun, X.; Lu, S.; Liu, S. Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies. Medicine 2018, 97, e0114. [Google Scholar] [CrossRef]
- Fan, H.; Kou, J.; Han, D.; Li, P.; Zhang, D.; Wu, Q.; He, Q. Association between vitamin C intake and the risk of pancreatic cancer: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 13973. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, X.; Lu, Q.; Tang, T.; Yang, Z. Vitamin E intake and pancreatic cancer risk: A meta-analysis of observational studies. Med. Sci. Monit. 2015, 21, 1249–1255. [Google Scholar]
- Gong, Z.; Holly, E.A.; Bracci, P.M. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control 2009, 20, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, J.; Brasky, T.M.; Xun, P.; Stevens, J.; White, E.; Gammon, M.D.; He, K. Antioxidant intake and pancreatic cancer risk: The Vitamins and Lifestyle (VITAL) Study. Cancer 2013, 119, 1314–1320. [Google Scholar] [CrossRef]
- Ding, Y.; Mullapudi, B.; Torres, C.; Mascariñas, E.; Mancinelli, G.; Diaz, A.M.; McKinney, R.; Barron, M.; Schultz, M.; Heiferman, M.; et al. Omega-3 Fatty Acids Prevent Early Pancreatic Carcinogenesis via Repression of the AKT Pathway. Nutrients 2018, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska-Ogłaza, A.; Zarzycka-Lindner, G.; Olejniczak, H.; Polaszewska-Muszyńska, M.; Junik, R. Use of metformin is associated with lower incidence of cancer in patients with type 2 diabetes. Endokrynol. Pol. 2017, 68, 652–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, B.; Yang, J.; Qiu, F.B.; Hu, S.Y.; Zhan, H.X. Can aspirin use reduce the risk of pancreatic cancer: An updated systematic review and meta-analysis. J. Pancreatol. 2020, 3, 201–210. [Google Scholar] [CrossRef]
- Bosetti, C.; Santucci, C.; Gallus, S.; Martinetti, M.; La Vecchia, C. Aspirin and the risk of colorectal and other digestive tract cancers: An updated meta-analysis through 2019. Ann. Oncol. 2020, 31, 558–568. [Google Scholar] [CrossRef]
- Sadr-Azodi, O.; Konings, P.; Brusselaers, N. Menopausal hormone therapy and pancreatic cancer risk in women: A population-based matched cohort study. United Eur. Gastroenterol. J. 2017, 5, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rubio, P.; Zock, J.P.; Rava, M.; Marquez, M.; Sharp, L.; Hidalgo, M.; Carrato, A.; Ilzarbe, L.; Michalski, C.; Molero, X.; et al. PanGenEU Study Investigators. Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut 2017, 66, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Li, Q.J.; Hao, F.B.; Wu, Y.Q.; Liu, S.; Zhong, G.C. Adherence to the 2018 World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and pancreatic cancer incidence and mortality: A prospective cohort study. Cancer Med. 2020, 9, 6843–6853. [Google Scholar] [CrossRef] [PubMed]
| Category | Modifiable Risk Factor of PC (Risk Level) | Non-Modifiable Risk Factor of PC (Risk Level) |
|---|---|---|
| Increased risk | Chronic pancreatitis (3.0–16.0×) | Age (50–70 years old) (6.8×) |
| Tobacco smoking (up to 2.5×) | Male sex (1.0–1.4×) | |
| High ethanol intake (up to 2.0×) | Ethnicity (Afro-Americans vs. Caucasian) (1.3×) | |
| Acute pancreatitis (2.0×) | Non “0” blood type (1.3–1.7×) | |
| Type 2 diabetes mellitus (1.5–2.0×) | ||
| Periodontal disease (1.5–1.7×) | ||
| Metabolic syndrome (up to 1.6×) | ||
| HBV/HCV infection (1.4–1.5×) | ||
| H. pylori infection (1.4–1.5×) | ||
| Frequent red meat consumption (1.2×) | ||
| Fructose (1.2×) | ||
| Obesity—per 5 kg/m2 (1.1×) | ||
| Microbiome (pending) | ||
| Occupational hazard (varied) | ||
| Reduced risk | Tea consumption (0.5×) | |
| Diet rich in fruits (0.6×) | ||
| Allergy—nasal (0.6×) | ||
| Physical activity (pending) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olakowski, M.; Bułdak, Ł. Modifiable and Non-Modifiable Risk Factors for the Development of Non-Hereditary Pancreatic Cancer. Medicina 2022, 58, 978. https://doi.org/10.3390/medicina58080978
Olakowski M, Bułdak Ł. Modifiable and Non-Modifiable Risk Factors for the Development of Non-Hereditary Pancreatic Cancer. Medicina. 2022; 58(8):978. https://doi.org/10.3390/medicina58080978
Chicago/Turabian StyleOlakowski, Marek, and Łukasz Bułdak. 2022. "Modifiable and Non-Modifiable Risk Factors for the Development of Non-Hereditary Pancreatic Cancer" Medicina 58, no. 8: 978. https://doi.org/10.3390/medicina58080978
APA StyleOlakowski, M., & Bułdak, Ł. (2022). Modifiable and Non-Modifiable Risk Factors for the Development of Non-Hereditary Pancreatic Cancer. Medicina, 58(8), 978. https://doi.org/10.3390/medicina58080978





