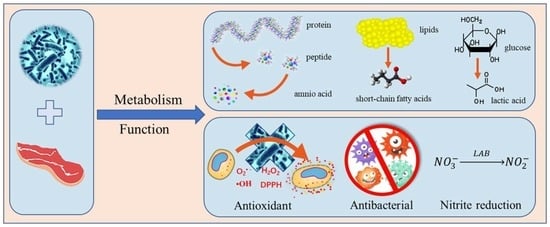
Research Update on the Impact of Lactic Acid Bacteria on the Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products
Abstract
1. Introduction
2. Effects of LAB on Substance Metabolism and the Flavor of Fermented Meat Products
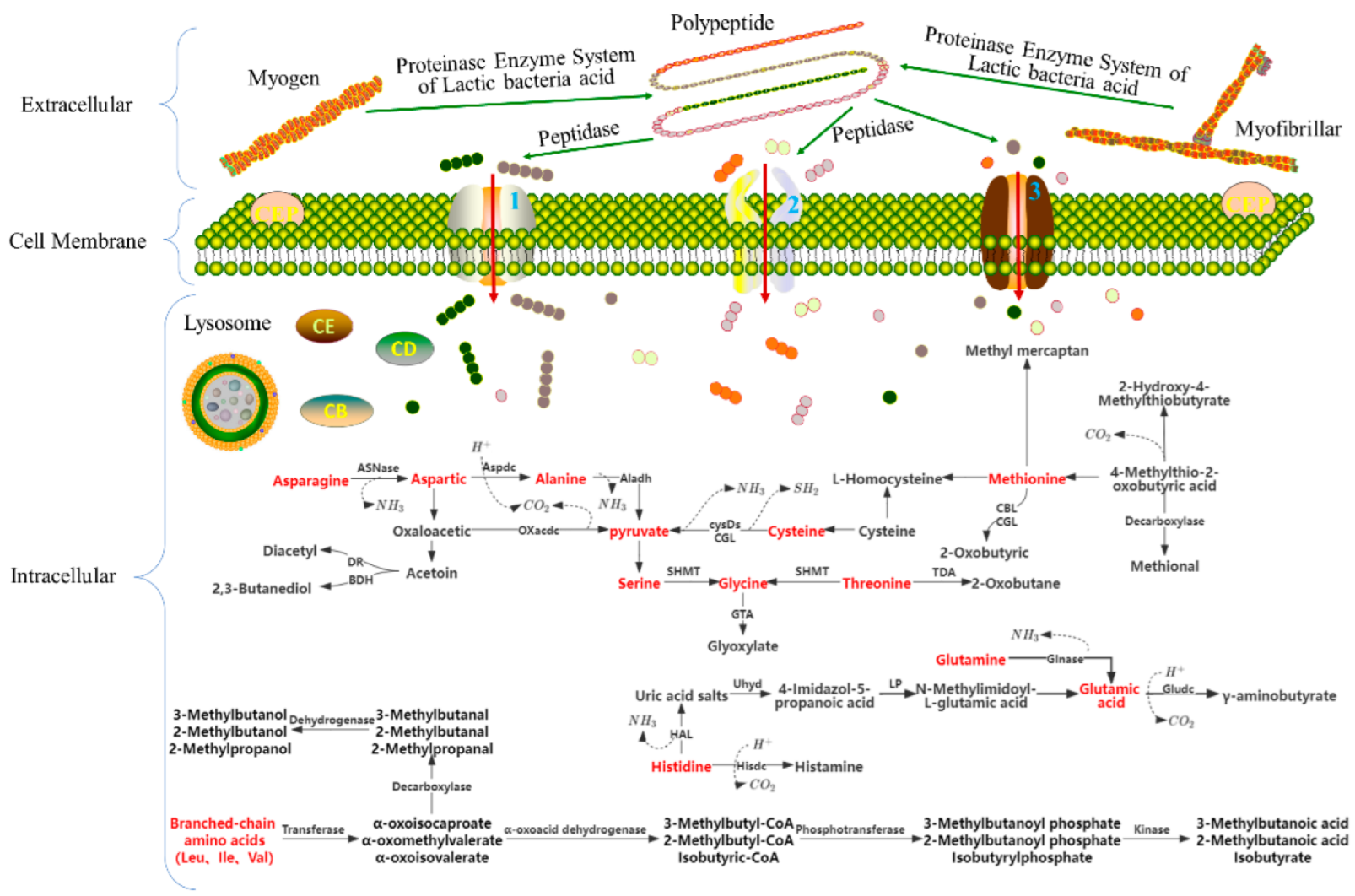
2.1. Effects of LAB on Protein Metabolism and Flavor
2.1.1. Effects of LAB on Protein Metabolism and on the Flavor and Physicochemical Properties of Meat Products
2.1.2. Improvement in the Tenderness and Color of Meat Products by LAB
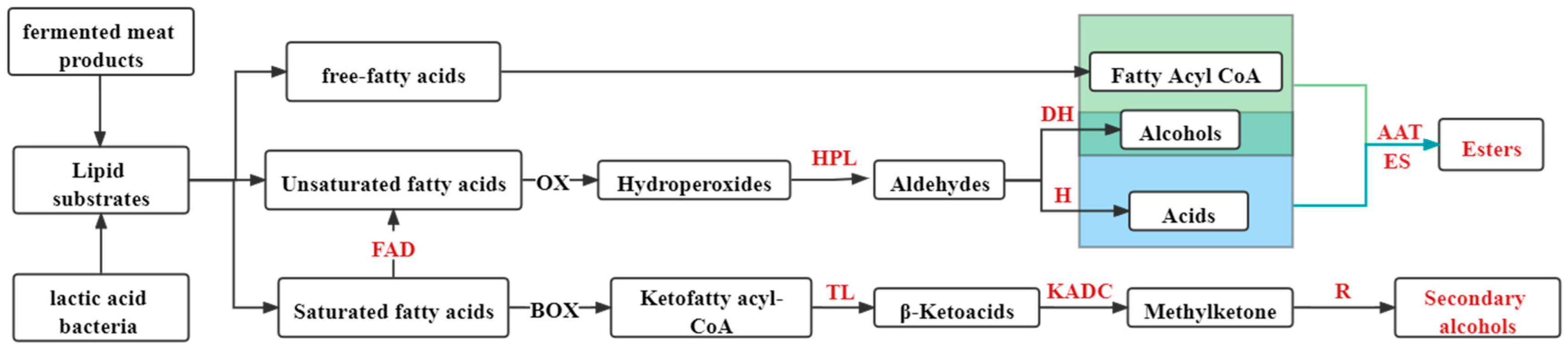
2.2. Effects of LAB on Fat Metabolism and Flavor
2.2.1. Fatty Acids and Their Oxidation Products
2.2.2. Potential of LAB in Cholesterol Degradation
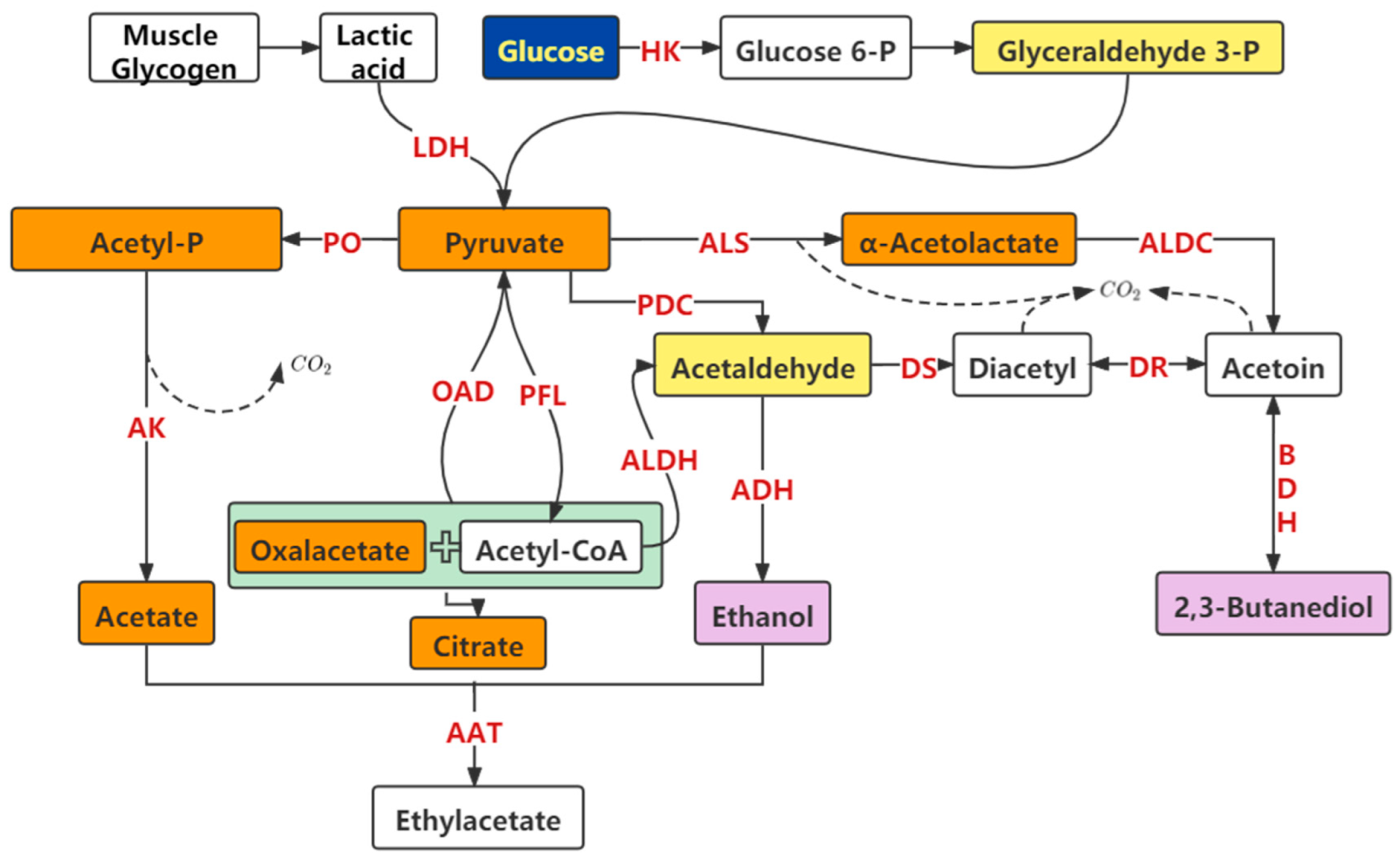
2.3. Effects of LAB on Carbohydrate Metabolism and Flavor
Metabolism of Glycogen and the Production of Flavor Substances in Meat Products by LAB
3. Application of LAB in the Fermented Meat Products
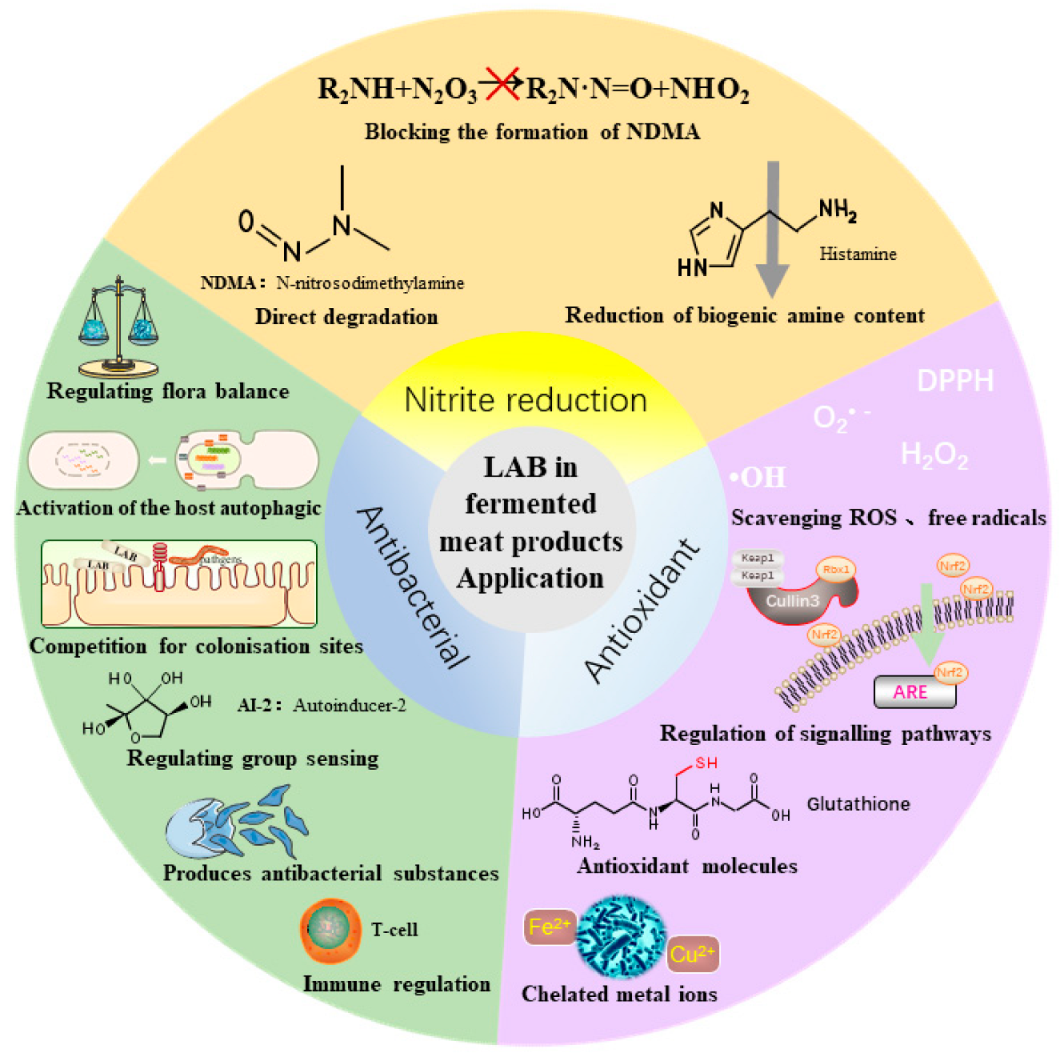
3.1. Nitrite Degradation
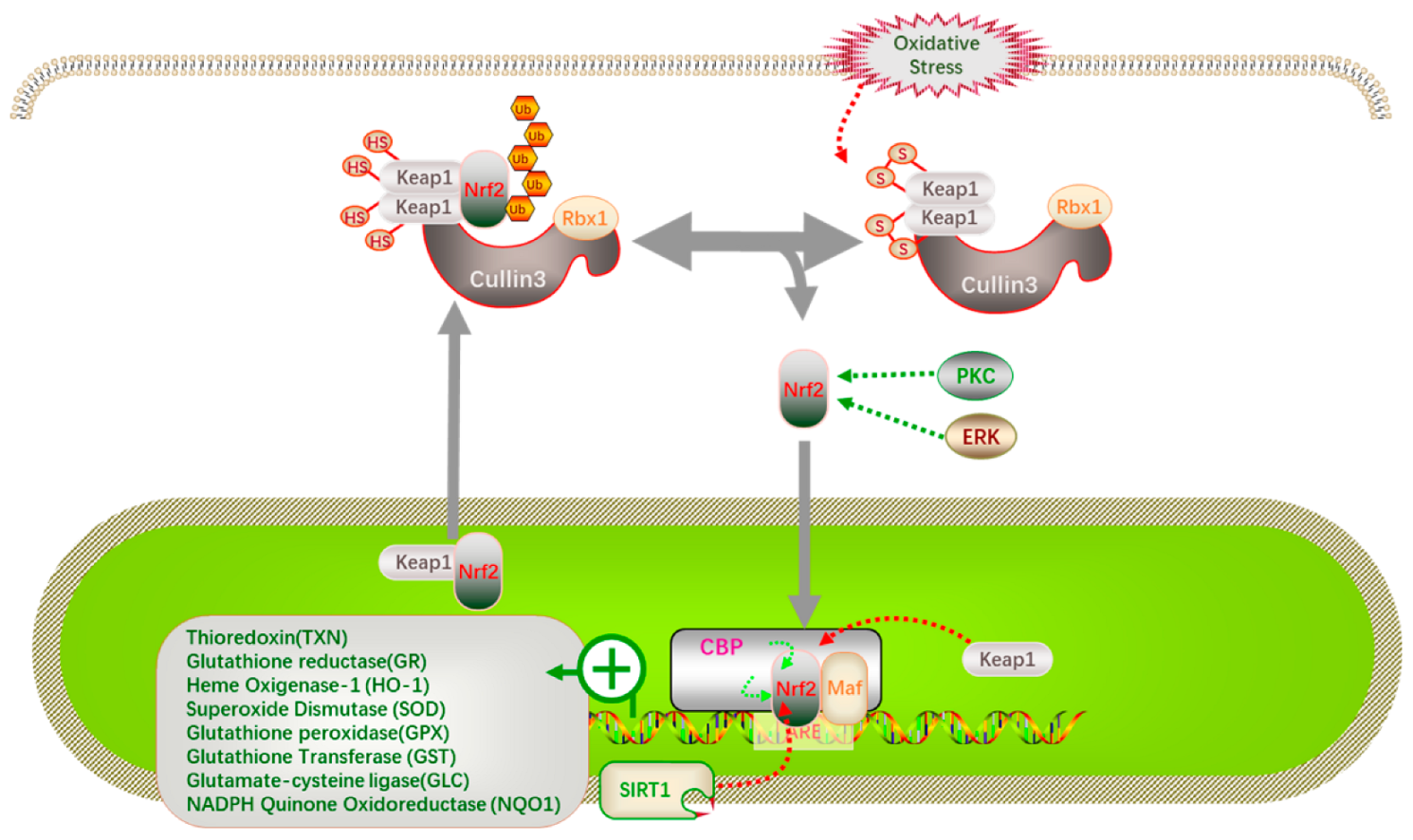
3.2. Antioxidant Properties of LAB
3.3. Antibacterial Properties of LAB
4. Effects of LAB on the Structure of Bacterial Flora in Fermented Meat Products
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorenzo, J.M.; Franco, D. Fat effect on physico-chemical, microbial and textural changes through the manufactured of dry-cured foal sausage lipolysis, proteolysis and sensory properties. Meat Sci. 2012, 92, 704–714. [Google Scholar] [CrossRef]
- Charmpi, C.; Van Reckem, E.; Sameli, N.; Van der Veken, D.; De Vuyst, L.; Leroy, F. The Use of Less Conventional Meats or Meat with High pH Can Lead to the Growth of Undesirable Microorganisms during Natural Meat Fermentation. Foods 2020, 9, 1386. [Google Scholar] [CrossRef]
- Cruxen, C.; Funck, G.D.; Haubert, L.; Dannenberg, G.D.S.; Marques, J.L.; Chaves, F.C.; da Silva, W.P.; Fiorentini, A.M. Selection of native bacterial starter culture in the production of fermented meat sausages: Application potential, safety aspects, and emerging technologies. Food Res. Int. 2019, 122, 371–382. [Google Scholar] [CrossRef]
- Besser, M.; Terberger, J.; Weber, L.; Ghebremedhin, B.; Naumova, E.A.; Arnold, W.H.; Stuermer, E.K. Impact of probiotics on pathogen survival in an innovative human plasma biofilm model (hpBIOM). J. Transl. Med. 2019, 17, 243. [Google Scholar] [CrossRef]
- Yao, K.; Liu, D.-m.; Liang, M.-h.; Brennan, C.S.; Brennan, M. Detection of nitrite degradation by Lactobacillus plantarum DMDL9010 through the anaerobic respiration electron transport chain using proteomic analysis. Int. J. Food Sci. Technol. 2020, 56, 1608–1622. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Verma, A.K.; Mehta, N.; Malav, O.P.; Kumar, D.; Sharma, N. Quality, functionality, and shelf life of fermented meat and meat products: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2844–2856. [Google Scholar] [CrossRef]
- Singh, V.; Pathak, V.; Verma, A.K. Fermented Meat Products: Organoleptic Qualities and Biogenic Amines—A Review. Am. J. Food Technol. 2012, 7, 278–288. [Google Scholar] [CrossRef]
- Zhong, A.; Chen, W.; Duan, Y.; Li, K.; Tang, X.; Tian, X.; Wu, Z.; Li, Z.; Wang, Y.; Wang, C. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT 2021, 149, 111873. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yang, K.; Liu, M.; Zhang, J.; Wei, X.; Fan, M. Screening for potential probiotic from spontaneously fermented non-dairy foods based on in vitro probiotic and safety properties. Ann. Microbiol. 2018, 68, 803–813. [Google Scholar] [CrossRef]
- Moreno, I.; Marasca, E.T.G.; de Sa, P.; de Souza Moitinho, J.; Marquezini, M.G.; Alves, M.R.C.; Bromberg, R. Evaluation of Probiotic Potential of Bacteriocinogenic Lactic Acid Bacteria Strains Isolated from Meat Products. Probiotics Antimicrob. Proteins 2018, 10, 762–774. [Google Scholar] [CrossRef]
- Dias, F.S.; Duarte, W.F.; Santos, M.R.; Ramos, E.M.; Schwan, R.F. Screening of Lactobacillus isolated from pork sausages for potential probiotic use and evaluation of the microbiological safety of fermented products. J. Food Prot. 2013, 76, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Klayraung, S. Probiotic Properties of Lactobacilli Isolated from Thai Traditional Food. Sci. Pharm. 2008, 76, 485–503. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Axelsson, L.; Naterstad, K.; Elsser, D.; Budde, B.B. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int. J. Food Microbiol. 2005, 105, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Landeta, G.; Curiel, J.A.; Carrascosa, A.V.; Munoz, R.; de las Rivas, B. Technological and safety properties of lactic acid bacteria isolated from Spanish dry-cured sausages. Meat Sci. 2013, 95, 272–280. [Google Scholar] [CrossRef]
- Federici, S.; Ciarrocchi, F.; Campana, R.; Ciandrini, E.; Blasi, G.; Baffone, W. Identification and functional traits of lactic acid bacteria isolated from Ciauscolo salami produced in Central Italy. Meat Sci. 2014, 98, 575–584. [Google Scholar] [CrossRef]
- Topçu, K.C.; Kaya, M.; Kaban, G. Probiotic properties of lactic acid bacteria strains isolated from pastırma. LWT 2020, 134, 110216. [Google Scholar] [CrossRef]
- Yuksekdag, Z.; Aslim, B. Assessment of potential probiotic- and starter properties of Pediococcus spp. isolated from Turkish-type fermented sausages (sucuk). J. Microbiol. Biotechnol. 2010, 20, 161–168. [Google Scholar] [CrossRef]
- Ratanaburee, A.; Kantachote, D.; Charernjiratrakul, W.; Sukhoom, A. Selection of γ-aminobutyric acid-producing lactic acid bacteria and their potential as probiotics for use as starter cultures in Thai fermented sausages (Nham). Int. J. Food Sci. Technol. 2013, 48, 1371–1382. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Teixeira, P. Selection of potential probiotic Enterococcus faecium isolated from Portuguese fermented food. Int. J. Food Microbiol. 2014, 191, 144–148. [Google Scholar] [CrossRef]
- Zugic Petrovic, T.D.; Ilic, P.D.; Grujovic, M.Z.; Mladenovic, K.G.; Kocic-Tanackov, S.D.; Comic, L.R. Assessment of safety aspect and probiotic potential of autochthonous Enterococcus faecium strains isolated from spontaneous fermented sausage. Biotechnol. Lett. 2020, 42, 1513–1525. [Google Scholar] [CrossRef]
- Zommiti, M.; Cambronel, M.; Maillot, O.; Barreau, M.; Sebei, K.; Feuilloley, M.; Ferchichi, M.; Connil, N. Evaluation of Probiotic Properties and Safety of Enterococcus faecium Isolated From Artisanal Tunisian Meat “Dried Ossban”. Front. Microbiol. 2018, 9, 1685. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alcántara, A.M.; Wacher, C.; Llamas, M.G.; López, P.; Pérez-Chabela, M.L. Probiotic properties and stress response of thermotolerant lactic acid bacteria isolated from cooked meat products. LWT 2018, 91, 249–257. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martin, A.; Benito, M.J.; Nevado, F.P.; de Guia Cordoba, M. Screening of lactic acid bacteria and bifidobacteria for potential probiotic use in Iberian dry fermented sausages. Meat Sci. 2008, 80, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Rongxin, W.; Fangda, S.; Yan, W.; Qian, C.; Kong, B. Evaluation the potential of lactic acid bacteria isolates from traditional beef jerky as starter cultures and their effects on flavor formation. LWT 2021, 142, 110982. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Ji, L.; Zhang, J.; Zhao, Z.; Zhang, R.; Bai, T.; Hou, B.; Zhang, Y.; Liu, D.; et al. A Review: Microbial Diversity and Function of Fermented Meat Products in China. Front. Microbiol. 2021, 12, 645435. [Google Scholar] [CrossRef]
- Mireille, Y.; Liesbeth, R. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Carretta, M.D.; Hidalgo, A.I.; Burgos, J.; Opazo, L.; Castro, L.; Hidalgo, M.A.; Figueroa, C.D.; Taubert, A.; Hermosilla, C.; Burgos, R.A. Butyric acid stimulates bovine neutrophil functions and potentiates the effect of platelet activating factor. Vet. Immunol. Immunopathol. 2016, 176, 18–27. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.; Wen, R.; Wang, Y.; Qin, L.; Kong, B. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108338. [Google Scholar] [CrossRef] [PubMed]
- Fadda, S.; Sanz, Y.; Vignolo, G.; Aristoy, M.-C.; Oliver, G.; Toldrá, F. Characterization of Muscle Sarcoplasmic and Myofibrillar Protein Hydrolysis Caused by Lactobacillus plantarum. Appl. Environ. Microbiol. 1999, 65, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Fadda, S.; Vignolo, G.; Aristoy, M.-C.; Oliver, G.; Toldrá, F. Hydrolytic Action of Lactobacillus casei CRL 705 on Pork Muscle Sarcoplasmic and Myofibrillar Proteins. J. Agric. Food Chem. 1999, 47, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, Q.; Zhang, A.; Liu, Y.; Li, Z.; Tang, H.; Cao, D.; Zhang, D. Revealing the Hypoglycemic Effects and Mechanism of GABA-Rich Germinated Adzuki Beans on T2DM Mice by Untargeted Serum Metabolomics. Front. Nutr. 2021, 8, 791191. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xiong, S.B.; Xie, B.J.; Qin, L.H. Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Sci. 2008, 80, 632–639. [Google Scholar] [CrossRef]
- Du, S.; Cheng, H.; Ma, J.K.; Li, Z.J.; Wang, C.H.; Wang, Y.L. Effect of starter culture on microbiological, physiochemical and nutrition quality of Xiangxi sausage. J. Food Sci. Technol. 2019, 56, 811–823. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Yang, Q. Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 2020, 137, 109351. [Google Scholar] [CrossRef]
- Gou, M.; Liu, X.; Qu, H. The role of nitric oxide in the mechanism of lactic acid bacteria substituting for nitrite. CyTA—J. Food 2019, 17, 593–602. [Google Scholar] [CrossRef]
- Salama, H.A.; Badr, A.N.; Elkhadragy, M.F.; Hussein, A.; Yehia, H.M.J.M. New Antifungal Microbial Pigment Applied to Improve Safety and Quality of Processed Meat-Products. Microorganisms 2021, 9, 989. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y. Complete Replacement of Nitrite With a Lactobacillus fermentum on the Quality and Safety of Chinese Fermented Sausages. Front. Microbiol. 2021, 12, 704302. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, Y.; Zhou, Z. Lactic acid bacteria in traditional fermented Chinese foods. Food Res. Int. 2011, 44, 643–651. [Google Scholar] [CrossRef]
- Bozkurt, H.; Erkmen, O. Effects of starter cultures and additives on the quality of Turkish style sausage (sucuk). Meat Sci. 2002, 61, 149–156. [Google Scholar] [CrossRef]
- Uppada, S.R.; Akula, M.; Bhattacharya, A.; Dutta, J.R. Immobilized lipase from Lactobacillus plantarum in meat degradation and synthesis of flavor esters. J. Genet. Eng. Biotechnol. 2017, 15, 331–334. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, L.; Yang, X.; Wang, X.; Cai, q.; Zhao, Y.; Wei, Y. Effect of Antioxidant Lactic Acid Bacteria on Lipid Hydrolysis and Oxidation of Dry-Cured Hairtail: A Study Using Principal Components Analysis. Food Sci. 2017, 38, 231–238. [Google Scholar] [CrossRef]
- Jesch, E.D.; Carr, T.P. Food Ingredients That Inhibit Cholesterol Absorption. Prev. Nutr. Food Sci. 2017, 22, 67–80. [Google Scholar] [CrossRef]
- Chimento, A.; Casaburi, I.; Avena, P.; Trotta, F.; De Luca, A.; Rago, V.; Pezzi, V.; Sirianni, R. Cholesterol and Its Metabolites in Tumor Growth: Therapeutic Potential of Statins in Cancer Treatment. Front. Endocrinol. 2018, 9, 807. [Google Scholar] [CrossRef]
- Khare, A.; Gaur, S. Cholesterol-Lowering Effects of Lactobacillus Species. Curr. Microbiol. 2020, 77, 638–644. [Google Scholar] [CrossRef]
- Daliri, E.B.; Kim, Y.; Do, Y.; Chelliah, R.; Oh, D.H. In Vitro and In Vivo Cholesterol Reducing Ability and Safety of Probiotic Candidates Isolated from Korean Fermented Soya Beans. Probiotics Antimicrob. Proteins 2022, 14, 87–98. [Google Scholar] [CrossRef]
- Yin, B.; Wa, Y.; Huang, Y.; Xu, G.; Gu, R. Study on cholesterol reduction in viro by single and mixed bacteria. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2020, 41, 44–49, 61. [Google Scholar] [CrossRef]
- Mahmoudi, I.; Moussa, O.B.; Hassouna, M. Symbiotic, Hypocholesterolemic and Antioxidant Effects of Potential Probiotic Lactobacilli Strains Isolated from Tunisian Camel Milk. Adv. Microbiol. 2017, 7, 328–342. [Google Scholar] [CrossRef]
- Ding, W.; Shi, C.; Chen, M.; Zhou, J.; Long, R.; Guo, X. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J. Funct. Foods 2017, 32, 324–332. [Google Scholar] [CrossRef]
- Guo, L.; Yang, L.; Huo, G. Cholesterol Removal by Lactobacillus plantarum Isolated from Homemade Fermented Cream in Inner Mongolia of China. Czech J. Food Sci. 2011, 29, 219–225. [Google Scholar] [CrossRef]
- MaLina, K.; Bao, Y. Screening, Identification of Cholesterol-lowering Abilities of Strains from Traditional Ferments and Analysis of Bile Salt Hydrolase Activity. J. Chin. Inst. Food Sci. Technol. 2021, 21, 266–275. [Google Scholar] [CrossRef]
- Liao, X.; Guo, L.; Qiu, L.; Gu, F.; Lin, J. Characterization and Mechanism of Lactic Acid Bacteria Isolated from Mango, with Cholesterol-Lowering Effects. J. Chin. Inst. Food Sci. Technol. 2016, 16, 10–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, X.; Sun, W.; Wang, C.; Li, C.; He, L.; Wang, X.; Tao, H.; Zeng, X. Evaluation and Application of Different Cholesterol-Lowering Lactic Acid Bacteria as Potential Meat Starters. J. Food Prot. 2021, 84, 63–72. [Google Scholar] [CrossRef]
- Yusuf, D.; Nuraida, L.; Dewanti-Hariyadi, R.; Hunaefi, D. In Vitro Characterization of Lactic Acid Bacteria from Indonesian Kefir Grains as Probiotics with Cholesterol-Lowering Effect. J. Microbiol. Biotechnol. 2019, 30, 726–732. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, K.; Zhao, Y.; Du, R.; Tian, J.; Jin, Y. Research Progress of Regulatory Key Factors Involved in Cholesterol Metabolism by Lactic Acid Bacteria. J. Chin. Inst. Food Sci. Technol. 2021, 21, 341–350. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, K.; Ma, M.; Ma, J.; Tian, J.; Jin, Y. Lactobacillus mediates the expression of NPC1L1, CYP7A1, and ABCG5 genes to regulate cholesterol. Food Sci. Nutr. 2021, 9, 6882–6891. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Z.; Lv, Y.; Tong, L.; Liu, T.; Yi, H.; Zhou, X.; Yu, Z.; Tian, X.; Cui, Q.; et al. Reduction of intestinal trimethylamine by probiotics ameliorated lipid metabolic disorders associated with atherosclerosis. Nutrition 2020, 79–80, 110941. [Google Scholar] [CrossRef] [PubMed]
- Illeghems, K.; De Vuyst, L.; Weckx, S. Comparative genome analysis of the candidate functional starter culture strains Lactobacillus fermentum 222 and Lactobacillus plantarum 80 for controlled cocoa bean fermentation processes. BMC Genom. 2015, 16, 766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ruan, L.; Sun, M.; Applied, M.G.J.; Microbiology, E. A Genomic View of Lactobacilli and Pediococci Demonstrates that Phylogeny Matches Ecology and Physiology. Appl. Environ. Microbiol. 2015, 81, 7233–7243. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int. J. Food Microbiol. 2003, 83, 115–131. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Sun, Q.; Dong, F.; Liu, Q. Antioxidant potential of a unique LAB culture isolated from Harbin dry sausage: In vitro and in a sausage model. Meat Sci. 2015, 110, 180–188. [Google Scholar] [CrossRef]
- Mei, L.; Pan, D.; Guo, T.; Ren, H.; Wang, L. Role of Lactobacillus plantarum with antioxidation properties on Chinese sausages. LWT 2022, 162, 113427. [Google Scholar] [CrossRef]
- Fernandez, A.; Ogawa, J.; Penaud, S.; Boudebbouze, S.; Ehrlich, D.; van de Guchte, M.; Maguin, E. Rerouting of pyruvate metabolism during acid adaptation in Lactobacillus bulgaricus. Proteomics 2008, 8, 3154–3163. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Jia, X.-Z.; Yu, J.-J.; Chen, Y.-H.; Liu, D.-M.; Liang, M.-H. Effect of different lactic acid bacteria on nitrite degradation, volatile profiles, and sensory quality in Chinese traditional paocai. LWT 2021, 147, 111597. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Yan, L.; Yang, H.; Wang, Y.; Ma, L. Inhibition Mechanism and Application of Lactic Acid Bacteria on N-Nitrosamine Formation. Food Sci. 2020, 41, 141–147. [Google Scholar] [CrossRef]
- Liao, E.; Xu, Y.; Jiang, Q.; Xia, W. Effects of inoculating autochthonous starter cultures on N-nitrosodimethylamine and its precursors formation during fermentation of Chinese traditional fermented fish. Food Chem. 2019, 271, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, Q.; Li, F.; Zheng, D.; Kong, B. Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum. Food Control 2016, 68, 358–366. [Google Scholar] [CrossRef]
- Sun, F.; Kong, B.; Chen, Q.; Han, Q.; Diao, X. N-nitrosoamine inhibition and quality preservation of Harbin dry sausages by inoculated with Lactobacillus pentosus, Lactobacillus curvatus and Lactobacillus sake. Food Control 2017, 73, 1514–1521. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, C.; Chen, H. Studies on Screening, Identification and Functional Properties of Excellent Lactic Acid Bacteria in Kombucha]. Food Sci. 2021, 21, 309–316. [Google Scholar] [CrossRef]
- Giudice, A.; Arra, C.; Turco, M.C. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol. Biol. 2010, 647, 37–74. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Sekhar, K.R.; Rachakonda, G.; Freeman, M.L. Cysteine-based regulation of the CUL3 adaptor protein Keap1. Toxicol. Appl. Pharmacol. 2010, 244, 21–26. [Google Scholar] [CrossRef]
- Yang, M.; Tian, J.; Jing, Z.; Zhao, L.; Zhang, K.; Jin, Y. Progress in Understanding the Antioxidant Regulation System of Lactic Acid Bacteria. Food Sci. 2018, 39, 290–295. [Google Scholar] [CrossRef]
- Feng, M.; Luan, X.; Sun, J. Comparative Study on the in Vitro Functional Characteristics of Three Strains of Lactic Acid Bacteria Isolated from Fermented Sausages. Food Sci. 2020, 41, 39–45. [Google Scholar] [CrossRef]
- Wen, R.; Kong, B.; Yin, X.; Zhang, H.; Chen, Q. Characterisation of flavour profile of beef jerky inoculated with different autochthonous lactic acid bacteria using electronic nose and gas chromatography-ion mobility spectrometry. Meat Sci. 2022, 183, 108658. [Google Scholar] [CrossRef]
- Yang, B.; Bai, J.; Jin, Y.; Wang, H.; Ni, Y.; Li, X. Screening of Lactic Acid Bacteria from Donkey Milk in Xinjiang for Use as Starter Culture and Their Probiotic Characteristics. Food Sci. 2022, 43, 224–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Lou, L.; Zhan, J.; Fan, M.; Li, D.; Liao, Q. Antioxidant Activities of Lactic Acid Bacteria for Quality Improvement of Fermented Sausage. J. Food Sci. 2017, 82, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Khaneghah, A.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.A.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.; Xu, Z. Identification of antibacterial substances of Lactobacillus plantarum DY-6 for bacteriostatic action. Food Sci. Nutr. 2020, 8, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Ren, H.Y.; Liu, D.Y.; Zhu, W.Y.; Wang, W. Effects of inoculating Lactobacillus sakei starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control 2013, 32, 591–596. [Google Scholar] [CrossRef]
- Lv, X.; Lin, Y.; Jie, Y.; Sun, M.; Zhang, B.; Bai, F.; Zhao, H.; Li, J. Purification, characterization, and action mechanism of plantaricin DL3, a novel bacteriocin against Pseudomonas aeruginosa produced by Lactobacillus plantarum DL3 from Chinese Suan-Tsai. Eur. Food Res. Technol. 2018, 244, 323–331. [Google Scholar] [CrossRef]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of Starter Cultures on the Safety of Fermented Meat Products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef]
- Du, R.; Wang, B.; Luo, Y.; Wang, Y.; Tian, J.; Sha, R.; Jin, Y. Comparison of Bacterial Diversity in Traditional Fermented Dairy and Meat Products by Illumina MiSeq Sequencing. J. Chin. Inst. Food Sci. Technol. 2021, 21, 269–277. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, K.; Yang, M.; Jing, Z.; Li, Q.; Zhao, L.; Jin, Y. Comparative Bacterial Diversity Analysis and Microbial Safety Assessment of Air-Dried Meat Products by Illumina MiSeq Sequencing Technology. Food Sci. 2019, 40, 33–40. [Google Scholar] [CrossRef]
- Diana, D.G.; Giuseppe, M.; Ivana, N.; Irene, A.; Tomaz, L.; Maddalena, R.; Stefano, R.; Beatriz, M.; Jordi, R. Lactic acid bacteria as protective cultures in fermented pork meat to prevent Clostridium spp. growth. Int. J. Food Microbiol. 2016, 235, 53–59. [Google Scholar]
- Tu, R.J.; Wu, H.Y.; Lock, Y.S.; Chen, M.J. Evaluation of microbial dynamics during the ripening of a traditional Taiwanese naturally fermented ham. Food Microbiol. 2010, 27, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Parlindungan, E.; Lugli, G.A.; Ventura, M.; van Sinderen, D.; Mahony, J. Lactic Acid Bacteria Diversity and Characterization of Probiotic Candidates in Fermented Meats. Foods 2021, 10, 1519. [Google Scholar] [CrossRef]
- Ge, Q.; Gu, Y.; Zhang, W.; Yin, Y.; Yu, H.; Wu, M.; Wang, Z.; Zhou, G. Comparison of microbial communities from different Jinhua ham factories. AMB Express 2017, 7, 37. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Q.; Sun, Q.; Kong, B.; Xiong, Y. Flavour formation from hydrolysis of pork sarcoplasmic protein extract by a unique LAB culture isolated from Harbin dry sausage. Meat Sci. 2015, 100, 110–117. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Zhao, H. Unraveling microbial community diversity and succession of Chinese Sichuan sausages during spontaneous fermentation by high-throughput sequencing. J. Food Sci. Technol. 2019, 56, 3254–3263. [Google Scholar] [CrossRef]
- Mu, Y.; Su, W.; Mu, Y.; Jiang, L. Combined Application of High-Throughput Sequencing and Metabolomics Reveals Metabolically Active Microorganisms During Panxian Ham Processing. Front. Microbiol. 2020, 10, 3012. [Google Scholar] [CrossRef]
- Madoroba, E.; Magwedere, K.; Chaora, N.S.; Matle, I.; Muchadeyi, F.; Mathole, M.A.; Pierneef, R. Microbial Communities of Meat and Meat Products: An Exploratory Analysis of the Product Quality and Safety at Selected Enterprises in South Africa. Microorganisms 2021, 9, 507. [Google Scholar] [CrossRef]
- Coppola, S.; Mauriello, G.; Aponte, M.; Moschetti, G.; Villani, F. Microbial succession during ripening of Naples-type salami, a southern Italian fermented sausage. Meat Sci. 2000, 56, 321–329. [Google Scholar] [CrossRef]
| Lactic Acid Bacteria | Source | Strains | Cholesterol Reduction Rate | References |
|---|---|---|---|---|
| Lactobacillus rhamnosus | Human; korean fermented soya beans | LV108; JDFM6 | 51.93%; 79.00% | [50,51] |
| Lactobacillus fermentum | Human; Tunisian camel milk | grx08; CABA16 | 38.79%; 58.00% | [51,52] |
| Lactobacillus casei | Human | grx12 | 38.2% | [51] |
| Lactobacillus plantarum | Fermented Tibetan yak milk; fermented cream in Inner Mongolia | Lp3; KLDS 1.0344 | 73.30%; 54.08% | [53,54] |
| Human Staphylococcus | Traditional enzymes | M-16 | 47.41% | [55] |
| Enterococcus lumbricoides | Hainan big fragrant mango | MPL1 | 57.62% | [56] |
| Pediococcus pentosaceus | Traditional fermented foods in Guizhou Province | MT-4 | 25.66 | [57] |
| Lactobacillus kefir | Indonesian kefir grains | JK17 | 68.75% | [58] |
| Category | Strains | References |
|---|---|---|
| Traditional Taiwanese naturally fermented ham | Lactobacillus fuchuensis, Lactobacillus sakei, Lactococcus lactis subsp. Cremoris, Lactococcus lactis, Lactococcus garvieae, Lactococcus lactis subsp. Lactis, Leuconostoc. Mesenteroides, Leuconostoc citreum, Leuconostoc carnosum, Enterococcus faecium, Enterococcus faecalis | [92] |
| Pancetta | Lactobacillus sakei, Lactobacillus curvatus, Loigo lactobacillus coryniformis subsp.torquens | [93] |
| Prosciutto | Loigo lactobacillus coryniformis subsp.torquens, P. acidilactici, Lactobacillus curvatus, Lactobacillus plantarum, Lactobacillus sakei | [93] |
| Jinhua Ham | Staphylococcus equorum, Staphylococcus lugdunensis, Lactobacillus Alimentarius, Diplococcus lurea, Pediococcus pentosaceus, Micrococcus mutans | [94] |
| Harbin dry sausage | Pediococcus pentosaceus, Lactobacillus brevis, Lactobacillus curvatus, lactobacillus fermenti, Staphylococcus xylosus, Lactobacillus sakei, Weissella hellenica, Leuconostoc citreum, Lactococcus raffinolactis and Lactobacillus plantarum | [95,96] |
| Sichuan sausages | Lactobacillus spp., Weissella spp., Pediococcus spp. | [97] |
| Panxian Ham | Staphylococcus, Micrococcus | [98] |
| South Africa sausages | Enterococcus, Staphylococcus | [99] |
| Naples-type salami | Lactobacillus Alimentarius, Lactobacillus sakei, Staphylococcus, Lactobacillus curvatus, Staphylococcus xylosus, Lactobacillus casei | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research Update on the Impact of Lactic Acid Bacteria on the Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products. Foods 2022, 11, 2090. https://doi.org/10.3390/foods11142090
Wang Y, Han J, Wang D, Gao F, Zhang K, Tian J, Jin Y. Research Update on the Impact of Lactic Acid Bacteria on the Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products. Foods. 2022; 11(14):2090. https://doi.org/10.3390/foods11142090
Chicago/Turabian StyleWang, Yi, Jun Han, Daixun Wang, Fang Gao, Kaiping Zhang, Jianjun Tian, and Ye Jin. 2022. "Research Update on the Impact of Lactic Acid Bacteria on the Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products" Foods 11, no. 14: 2090. https://doi.org/10.3390/foods11142090
APA StyleWang, Y., Han, J., Wang, D., Gao, F., Zhang, K., Tian, J., & Jin, Y. (2022). Research Update on the Impact of Lactic Acid Bacteria on the Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products. Foods, 11(14), 2090. https://doi.org/10.3390/foods11142090