The Cholesterol-Modulating Effect of the New Herbal Medicinal Recipe from Yellow Vine (Coscinium fenestratum (Goetgh.)), Ginger (Zingiber officinale Roscoe.), and Safflower (Carthamus tinctorius L.) on Suppressing PCSK9 Expression to Upregulate LDLR Expression in HepG2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cell Line, Chemicals, and Computer Software
2.1.2. Herb Material
2.2. Extraction and Isolation
2.2.1. Water Extraction
2.2.2. Ethanol Extraction
2.3. GC-MS/MS Analysis
2.4. Treatment of HepG2 Cells
2.4.1. Cell Viability Analysis
2.4.2. Quantitative Reverse Transcription PCR (RT-qPCR) Analysis
2.4.3. Oil Red O Staining
2.5. Molecular Docking
2.6. Binding Site Analysis
2.7. Statistical Analysis
3. Results
3.1. GC-MS/MS Analysis
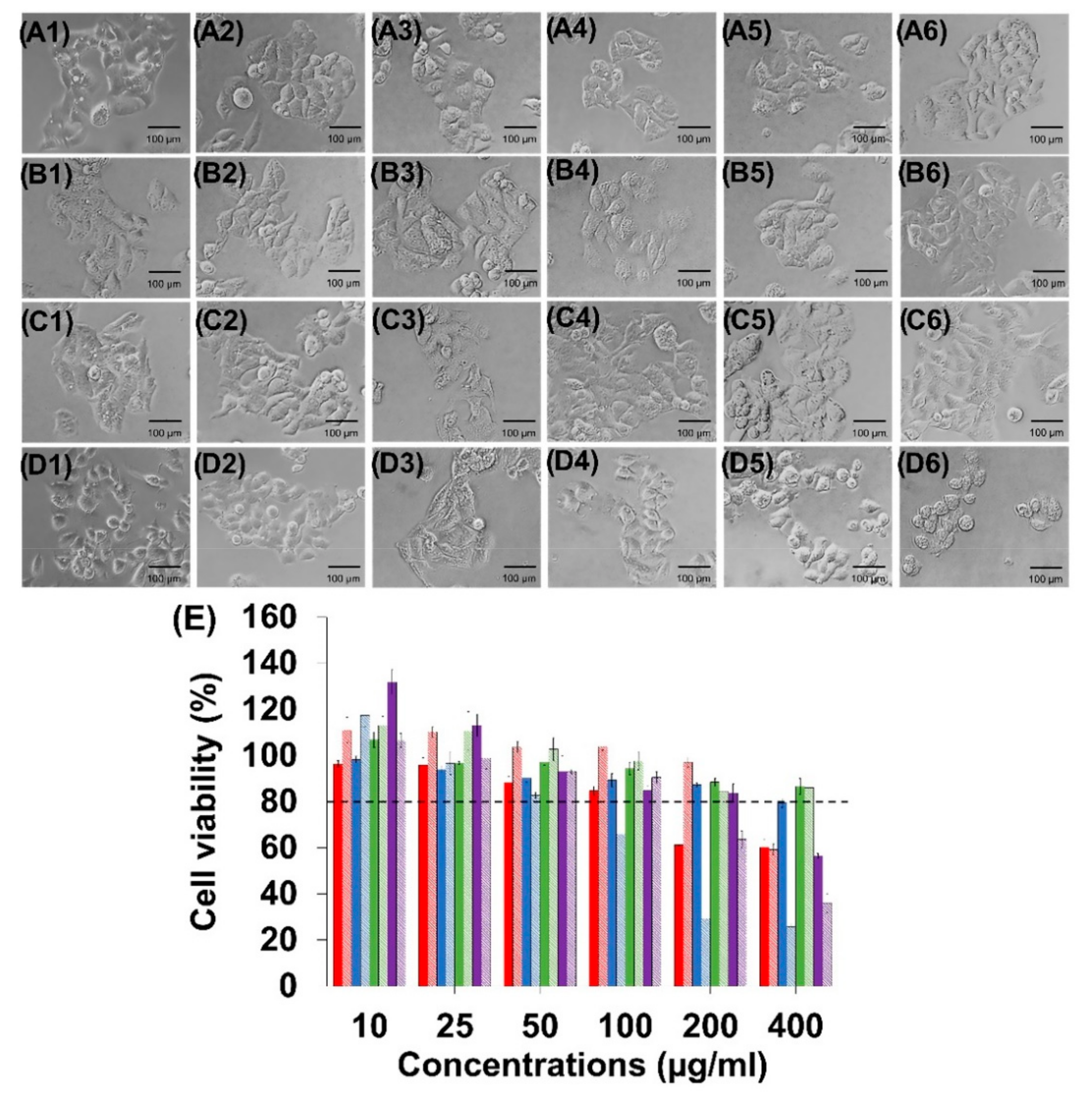
3.2. Determination of Maximum Dose for HepG2
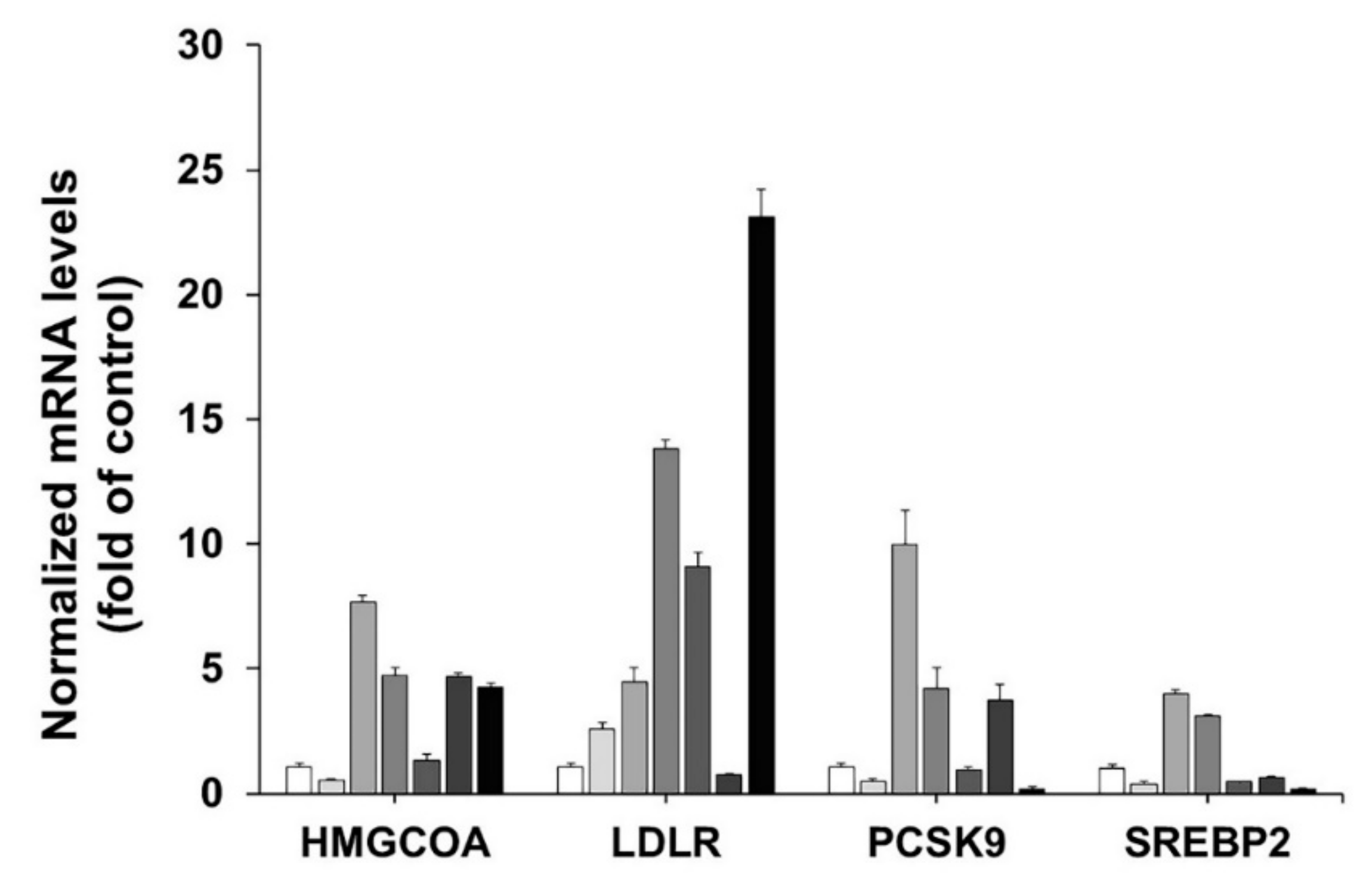
3.3. Effect of the C. fenestratum, Z. officinale, and C. tinctorius on Transcriptional Activity of HMGCR, LDLR, PCSK9, and SREBP2
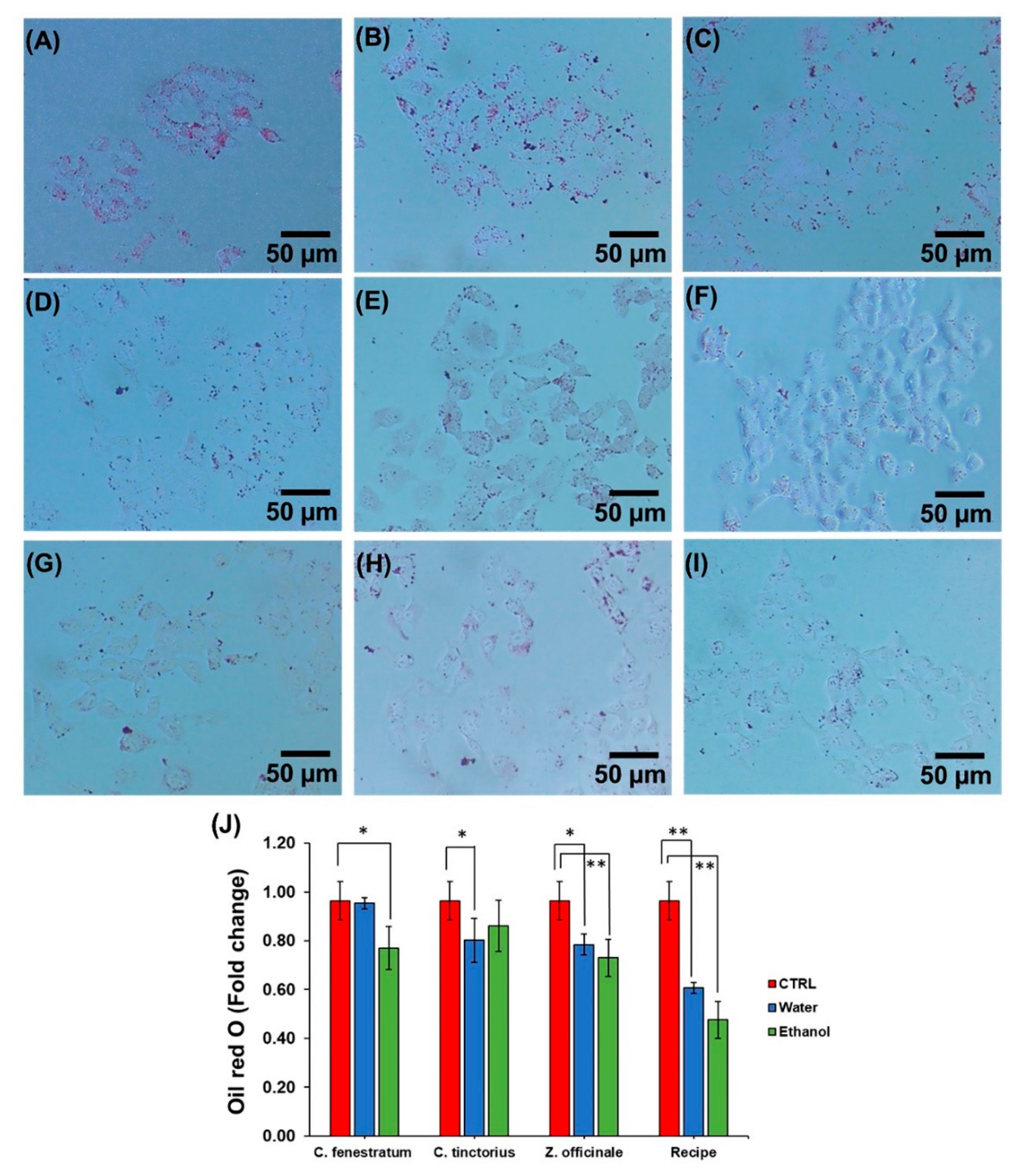
3.4. Effect of Lipid Deposition in HepG2
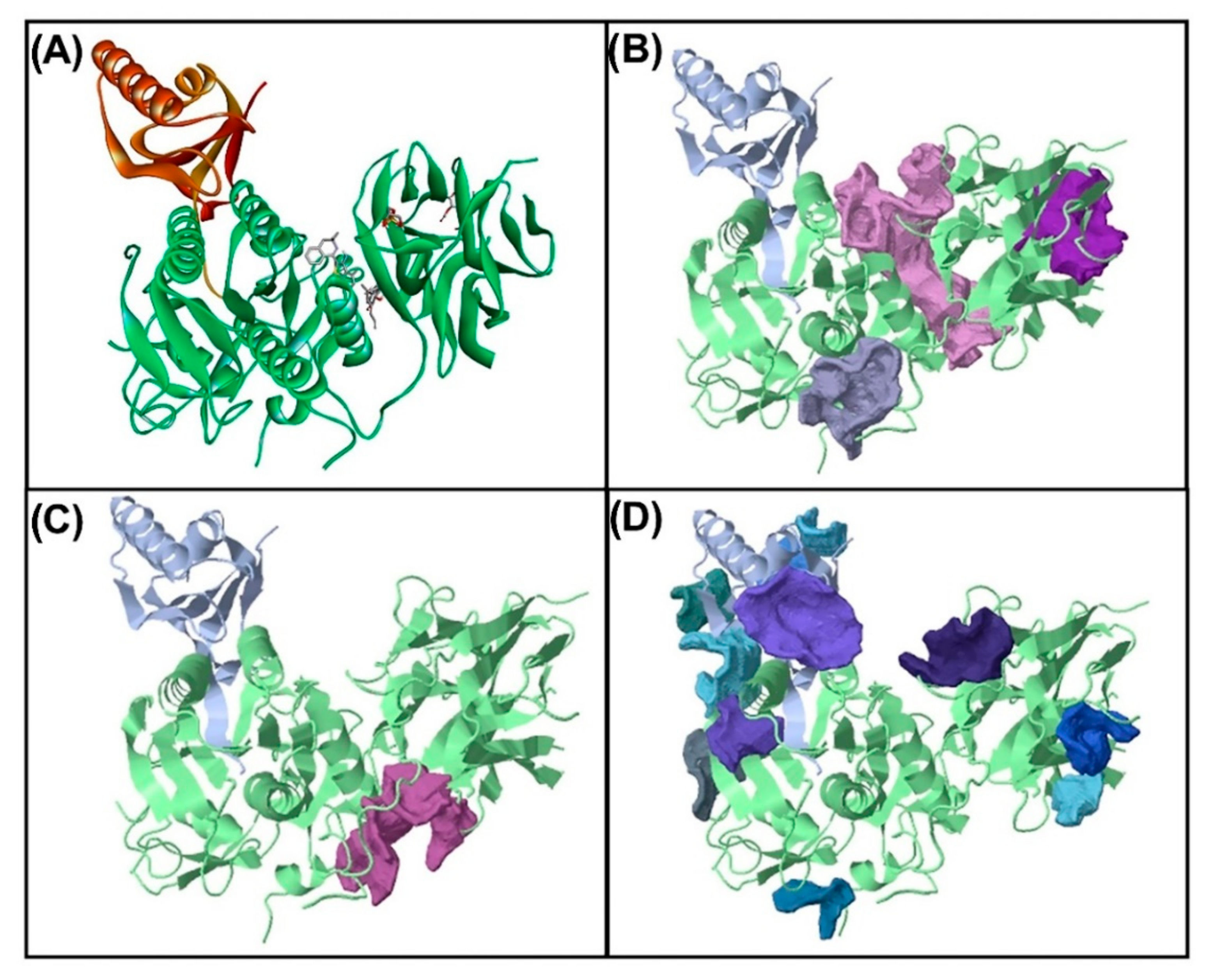
3.5. Molecular Docking for the Top 5 Highest Amounts of the Compound from Each Herb
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Istvan, E. Statin inhibition of HMG-CoA reductase: A 3-dimensional view. Atheroscler. Suppl. 2003, 4, 3–8. [Google Scholar] [CrossRef]
- Liu, L.; Yeh, Y.-Y. S-alk (en) yl cysteines of garlic inhibit cholesterol synthesis by deactivating HMG-CoA reductase in cultured rat hepatocytes. J. Nutr. 2002, 132, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, A.; Bagherniya, M.; Fakheran, O.; Reiner, Ž.; Xu, S.; Sahebkar, A. Medicinal plants and bioactive natural compounds as inhibitors of HMG-CoA reductase: A literature review. BioFactors 2020, 46, 906–926. [Google Scholar] [CrossRef] [PubMed]
- Pearlstein, R.A.; Hu, Q.Y.; Zhou, J.; Yowe, D.; Levell, J.; Dale, B.; Kaushik, V.K.; Daniels, D.; Hanrahan, S.; Sherman, W. New hypotheses about the structure-function of proprotein convertase subtilisin/kexin type 9: Analysis of the epidermal growth factor-like repeat A docking site using WaterMap. Proteins Struct. Funct. Bioinform. 2010, 78, 2571–2586. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.I.; Swedberg, J.E.; Withka, J.M.; Rosengren, K.J.; Akcan, M.; Clayton, D.J.; Daly, N.L.; Cheneval, O.; Borzilleri, K.A.; Griffor, M. Design and synthesis of truncated EGF-A peptides that restore LDL-R recycling in the presence of PCSK9 in vitro. Chem. Biol. 2014, 21, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.-W.; Lagace, T.A.; Garuti, R.; Zhao, Z.; McDonald, M.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 2007, 282, 18602–18612. [Google Scholar] [CrossRef] [Green Version]
- Lagace, T.A. PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef]
- Nassoury, N.; Blasiole, D.A.; Tebon Oler, A.; Benjannet, S.; Hamelin, J.; Poupon, V.; McPherson, P.S.; Attie, A.D.; Prat, A.; Seidah, N.G. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic 2007, 8, 718–732. [Google Scholar] [CrossRef]
- Tushar, K.; George, S.; Remashree, A.; Balachandran, I. Coscinium fenestratum (Gaertn.) Colebr.—A review on this rare, critically endangered and highly-traded medicinal species. J. Plant Sci. 2008, 3, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Ved, D.; Saha, D.; Ravikumar, K.; Haridasan, K. Coscinium fenestratum. IUCN Red List Threat. Species 2015, T50126585A50131325. [Google Scholar]
- Rojsanga, P.; Gritsanapan, W.; Suntornsuk, L. Determination of berberine content in the stem extracts of Coscinium fenestratum by TLC densitometry. Med. Princ. 2006, 15, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Kapoor, N.; Kale, R.D. Coscinium fenestratum: Callus and suspension cell culture of the endangered medicinal plant using vermicompost extract and coelomic fluid as plant tissue culture media. Am. J. Plant Sci. 2016, 7, 899. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhao, Y.; Zhao, L.; Lu, F. The effects of berberine on blood lipids: A systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013, 79, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tong, Q.; Shou, J.-W.; Zhao, Z.-X.; Li, X.-Y.; Zhang, X.-F.; Ma, S.-R.; He, C.-Y.; Lin, Y.; Wen, B.-Y. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics 2017, 7, 2443. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-G.; Park, H.-J.; Kim, J.-W.; Jung, J.-M.; Kim, M.-J.; Jegal, H.-G.; Kim, I.-S.; Kang, M.-J.; Wee, G.; Yang, H.-Y. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 2018, 8, 10130. [Google Scholar] [CrossRef] [PubMed]
- Račková, L.; Cupáková, M.; Ťažký, A.; Mičová, J.; Kolek, E.; Košt’álová, D. Redox properties of ginger extracts: Perspectives of use of Zingiber officinale Rosc. as antidiabetic agent. Interdiscip. Toxicol. 2013, 6, 26. [Google Scholar] [CrossRef]
- Bhandari, U.; Ahmed, J.; Pillai, K. An overview of Zingiber officinale (ginger); Chemistry and pharmacological profile. Hamdard Med. 2001, 44, 28–32. [Google Scholar]
- Mascolo, N.; Jain, R.; Jain, S.; Capasso, F. Ethnopharmacologic investigation of ginger (Zingiber officinale). J. Ethnopharmacol. 1989, 27, 129–140. [Google Scholar] [CrossRef]
- Jitoe, A.; Masuda, T.; Tengah, I.; Suprapta, D.N.; Gara, I.; Nakatani, N. Antioxidant activity of tropical ginger extracts and analysis of the contained curcuminoids. J. Agric. Food Chem. 1992, 40, 1337–1340. [Google Scholar] [CrossRef]
- Krishnakantha, T.; Lokesh, B.R. Scavenging of superoxide anions by spice principles. Indian J. Biochem. Biophys. 1993, 30, 133–134. [Google Scholar]
- Pulla Reddy, A.C.; Lokesh, B. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124. [Google Scholar] [CrossRef]
- Bhandari, U.; Pillai, K. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J. Ethnopharmacol. 2005, 97, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Bhagyawant, S.S.; Srivastava, N. Detailed study on therapeutic properties, uses and pharmacological applications of safflower (Carthamus tinctorius L.). Int. J. Ayurveda Pharma Res. 2014, 2, 1–4. [Google Scholar]
- COŞGE, B.; GÜRBÜZ, B.; KIRALAN, M. Oil content and fatty acid composition of some safflower (Carthamus tinctorius L.) varieties sown in spring and winter. Int. J. Nat. Eng. Sci. 2007, 1, 11–15. [Google Scholar]
- Katkade, M.; Syed, H.; Andhale, R.; Sontakke, M. Fatty acid profile and quality assessment of safflower (Carthamus tinctorius) oil. J. Pharmacogn. Phytochem. 2018, 7, 3581–3585. [Google Scholar]
- Salem, M.A.; Zayed, A.; Alseekh, S.; Fernie, A.R.; Giavalisco, P. The integration of MS-based metabolomics and multivariate data analysis allows for improved quality assessment of Zingiber officinale Roscoe. Phytochemistry 2021, 190, 112843. [Google Scholar] [CrossRef]
- Bhagyalakshmi, B.; Singh, N.S. Meristem culture and micropropagation of a variety of ginger (Zingiber officinale Rosc.) with a high yield of oleoresin. J. Hortic. Sci. 1988, 63, 321–327. [Google Scholar] [CrossRef]
- Nair, K.P. Production, Marketing, and Economics of Ginger. In Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.)—World’s Invaluable Medicinal Spices: The Agronomy and Economy of Turmeric and Ginger; Nair, K.P., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 493–518. [Google Scholar]
- Arablou, T.; Aryaeian, N. The effect of ginger (Zingiber Officinale) as an ancient medicinal plant on improving blood lipids. J. Herb. Med. 2018, 12, 11–15. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the Chemopreventive Effects of Ginger and its Phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Mowrey, D.; Clayson, D. Motion Sickness, Ginger, and Psychophysics. Lancet 1982, 319, 655–657. [Google Scholar] [CrossRef]
- Vijay, D.; Haleshi, C.; Sringeswara, A.N. Endangered Medicinal Plant Coscinium fenestratum (Gaertn.) Colebr A Review. Pharmacogn. J. 2020, 12, 1077–1085. [Google Scholar]
- Goveas, S.W.; Abraham, A.; Research. Extraction and secondary metabolite analysis of Coscinium fenestratum (Gaertn.) Colebr: An important medicinal plant of western ghats. Int. J. Pharm. Sci. Res. 2014, 5, 3484. [Google Scholar]
- Kothalawala, S.D.; Edward, D.; Harasgama, J.C.; Ranaweera, L.; Weerasena, O.V.D.S.J.; Niloofa, R.; Ratnasooriya, W.D.; Premakumara, G.A.S.; Handunnetti, S.M. Immunomodulatory Activity of a Traditional Sri Lankan Concoction of Coriandrum sativum L. and Coscinium fenestratum G. Evid. Based Complement. Altern. Med. 2020, 2020, 9715060. [Google Scholar] [CrossRef]
- Reuter, J.; Huyke, C.; Casetti, F.; Theek, C.; Frank, U.; Augustin, M.; Schempp, C. Anti-inflammatory potential of a lipolotion containing coriander oil in the ultraviolet erythema test. Der Dtsch. Dermatol. Ges. 2008, 6, 847–851. [Google Scholar] [CrossRef]
- Harisaranraj, R.; Babu, S.; Suresh, K. Antimicrobial properties of selected Indian medicinal plants against acne-inducing bacteria. Ethnobot. Leafl. 2010, 14, 84–94. [Google Scholar]
- Suseela, V.; Poornima, K. Free radical scavenging activity of tree turmeric (Coscinium fenestratum). Indian J. Nutr. Diet. 2009, 46, 199–203. [Google Scholar]
- Wongcome, T.; Panthong, A.; Jesadanont, S.; Kanjanapothi, D.; Taesotikul, T.; Lertprasertsuke, N. Hypotensive effect and toxicology of the extract from Coscinium fenestratum (Gaertn.) Colebr. J. Ethnopharmacol. 2007, 111, 468–475. [Google Scholar] [CrossRef]
- Lu, J.-X.; Zhang, C.-X.; Hu, Y.; Zhang, M.-H.; Wang, Y.-N.; Qian, Y.-X.; Yang, J.; Yang, W.-Z.; Jiang, M.-M.; Guo, D.-A. Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites. Phytomedicine 2019, 58, 152826. [Google Scholar]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Eawsakul, K.; Chinavinijkul, P.; Saeeng, R.; Chairoungdua, A.; Tuchinda, P.; Nasongkla, N. Preparation and characterizations of RSPP050-loaded polymeric micelles using poly (ethylene glycol)-b-poly (ε-caprolactone) and poly (ethylene glycol)-b-poly (D, L-lactide). Chem. Pharm. Bull. 2017, 65, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Nasongkla, N.; Tuchinda, P.; Munyoo, B.; Eawsakul, K. Preparation and Characterization of MUC-30-Loaded Polymeric Micelles against MCF-7 Cell Lines Using Molecular Docking Methods and In Vitro Study. Evid. Based Complementary Altern. Med. 2021, 2021, 5597681. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Chen, P.-Y.; Wu, M.-J.; Tai, M.-H.; Yen, J.-H. Tanshinone IIA modulates low density lipoprotein uptake via down-regulation of PCSK9 gene expression in HepG2 cells. PLoS ONE 2016, 11, e0162414. [Google Scholar]
- Leng, E.; Xiao, Y.; Mo, Z.; Li, Y.; Zhang, Y.; Deng, X.; Zhou, M.; Zhou, C.; He, Z.; He, J. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complement. Altern. Med. 2018, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Zainab, R.; Kaleem, A.; Ponczek, M.B.; Abdullah, R.; Iqtedar, M.; Hoessli, D.C. Finding inhibitors for PCSK9 using computational methods. PLoS ONE 2021, 16, e0255523. [Google Scholar] [CrossRef]
- Shamsara, J. CrossDocker: A tool for performing cross-docking using Autodock Vina. SpringerPlus 2016, 5, 344. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Huey, R.; Hart, W.E.; Halliday, S.; Belew, R.; Olson, A.J. AutoDock. In Automated Docking of Flexible Ligands to Receptor-User Guide; The Scripps Research Institute, Molecular Graphics Laboratory, Department of Molecular Biology: La Jolla, CA, USA, 2001. [Google Scholar]
- Roth, E.M.; Diller, P. Alirocumab for hyperlipidemia: Physiology of PCSK9 inhibition, pharmacodynamics and Phase I and II clinical trial results of a PCSK9 monoclonal antibody. Future Cardiol. 2014, 10, 183–199. [Google Scholar] [CrossRef]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef]
- Eawsakul, K.; Panichayupakaranant, P.; Ongtanasup, T.; Warinhomhoun, S.; Noonong, K.; Bunluepuech, K. Computational study and in vitro alpha-glucosidase inhibitory effects of medicinal plants from a Thai folk remedy. Heliyon 2021, 7, e08078. [Google Scholar] [CrossRef]
- Sasaki, M.; Terao, Y.; Ayaori, M.; Uto-Kondo, H.; Iizuka, M.; Yogo, M.; Hagisawa, K.; Takiguchi, S.; Yakushiji, E.; Nakaya, K. Hepatic Overexpression of Idol Increases Circulating Protein Convertase Subtilisin/Kexin Type 9 in Mice and Hamsters via Dual Mechanisms: Sterol Regulatory Element–Binding Protein 2 and Low-Density Lipoprotein Receptor–Dependent Pathways. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1171–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bursill, C.; Roach, P.D.; Bottema, C.D.; Pal, S. Green tea upregulates the low-density lipoprotein receptor through the sterol-regulated element binding protein in HepG2 liver cells. J. Agric. Food Chem. 2001, 49, 5639–5645. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Shimokawa, T.; Kobayashi, T.; Okuyama, H. Lipid lowering effects of high linoleate and high α-linolenate diets in rats and mice. Consequence of long-term feedings. Chem. Pharm. Bull. 1992, 40, 2129–2132. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.-Y.; Li, H.; Tang, J.-J.; Wang, J.; Luo, J.; Liu, B.; Wang, J.-K.; Shi, X.-J.; Cui, H.-W.; Tang, J. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 2018, 9, 5138. [Google Scholar] [CrossRef] [Green Version]
- Bálint, M.; Jeszenői, N.; Horváth, I.; van der Spoel, D.; Hetényi, C. Systematic exploration of multiple drug binding sites. J. Chemin. 2017, 9, 65. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Mora, S.; Glynn, R.J.; Boekholdt, S.M.; Nordestgaard, B.G.; Kastelein, J.J.P.; Ridker, P.M. On-Treatment Non–High-Density Lipoprotein Cholesterol, Apolipoprotein B, Triglycerides, and Lipid Ratios in Relation to Residual Vascular Risk after Treatment with Potent Statin Therapy: JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). J. Am. Coll. Cardiol. 2012, 59, 1521–1528. [Google Scholar] [PubMed] [Green Version]
- Ghadimi, S.; Asad-Samani, K.; Ebrahimi-Valmoozi, A.A. Synthesis, spectroscopic characterization and structure-activity relationship of some phosphoramidothioate pesticides. J. Iran. Chem. Soc. 2011, 8, 717–726. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the General Solubility Equation: In Silico Prediction of Aqueous Solubility Incorporating the Effect of Topographical Polar Surface Area. J. Chem. Inf. Modeling 2012, 52, 420–428. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
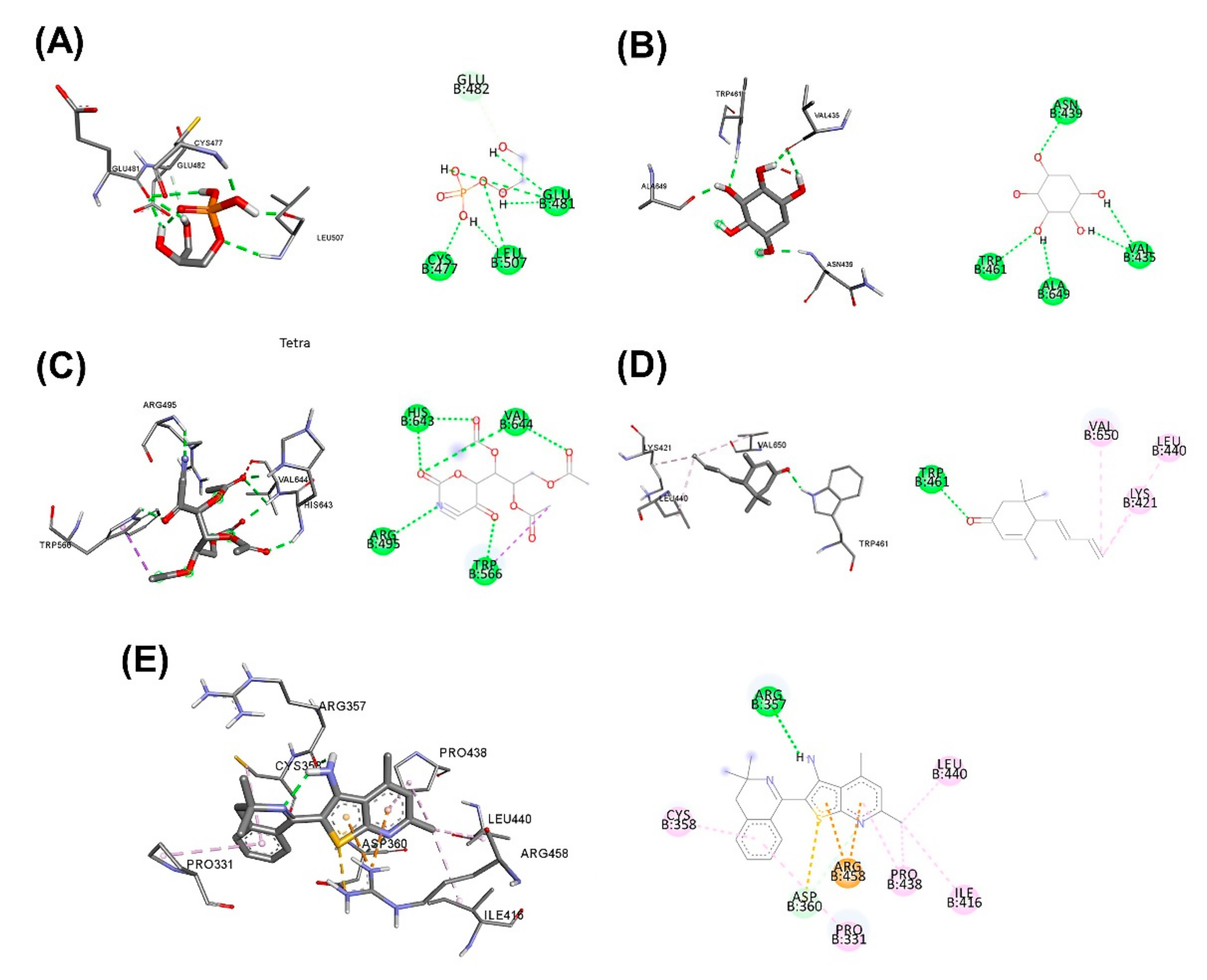
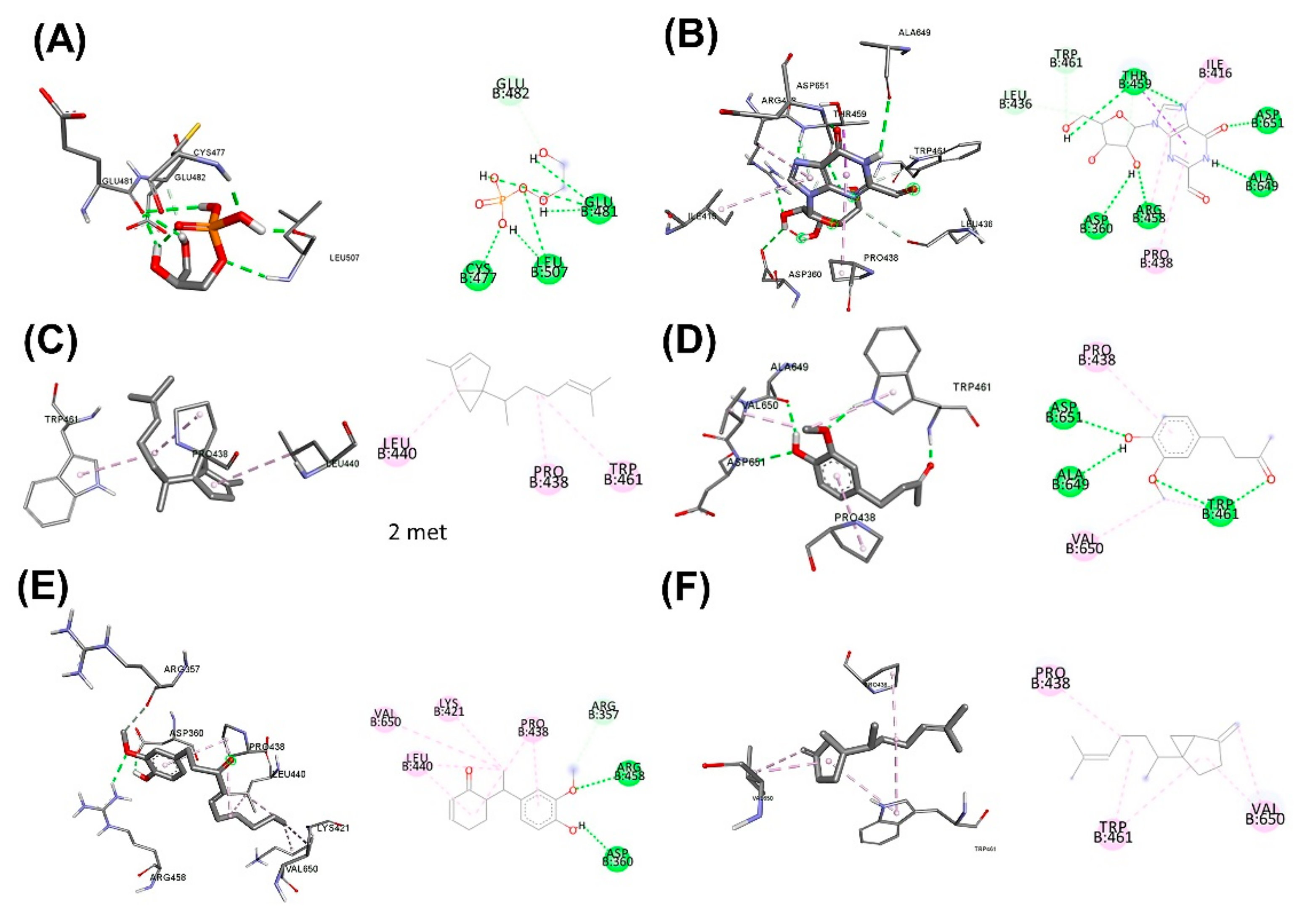
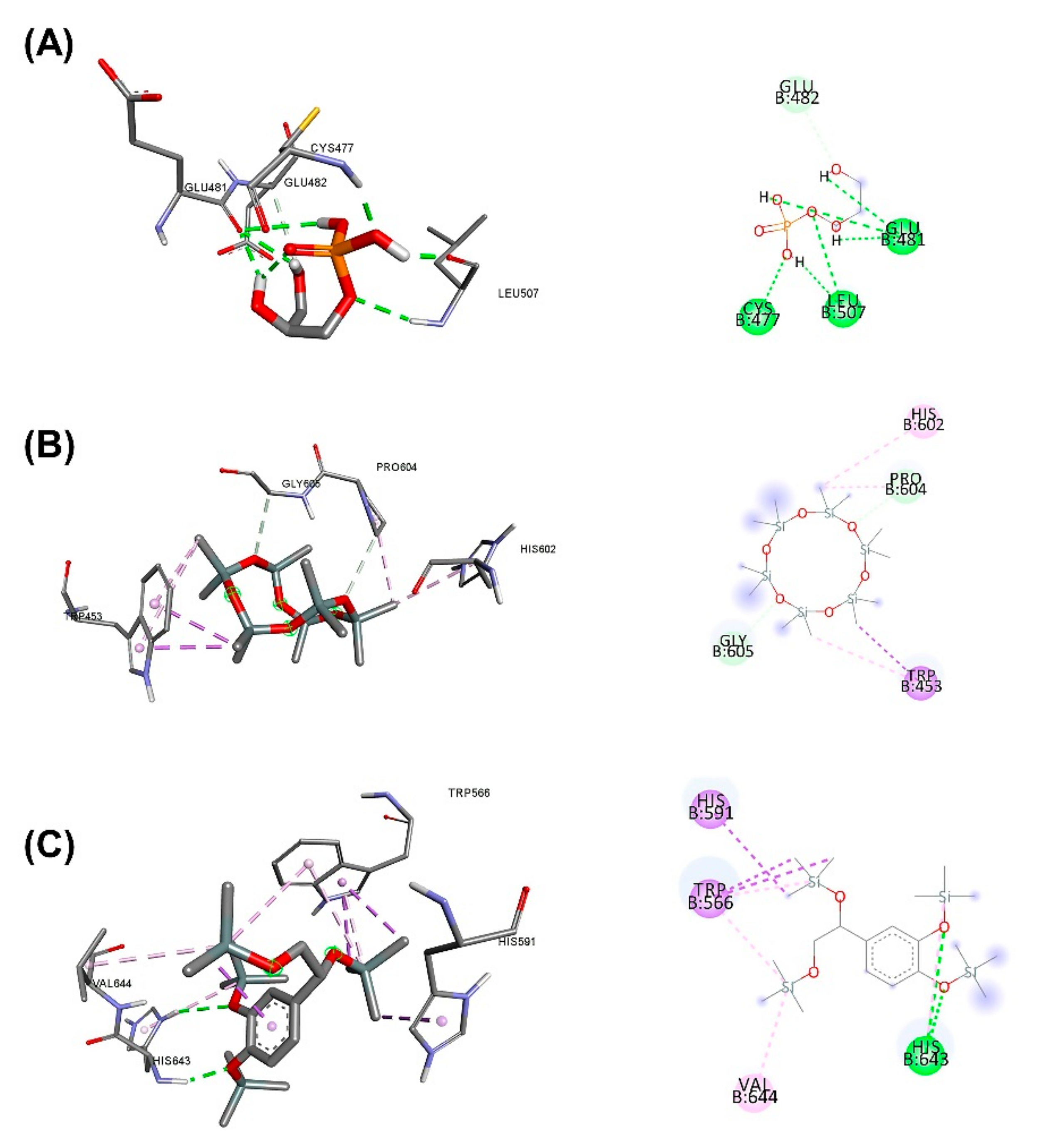
| Scientific Name | Primary Metabolite | Secondary Metabolite | Uses of Plants in Different Countries | Preparations/Therapeutic Uses |
|---|---|---|---|---|
| Z. officinale | carbohydrate, lipids, amino acids, cinnamic acid, and vitamins [26] | oleoresin, phenolics, zingiberene, gingerols, shogaols, aromatic alcohol, and terpenoids [27] | It is distributed all over the world, such as in European countries, America, China, Japan, and India [28] with the following benefits: | |
| C.fenestratum | carbohy-drate, lipids, amino acids, and vitamins [34] | alkaloids, tannins, saponins, flavonoids, phenolic compounds [35] | It is distributed all over the world, such as in Sri Lanka, India, and Thailand with the following benefits: antidiabetic, diuretic, cholesterol lowering, anticancer, anti-inflammatory, antifungal, antihelmintic, antioxidant, and antimicrobial effects [36,37] | Use stem and dried preparation with solvent extractions such as |
| C. tinctorius | formic acid, acetic acid, succinic acid, glucose, fructose, asparagine, proline, alanine, glutamine, valine, uridine, trigonelline, and choline [41] | saffloquinoside C, saffloquinoside A, anhydrosafflor yellow B, rutin, (2S)−4′,5,6,7-tetrahydroxyflavanone 6-O-β-D-glucoside, 5,7,4′-trihydroxy-6-methoxyflavone3-O-β-D-rutinoside, kaempferol-3-O-β-D-glucoside, kaempferol-3-O-rutinoside, (2S)−4′,5,7,8-tetrahydroxy-flavanone-8-O-βD-glucoside, 6-hydroxykaempferol-3,6,7-tri-O-β-D-glucoside, and kaempferol-3-O-β-D-glucosyl-(1→2)-β-D-glucoside | It is distributed all over the world, such as in India, Mexico, America, Spain, Australia, and China with the following benefits:
|
|
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | 5′-CATGAGAAGTATGACAACAGCCT-3′ | 5′-AGTCCTTCCACGATACCAAAGT-3′ |
| PCSK9 | 5′-GCTGAGCTGCTCCAGTTTCT-3′ | 5′-AATGGCGTAGACACCCTCAC-3′ |
| LDLR | 5′-AGTTGGCTGCGTTAATGTGA-3′ | 5′-TGATGGGTTCATCTGACCAGT-3′ |
| HMGCR | 5′-TGATTGACCTTTCCAGAGCAAG-3′ | 5′-CTAAAATTGCCATTCCACGAGC-3′ |
| Gene | Grid Position | Grid Size |
|---|---|---|
| PCSK9 | 34.025 × 23.492 × 25.638 | 110 × 82 × 126 |
| HMGCR | 73.702 × 0.468 × 18.849 | 122 × 78 × 126 |
| S. No. | RT | Name of the Compound | Molecular Formulae | MW | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 6.10 | D-Alanine, N-propargyloxycarbonyl-, isohexyl ester | C13H21NO4 | 255 | 3.14 |
| 2 | 7.72 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 8.56 |
| 3 | 9.05 | Acetic anhydride | C4H6O3 | 102 | 5.72 |
| 4 | 9.38 | Benzofuran, 2,3-dihydro- | C8H8O | 120 | 23.24 |
| 5 | 11.14 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 444 | 6.96 |
| 6 | 14.59 | Sucrose | C12H22O11 | 342 | 6.08 |
| 7 | 14.97 | 3,5-Dimethoxy-4-hydroxytoluene | C9H12O3 | 168 | 2.46 |
| 8 | 15.22 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | C18H52O7Si7 | 576 | 13.73 |
| 9 | 16.45 | 2,4-Di-tert-butylphenol | C14H22O | 206 | 4.68 |
| 10 | 16.83 | Methyl 4-O-acetyl-2,3,6-tri-O-ethyl-.alpha.-d-galactopyranoside | C15H28O7 | 320 | 2.57 |
| 11 | 18.99 | 3,4-Dihydroxyphenylglycol, 4TMS derivative | C20H42O4Si4 | 458 | 8.94 |
| 12 | 22.27 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane-Dup1 | C18H52O7Si7 | 576 | 4.79 |
| 13 | 25.21 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane-Dup2 | C18H52O7Si7 | 576 | 2.71 |
| 14 | 27.89 | Heptasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13-tetradecamethyl- | C18H44O6Si7 | 504 | 1.79 |
| 15 | 29.52 | Ethanol, 2,2′-(dodecylimino)bis- | C16H35NO2 | 273 | 2.34 |
| 16 | 39.48 | Heptacosane | C27H56 | 380 | 1.36 |
| 17 | 41.25 | Octacosane | C28H58 | 394 | 0.92 |
| S. No. | RT | Name of the Compound | Molecular Formulae | MW | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 6.72 | Tert.-butylaminoacrylonitryl | C7H12N2 | 124 | 1.67 |
| 2 | 7.22 | N-(Trimethylsilyl)pyridin-4-amine | C8H14N2Si | 166 | 0.36 |
| 3 | 7.75 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 0.22 |
| 4 | 8.79 | Catechol | C6H6O2 | 110 | 0.9 |
| 5 | 9.06 | Acetic anhydride | C4H6O3 | 102 | 0.49 |
| 6 | 10.67 | Hydroquinone | C6H6O2 | 110 | 0.33 |
| 7 | 11.17 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 444 | 0.38 |
| 8 | 12.67 | Phenol, 2,6-dimethoxy- | C8H10O3 | 154 | 1.72 |
| 9 | 13.92 | Benzaldehyde, 3-hydroxy-4-methoxy- | C8H8O3 | 152 | 0.24 |
| 10 | 15.22 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | C18H52O7Si7 | 576 | 0.32 |
| 11 | 16.09 | beta.-D-Glucopyranose, 1,6-anhydro- | C6H10O5 | 162 | 0.78 |
| 12 | 16.45 | 2,4-Di-tert-butylphenol | C14H22O | 206 | 0.93 |
| 13 | 16.6 | 2-Methoxy-6-methoxycarbonyl-4-pyrone | C8H8O5 | 184 | 0.12 |
| 14 | 16.72 | Benzoic acid, 4-hydroxy-3-methoxy-, methyl ester | C9H810O4 | 182 | 0.22 |
| 15 | 16.83 | Methyl 4-O-acetyl-2,3,6-tri-O-ethyl-.alpha.-d-galactopyranoside | C15H28O7 | 320 | 2.1 |
| 16 | 16.96 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | C10H12O3 | 180 | 0.7 |
| 17 | 17.81 | Megastigmatrienone | C13H18O | 190 | 0.31 |
| 18 | 18.25 | Megastigmatrienone-Dup1 | C13H18O | 190 | 0.99 |
| 19 | 19.02 | Tetraacetyl-d-xylonic nitrile | C14H17NO9 | 343 | 27.92 |
| 20 | 19.31 | Megastigmatrienone-Dup2 | C13H18O | 190 | 4.26 |
| 21 | 19.63 | d-Gala-l-ido-octonic amide | C8H17NO8 | 255 | 0.19 |
| 22 | 19.81 | 2,6-Dimethoxyhydroquinone | C8H10O4 | 170 | 1.47 |
| 23 | 19.98 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- | C9H10O4 | 182 | 3.35 |
| 24 | 20.28 | d-Gala-l-ido-octonic amide-Dup1 | C8H17NO8 | 255 | 9.75 |
| 25 | 20.64 | Inositol, 1-deoxy- | C6H12O5 | 164 | 15.58 |
| 26 | 20.73 | Inositol, 1-deoxy--Dup1 | C6H12O5 | 164 | 9.31 |
| 27 | 21.11 | 3,4-Dihydrocoumarin, 4,4-dimethyl-6-hydroxy- | C11H12O3 | 192 | 0.14 |
| 28 | 21.75 | (E)-4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenol | C10H12O3 | 180 | 1.14 |
| 29 | 22.36 | Benzoic acid, 4-hydroxy-3,5-dimethoxy-, methyl ester | C10H12O5 | 212 | 0.24 |
| 30 | 26.84 | trans-Sinapyl alcohol | C11H14O4 | 210 | 1.66 |
| 31 | 29.52 | Ethanol, 2,2′-(dodecylimino)bis- | C16H35NO2 | 273 | 0.66 |
| 32 | 35.65 | Hentriacontane | C31H64 | 436 | 0.39 |
| 33 | 37.8 | Octacosane, 2-methyl- | C29H60 | 408 | 0.54 |
| 34 | 39.48 | Heptacosane | C27H56 | 380 | 0.69 |
| 35 | 40.48 | Octacosane, 2-methyl-Dup1 | C29H60 | 408 | 0.72 |
| 36 | 41.25 | Hentriacontane-Dup1 | C31H64 | 436 | 0.66 |
| 37 | 41.45 | Doxepin | C19H21NO | 279 | 0.11 |
| 38 | 42.02 | Tetratetracontane | C44H90 | 618 | 0.36 |
| 39 | 42.31 | 1,4-Methano-2H-cyclopent[d]oxepin-2,5(4H)-dione, 6-[(dimethylamino)methyl]hexahydro-8a-hydroxy-5a-methyl-9-(1-methylethyl)-, [1R-(1.alpha.,4.alpha.,5a.alpha.,6.beta.,8a.alpha.,9S*)]- | C17H27NO4 | 309 | 0.66 |
| 40 | 42.66 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | C20H21N3S | 335 | 5.87 |
| 41 | 42.84 | Octacosane | C28H58 | 394 | 0.28 |
| 42 | 46.12 | 1(4H)-naphthalenone, 4-[[4-(diethylamino)phenyl]imino]-2-hydroxy- | C20H20N2O2 | 320 | 0.21 |
| 43 | 49.16 | Olean-12-en-28-oic acid, 3-hydroxy-, methyl ester, (3.beta.)- | C31H50O3 | 470 | 0.14 |
| S. No | RT | Name of the Compound | Molecular Formulae | MW | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 5.45 | 3(2H)-Furanone, 4-hydroxy-5-methyl- | C5H6O3 | 114 | 0.55 |
| 2 | 6.13 | Maltol | C6H10O3 | 126 | 2.78 |
| 3 | 6.73 | Tert.-butylaminoacrylonitryl | C7H12N2 | 124 | 2.17 |
| 4 | 7.45 | 2-Propanamine, N-methyl-N-nitroso- | C4H10N2O | 102 | 0.23 |
| 5 | 7.75 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 2.14 |
| 6 | 8.76 | Catechol | C6H6O2 | 110 | 0.8 |
| 7 | 9.13 | Decanal | C10H20O | 156 | 1.64 |
| 8 | 10.65 | Cyclobuta[1,2:3,4]dicyclooctene, hexadecahydro- | C16H28 | 220 | 0.44 |
| 9 | 11.17 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 444 | 0.89 |
| 10 | 11.79 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150 | 0.48 |
| 11 | 14.09 | 10-Methyl-8-tetradecen-1-ol acetate | C17H32O2 | 268 | 0.53 |
| 12 | 14.72 | 2-Formyl-9-[.beta.-d-ribofuranosyl]hypoxanthine | C11H12N4O6 | 296 | 4.37 |
| 13 | 14.93 | Cyclopentanecarboxaldehyde | C6H10O | 98 | 0.52 |
| 14 | 15.22 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | C18H52O7Si7 | 576 | 0.48 |
| 15 | 15.83 | trans-Sesquisabinene hydrate | C15H26O | 222 | 0.40 |
| 16 | 15.9 | Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- | C15H22 | 202 | 2.8 |
| 17 | 16.15 | Octanal, 7-hydroxy-3,7-dimethyl- | C10H20O2 | 172 | 0.26 |
| 18 | 16.24 | (1S,5S)-2-Methyl-5-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hex-2-ene | C15H24 | 204 | 9.06 |
| 19 | 16.39 | Alpha.-Farnesene | C15H24 | 204 | 2.1 |
| 20 | 16.46 | Phenol, 2,5-bis(1,1-dimethylethyl)- | C14H22O | 206 | 3.71 |
| 21 | 16.54 | Beta.-Bisabolene | C15H24 | 204 | 2.33 |
| 22 | 16.81 | 3-Cyclohexene-1-methanol, 2-hydroxy-.alpha.,.alpha.,4-trimethyl- | C10H8O2 | 170 | 1.14 |
| 23 | 16.95 | (1S,5S)-4-Methylene-1-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hexane | C15H24 | 204 | 3.77 |
| 24 | 17.54 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- | C10H18O2 | 170 | 1.05 |
| 25 | 18.07 | 4-(1-Hydroxyallyl)-2-methoxyphenol | C10H12O3 | 180 | 1.39 |
| 26 | 18.58 | Ethyl N-(o-anisyl)formimidate | C10H13NO2 | 179 | 0.49 |
| 27 | 18.99 | Ethyl .alpha.-d-glucopyranoside | C8H16O6 | 208 | 3.1 |
| 28 | 19.7 | 2-Butanone, 4-(4-hydroxy-3-methoxyphenyl)- | C11H14O3 | 194 | 38.21 |
| 29 | 20.65 | 4-(3,4-Dimethoxyphenyl)butan-2-one | C12H16O3 | 208 | 0.17 |
| 30 | 20.86 | (1R,2R,4S,6S,7S,8S)-8-Isopropyl-1-methyl-3-methylenetricyclo[4.4.0.02,7]decan-4-ol | C15H24O | 220 | 0.26 |
| 31 | 23.26 | cis-Z-.alpha.-Bisabolene epoxide | C15H24O | 220 | 0.49 |
| 32 | 23.59 | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- | C8H26O | 222 | 0.47 |
| 33 | 24.41 | trans-Z-.alpha.-Bisabolene epoxide | C15H24O | 220 | 0.43 |
| 34 | 25.48 | Hexadecanoic acid, methyl ester | C17H32O2 | 270 | 0.2 |
| 35 | 29.52 | Ethanol, 2,2′-(dodecylimino)bis- | C16H35NO2 | 273 | 0.89 |
| 36 | 31 | (E)-1-(4-Hydroxy-3-methoxyphenyl)dec-3-en-5-one | C17H24O3 | 276 | 2.08 |
| 37 | 32.25 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | C17H24O3 | 276 | 5.89 |
| 38 | 35.44 | (E)-4-(2-(2-(2,6-Dimethylhepta-1,5-dien-1-yl)-6-pentyl-1,3-dioxan-4-yl)ethyl)-2-methoxyphenol | C27H42O4 | 430 | 0.35 |
| 39 | 35.85 | (3R,5S)-1-(4-Hydroxy-3-methoxyphenyl)decane-3,5-diyl diacetate | C21H42O4 | 380 | 0.69 |
| 40 | 39.74 | 1-(4-Hydroxy-3-methoxyphenyl)tetradec-4-en-3-one | C21H32O3 | 332 | 0.25 |
| S. No. | RT | Name of the Compound | Molecular Formulae | MW | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 5.45 | 3(2H)-Furanone, 4-hydroxy-5-methyl- | C5H6O3 | 114 | 2.82 |
| 2 | 5.62 | Acetic anhydride | C4H6O3 | 102 | 1.41 |
| 3 | 5.77 | .gamma.-Dodecalactone | C12H22O2 | 198 | 4.31 |
| 4 | 6.13 | Maltol | C6H6O3 | 126 | 4.2 |
| 5 | 6.74 | Cyclopentanol | C5H10O | 86 | 6.74 |
| 6 | 7.45 | 2-Propanamine, N-methyl-N-nitroso- | C4H10N2O | 102 | 2.18 |
| 7 | 7.75 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 7.76 |
| 8 | 7.87 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl--Dup1 | C6H8O4 | 144 | 4.84 |
| 9 | 8.37 | 2H-Pyran, 3,4-dihydro- | C5H8O | 84 | 2.42 |
| 10 | 8.58 | 5,8,11,14-Eicosatetraenoic acid, phenylmethyl ester, (all-Z)- | C27H38O2 | 394 | 0.69 |
| 11 | 8.76 | Catechol | C6H6O2 | 110 | 4.27 |
| 12 | 9.06 | Acetamide, N-[4-(4-nitrobenzylidenamino)-3-furazanyl]- | C11H9N5O4 | 275 | 3.43 |
| 13 | 9.4 | Benzofuran, 2,3-dihydro- | C8H8O | 120 | 3.51 |
| 14 | 9.61 | 5-Hydroxymethylfurfural | C6H6O3 | 126 | 1.33 |
| 15 | 10.67 | Hydroquinone | C6H6O2 | 110 | 0.82 |
| 16 | 10.97 | 2-Butanone, 4-(ethylthio)- | C6H12OS | 132 | 0.97 |
| 17 | 11.15 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 444 | 3.31 |
| 18 | 11.77 | 2-Methyl-9-.beta.-d-ribofuranosylhypoxanthine | C11H14N4O5 | 282 | 2.04 |
| 19 | 12.66 | Phenol, 2,6-dimethoxy- | C8H10O3 | 154 | 0.43 |
| 20 | 13.31 | DL-Proline, 5-oxo-, methyl ester | C6H9NO3 | 143 | 1.76 |
| 21 | 13.9 | 4-Methyl(trimethylene)silyloxyoctane | C12H26OSi | 214 | 1.72 |
| 22 | 14.16 | 3,7-Diacetamido-7H-s-triazolo[5,1-c]-s-triazole | C7H9N7O2 | 223 | 2.54 |
| 23 | 14.89 | l-Pyrrolid-2-one, N-carboxyhydrazide | C5H9N3O2 | 143 | 6.13 |
| 24 | 14.97 | Guanosine | C10H13N5O5 | 283 | 6.58 |
| 25 | 15.22 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | C18H52O7 Si7 | 576 | 1.29 |
| 26 | 16.45 | 2,4-Di-tert-butylphenol | C14H22O | 206 | 1.41 |
| 27 | 18.72 | d-Glycero-d-ido-heptose | C7H14O7 | 210 | 1.42 |
| 28 | 19.44 | 3-Deoxy-d-mannonic acid | C6H12O6 | 180 | 7.85 |
| 29 | 19.68 | d-Glycero-d-ido-heptose-Dup1 | C7H14O7 | 210 | 3.94 |
| 30 | 19.87 | 2-Methyl-9-.beta.-d-ribofuranosylhypoxanthine-Dup1 | C11H14N4O5 | 282 | 2.17 |
| 31 | 22.29 | Heptasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13-tetradecamethyl- | C14H44O6Si7 | 504 | 0.33 |
| 32 | 25.48 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 0.76 |
| 33 | 29.52 | Ethanol, 2,2′-(dodecylimino)bis- | C16H35NO2 | 273 | 1.2 |
| 34 | 32.25 | Heptacosane | C27H56 | 380 | 0.86 |
| 35 | 39.48 | Heptacosane-Dup1 | C27H56 | 380 | 1.45 |
| 36 | 40.34 | 9-Octadecenamide, (Z)- | C18H35NO | 281 | 0.76 |
| 37 | 41.25 | Heptacosane-Dup2 | C27H56 | 380 | 0.35 |
| S. No | RT | Name of the Compound | Molecular Formulae | MW | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 6.11 | 3-Acetylthymine | C7H8N2O3 | 168 | 0.26 |
| 2 | 6.72 | Tert.-butylaminoacrylonitryl | C7H12N2 | 124 | 1.1 |
| 3 | 7.22 | 4-Isopropylbenzenethiol, S-methyl- | C10H14S | 166 | 0.34 |
| 4 | 7.75 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 0.15 |
| 5 | 8.78 | Catechol | C6H8O2 | 110 | 0.8 |
| 6 | 9.06 | 1-[3-(4-Bromophenyl)-2-thioureido]-1-deoxy-b-d-glucopyranose 2,3,4,6-tetraacetate | C21H25BrN2O9S | 560 | 0.26 |
| 7 | 11.15 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 444 | 0.09 |
| 8 | 11.79 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150 | 0.17 |
| 9 | 12.68 | Phenol, 2,6-dimethoxy- | C9H10O3 | 154 | 0.09 |
| 10 | 13.31 | 2-Pyrrolidinone, 5-(cyclohexylmethyl)- | C11H19NO | 181 | 0.18 |
| 11 | 13.92 | Benzaldehyde, 3-hydroxy-4-methoxy- | C8H8O3 | 152 | 0.15 |
| 12 | 15.23 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | C18H52O7Si7 | 576 | 0.4 |
| 13 | 16.11 | .beta.-D-Glucopyranose, 1,6-anhydro- | C6H10O5 | 162 | 0.67 |
| 14 | 16.46 | 2,4-Di-tert-butylphenol | C14H22O | 206 | 0.37 |
| 15 | 16.59 | 2-Methoxy-6-methoxycarbonyl-4-pyrone | C8H8O5 | 184 | 0.1 |
| 16 | 16.73 | Benzoic acid, 4-hydroxy-3-methoxy-, methyl ester | C9H10O4 | 182 | 0.11 |
| 17 | 16.84 | Methyl 4-O-acetyl-2,3,6-tri-O-ethyl-.alpha.-d-galactopyranoside | C15H28O7 | 320 | 0.19 |
| 18 | 16.97 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | C10H12O3 | 180 | 0.26 |
| 19 | 17.8 | Megastigmatrienone | C13H18O | 190 | 0.19 |
| 20 | 18.24 | Megastigmatrienone-Dup1 | C13H18O | 190 | 0.86 |
| 21 | 18.71 | 3,4,5-Trimethoxyphenol | C9H12O4 | 184 | 1.91 |
| 22 | 19.01 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl ester | C26H50O2 | 394 | 7.19 |
| 23 | 19.18 | Tetraacetyl-d-xylonic nitrile | C14H17NO9 | 343 | 9.47 |
| 24 | 19.32 | Megastigmatrienone-Dup2 | C13H18O | 190 | 11.58 |
| 25 | 19.81 | 2-Oxa-3-azabicyclo[4.4.0]dec-3-ene, 5-methyl-1-trimethylsilyloxy-, N-oxide | C12H23NO3Si | 257 | 2 |
| 26 | 19.98 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- | C9H10O4 | 182 | 3 |
| 27 | 20.07 | .alpha.-l-Mannose semicarbazone pentaacetate | C18H25N3O12 | 475 | 1.48 |
| 28 | 20.28 | d-Gala-l-ido-octonic amide | C8H17NO8 | 255 | 7 |
| 29 | 20.6 | Shikimic acid | C7H10O5 | 174 | 4.84 |
| 30 | 20.9 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | C11H14O3 | 194 | 8.69 |
| 31 | 21.13 | Inositol, 1-deoxy- | C6H12O5 | 164 | 6.02 |
| 32 | 21.46 | Inositol, 1-deoxy--Dup1 | C6H12O5 | 164 | 15.44 |
| 33 | 21.75 | (E)-4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenol | C10H12O3 | 180 | 1.26 |
| 34 | 22.28 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane-Dup1 | C18H52O7Si7 | 576 | 0.2 |
| 35 | 22.37 | Benzoic acid, 4-hydroxy-3,5-dimethoxy-, methyl ester | C10H12O5 | 212 | 0.72 |
| 36 | 22.82 | 4-Hydroxy-4a,8-dimethyl-3-methylene-3,3a,4,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione | C15H18O4 | 262 | 0.12 |
| 37 | 25.48 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 0.19 |
| 38 | 26.83 | trans-Sinapyl alcohol | C11H14O4 | 210 | 0.43 |
| 39 | 27.89 | 1,3-Dioxolo[4,5-g]isoquinolin-5(6H)-one, 7,8-dihydro- | C10H9NO3 | 191 | 0.1 |
| 40 | 28.68 | 9,12-Octadecadienoic acid, methyl ester, (E,E)- | C19H34O2 | 294 | 0.08 |
| 41 | 28.8 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 296 | 0.15 |
| 42 | 29.52 | Ethanol, 2,2′-(dodecylimino)bis- | C16H35NO2 | 273 | 0.25 |
| 43 | 40.47 | 7-Isoquinolinol, 1,2,3,4-tetrahydro-1-[(3-hydroxy-4-methoxyphenyl)methyl]-6-methoxy-2-methyl-, (S)- | C19H23NO4 | 329 | 0.09 |
| 44 | 41.05 | Corydine | C20H23NO4 | 341 | 0.06 |
| 45 | 41.45 | Ethylamine, 2-((p-bromo-.alpha.-methyl-.alpha.-phenylbenzyl)oxy)-N,N-dimethyl- | C18H22BrNO | 347 | 0.06 |
| 46 | 42.3 | 1-Undecanamine, N,N-dimethyl- | C13H29N | 199 | 0.32 |
| 47 | 42.67 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | C20H21N3S | 335 | 4.26 |
| 48 | 44.57 | Berbine, 13,13a-didehydro-9,10-dimethoxy-2,3-(methylenedioxy)- | C20H19NO4 | 337 | 1.3 |
| 49 | 44.69 | Ergosta-5,22-dien-3-ol, acetate, (3.beta.,22E)- | C30H48O2 | 440 | 0.28 |
| 50 | 45.31 | Thalictricavine | C21H23NO4 | 353 | 0.12 |
| 51 | 45.42 | .beta.-Sitosterol | C29H50O | 414 | 0.21 |
| 52 | 46.15 | 1(4H)-naphthalenone, 4-[[4-(diethylamino)phenyl]imino]-2-hydroxy- | C20H20N2O2 | 320 | 1.29 |
| 53 | 49.19 | Olean-12-en-28-oic acid, 3-hydroxy-, methyl ester, (3.beta.)- | C31H50O3 | 470 | 2.15 |
| 54 | 50.18 | Urs-12-en-28-oic acid, 3-hydroxy-, methyl ester, (3.beta.)- | C31H50O3 | 470 | 0.23 |
| 55 | 50.35 | Urs-12-en-28-oic acid, 3-hydroxy-, methyl ester, (3.beta.)-Dup1 | C31H50O3 | 470 | 0.78 |
| S. No. | RT | Name of the Compound | Molecular Formulae | MW | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 9.12 | Decanal | C10H20O | 156 | 3.1 |
| 2 | 10.18 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | C10H18O | 154 | 0.91 |
| 3 | 15.91 | Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- | C15H22 | 202 | 1.28 |
| 4 | 16.23 | 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | C15H24 | 204 | 3.79 |
| 5 | 16.4 | .alpha.-Farnesene | C15H24 | 204 | 1.19 |
| 6 | 16.55 | .beta.-Bisabolene | C15H24 | 204 | 0.93 |
| 7 | 16.94 | Cyclohexene, 3-(1,5-dimethyl-4-hexenyl)-6-methylene-, [S-(R*,S*)]- | C15H24 | 204 | 2.03 |
| 8 | 17.74 | Nerolidol | C15H26O | 222 | 0.89 |
| 9 | 18.07 | 4-(1-Hydroxyallyl)-2-methoxyphenol | C10H12O3 | 180 | 1.35 |
| 10 | 19.8 | Butan-2-one, 4-(3-hydroxy-2-methoxyphenyl)- | C11H14O3 | 194 | 33.27 |
| 11 | 20.11 | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- | C15H26O | 222 | 1.4 |
| 12 | 20.66 | (1S,2R,5R)-2-Methyl-5-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hexan-2-ol | C15H26O | 222 | 0.99 |
| 13 | 20.87 | 1H-3a,7-Methanoazulen-5-ol, octahydro-3,8,8-trimethyl-6-methylene- | C15H24O | 220 | 1.63 |
| 14 | 23.27 | cis-Z-.alpha.-Bisabolene epoxide | C15H24O | 220 | 1.83 |
| 15 | 24.43 | trans-Z-.alpha.-Bisabolene epoxide | C15H24O | 220 | 0.83 |
| 16 | 24.55 | Acetic acid, 3-hydroxy-6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-yl ester | C17H26O3 | 278 | 0.65 |
| 17 | 25.48 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 0.42 |
| 18 | 31.02 | (E)-1-(4-Hydroxy-3-methoxyphenyl)dec-3-en-5-one | C17H24O3 | 276 | 4.96 |
| 19 | 31.2 | 3-Decanone, 1-(4-hydroxy-3-methoxyphenyl)- | C17H26O3 | 278 | 1.5 |
| 20 | 32.33 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | C17H24O3 | 276 | 24.37 |
| 21 | 35.48 | (E)-4-(2(2-(2,6-Dimethylhepta-1,5-dien-1-yl)-6-pentyl-1,3-dioxan-4-yl)ethyl)-2-methoxyphenol | C27H42O4 | 430 | 1.13 |
| 22 | 35.87 | 1-(4-Hydroxy-3-methoxyphenyl)dodec-4-en-3-one | C19H28O3 | 304 | 5.23 |
| 23 | 38.64 | (E)-1-(4-Hydroxy-3-methoxyphenyl)tetradec-3-en-5-one | C21H32O3 | 332 | 0.74 |
| 24 | 39.74 | 1-(4-Hydroxy-3-methoxyphenyl)tetradec-4-en-3-one | C21H32O3 | 332 | 3.24 |
| 25 | 40.14 | 1-(4-Hydroxy-3-methoxyphenyl)tetradecane-3,5-dione | C21H32O4 | 348 | 0.35 |
| 26 | 42.79 | (E)-4-(2(2-(2,6-Dimethylhepta-1,5-dien-1-yl)-6-pentyl-1,3-dioxan-4-yl)ethyl)-2-methoxyphenol-Dup1 | C27H42O4 | 430 | 0.61 |
| 27 | 45.43 | .beta.-Sitosterol | C29H50O | 414 | 1.35 |
| No. | Binding Site | Amino Acid |
|---|---|---|
| 1 | Strong No. 1 | ILE:154, PRO:155, ASN:157, LEU:158, GLU:159, ARG:160, ILE:161, THR:162, PRO:163, ARG:165, TYR:166, ARG:167, ARG:237, ASP:238, ALA:239, GLY:240, VAL:241, ALA:242, LYS:243, GLY:244, GLY:394, ILE:395, ALA:397, MET:398, MET:399, LEU:400, SER:401, ALA:402, GLU:403, LEU:406, ARG:414, PHE:418, ALA:443, LEU:444, PRO:445, PRO:446, SER:447, THR:448, HIS:449, GLY:450, ALA:451 |
| 2 | Strong No. 2 | ALA:68:A, LYS:69:A, GLY:292, TYR:293, SER:294, ARG:295, LEU:297, ASN:298, ALA:299, ALA:300, CYS:301, GLN:302, ARG:303, LEU:304, ALA:305, ARG:306, ALA:307, GLY:308, VAL:309, THR:313, ASP:321, ALA:322, CYS:323, LEU:324, TYR:325, SER:326, PRO:327, ALA:328, SER:329, ALA:330, PRO:331, GLU:332, VAL:333, ILE:334, THR:335, GLY:356, ARG:357, CYS:358, VAL:359, ASP:360, LEU:361, THR:407, LEU:408, ALA:409, GLU:410, ARG:412, GLN:413, ILE:416, HIS:417, SER:419, ALA:420, LYS:421, ASP:422, VAL:423, ILE:424, ASN:425, GLU:426, ALA:427, PHE:429, GLU:431, ASP:432, GLN:433, ARG:434, VAL:435, LEU:436, THR:437, PRO:438, ASN:439, LEU:440, CYS:457, ARG:458, THR:459, VAL:460, TRP:461, SER:462, ALA:463, HIS:464, SER:465, GLY:466, ALA:471, THR:472, ALA:473, ILE:474, ALA:475, ARG:476, CYS:477, ALA:478, PRO:479, ASP:480, GLU:481, GLU:482, LEU:483, PHE:489, ARG:491, GLU:501, GLY:505, LYS:506, LEU:507, VAL:508, ARG:510, VAL:520, TYR:521, ALA:522, ILE:523, ARG:525, CYS:526, GLU:620, GLN:621, THR:623, VAL:624, ALA:625, CYS:626, TYR:648, ALA:649, VAL:650, ASP:651, ASN:652, THR:653, CYS:654, VAL:655, ARG:657 |
| 3 | Strong No. 3 | CYS:486, SER:487, SER:488, GLY:493, LYS:494, ARG:495, ARG:496, GLY:497, GLU:498, ALA:514, PHE:515, ARG:549, LEU:559, GLY:561, CYS:562, SER:563, SER:564, HIS:565, TRP:566, GLU:567, VAL:568, GLU:569, ASP:570, GLN:584, PRO:585, ASN:586, GLN:587, CYS:588, VAL:589, GLY:590, HIS:591, ARG:592, GLU:593, ALA:594, SER:595, ILE:596, HIS:597, LYS:609, VAL:610, LYS:611, GLU:612, GLY:634, CYS:635, SER:636, ALA:637, LEU:638, PRO:639, SER:642, HIS:643, VAL:644, LEU:645, GLY:646, ALA:647, TYR:648, VAL:656, ALA:671, ALA:674, VAL:675, ALA:676, ILE:677 |
| 4 | Medium | GLU:159, ARG:160, ILE:161, THR:162, PRO:163, PRO:164, ARG:165, TYR:166, ASP:343, GLU:403, GLN:413, ARG:414, ILE:416, HIS:417, PHE:418, SER:419, ALA:420, LYS:421, ASP:422, VAL:423, LEU:440, VAL:441, ALA:442, ALA:443, LEU:444, PRO:445, PRO:446, SER:447, THR:448, HIS:449, GLY:450, ALA:451, GLY:452, TRP:453, GLN:454, LEU:455, PHE:456, CYS:457, ARG:458, ARG:525, LEU:606, LYS:611, ALA:625, CYS:626, GLU:627, GLU:628, GLY:629, TRP:630, THR:631, LEU:632, VAL:650, ASP:651, ASN:652, THR:653, CYS:679, ARG:680, SER:681, ARG:682 |
| No. | Herb | Compound Name | GC-MS/MS | ArgusLab | Autodock | |
|---|---|---|---|---|---|---|
| % Peak Area | Binding Energy (kcal/mol) | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | |||
| 1 | Alirocumab (Positive control) | −7.59 | −5.61 | 77.42 µM | ||
| 2 | C. tinctorius | Benzofuran, 2,3-dihydro- | 23.24 | −8.90 | −5.43 | 104.25 µM |
| 3 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | 21.23 | N/B | −5.47 | 97.4 µM | |
| 4 | 3,4-Dihydroxyphenylglycol, 4TMS derivative | 8.94 | −8.63 | −7.54 | 2.96 µM | |
| 5 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 8.56 | −6.19 | −6.99 | 7.46 µM | |
| 6 | Cyclohexasiloxane, dodecamethyl- | 6.96 | −8.34 | −7.88 | 1.69 µM | |
| 7 | C. fenestratum | d-Gala-l-ido-octonic amide | 9.94 | −7.15 | −6.46 | 18.3 µM |
| 8 | Inositol, 1-deoxy- | 24.89 | −8.33 | −7.30 | 4.48 µM | |
| 9 | Tetraacetyl-d-xylonic nitrile | 27.92 | −8.26 | −6.76 | 11.05 µM | |
| 10 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | 5.87 | −11.14 | −10.15 | 36.5 nM | |
| 11 | Megastigmatrienone | 5.56 | −10.83 | −7.87 | 1.7 µM | |
| 12 | Z. officinale | 2-Butanone, 4-(4-hydroxy-3-methoxyphenyl)- | 38.21 | −8.73 | −7.66 | 2.42 µM |
| 13 | (1S,5S)-2-Methyl-5-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hex-2-ene | 9.06 | −10.26 | −7.25 | 4.82 µM | |
| 14 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | 5.89 | −10.32 | −8.35 | 754.12 nM | |
| 15 | 2-Formyl-9-[.beta.-d-ribofuranosyl]hypoxanthine | 4.37 | −7.62 | −10.79 | 12.4 nM | |
| 16 | (1S,5S)-4-Methylene-1-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hexane | 3.77 | −11.26 | −7.40 | 3.78 µM | |
| No. | Herb | Compound Name | GC-MS/MS | ArgusLab | Autodock | |
|---|---|---|---|---|---|---|
| % Peak Area | Binding Energy (kcal/mol) | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | |||
| 1 | Alirocumab (Positive control) | −7.59 | −5.61 | 77.42 µM | ||
| 2 | C. tinctorius | Cyclopentanol | 6.74 | −8.29 | −5.27 | 137.36 µM |
| 3 | 3-Deoxy-d-mannonic acid | 7.85 | −7.43 | −6.93 | 8.27 µM | |
| 4 | Guanosine | 6.58 | −7.47 | −11.31 | 5.16 nM | |
| 5 | l-Pyrrolid-2-one, N-carboxyhydrazide | 6.13 | −7.27 | −7.38 | 3.89 µM | |
| 6 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 12.60 | −6.19 | −6.99 | 7.46 µM | |
| 7 | C. fenestratum | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | 8.69 | −8.76 | −7.51 | 3.11 µM |
| 8 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl ester | 7.19 | −12.56 | −5.14 | 169.81 µM | |
| 9 | Megastigmatrienone | 12.63 | −10.83 | −7.87 | 1.7 µM | |
| 10 | Inositol, 1-deoxy- | 21.46 | −8.33 | −7.30 | 4.48 µM | |
| 11 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | 4.26 | −11.14 | −10.15 | 36.5 nM | |
| 12 | Z. officinale | 1,3-Cyclohexadiene, 5-)1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)] | 3.79 | −10.91 | −7.36 | 4.0 µM |
| 13 | 1-(4-Hydroxy-3-methoxyphenyl)dodec-4-en-3-one | 5.23 | −11.29 | −8.69 | 428.1 nM | |
| 14 | (E)-1-(4-Hydroxy-3-methoxyphenyl)dec-3-en-5-one | 4.96 | −10.40 | −8.8 | 351.96 nM | |
| 15 | Butan-2-one, 4-(3-hydroxy-2-methoxyphenyl)- | 33.27 | −8.25 | −7.44 | 3.54 µM | |
| 16 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | 24.37 | −10.32 | −8.35 | 754.12 nM | |
| No. | Herb | Compound Name | GC-MS/MS | ArgusLab | Autodock | |
|---|---|---|---|---|---|---|
| % Peak Area | Binding Energy (kcal/mol) | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | |||
| 1 | Lovastatin (Positive control) | −9.23012 | −8.55 | 540.36 nM | ||
| 2 | C. tinctorius | Benzofuran, 2,3-dihydro- | 23.24 | −8.19673 | −5.91 | 46.78 μM |
| 3 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | 21.23 | N/B | −5.15 | 168.03 μM | |
| 4 | 3,4-Dihydroxyphenylglycol, 4TMS derivative | 8.94 | −7.66333 | −6.60 | 14.6 μM | |
| 5 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 8.56 | −6.64198 | −7.22 | 5.07 μM | |
| 6 | Cyclohexasiloxane, dodecamethyl- | 6.96 | −7.98578 | −7.59 | 2.75 μM | |
| 7 | C. fenestratum | d-Gala-l-ido-octonic amide | 9.94 | −7.64931 | −5.85 | 51.27 μM |
| 8 | Inositol, 1-deoxy- | 24.89 | −8.28603 | −7.34 | 4.15 μM | |
| 9 | Tetraacetyl-d-xylonic nitrile | 27.92 | −7.88168 | −6.49 | 17.48 μM | |
| 10 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | 5.87 | −10.0154 | −7.75 | 2.07 μM | |
| 11 | Megastigmatrienone | 5.56 | −9.73578 | −6.04 | 37.12 μM | |
| 12 | Z. officinale | 2-Butanone, 4-(4-hydroxy-3-methoxyphenyl)- | 38.21 | −9.35038 | −5.90 | 47.54 μM |
| 13 | (1S,5S(-2-Methyl-5-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hex-2-ene | 9.06 | −10.7714 | −5.82 | 54.27 μM | |
| 14 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | 5.89 | −10.5172 | −6.10 | 33.53 μM | |
| 15 | 2-Formyl-9-[.beta.-d-ribofuranosyl]hypoxanthine | 4.37 | −7.52531 | −7.96 | 1.47 μM | |
| 16 | (1S,5S)-4-Methylene-1-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hexane | 3.77 | −10.1426 | −5.41 | 108.68 μM | |
| No. | Herb | Compound Name | GC-MS/MS | ArgusLab | Autodock | |
|---|---|---|---|---|---|---|
| % Peak Area | Binding Energy (kcal/mol) | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | |||
| 1 | Lovastatin (Positive control) | −9.23012 | −8.55 | 540.36 nM | ||
| 2 | C. tinctorius | Cyclopentanol | 6.74 | −8.37591 | −4.72 | 345.87 μM |
| 3 | 3-Deoxy-d-mannonic acid | 7.85 | −7.71546 | −4.19 | 845.72 μM | |
| 4 | Guanosine | 6.58 | −8.31259 | −7.77 | 2 μM | |
| 5 | l-Pyrrolid-2-one, N-carboxyhydrazide | 6.13 | −7.38878 | −6.99 | 7.54 μM | |
| 6 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 12.60 | −6.64198 | −7.22 | 5.07 μM | |
| 7 | C. fenestratum | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | 8.69 | −8.90424 | −6.69 | 12.52 μM |
| 8 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl ester | 7.19 | −11.2679 | −3.62 | 2.22 mM | |
| 9 | Megastigmatrienone | 12.63 | −9.73578 | −6.04 | 37.12 μM | |
| 10 | Inositol, 1-deoxy- | 21.46 | −8.28603 | −7.34 | 4.15 μM | |
| 11 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | 4.26 | −10.0154 | −7.75 | 2.07 μM | |
| 12 | Z. officinale | 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | 3.79 | −10.5606 | −5.80 | 56.41 μM |
| 13 | 1-(4-Hydroxy-3-methoxyphenyl)dodec-4-en-3-one | 5.23 | −10.681 | −5.43 | 104.75 μM | |
| 14 | (E)-1-(4-Hydroxy-3-methoxyphenyl)dec-3-en-5-one | 4.96 | −10.2192 | 6.04 | 37.24 μM | |
| 15 | Butan-2-one, 4-(3-hydroxy-2-methoxyphenyl)- | 33.27 | −8.67751 | −5.68 | 69.13 μM | |
| 16 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | 24.37 | −10.5172 | −6.10 | 33.53 μM | |
| No. | Herb | Compound Name | GC MS/MS | ArgusLab | Autodock | |
|---|---|---|---|---|---|---|
| % Peak Area | Binding Energy (kcal/mol) | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | |||
| 1 | Metformin (Positive control) | −5.87716 | −5.56 | 84.25 μM | ||
| 2 | C. tinctorius | Benzofuran, 2,3-dihydro- | 23.24 | −8.62431 | −5.08 | 189.21 μM |
| 3 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | 21.23 | N/B | −5.85 | 51.15 μM | |
| 4 | 3,4-Dihydroxyphenylglycol, 4TMS derivative | 8.94 | −7.48581 | −4.38 | 615.35 μM | |
| 5 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 8.56 | −6.69362 | −6.49 | 17.62 μM | |
| 6 | Cyclohexasiloxane, dodecamethyl- | 6.96 | −7.32463 | −7.03 | 7.06 μM | |
| 7 | C. fenestratum | d-Gala-l-ido-octonic amide | 9.94 | −7.16144 | −5.95 | 43.16 μM |
| 8 | Inositol, 1-deoxy- | 24.89 | −7.83301 | −6.89 | 8.83 μM | |
| 9 | Tetraacetyl-d-xylonic nitrile | 27.92 | −7.59524 | −5.13 | 173.8 μM | |
| 10 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | 5.87 | −9.68843 | −9.91 | 54.25 nM | |
| 11 | Megastigmatrienone | 5.56 | −11.7348 | −7.37 | 3.97 μM | |
| 12 | Z. officinale | 2-Butanone, 4-(4-hydroxy-3-methoxyphenyl)- | 38.21 | −8.93613 | −7.39 | 3.82 μM |
| 13 | (1S,5S)-2-Methyl-5-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hex-2-ene | 9.06 | −12.9835 | −7.32 | 4.3 μM | |
| 14 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | 5.89 | −11.3944 | −8.62 | 476.42 nM | |
| 15 | 2-Formyl-9-[.beta.-d-ribofuranosyl]hypoxanthine | 4.37 | −7.4906 | −8.69 | 425.74 nM | |
| 16 | (1S,5S)-4-Methylene-1-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hexane | 3.77 | −12.7577 | −7.41 | 3.72 μM | |
| No. | Herb | Compound Name | GC MS/MS | ArgusLab | Autodock | |
|---|---|---|---|---|---|---|
| % Peak Area | Binding Energy (kcal/mol) | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | |||
| 1 | Metformin (Positive control) | −5.87716 | −5.56 | 84.25 μM | ||
| 2 | C. tinctorius | Cyclopentanol | 6.74 | −7.35609 | −4.51 | 498.19 μM |
| 3 | 3-Deoxy-d-mannonic acid | 7.85 | −7.11679 | −5.37 | 115.85 μM | |
| 4 | Guanosine | 6.58 | −7.51631 | −9.56 | 99.06 nM | |
| 5 | l-Pyrrolid-2-one, N-carboxyhydrazide | 6.13 | −6.78964 | −6.51 | 16.87 μM | |
| 6 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 12.60 | −6.69362 | −6.49 | 17.62 μM | |
| 7 | C. fenestratum | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | 8.69 | −9.32055 | −7.45 | 3.49 μM |
| 8 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl ester | 7.19 | −11.2105 | −4.97 | 227.42 μM | |
| 9 | Megastigmatrienone | 12.63 | −11.7348 | −7.37 | 3.97 μM | |
| 10 | Inositol, 1-deoxy- | 21.46 | −7.83301 | −6.89 | 8.83 μM | |
| 11 | Thieno[2,3-b]pyridine, 3-amino-2-(3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)-4,6-dimethyl- | 4.26 | −9.68843 | −9.91 | 54.25 nM | |
| 12 | Z. officinale | 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | 3.79 | −11.7619 | −7.05 | 6.75 μM |
| 13 | 1-(4-Hydroxy-3-methoxyphenyl)dodec-4-en-3-one | 5.23 | −11.602 | −4.88 | 265.67 μM | |
| 14 | (E)-1-(4-Hydroxy-3-methoxyphenyl)dec-3-en-5-one | 4.96 | −10.7057 | −6.15 | 30.88 μM | |
| 15 | Butan-2-one, 4-(3-hydroxy-2-methoxyphenyl)- | 33.27 | −9.25557 | −5.93 | 45.14 μM | |
| 16 | 1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | 24.37 | −11.3944 | −4.88 | 265.67 μM | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ongtanasup, T.; Prommee, N.; Jampa, O.; Limcharoen, T.; Wanmasae, S.; Nissapatorn, V.; Paul, A.K.; Pereira, M.d.L.; Wilairatana, P.; Nasongkla, N.; et al. The Cholesterol-Modulating Effect of the New Herbal Medicinal Recipe from Yellow Vine (Coscinium fenestratum (Goetgh.)), Ginger (Zingiber officinale Roscoe.), and Safflower (Carthamus tinctorius L.) on Suppressing PCSK9 Expression to Upregulate LDLR Expression in HepG2 Cells. Plants 2022, 11, 1835. https://doi.org/10.3390/plants11141835
Ongtanasup T, Prommee N, Jampa O, Limcharoen T, Wanmasae S, Nissapatorn V, Paul AK, Pereira MdL, Wilairatana P, Nasongkla N, et al. The Cholesterol-Modulating Effect of the New Herbal Medicinal Recipe from Yellow Vine (Coscinium fenestratum (Goetgh.)), Ginger (Zingiber officinale Roscoe.), and Safflower (Carthamus tinctorius L.) on Suppressing PCSK9 Expression to Upregulate LDLR Expression in HepG2 Cells. Plants. 2022; 11(14):1835. https://doi.org/10.3390/plants11141835
Chicago/Turabian StyleOngtanasup, Tassanee, Nuntika Prommee, Onkamon Jampa, Thanchanok Limcharoen, Smith Wanmasae, Veeranoot Nissapatorn, Alok K. Paul, Maria de Lourdes Pereira, Polrat Wilairatana, Norased Nasongkla, and et al. 2022. "The Cholesterol-Modulating Effect of the New Herbal Medicinal Recipe from Yellow Vine (Coscinium fenestratum (Goetgh.)), Ginger (Zingiber officinale Roscoe.), and Safflower (Carthamus tinctorius L.) on Suppressing PCSK9 Expression to Upregulate LDLR Expression in HepG2 Cells" Plants 11, no. 14: 1835. https://doi.org/10.3390/plants11141835
APA StyleOngtanasup, T., Prommee, N., Jampa, O., Limcharoen, T., Wanmasae, S., Nissapatorn, V., Paul, A. K., Pereira, M. d. L., Wilairatana, P., Nasongkla, N., & Eawsakul, K. (2022). The Cholesterol-Modulating Effect of the New Herbal Medicinal Recipe from Yellow Vine (Coscinium fenestratum (Goetgh.)), Ginger (Zingiber officinale Roscoe.), and Safflower (Carthamus tinctorius L.) on Suppressing PCSK9 Expression to Upregulate LDLR Expression in HepG2 Cells. Plants, 11(14), 1835. https://doi.org/10.3390/plants11141835










