Abstract
Plants of the genus Opuntia spp are widely distributed in Africa, Asia, Australia and America. Specifically, Mexico has the largest number of wild species; mainly O. streptacantha, O. hyptiacantha, O. albicarpa, O. megacantha and O. ficus-indica. The latter being the most cultivated and domesticated species. Its main bioactive compounds include pigments (carotenoids, betalains and betacyanins), vitamins, flavonoids (isorhamnetin, kaempferol, quercetin) and phenolic compounds. Together, they favor the different plant parts and are considered phytochemically important and associated with control, progression and prevention of some chronic and infectious diseases. Part 1 collected information on its preventive actions against atherosclerotic cardiovascular diseases, diabetes and obesity, hepatoprotection, effects on human infertility and chemopreventive capacity. Now, this second review (Part 2), compiles the data from published research (in vitro, in vivo, and clinical studies) on its neuroprotective, anti-inflammatory, antiulcerative, antimicrobial, antiviral potential and in the treatment of skin wounds. The aim of both reviews is to provide scientific evidences of its beneficial properties and to encourage health professionals and researchers to expand studies on the pharmacological and therapeutic effects of Opuntia spp.
1. Introduction
The Traditional Medicine/Complementary and Alternative Medicine (TCAM) concept includes any practice, knowledge and belief in health that incorporates medicine based on plants, animals and/or minerals, spiritual therapies, manual techniques and exercises applied individually or in combination to improve human health. The World Health Organization (WHO) considers that TCAM have shown favorable factors that contribute to an increasing acceptance worldwide, such as easy access, diversity, relatively low cost and, most importantly, relatively low adverse toxic effects in comparison with allopathic medicine where these effects are frequently attributed to synthetic drugs. For this reason, TCAM continues to be used by different populations to treat and/or prevent the onset and progression of chronic and infectious diseases [,,].
Throughout human history, plants and their phytochemicals have played an important role at improving human health care. Opuntia species have specifically shown many beneficial properties and high biotechnological capacity. These plants classified as angiosperm dicotyledonous are the most abundant of the Cactaceae family and are importantly distributed in America, Africa, Asia, Australia, and in the central Mediterranean area. Due to their capacity to store water in one or more of their organs, they are considered succulent plants whose cultivation is ideal in arid areas since they are very efficient to generate biomass in water scarcity conditions [,,,].
Most opuntioid cacti have flat and edible stems called cladodes (CLDs), paddles, nopales or stalks. Generally, young CLDs (also called nopalitos) are eaten as a vegetable in salads, while their fruits (called cactus pear fruits, tunas or prickly pear fruits (PPFs)) are widely eaten as fresh seasonal fruit. PPFs are oval berries with lots of seeds throughout all the pulp and a semi-hard bark that contains thorns. They are grouped in different colors (red, purple, orange/yellow, and white). Generally, the fruit with white flesh and green skin is the most consumed as food [,,,]. Some evidences indicate that Opuntia plants have been consumed by humans for more than 8000 years and due to their easy adaptation and spread in different types of soil, their domestication process has favored the constant collection of CLDs and PPFs by man [,,,].
2. Impact of the Opuntia Genus in Mexico and Other Countries
The Cactaceae family includes about 200 genera and 2000 species classified into three to six subfamilies. The Opuntioideae subfamily comprises between 15 and 18 genera, Opuntia being the most diverse and widely distributed genus in the American continent [,,]. However, Mexico has the largest number of wild species. The most representaive are O. streptacantha (OS), O. hyptiacantha (OH), O. albicarpa (OA), O. megacantha (OM) and O. ficus-indica (Figure 1). The latter is highly cultivated and domesticated species due to its nutritional, medicinal, pharmaceutical, and economic impacts. It is believed to be a secondary crop with fewer thorns derived from OM, (a native species from central Mexico) [,,,,,]. Currently, O. ficus-indica (OFI) has become as important a vegetable crop as corn and agave-tequila; its economic relevance is significantly increasing in our country and in other parts of the world, especially for improving health when nopal and prickly pear are included in a diet. Therefore, the OFI domestication process has favored changes in the texture, flavor, size, color, quantity and quality of the cladodes and their fruits [,,]. Mexico and Italy are the main producing and consuming countries of the approximately 590,000 ha cultivated around the world. The Annual Mexican production can reach 350,000 tons; for this reason, our country represents approximately 90% of the total production worldwide. In addition, Mexico is the main producer of prickly pear fruits, representing more than 45% of world production; however, only 1.5% of this production is exported, due to various factors such as a low level of technology in its production, climatic changes, the lack of a marketing plan (supply/demand) and that it is a seasonal product (it is obtained between the months of June to November) [,,,].
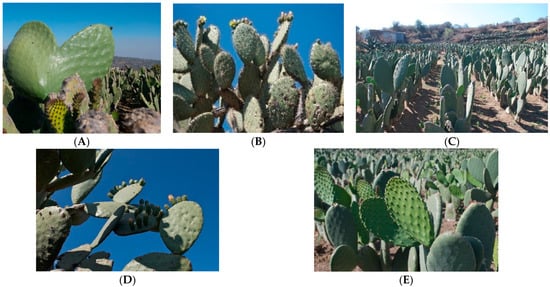
Figure 1.
Mexican cultivars of O. Streptacantha (A), O. megacantha (B) and O. ficus-indica (C), O. hyptiacantha (D), and O. albicarpa (E).
In relation to the impact of Opuntia species in other countries, there is evidence of positive and negative aspects. In the first case, due to their adaptability, which allows them to grow where no other crop can, their plants and fruits (specifically, PPFs) have become an essential and reliable crop for the diet of the inhabitants (As it happens in Ethiopia). Likewise, healers of the Kani tribes in the Tirunelveli hills of the Western Ghats of India, consider the opuntia species spiritual and esential for their first aid remedies; especially O. dillenii which is used to treat cough, headache, poisonous animal bites, cold and fever control [,].
Unfortunately, because they can survive in arid/semi-arid environments, high temperatures, little rainfall and limited nutrient supply, they are considered an invasive alien species in some South African and Kenyan communities that can threaten biodiversity and food security. For this reason, Opuntia (especially O. stricta) is included in the diet of animals (forage for livestock), as a measure to control its spread, mainly in dry seasons [,].
In general, in Mexico and other countries, Opuntia is a resource that has high agrotechnological potential, both as a food crop and as a base element to obtain derived products, which are used in the food industry (human and animal), medicine, agricultural industry and cosmetology (Table 1).

Table 1.
Main products and by-products obtained from Opuntia (Nopal).
3. Nutritionalcomposition and Mechanisms of Pharmacological Action of the Opuntia Genus
Different methods have documented the nutritional value of Opuntia spp. Most of these studies coincide in the differences among the phytochemical composition of their plant parts (fruits, roots, cladodes, flowers, seeds and stems) and the wild and domesticated species. These can be attributed to environmental conditions (climate, humidity), the type of soil that prevails in the cultivation sites, the age of maturity of the cladodes, and the harvest season [,,]. The nutritional composition of the different parts of Opuntia ficus-indica (L.) Mill. is summarized in Table 2. In general, opuntioid cacti contain a large amount of water (80 and 95%), carbohydrates (3–7%), proteins (0.5–1%), soluble fiber (1–2%), fatty acids (palmitic, stearic, oleic, vaccenic and linoleic) and minerals (Potassium (K), calcium (Ca), phosphorus (P), magnesium (Mg), chrome (Cr) and sodium (Na)). They also have viscous and/or mucilaginous materials (made up of D-glucose, D-galactose, L-arabinose, D-xylose and polymers such as β-D-galacturonic acid linked to (1–4) and L-rhamnose residues linked with R (1–2)) whose function is to absorb and regulate the amount of cellular water in dry seasons [,,,]. Among the main bioactive compounds of prickly pear highlight the pigments (carotenoids, betalains, betaxanthins and betacyanins), vitamins (B1, B6, E, A, and C), flavonoids (isorhamnetin, kaempferol, quercetin, nicotiflorine, dihydroquercetin, penduletin, lutein), rutin, aromadendrine, myricetin vitexin, flavonones and flavanonols) and phenolic compounds (ferulic acid, feruloyl-sucrose and synapoyl-diglycoside) [,,,,,,].

Table 2.
Nutritional composition in different anatomical parts of Opuntia ficus-indica (L.) Mill.
Specifically, the cladodes and prickly pears fruits of OFI have shown several kinds of bioactive compounds, among which flavonoids (such as quercetin, kaempferol, isorhamnetin), essential amino acids (Glutamine, arginine, leucine, isoleucine, lysine, valine and phenylalanine), vitamins (B1, B6, E, A, and C), minerals (mainly K and Ca), and betalains [such as betaxanthins (betanin and indicaxanthin) and betacyanins (betanidin, isobetanin, isobetanidine, and neobetanin) [,,,,,,].
As mentioned in part 1 [], various studies have shown the action of phytochemicals as substrates to activate different biochemical reactions that provide important health benefits. For that reason, they could be included in the definition of nutraceutical: “Any non-toxic food extract supplement that has been scientifically proven to be beneficial to health both intreating and preventing diseases” [,,]. Different authors agree that the carotenoids are important compounds with great benefits for human health, related to the prevention and reduction of the development of some diseases, such as cardiovascular diseases, cancer and macular degeneration and that taurine (semi-essential amino acid) is involved in the modulation of the inflammatory response with potential antioxidant. As well as, that some plant sterols are incorporated into foods intended for human consumption to lower blood cholesterol levels [,]. On the other hand, the scientific evidence suggests that the phenolic acids (hydroxycinnamic acids and hydroxybenzoic acids), flavonoids, lignins and stilbenes have a high antioxidant potential that has been related in many health benefits such as prevention of inflammation, cardiovascular dysregulation, and neurodegenerative diseases [,]. In this same approach, it is known that the flavonoids are a group of bioactive compounds that exhibit many effects in the protection of the body, and their regular consumption is associated with reduced risk of several chronic diseases (especially, for its antioxidant, antiviral and antibacterial capacities). Finally, the Betalains are powerful radical eliminators in chemical systems and has act as efficient antioxidants in several biological models. Potential related as a possible strategy for intestinal inflammation [,,]
In this context, opuntioid cacti reveal different mechanisms of action that can be interrelated and favor their biological effects. In general, they are organized in 7 groups: (I) Inhibition of the absorption of substances, favoring the absorption of protective agents and/or modification of the intestinal flora (action of soluble fiber and ascorbic acid), (II) Scavenging of reactive oxygen species and/or protection of DNA nucleophilic sites (antioxidant action), (III) Anti-inflammatory activity, (IV) Modification of transmembrane transport (effect of short-chain fatty acids and calcium in the diet), (V) Modulation of xenobiotic metabolising enzymes, inhibition of mutagen agents activation and induction of detoxification pathways (flavonoids, polyphenols and índoles), (VI) Enhancement of apoptosis (action of some flavonoids), and (VII) Maintenance of genomic stability (effect of some vitamins, minerals and polyphenols) [,,,,,,,,].
Together, the bioactive compounds of Opuntia spp. favor its different plant parts to be considered phytochemically important and associated with the control, progression and prevention of some chronic and infectious diseases. This second review (Part 2) focuses on information from published research (in vitro, in vivo and clinical studies) on its anti-inflammatory, antiulcerative, neuroprotective, antimicrobial, antiviral properties and on the treatment of skin wounds; which will be discussed below.
4. Pharmacological, Therapeutic and Preventive Properties
4.1. Anti-Inflammator and Antiulcerative Effects
The inflammatory cascade includes a long chain of molecular reactions and cellular processes (highlighting phagocytosis, chemotaxis, mitosis, and cell differentiation) designed as a biological response to different noxious stimuli, including dust particles, chemical substances, physical injuries, bacteria, viruses, and parasites. This cascade is an important factor for the progression of various chronic disorders, such as obesity, arthritis, diabetes, cancer, cardiovascular diseases, eye disorders, autoimmune diseases and inflammatory bowel disorders; therefore, in recent decades it has become a highly studied field of research [,]. The stages of inflammation depend on the duration of the process and various immunological factors, classifying them into acute and chronic. The first is characterized by a rapid initial response that can last minutes and/or a few days. There is accumulation of plasma proteins and leukocytes, presenting increased blood flow, swelling, redness, pain and heat. When this response is prolonged, chronicity is established, which leads to an increase in the presence of lymphocytes, macrophages, and mast cells at the site of infection and/or damage. Simultaneously, repair mechanisms that can generate fibrosis and overproduction of connective tissue are activated. In general, inflammation is a vital response of the immune system; however, a chronic process can induce secondary consequences in the biological response associated with an increased risk of chronic diseases. This usually occurs through infections that are not cleared by endogenous protective mechanisms or by some type of genetic susceptibility [,].
Various investigations have confirmed that inflammation is related to the induction of oxidative stress (OXs) due to the increase in cells (lymphocytes, macrophages, and mast cells) that leads to greater oxygen uptake, increasing the production and release of reactive oxygen species (ROS) in the damaged area. In addition, the activation of signal transduction cascades and alteration in transcription factors (such as nuclear factor kappa B (NF-κB), signal transducer and activator of transcription 3, activator protein-1, factor 2 related to NF-E2, activated T-cell nuclear factor, and hypoxia-inducible factor-1α (HIF1-α)) [,]. Likewise, cyclooxygenase-2 (COX-2), nitric oxide synthase (iNOS), expression of inflammatory cytokines (such as tumor necrosis factor alpha (TNF-α)), interleukins (IL-1β, IL-6) and chemokines, are induced. Scientific findings of different anti-inflammatory agents have shown that bioactive extracts and their natural compounds exert their biological properties by blocking signaling pathways, such as NF-κB and mitogen-activated protein kinases (MAPK) [,].
On the other hand, Gastric Ulcers (GU) are open sores in the mucosa lining the stomach and/or duodenum. The most frequent symptomatology is pain and burning that can occur between meals or at night, with different durations (minutes and/or days). The worldwide incidence of GU varies depending on age, sex, and geographic location, but it remains a common condition and a major public health problem due to high healthcare costs and life-threatening complications (bleeding, perforation, and obstruction), that favor its high morbidity and mortality []. The pathophysiology of GU is multifactorial but is generally associated with the result of an imbalance between the protective and aggressive factors of the gastric mucosae; that is, when there is a significant increase in the acids that help digest food, damaging the walls of the stomach and/or duodenum. Among the harmful factors that favor its incidence are excessive gastric acid and pepsin secretion, increased ROS, Helicobacter pylori infection, constant alcohol consumption, and prolonged ingestion of nonsteroidal anti-inflammatory drugs (NSAIDs) [,,]. While gastrointestinal defense mechanisms include mucus secretion, bicarbonate production, nitric oxide (NO), prostaglandin synthesis, normal gastric motility, and adequate tissue microcirculation. Currently, GU treatments are aimed at improving the defenses of the gastric mucosae or counteracting harmful factors. Among the most used are those that reduce gastric acid secretion (H2 receptor antagonists), those that inhibit the proton pump (omeprazole); and antibiotics that control H. pylori. However, its high costs and side effects of long-term treatments combined with recurrence of ulcers and some cases of rejection to conventional therapies have motivated the search for new antiulcer agents. In particular, those that improve the quality of ulcer healing to prevent abnormalities in mucosal regeneration and the persistence of chronic inflammation by reducing the infiltration of neutrophils and macrophages (i.e., prevent ulcer recurrence) [,,].
Like anti-inflammatory agents extracted from natural compounds, herbal anti-ulcer medications have also become an excellent source to obtain them. In this sense, Opuntia spp. is no exception, and possibly, after the studies related to atherosclerotic cardiovascular diseases, diabetes, obesity and chemopreventive capacity (included in part 1) [], the anti-inflammatory and antiulcerative evaluation field is of equal relevance to researchers.
Probably, the first study to break into this field of evaluation was aimed to confirm in rodents that a preparation of dried flowers of OFI reduced the discomfort of benign prostatic hypertrophy by suppressing the release of beta-glucuronidase (lysosomal enzyme of the neutrophils) []. Subsequently, two research groups, Park et al., (1998) [] and Loro et al., (1998) [], continued the studies. In the first, ethanolic extracts of fruits (EEOF) and stems (EEOS) of OFI were analyzed on the acetic acid writhing syndrome and paw edema induced by carrageenan (CRRG) in Sprague Dawley rats. Both extracts decreased writhing and edema; as well as the release of the same lysosomal enzyme. Their results suggested that EEOF and EEOS have analgesic and anti-inflammatory actions, and a possible protective effect against gastric injury []. Using similar techniques, the second group of scientists evaluated different lyophilisates (50–400 mg/kg, i.p.) from the fruits of O. dillenii Haw (OdHw); the result was similar when the chemical stimuli were dose-dependently inhibited (writhing test) and thermal (hot plate test) in Wistar rats; mainly in doses of 50 and 100 mg/kg []. The results of the previous studies motivated the fractionation of a methanolic extract of OFI stems and, together with a model of chronic inflammation induced by adjuvants in mice, β-sitosterol was isolated and identified as a possible anti-inflammatory active ingredient [].
In relation to the first evidence of the anti-ulcer effect, Galati et al. [,,] found that by previously administering lyophilized CLDs and/or OFI whole fruit juice on experimental ulcers induced by ethanol (EtOH) in rats, a cytoprotective action related to an increase in the production of gastric mucus was exerted. They attributed such effect to the mixture of mucilage and pectin present in OFI [,,]. Table 3 shows the main studies that evidence the anti-inflammatory and antiulcerative effects of Opuntia spp. In summary, from 1993 to date, 16 of 39 have been in vitro studies; 20, using laboratory animals (mainly rodents) and 3, developed with patients (clinical studies). Mainly, different types of extracts (methanol (MeOH), hexane (Hx), chloroform (Chl), ethyl acetate (EtOAc), butanol and aqueous) obtained from CLDs and roots of OFI, OdHw and O. humifusa (OHF) have been analyzed. In addition, powders from the stems, juices, vinegars and oils of PPFs and/or their seeds extracted from O. elatio Mill, O. macrorhiza Engelm (OME), OFI and OdHw have been explored. Most studies confirm and agree that the main mechanism of action of both properties (anti-inflammatory and anti-ulcer) is related to its antioxidant capacity, which is attributed to a possible synergistic and/or combined effect between the different bioactive compounds (phenols, flavonoids (such as quercetin, kaempferol, isorhamnetin), betalains (betanin and indicaxanthin), betacyanins, α-pyrones (opuntiol and opuntioside glucoside), pectin and mucilage) present in the chemical composition of the Opuntia genus [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].

Table 3.
Studies testing for anti-inflammatory and antiulcerative effects of Opuntia spp.
4.2. Neuroprotective Effect
Neurodegenerative disease (ND) is a progressive dysfunction and/or loss of neuronal structure and function, generally irreversible, that alters intellectual and cognitive faculties. This disorder occurs in various diseases that affect the central nervous system (CNS) and may be acute or chronic [,,,]. The first case refers to a condition where neurons are rapidly damaged and can die in response to a sudden insult or traumatic event (such as head injury, stroke, traumatic brain injury, brain hemorrhage, or ischemic brain damage). On the other hand, chronic neurodegeneration is a state where a degenerative process that begins slowly and worsens over time due to multifactorial causes, is experienced; resulting in the progressive and irreversible destruction of specific neuronal populations. Among the most significant chronic neurodegenerative disorders are Alzheimer’s disease (AD), Huntington’s disease, Parkinson’s disease (PD), and amyotrophic lateral sclerosis. As mentioned, the causes of ND are multifactorial and are associated with different types of biological mechanisms, which in general can be summarized as: (a) oxidative stress (EOx), (b) neuroinflammation, (c) excitotoxicity, (d) mitochondrial dysfunction, (e) induction of apoptosis and (f) abnormal protein folding and aggregation [,,,].
Specifically, imbalanced ROS production and poor antioxidant defense (endogenous and/or exogenous) cause EOx resulting in cell damage, impaired DNA repair system, and mitochondrial dysfunction; accelerating the neurodegenerative process. On the other hand, neuroinflammation involves both the innate and adaptive CNS immune systems, playing an important role in the pathophysiology of DN. Since microglia are the main components of the innate immune defense, and if there are pathological changes within the CNS, they secrete inflammatory mediators (cytokines, chemokines, COX-2), which activate astrocytes to induce a secondary inflammatory response in a population of neurons that respond to a survival process [,,,].
Excitotoxicity [neuronal death caused by excessive or prolonged activation of glutamate (Glu) receptors by excitatory amino acids or CNS excitotoxins] is also involved in degenerative pathogenesis. Excitotoxins that bind to Glu receptors, as well as pathologically high levels of their release, are known to cause toxicity by allowing a rapid entry of calcium ions (Ca2+) into the cell, activating various Ca2+-dependent enzymes (iNOS, phospholipases, lipase endonucleases, xanthine oxidase, protein phosphatases, proteases, and protein kinase). These enzymes continue to damage cellular structures (such as components of the cytoskeleton, membrane, and DNA) and/or generate ROS, mitochondrial dysfunction, and other inflammatory responses, which together lead to neuronal death [,,,].
Finally, combining apoptosis (a highly regulated form of cell death that is triggered by intrinsic and extrinsic signals) and the mitochondria (site of oxidative phosphorylation and cellular respiration), a significant role is played in maintaining a low concentration of Ca2+ cytosolic [,,,]. Excessive uptake of this ion and generation of ROS cause the opening of mitochondrial permeability transition pores, inducing matrix inflammation, mitochondrial uncoupling, and membrane rupture that releases cytochrome-c (Cyt-c) and apoptosis inducing factor. Cyt-c, a caspase-dependent pathway, binds to apoptotic protease-activating factor 1 and procaspase-9 to form an apoptosome complex and activate the caspase-3 pathway, producing apoptotic neuronal death. While the apoptosis-inducing factor, a caspase-independent mechanism, moves to the nucleus and induces the DNA fragmentation, chromatin condensation and subsequently cell collapse [,,,].
Progressive degeneration and/or neuronal death causes characteristic symptoms such as problems with movement (ataxia) or alterations in cognitive functioning (dementia), and since, unfortunately, most NDs have no cure, conventional treatments focuson improving symptoms and relieving pain. For example, in individuals with PD there are low concentrations of dopamine (DA) in the brain, so the main drugs (Carbidopa-levodopa, Dopamine agonists, Inhibitors of the enzyme monoamine oxidase type B or Inhibitors of catechol -O-methyltransferase), mimic, increase or replace this neurotransmitter (NT) [,,,]. In the case of AD, cholinesterase inhibitors are prescribed to patients with mild and/or moderate symptoms to prevent the breakdown of Acetylcholine (Ach), a brain neurotransmitter that is related to memory and thought. Unfortunately, these medications often lose their therapeutic effect over time. Another example is the so-called “disease-modifying agent”, “aducanumab”, a human antibody that targets β-amyloid protein (Aβ) to reduce brain lesions (amyloid plaques) associated with AD [,,,].
Various studies have tried to elucidate mechanisms and possible therapeutic objectives to combat NDs, in order to avoid neuronal damage and preserve the integrity and functionality of these cells; all resultingin a concept known as Neuroprotection. A strategy that includes three approaches: (1) before the onset of the disease to avoid any risk factors might affect the neurons. (2) during the progression of the disease to avoid the spread of the lesion from one neuron to another; and (3) to try to delay and /or stop progressive neurodegeneration [,,,].
Again, natural products and their bioactive compounds are an excellent source of neuroprotective agents for the treatment of ND. The early agents studied limited their mechanism of action to intervene in NT receptors through agonists and antagonists; the best known example was caffeine, adenosine A2 receptor antagonist, which has been shown to protect dopaminergic neurons in an experimental model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. It is now known that microglial cells also express the A2 receptor, and A2 receptor antagonist or caffeine can reduce the activation of these cells. Therefore, drinking coffee, maintaining a healthy lifestyle and having moderate physical activity has been considered a neuroprotective strategy, and added to the fact that A2 antagonists can protect neurons and minimize the activation of microglial cells, the field of research of phytotherapy continues to be active in the search for new and innovative neuroprotective agents [,,,].
Since multifactorial pathological mechanisms (EOx, neuroinflammation, excitotoxicity, mitochondrial dysfunction, and apoptosis) are associated with neurodegeneration, current research look for multiplemechanisms of action that intervene in the complexity of the disease with natural neuroprotective agents instead of looking for a single biological objective.
Table 4 shows the main evidence of the neuroprotective effect of Opuntia spp. Since the year 2000, when the exploration of this scientific field began, most of the studies have been carried out in vitro (mouse cortical cells, primary cultured rat cortical cells, PC12 cells) where extracts of CLDs and prickly pear fruits have been evaluated. Some phytochemicals (Quercetin, quercetin 3-methyl ether, indicaxanthin, polysaccharides) from 6 species of Opuntia; where the most studied are OFI and OFI var. Saboten (OFIS). Basically, neuronal damage and toxicity have been induced by different agents and/or substances; such as xanthine/xanthine oxidase (X/XO), FeCl2/ascorbicFeCl2/ascorbic acid, N-methyl-d-aspartate (NMDA), kainate (KA), oxygen-glucose deprivation (OGD, LPS, AlCl3 and Aβ. Although, probably, the neuroprotective effect of Opuntia spp. can be carried out through multiple mechanisms, most authors agree that the antioxidant capacity is the most significant and/or representative [,,,,,,,,,,,,,]

Table 4.
Scientific evidence of the neuroprotective effect of Opuntia spp.
4.3. Antiviral and Antimicrobial Effects
Despite the incredible progress in human medicine, Viral Infections (VI) continue to be responsible for various chronic and acute diseases. Diseases such as Acquired Immunodeficiency Syndrome (AIDS), hepatitis, and respiratory syndromes, especially the one caused by the severe acute respiratory syndrome virus type-2 (SARS-CoV-2) are associated with high rates of morbidity and mortality [,]. Again, natural products are a rich source of bioactive compounds with possible antiviral effects; thus, identifying them is of critical importance. A wide variety of phytochemicals, including coumarins, flavonoids, terpenoids, organosulfur compounds, lignans, polyphenols, saponins, proteins, and peptides, have been found to influence cell functions, membrane permeability, and viral replication. Therefore, natural-based pharmacotherapy may be a good alternative for VI treatment. Antiviral agents can be classified according to their chemical nature or their activity against viral proteins and/or host cellular proteins. Particularly, the antiviral activity can be exerted based on its ability to inhibit any viral entry, viral DNA and RNA synthesis, as well as viral replication/reproduction. Differences in viral structure and replication cycle are crucial to the design of any antiviral medication [,].
The antiviral activity of phytochemicals can be established by different biological assays, commonly used to assess cytotoxicity, cytopathic effect, and the ability to block viral cell-to-cell propagation. Purified natural products are considered a rich resource for the development of new antiviral drugs. However, their extraction and isolation can be a difficult process, since many bioactive compounds are present in low concentration in the natural source and due to their complex chemical structures they are not easy to synthesize. In addition, the majorityof the natural compounds are used as unpurified crude extracts, which makes it very important to isolate each biomolecule individually and to establish their pharmacokinetics (absorption, distribution, metabolism, excretion), therapeutic effects, dose and possible toxicity events [,].
Among the isolated bioactive compounds with recognized antiviral action is rutin (known as quercetin-3-rutinoside), a flavonoid glycoside effective against avian influenza virus, herpes simplex virus 1 and 2 (HSV-1, HSV-2) and parainfluenza -3 virus [,]. Quercetin, an aglycone of rutin, has demonstrated its therapeutic potential against influenza A virus (IFV-A), rhinovirus, dengue virus type 2 (DENV-2), HSV-1, poliovirus type 1 (PV- 1), adenovirus, Epstein-Barr virus, Mayaro virus, Japanese encephalitis virus, Respiratory Syncytial Virus (RSV), and Hepatitis C virus (HCV) [,]. Among the mechanisms of action of quercetin are limiting the activity of some thermal shock proteins involved in viral translation (Internal Ribosome Entry Site or IRES) mediated by the non-structural protein 5A (NS5A), inhibiting NS3 protease and viral replication of HCV, reducing endocytosis, blocking viral genome transcription and rhinovirus protein synthesis, and decreasing DENV-2 replication [,]. Other flavonoids, such as myricetin (3,3′,4′,5,5′,7-hexahydroxyflavone), quercetagetin (3,3′,4′,5,6,7-hexahydroxyflavone), and Baicalein (5,6,7-trihydroxyflavone) block the reverse transcriptase of the Rauscher murine leukemia virus and the human immunodeficiency virus (HIV). Finally, Baicalin (the glucuronide of baicalein) inhibits the synthesis of DNA and viral proteins of the hepatitis B virus (HBV); it is also active against HIV, DENV, RSV, enterovirus, and Newcastle disease virus [,].
Thus far, only three investigations have evaluated the antiviral effect of Opuntia spp. In the first, administration of an OS stem extract to mice, horses, and humans inhibited the intracellular replication of several DNA and RNA viruses, such as HSV-2, RSV, HIV, IFV-A, equine herpes and pseudorabies virus. Although a viral inactivation at the extracellular level was also observed, there were no answers about the possible inhibitory components of the extract []. On the other hand, Bouslama et al., (2011) analyzed the inhibitory effect of two extracts (aqueous and/or EtOH) from OFI stems on the replication of two enveloped viruses (HSV-2 and IFV-A) and a non-enveloped virus (PV-1). Given that only the EtOH extract showed significant antiviral activity in vitro, two stem chlorophyll derivatives (pheophorbide a and pyropheophorbide a) were isolated; which demonstrated a virucidal effect only on both enveloped viruses. These findings suggest that both phytochemicals could recognize specific glycoproteins of enveloped viruses, preventing their binding to the host cell receptors and inhibiting VI []. In the latest study, an antiviral protein (named Opuntin B) from OFI was purified; which shows the total degradationof genomic RNA of the plant and causes a displacement of the electrophoretic mobility of the RNAs of the cucumber mosaic virus (CMV). Using CMV as prey protein and Opuntin B as bait protein, far western dot blot analysis showed no interaction between antiviral protein and viral coat protein [].
On the other hand, microbial pathogens (MP) can enter a host using different transmission mechanisms, which are generally classified as: (a) direct contact (cutaneous lesions, urogenital tract and/or sexual transmission), (b) indirect contact (contaminated hands and/or inanimate instruments), and (c) airway (inhalation of droplets of different diameters through the respiratory tract and/or ingestion of contaminated food or drink). Regardless of the route of transmission, MPs are responsible of producing various diseases that generate public health problems and cause excessive economic costs [,]. Unfortunately, antimicrobial resistance has also become an increasingly important and pressing global problem, as of the millions of people who acquire bacterial infections, approximately 70% of cases involve strains that are resistant at least to one drug [,,]. Therefore, in response to this problem, pharmaceutical companies are focusing their efforts on improving antimicrobial agents; however, the researchers acknowledge that they are reaching the end game in terms of alterations to their chemical structures. For this reason, natural products can be a rich source of anti-infective agents that work at different target sites and can replace synthetic compounds [,,].
Obtaining natural antimicrobial agents also has an impact on food preservation to prevent disease transmission after ingestion of contaminated food and/or beverages. These bioconservatives must keep and preserve nutritional values and/or guarantee food safety; all these aspects are not usually met with synthetic conservation methods (nitrates, benzoates, sulfites, sorbates, formaldehyde) that despite being approved by government agencies continue to threaten health, by frequently inducing allergic reactions.
Some studies suggest that natural antimicrobials may be safer than synthetic ones; therefore, obtaining anti-infective agents from plants and algae can be an alternative strategy to develop new drugs that are safer, more effective and avoid bacterial resistance [,].
In general, the mechanisms of action of natural antimicrobials include disruption of the cytoplasmic membrane, inhibition of nucleic acid synthesis, decrease in proton motive force, and inhibition of energy metabolism (ATP depletion) [,]. Most antimicrobials derived from plants have been found in herbs and spices. These agents have different structural configurations that provide their antimicrobial action; the presence of hydroxyl groups (-OH) is believed to be the main cause of this property. Possibly due to the interaction of the -OH groups with the bacterial cell membrane that alters its structures and causes the leakage of its components. The antioxidant capacity is usually linked to the antimicrobial effect, which together (antioxidant/-OH groups) makes the compound more effective [,].
Currently, more than 1300 plants have shown antimicrobial activity, from which more than 30,000 compounds with this characteristic have been extracted. Plants and herbs (such as oregano, garlic, parsley, sage, coriander, rosemary, lemongrass, ginger, and chili), spices (cinnamon, cloves, curry, and pepper), and some essential oils (such as citral) have been shown to be effective against Escherichia coli, Listeria monocytogenes, Campylobacter spp., Staphylococcus aureus, Salmonella spp., Pseudomonas aeruginosa, Vibrio cholerae and Bacillus cereus [,,,,]. Among the most relevant antimicrobial phytochemicals are thiosulfinates, glucosinolates, phenols, organic acids, flavonoids and saponins. However, those with the highest activity are phenols (terpenes, aliphatic alcohols, aldehydes, ketones, acids and isoflavonoids [,,,,].
Although Ginestra et al., (2009) indicated that cladodes of O. ficus indica contain glucose, kaempherol and isorhamnetin, and apparently do not have antimicrobial activity, even after an enzymatic treatment; it was not a reason to rule out the development of further investigations []. In this sense, Sánchez et al., (2010) measured the synthesis of ATP, minimal bactericidal concentrations (MBCs), and changes in the integrity and potential of the Vibrio cholerae membrane after exposing it to methanolic, ethanolic and aqueous extracts of OFI var. Villanueva L. The three types of extracts were active against the bacteria (MBCs ranged between 0.5 and 3.0 mg/mL), were able to break cell membranes and cause an increase in their permeability [].
These results opened the studies to the control of bacterial contamination in food and subsequently, the antimicrobial activity of non-polar extracts (petroleum ether and Chl) and polar extracts (MeOH and water) from the dried stems of OdHw and rhizome of Zingiber officinale were compared with Bacillus subtilis, Staphylococcus aureus and Salmonella typhi. The results also confirmed that this last bacterium is resistant to all extracts of both plants. Unlike E. coli and B. subtilis that were inhibited with the ether and chloroform extracts of OdHw, as well as with the MeOH and aqueous extracts of Z. officinale. These data suggest that the beneficial property of both plants is affected by the polarity of the extraction solvent [].
In general, the studies developed to date (Table 5) suggest that different extracts (Hx, MeOH, EtOH, Chl, EtOAc, acetone (Ace), aqueous, dichloromethane (DCM) and mucilage) and/or oils from PPFs, CLDs (ripe and nopalitos), flowers, seeds and fruit peel especially obtained from OFI, OdHw, O. xoconostle (OX), O. albicarpa (OA), O. stricta, O. microdasys (OMs) and O. macrorhiza Engelm (OME) have shown antimicrobial action against Gram-positive bacteria (such as Staphylococcus aureus, Staphylococcus haemolyticus, Listeria Monocytogenes, Bacillus cereus, Bacillus subtilis, Bacillus thuringiensis, Enterococcus faecalis, Streptococcus pneumoniae and Micrococcus flavus) and Gram-negative bacteria (Escherichia coli, Vibrio parahaemolyticus, Klebsiella pneumoniae, Pseudomonasaeruginosa, Pseudomonas fluorescens, Salmonella typhimurium, Acinetobacter lwoffii, Acinetobacter baumannii, Campylobacter coli, Campylobacter jejuni, Porphyromonas gingivalis, Prevotella intermedia, Enterobacter cloacae, Stenotrophomonas maltophilia, and Neisseria gonorrhoeae) [,,,,,,,,,,,,,,,,,,,,,,].

Table 5.
Scientific evidence of the antimicrobial effects of Opuntia spp.
4.4. Action in the Treatment of Skin Wounds
The skin is the largest organ of the human body that acts as a protective barrier against harmful agents from the external environment. It controls thermal regulation and homeostasis of water and electrolytes. When this barrier is damaged, the body promotes the healing and/or scarring process to regenerate the injured area, involving molecular, cellular, and biochemical mechanisms that are divided into four phases (hemostasis, inflammatory, proliferative, and remodeling) (Figure 2). Any disruption in the balance of these processes causes problems and delays in wound healing; deteriorations related to aging, pathological situations (such as diabetes, obesity and/or arterial diseases) and multiple local and systemic factors (hypoxia, OXs, diminished immune response, poor nutrition, medications and infectious agents) [].
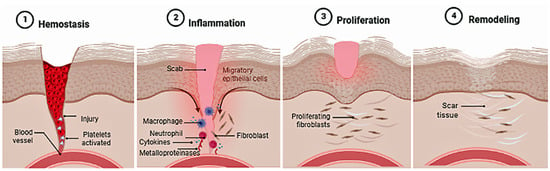
Figure 2.
Stages of skin healing.
Cicatrization is a physiological process that involves perfect interactions of numerous cells and molecules, so the imbalance of these interactions generates alterations during the process that are expressed as excessive yellow discharge, pain, swelling, redness and fever. Among the most relevant is the chronic inflammatory state, where pro- and anti-inflammatory mediators produce an exacerbated recruitment of neutrophils and macrophages with overexpression of inflammatory cytokines and excessive release of ROS, which together interfere with the proliferation/differentiation of keratinocytes and fibroblasts in the injured area and leads to cell apoptosis []. In addition, the increase in proinflammatory cytokines affects subsequent wound healing mechanisms, increasing matrix metalloproteinases (MMPs) and other proteases that alter cell proliferation/migration and reduce the accumulation of extracellular matrix components. Normally, there must be a balance between proliferation/activation and maturation/apoptosis of blood vessels; and if this is not done correctly, neovascularization and blood flow in the area are reduced, delaying the subsequent mechanisms of the proliferative and remodeling phase. Another mistake is the involvement of wound keratinocytes, which acquire a hyperproliferative state due to overexpression of the β-catenin/c-myc pathway, and express low levels of keratins 1, 2, and 10. This alters the migratory potential of these cells, which is related to the proteolytic degradation of growth factors and extracellular matrix proteins necessary for migration. Impaired remodeling is another major failure, as injured cells synthesize excessive amounts of MMPs and other proteases, degrading not only extracellular matrix components, but also cell surface receptors, growth factors, and the cytokines. In addition, inhibitors of metalloproteinases (TIMP) are reduced, contributing to the deregulation of proteases in these lesions, and consequently, the degradation of important molecules of the extracellular matrix such as collagen, elastin, fibronectin and chondroitin sulfate [].
Over the years, adequate therapies have been sought to improve or promote the wound healing process. Currently, there are several treatments that can be classified into surgical procedures (autografts, allografts and xenografts), non-surgical therapies (topical formulations, dressings and skin substitutes) and pharmacological agents. However, depending on the size, type of wound, and factors that caused the damage, existing therapies are not completely effective []. Once again, phytomedicine, being popular among the general population in different regions of the world, opens new avenues of pharmacological intervention for the healing of skin wounds. Among the known phytotherapeutic agents are Aloe vera, mimosa (Mimosa sensitive), grape vine (Vitis vinifera), chamomile (Matricaria chamomilla), ginseng (Panax ginseng), jojoba (Simmondsia chinensis), rosemary (Salvia rosmarinus), lemon (Citrus limon), comfrey (Symphytum officinale), papaya (Carica papaya), oats (Avena sativa), garlic (Allium sativum), ginkgo (Ginkgo biloba), ocimum (Ocimum basilicum), tree oil, and olive oil [].
In the case of Opuntia spp. and its extracts, there is various evidence of its use in traditional medicine for the treatment of burns, skin disorders and wound healing. The first study in this field of research compared the healing activity of a base cream containing lyophilized cladodes of OFI at 15% against a commercial ointment on wounds produced on the back of rats. After 5 days of treatment, the epithelialization process was evident and complete, suggesting that cladodes accelerate the proliferation and migration of keratinocytes in the cicatrization process []. The previous result was confirmed when two lyophilized polysaccharide extracts obtained from OFI were applied topically for 6 days and observed that they induce a beneficial effect (accelerate the re-epithelialization-remodeling phases and favor cell-matrix interactions) in the skin wounds of rats [].
Using benzopyrene- or TNF-α-stimulated keratinocytes, Nakahara et al., (2015) demonstrated that CLD extracts protect the epidermal barrier and keratinocyte function by increasing the expression of filaggrin and loricrin, two proteins present in keratinocytes and corneocytes differentiated. In addition, they attribute the protective effect to an inhibition of ROS production caused by inflammatory agents. This property is probably related to the activation of nuclear erythroid factor (Nrf2) and NAD(P)H:quinone oxidoreductase 1 []. It is considered that the cicatrizant properties of OFI cladodes may involve high molecular weight polysaccharide components (such as linear galactan polymer and highly branched xyloarabinan) as well as low molecular weight components [lactic acid, D-mannitol, piscidic, eucomic, and 2-hydroxy-4-(4′-hydroxyphenyl)-butanoic acid]. These extracts could accelerate cell regeneration in a keratinocyte monolayer, which suggest that OFI components exhibit high anti-inflammatory and wound-healing properties [].
Likewise, polysaccharides extracted from OFI stimulate the proliferation of fibroblasts and keratinocytes []. Among the protective agents present in Opuntia extracts, isorhamnetin glucoside components [such as isorhamnetin-glucosyl-rhamnoside diglucoside (IGR)], could inhibit COX-2, TNF-α and IL-6 production and induction of NO evoked by LPS [].Not only OFI has shown beneficial effects, O. humifusa (OHF) extracts regulate the production of hyaluronic acid (HA) by increasing the expression of HA synthase in keratinocytes exposed to UV-B treatment. Treatment with these extracts could decrease the increased expression of hyaluronidase UV-B. The same protective effect on HA has been observed in SKH-1 hairless mice exposed to UV-B, which indicates that OHF extracts have a great capacity for skin care []. Table 6 presents all the studies of Opuntia spp. that justify its efficacy at the molecular and cellular level for the healing of skin wounds, as well as its use in dermatological preparations.

Table 6.
Scientific evidence of Opuntia spp. on the healing of skin wounds.
5. Toxic Evidence of the Genus Opuntia
The cacti family contains approximately 200 genera and 2000 species, which favors a wide genetic diversity that, together with environmental conditions (climate, humidity), type of soil, age of maturity of the cladodes and the harvest season, generates differences in the phytochemical composition of its vegetable parts (PPFs, CLDs, roots, flowers, seeds and stems) between wild and domesticated species, inducing changes in its nutritional values and undoubtedly in its functional and therapeutic properties. In this sense, although the public and some health professionals consider herbal medicines to be relatively safe because they are “natural”, there is very little data to support this assumption. Therefore, Opuntia spp. species are not exempt from possible adverse and toxic effects.
Saleem et al., (2005) evaluated for the first time its toxicological safety by determining the hypotensive activity of a methanolic extract of OdHw and its alpha-pyrone glycoside (opuntioside-I) in normotensive rats. At the end of their study, they observed no mortality with the extract and/or opuntioside-I orally administered, even at high doses of 1000 mg/kg/day. However, histopathological analysis revealed slight changes in the liver and spleen of the animals []. Subsequently, in 2012, the physicochemical characteristics (acidity, percentage of free fatty acids, saponification value, refractive index and density), lethal dose 50 (LD50) and toxicity of an OFI seed oil in mice were determined. Finding that the LD50 values ranged between 40.7 and 45.4 mL/kg body wt for oral administration and 2.52–2.92 mL/kg body wt for intraperitoneal administration [].
These results and variations in doses called the attention to analyze other species of Opuntia, e.g., Osorio-Esquivel et al., (2012) who determined the acute toxicity of a MeOH extract of O. xoconostle (OX) seeds in mice fed with a hypercholesterolemic diet; finding that it was greater than 5000 mg/kg of body weight without the presence of apparent toxic manifestations []. Similar data on the absence of any sign of acute toxicity were observed in two other studies; the first, when orally administering up to 5 mL/kg of cactus pear seed oil (CPSO) to Wistar rats to determine its hypoglycemic effect [] and/or by evaluating the in vitro and in vivo bioactivities of O. macrorhiza Engelm seed oil (OMESO) [].
Considering that OFI is an important dietary source, a toxicological evaluation of aqueous extracts from different parts of the plant was performed and compared using three types of assays (MTT, Comet and the γH2AX In-Cell Western). The conclusion was that the fruit pulp extracts showed the best antigenotoxic effect against H2O2 and that no extract induced genotoxicity and/or cytotoxicity in the cell lines used [].
To confirm the above findings, Han et al. (2019) investigated the genotoxicity of three doses of an OFIS extract (500, 1000 and 2000 mg/kg/day) orally administered for one week in rodents using the Ames test (S. typhimurium strains TA100, TA1535, TA98 and TA153 and E. coli strain WP2 urvA), chromosomal aberration assay in Chinese hamster lung cells and micronucleus test in bone marrow cells. In summary, it was observed that: (a) OFIS did not alter normal animal behavior or body weight gain, (b) mutagenicity was not present in both bacterial strains with or without S9 activation and (c) the number of micronucleated polychromatic erythrocytes (MPE) was not increased [].
Recently, two groups of researchers addressed the safety of OdHw, considering that it is a cactaceae traditionally used in several countries to treat ailments such as inflammation, gastric ulcers, diabetes, hepatitis, asthma, and intestinal spasms. In the first study, the acute toxicity of the oil obtained from its seeds was evaluated in albino mice and Wistar rats. After a single administration of the established doses (1.0, 2.0, 3.0, 5.0 and 7.0 mL/kg), adverse signs and/or mortality were observed for four weeks. The conclusion was that the oil produced no variations in the body weight of the animals and no mortality or signs of toxicity during the entire monitoring period. In addition, cell viability was not affected when human hepatoma HepG2 culture was analyzed [,].
Finally, when evaluating a MeOH extract of cladodes by MTT assay in human embryonic kidney cell line, genomic DNA fragmentation using agarose gel electrophoresis and bone marrow micronuclei frequency, it was proved that a 7-day treatment of 5 g/kg of the extract orally had no effect on DNA integrity, neither did it induce cytotoxicity or stimulate MPE formation []. Unfortunately, although Opuntia spp. could be considered a reliable and safe plant, some authors have identified and reported the presence of certain side effects during oral consumption of OFI, such as mild diarrhea, increased stool volume and frequency, nausea, headache and lower colonic obstruction [,,,].
Despite these secondary effects, plants of the Opuntia genus are traditional foods frequently consumed and their cladodes and fruits are still considered with high agrotechnological potential. Besides, studies suggesting an LD₅₀, above 5000 mg/kg are safe levels. To date, there is no established dose and/or concentration for its consumption and there are different intervals that depend on the route of administration, the species (humans/animals) in which they are used; the approach to use it whether it is food (fresh, juices or extracts) or for experimental evaluation.
Some authors recommend an intake between 10 to 17 g/person/day of Opuntia and/or prickly pear fruits (PPFs) to have a healthy life. Others suggest between 100 and 500 g/day of roasted CLDs to significantly reduce the complications of diabetes mellitus. There are products, such as PPFs, in commercial presentations of capsules, tablets, powders, and juices whose oral dosage regimens are established at 250 mg/3 times a day/every 8 h [,,].
In general, summarizing the information from both documents (part 1 and 2) it can be seen that the doses range from 50 mg/kg to 7 g/kg [].
6. Conclusions and Perspectives
Although modern medicine is available in most countries for the control and treatment of many diseases, phytomedicine and/or TCAM continue being popularly used in different populations for historical, cultural reasons, easy access, low cost, diversity, and especially, a relative lower quantity of adverse effects. The set of studies presented in both reviews (Part 1 and 2) demonstrate the beneficial properties of the different vegetative parts of Opuntia spp. (wild and domesticated). For this reason, scientific research on this genus of plants (known as succulents, due to their ability to generate biomass by storing water in one or more of their organs) has deepened and may continue to increase, in order to better understand their nutritional and therapeutic properties.
In general, most of the evidence confirms that CLDs, PPFs, oils and/or extracts (MeOH, Hx, EtOAc, Chl and aqueous) coming mainly from OFI, OS, OdHw, OHF, OX and O. macrorhiza Engelm have presented relevant therapeutic and/or pharmacological potentials; whose mechanisms of action are mainly related to the inhibition of the absorption of substances, modification of the intestinal flora, elimination of reactive oxygen species and/or protection of nucleophilic DNA sites, anti-inflammatory activity, induction of detoxification pathways, and activation of apoptosis. However, it is convenient to extend the investigations to other species in order to analyze and confirm their pharmacological capacities.
Likewise, the results of the investigations confirm and coincide that these beneficial properties are possible attributed to a synergistic and/or combined effect among the different bioactive compounds (vitamins, flavonoids (isorhamnetin, kaempferol, quercetin), phenolic compounds, pigments (carotenoids, betalains and betacyanins), α-pyrones (opunthiol and opuntioside glucoside), pectin and mucilage). Nonetheless, it is convenient to increase the individual studies of each phytochemical, to determine its protective action; given that as substrates they can activate different biochemical reactions to provide important health benefits and be recognized as significant nutraceutical agents. All of the above, added to the fact that several Opuntia plants have been consumed by humans for more than 8000 years, which are easily adapted and/or propagated in different types of soil. In addition to their relatively low presence of adverse and toxic effects, they favor their domestication process, the increase in economic interest and new advances in the field of biotechnology.
It is convenient to remember that the process to discover drugs and/or medications is complex and costly. In that process different types of studies converge (such as those presented in this document; in vitro, in vivo and clinical). In recent years, computational methods (also called in-silico) have been integrated into this multidisciplinary effort, contributing to efficient data analysis, filtering and/or selecting individual bioactive molecules for their subsequent experimental evaluation, and also to generate hypotheses that favor the understanding of its mechanism of action and the design of new chemical structures.Again, Opuntia species are not exempt from participating in this area of research. Among the most significant studies, those carried out by Elkady et al., (2020), who isolated and characterized constituents of the prickly pear peel to determine their antibacterial activity stand out. This latter assay revealed that quercetin 5,4′-dimethyl ether found in EtOAc fraction exerted an inhibitory effect against pneumonia pathogens. Virtual docking of the isolated compounds showed promise in silico anti-quorum sensing efficacy, suggesting that unused waste from fruits contains bioactive components with possible beneficial potential []. On the other hand, an In Silico Investigation on the Interaction of Chiral Phytochemicals from O. ficus-indica with SARS-CoV-2 Mpro (main viral protease) was developed. Using two web-based molecular docking programs (1-Click Mcule and COVID-19 Docking Server) several flavonols and flavonol glycosides were identified; highlighting the chiral compound astragalin with high binding affinity for Mpro and a low toxicity profile. Emerging the possibility of a protease inhibitor agent as an anti-COVID-19 strategy []. In the most recent study, the possible targets in the PI3K/Akt/mTOR pathway acted upon by an O. xoconostle extract were modeled and simulated in silico using the Big Data-Cellulat platform, as well as the concentration range of LD₅₀ to be used in breast cancer cells. The in silico results showed that the activation of I3K and Akt is related to angiogenesis and inhibition of apoptosis, and that the extract has an antiproliferative effect on cancer cells, causing the cells to interrupt in the G2/M phase of the cell cycle []. Taken together, these three studies demonstrate that the use of in silico tools is a valuable method for conducting virtual experiments and discovering new therapeutic agents.
In conclusion, there is still a long way to go on scientific research to understand in more detail the significant beneficial properties of all species of Opuntia spp.
Author Contributions
E.M.-S., E.M.-B., J.A.M.-G. and J.P.-R. designed the concept, wrote the majority of the paper and managed the authors; N.V.-M., J.A.I.-V., M.S.-G. and L.D.-O. conducted the literature search, wrote key sections of the paper; I.Á.-G., Á.M.-G. and J.I.-V. wrote sections of the paper and managed the reference list. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Florencia Ana María Talavera Silva for all her academic support. Her comments and observations in reviewing articles are always valuable and we give her immense recognition for her efforts.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mendoza-Pérez, J.; Fregoso-Aguilar, T. Chemistry of Natural Antioxidants and Studies Performed with Different Plants Collected in Mexico. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-González, J.A., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 59–85. ISBN 978-953-51-1123-8. [Google Scholar]
- Peltzer, K.; Pengpid, S. Utilization and Practice of Traditional/Complementary/Alternative Medicine (T/CAM) in Southeast Asian Nations (ASEAN) Member States. Stud. Ethno-Med. 2015, 9, 209–218. [Google Scholar] [CrossRef]
- López-Romero, D.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Alvarez-Gonzalez, I.; Morales-González, Á.; Madrigal-Santillán, E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients 2018, 10, 1954. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; García-Melo, F.; Morales-González, J.A.; Vázquez-Alvarado, P.; Muñoz-Juárez, S.; Zuñiga-Pérez, C.; Sumaya-Martínez, M.T.; Madrigal-Bujaidar, E.; Hernández-Ceruelos, A. Antioxidant and Anticlastogenic Capacity of Prickly Pear Juice. Nutrients 2013, 5, 4145–4158. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Martínez-Cruz, O.; Paredes-López, O. Phytochemical Content, Nutraceutical Potential and Biotechnological Applications of an Ancient Mexican Plant: Nopal (Opuntia ficus-indica). Curr. Nutr. Food. Sci. 2014, 10, 196–217. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Del Socorro Santos Díaz, M.; Barba de la Rosa, A.-P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med. Cell Longev. 2017, 2017, 8634249. [Google Scholar] [CrossRef]
- Griffith, M.P. The Origins of an Important Cactus Crop, Opuntia ficus-indica (Cactaceae): New Molecular Evidence. Am. J. Bot. 2004, 91, 1915–1921. [Google Scholar] [CrossRef]
- Ochoa, M.; Giuseppe Barbera, G. History and Economic and Agro-Ecological Importance. In Crop Ecology, Cultivation and Uses of Cactus Pear; Inglese, P., Mondragon, C., Eds.; Food and Agriculture Organization of the United: Rome, Italy, 2017; pp. 1–11. ISBN 978-92-5-109860-8. [Google Scholar]
- Kiesling, R.; Metzing, D. Origin and Taxonomy of Opuntia ficus-indica. In Crop Ecology, Cultivation and Uses of Cactus Pear; Inglese, P., Mondragon, C., Eds.; Food and Agriculture Organization of the United: Rome, Italy, 2017; pp. 13–16. ISBN 978-92-5-109860-8. [Google Scholar]
- Griffith, P.; Porter, M. Phylogeny of Opuntioideae (Cactaceae). Int. J. Plant Sci. 2009, 170, 107–116. [Google Scholar] [CrossRef]
- Reyes-Aguero, J.A.; Rivera, J.R.A. Agrobiodiversity of Cactus Pear (Opuntia, Cactaceae) in the Meridional Highlands Plateau of Mexico. J. Nat. Resour. Dev. 2011, 1, 1–9. [Google Scholar] [CrossRef][Green Version]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of Natural Products with Hepatoprotective Effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- Silva, M.A.; Gonçalves-Albuquerque, T.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, M.; Ignacimuthu, S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J. Ethnopharmacol. 2011, 134, 851–864. [Google Scholar] [CrossRef]
- Githae, E.W. Status of Opuntia invasions in the arid and semi-arid lands of Kenya. CAB Reviews. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Chakale, M.V.; Asong, J.A.; Struwig, M.; Mwanza, M.; Aremu, A.O. Ethnoveterinary Practices and Ethnobotanical Knowledge on Plants Used against Cattle Diseases among Two Communities in South Africa. Plants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef]
- El-Samahy, S.; El-Hady, E.A.; Habiba, R.; Moussa, T. Chemical and Rheological Characteristics of Orange-Yellow Cactus-Pear Pulp from Egypt. J. Prof. Assoc. Cact. Dev. 2006, 8, 39–51. [Google Scholar]
- Osuna-Martínez, U.; Reyes-Esparza, J.; Rodríguez-Fragoso, L. Cactus (Opuntia ficus-indica): A Review on Its Antioxidants Properties and Potential Pharmacological Use in Chronic Diseases. Nat. Prod. Chem. Res. 2014, 2, 153–159. [Google Scholar] [CrossRef]
- Kaur, M. Pharmacological Actions of Opuntia ficus indica: A Review. J. App. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a Source of Bioactivity Compounds for Health and Nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Mercado-Gonzalez, P.E.; Izquierdo Vega, J.; Vargas-Mendoza, N.; Álvarez-González, I.; Fregoso-Aguilar, T.; Delgado-Olivares, L.; et al. Opuntia Genus in Human Health: A Comprehensive Summary on Its Pharmacological, Therapeutic and Preventive Properties. Part 1. Horticulturae. 2022, 8, 88. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and Human Health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Deckelbaum, R.J. Stanol/Sterol ester-containing foods and blood cholesterol levels a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Am. Heart J. 2001, 103, 1177–1179. [Google Scholar]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids-Food sources and health benefits. Rocz. Pa’nstw. Zakł. Hig. 2014, 65, 79–85. [Google Scholar]
- Smeriglio, A.; De Francesco, C.; Denaro, M.; Trombetta, D. Prickly Pear Betalain-Rich Extracts as New Promising Strategy for Intestinal Inflammation: Plant Complex vs. Main Isolated Bioactive Compounds. Front. Pharmacol. 2021, 12, 722398. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Escobedo-Hinojosa, W.I.; Gomez-Chang, E.; García-Martínez, K.; Guerrero Alquicira, R.; Cardoso-Taketa, A.; Romero, I. Gastroprotective Mechanism and Ulcer Resolution Effect of Cyrtocarpa Procera Methanolic Extract on Ethanol-Induced Gastric Injury. Evid. Based Complement. Alternat. Med. 2018, 2018, 2862706. [Google Scholar] [CrossRef]
- Bi, W.-P.; Man, H.-B.; Man, M.-Q. Efficacy and Safety of Herbal Medicines in Treating Gastric Ulcer: A Review. World J. Gastroenterol. 2014, 20, 17020–17028. [Google Scholar] [CrossRef]
- Kangwan, N.; Park, J.-M.; Kim, E.-H.; Hahm, K.B. Quality of Healing of Gastric Ulcers: Natural Products beyond Acid Suppression. World J. Gastrointest. Pathophysiol. 2014, 5, 40–47. [Google Scholar] [CrossRef]
- Palevitch, D.; Earon, G.; Levin, I. Treatment of Benign Prostatic Hypertrophy with Opuntia ficus-indica (L.) Miller. J. Herbs Spices Med. Plants. 1993, 2, 45–49. [Google Scholar] [CrossRef]
- Park, E.H.; Kahng, J.H.; Paek, E.A. Studies on the Pharmacological Action of Cactus: Identification of Its Anti-Inflammatory Effect. Arch. Pharm. Res. 1998, 21, 30–34. [Google Scholar] [CrossRef]
- Loro, J.F.; del Rio, I.; Pérez-Santana, L. Preliminary Studies of Analgesic and Anti-Inflammatory Properties of Opuntia dillenii Aqueous Extract. J. Ethnopharmacol. 1999, 67, 213–218. [Google Scholar] [CrossRef]
- Park, E.H.; Kahng, J.H.; Lee, S.H.; Shin, K.H. An Anti-Inflammatory Principle from Cactus. Fitoterapia 2001, 72, 288–290. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Tripodo, M.M.; d’Aquino, A.; Mondello, M.R. Antiulcer Activity of Opuntia ficus indica (L.) Mill. (Cactaceae): Ultrastructural Study. J. Ethnopharmacol. 2001, 76, 1–9. [Google Scholar] [CrossRef]
- Galati, E.M.; Pergolizzi, S.; Miceli, N.; Monforte, M.T.; Tripodo, M.M. Study on the Increment of the Production of Gastric Mucus in Rats Treated with Opuntia ficus indica (L.) Mill. Cladodes. J. Ethnopharmacol. 2002, 83, 229–233. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical Characterization and Biological Effects of Sicilian Opuntia ficus indica (L.) Mill. Fruit Juice: Antioxidant and Antiulcerogenic Activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant Betalains from Cactus Pear (Opuntia ficus-indica) Inhibit Endothelial ICAM-1 Expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Ahmed, M.S.; El Tanbouly, N.D.; Islam, W.T.; Sleem, A.A.; El Senousy, A.S. Antiinflammatory Flavonoids from Opuntia dillenii (Ker-Gawl) Haw. Flowers Growing in Egypt. Phytother. Res. 2005, 19, 807–809. [Google Scholar] [CrossRef]
- Allegra, M.; Furtmüller, P.G.; Jantschko, W.; Zederbauer, M.; Tesoriere, L.; Livrea, M.A.; Obinger, C. Mechanism of Interaction of Betanin and Indicaxanthin with Human Myeloperoxidase and Hypochlorous Acid. Biochem. Biophys. Res. Commun. 2005, 332, 837–844. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, S.-C.; Kim, T.-W.; Kim, K.-S.; Song, J.-C.; Kim, S.-K.; Lee, H.-M.; Sung, H.-J.; Park, H.-J.; Song, Y.-B.; et al. Radical Scavenging and Anti-Inflammatory Activity of Extracts from Opuntia humifusa Raf. J. Pharm. Pharmacol. 2006, 58, 113–119. [Google Scholar] [CrossRef]
- Panico, A.M.; Cardile, V.; Garufi, F.; Puglia, C.; Bonina, F.; Ronsisvalle, S. Effect of Hyaluronic Acid and Polysaccharides from Opuntia ficus indica (L.) Cladodes on the Metabolism of Human Chondrocyte Cultures. J. Ethnopharmacol. 2007, 111, 315–321. [Google Scholar] [CrossRef]
- Jung, J.; Shin, J.H. Effect of Opuntia ficus-indica Extract on Anti-Inflammatory in Murine Macrophages. FASEB J. 2010, 24, 929.5. [Google Scholar] [CrossRef]
- Kim, J.; Jho, K.H.; Choi, Y.H.; Nam, S.-Y. Chemopreventive Effect of Cactus (Opuntia humifusa) Extracts: Radical Scavenging Activity, pro-Apoptosis, and Anti-Inflammatory Effect in Human Colon (SW480) and Breast Cancer (MCF7) Cells. Food Funct. 2013, 4, 681–688. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Indicaxanthin Inhibits NADPH Oxidase (NOX)-1 Activation and NF-ΚB-Dependent Release of Inflammatory Mediators and Prevents the Increase of Epithelial Permeability in IL-1β-Exposed Caco-2 Cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef]
- Allegra, M.; Ianaro, A.; Tersigni, M.; Panza, E.; Tesoriere, L.; Livrea, M.A. Indicaxanthin from Cactus Pear Fruit Exerts Anti-Inflammatory Effects in Carrageenin-Induced Rat Pleurisy. J. Nutr. 2014, 144, 185–192. [Google Scholar] [CrossRef]
- Matias, A.; Nunes, S.L.; Poejo, J.; Mecha, E.; Serra, A.T.; Madeira, P.J.A.; Bronze, M.R.; Duarte, C.M.M. Antioxidant and Anti-Inflammatory Activity of a Flavonoid-Rich Concentrate Recovered from Opuntia ficus-indica Juice. Food Funct. 2014, 5, 3269–3280. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Sheth, N.R.; Suhagia, B.N. Analgesic and Anti-Inflammatory Action of Opuntia elatior Mill Fruits. J. Ayurveda Integr. Med. 2015, 6, 75–81. [Google Scholar] [CrossRef][Green Version]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Martínez-Vitela, C.; Serna-Saldívar, S.O. Topical Anti-Inflammatory Effects of Isorhamnetin Glycosides Isolated from Opuntia ficus-indica. Biomed. Res. Int. 2015, 2015, 847320. [Google Scholar] [CrossRef]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; De Angelis, M.; Silano, M.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation of Cactus Cladodes (Opuntia ficusindica L.) Generates Flavonoid Derivatives with Antioxidant and Anti-Inflammatory Properties. PLoS ONE 2016, 11, e0152575. [Google Scholar] [CrossRef]
- Siddiqui, F.; Abidi, L.; Poh, C.F.; Faizi, S.; Farooq, A.D. Analgesic Potential of Opuntia dillenii and Its Compounds Opuntiol and Opuntioside against Pain Models in Mice. Rec. Nat. Prod. 2016, 10, 721. [Google Scholar]
- Siddiqui, F.; Naqvi, S.; Abidi, L.; Faizi, S.; Avesi, L.; Mirza, T.; Farooq, A.D. Opuntia dillenii Cladode: Opuntiol and Opuntioside Attenuated Cytokines and Eicosanoids Mediated Inflammation. J. Ethnopharmacol. 2016, 182, 221–234. [Google Scholar] [CrossRef]
- Sharma, B.R.; Park, C.M.; Choi, J.W.; Rhyu, D.Y. Anti-Nociceptive and Anti-Inflammatory Effects of the Methanolic Extract of Opuntia humifusa Stem. Avicenna J. Phytomed. 2017, 7, 366–375. [Google Scholar]
- Chahdoura, H.; El Bok, S.; Refifa, T.; Adouni, K.; Khemiss, F.; Mosbah, H.; Ben-Attia, M.; Flamini, G.; Achour, L. Activity of Anti-Inflammatory, Analgesic and Antigenotoxic of the Aqueous Flower Extracts of Opuntia microdasys Lem.Pfeiff. J. Pharm. Pharmacol. 2017, 69, 1056–1063. [Google Scholar] [CrossRef]
- Bounihi, A.; Bitam, A.; Bouazza, A.; Yargui, L.; Koceir, E.A. Fruit Vinegars Attenuate Cardiac Injury via Anti-Inflammatory and Anti-Adiposity Actions in High-Fat Diet-Induced Obese Rats. Pharm. Biol. 2017, 55, 43–52. [Google Scholar] [CrossRef]
- Aboura, I.; Nani, A.; Belarbi, M.; Murtaza, B.; Fluckiger, A.; Dumont, A.; Benammar, C.; Tounsi, M.S.; Ghiringhelli, F.; Rialland, M.; et al. Protective Effects of Polyphenol-Rich Infusions from Carob (Ceratonia siliqua) Leaves and Cladodes of Opuntia ficus-indica against Inflammation Associated with Diet-Induced Obesity and DSS-Induced Colitis in Swiss Mice. Biomed. Pharmacother. 2017, 96, 1022–1035. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Adouni, K.; Mhadhebi, L.; Calhelha, R.C.; Snoussi, M.; Majdoub, H.; Flamini, G.; Ferreira, I.C.F.R.; Achour, L. Bioactivity and Chemical Characterization of Opuntia macrorhiza Engelm. Seed Oil: Potential Food and Pharmaceutical Applications. Food Funct. 2017, 8, 2739–2747. [Google Scholar] [CrossRef]
- Saih, F.-E.; Andreoletti, P.; Mandard, S.; Latruffe, N.; El Kebbaj, M.S.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Protective Effect of Cactus Cladode Extracts on Peroxisomal Functions in Microglial BV-2 Cells Activated by Different Lipopolysaccharides. Molecules 2017, 22, 102. [Google Scholar] [CrossRef]
- Aiello, A.; Di Bona, D.; Candore, G.; Carru, C.; Zinellu, A.; Di Miceli, G.; Nicosia, A.; Gambino, C.M.; Ruisi, P.; Caruso, C.; et al. Targeting Aging with Functional Food: Pasta with Opuntia Single-Arm Pilot Study. Rejuvenation Res. 2018, 21, 249–256. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Vasto, S.; Pintaudi, A.M.; Livrea, M.A.; Allegra, M. Short-Term Cactus Pear [Opuntia ficus-indica (L.) Mill] Fruit Supplementation Ameliorates the Inflammatory Profile and Is Associated with Improved Antioxidant Status among Healthy Humans. Food Nutr. Res. 2018, 62, 1262. [Google Scholar] [CrossRef]
- Benattia, F.K.; Arrar, Z.; Dergal, F.; Khabbal, Y. Pharmaco-Analytical Study and Phytochemical Profile of Hydroethanolic Extract of Algerian Prickly Pear (Opuntia ficus-indica L.). Curr. Pharm. Biotechnol. 2019, 20, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Bardaa, S.; Turki, M.; Ben Khedir, S.; Mzid, M.; Rebai, T.; Ayadi, F.; Sahnoun, Z. The Effect of Prickly Pear, Pumpkin, and Linseed Oils on Biological Mediators of Acute Inflammation and Oxidative Stress Markers. Biomed. Res. Int. 2020, 2020, 5643465. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yang, W.K.; Park, Y.R.; Park, Y.C.; Park, I.J.; Lee, G.J.; Kang, H.S.; Kim, B.K.; Kim, S.H. Opuntia ficus-indica Alleviates Particulate Matter 10 Plus Diesel Exhaust Particles (PM10D)-Induced Airway Inflammation by Suppressing the Expression of Inflammatory Cytokines and Chemokines. Plants 2022, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Hyun, J.E.; Li, D.W.; Moon, Y.I. Effects of Opuntia ficus-indica Var. Saboten Stem on Gastric Damages in Rats. Arch. Pharm. Res. 2002, 25, 67–70. [Google Scholar] [CrossRef]
- Wiese, J.; McPherson, S.; Odden, M.C.; Shlipak, M.G. Effect of Opuntia ficus indica on Symptoms of the Alcohol Hangover. Arch. Intern. Med. 2004, 164, 1334–1340. [Google Scholar] [CrossRef]
- Vázquez-Ramírez, R.; Olguín-Martínez, M.; Kubli-Garfias, C.; Hernández-Muñoz, R. Reversing Gastric Mucosal Alterations during Ethanol-Induced Chronic Gastritis in Rats by Oral Administration of Opuntia ficus-indica Mucilage. World J. Gastroenterol. 2006, 12, 4318–4324. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Hfaiedh, M.; Sakly, M.; Zourgui, L.; Rhouma, K.B. Antioxidant and Antiulcerogenic Activities of Opuntia ficus indica f. Inermis Root Extract in Rats. Phytomedicine 2010, 17, 1120–1126. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Sakly, M.; Ben Rhouma, K. Evaluation of Antioxidant and Antiulcerogenic Activities of Opuntia ficus indica f. Inermis Flowers Extract in Rats. Environ. Toxicol. Pharmacol. 2011, 32, 406–416. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaeidh, N.; Bouoni, Z.; Sakly, M.; Rhouma, K.B. Ameliorative Effect of Opuntia ficus indica Juice on Ethanol-Induced Oxidative Stress in Rat Erythrocytes. Exp. Toxicol. Pathol. 2013, 65, 391–396. [Google Scholar] [CrossRef]
- Jouini, M.; Abdelhamid, A.; Chaouch, M.A.; le Cerf, D.; Bouraoui, A.; Majdoub, H.; Ben Jannet, H. Physico-Chemical Characterization and Pharmacological Activities of Polysaccharides from Opuntia microdasys Var. Rufida Cladodes. Int. J. Biol. Macromol. 2018, 107, 1330–1338. [Google Scholar] [CrossRef]
- Babitha, S.; Bindu, K.; Nageena, T.; Veerapur, V.P. Fresh Fruit Juice of Opuntia dillenii Haw. Attenuates Acetic Acid-Induced Ulcerative Colitis in Rats. J. Diet. Suppl. 2019, 16, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Elsawi, S.A.; Radwan, R.R.; Elbatanony, M.M.; El-Feky, A.M.; Sherif, N.H. Prophylactic Effect of Opuntia ficus indica Fruit Peel Extract against Irradiation-Induced Colon Injury in Rats. Planta Med. 2020, 86, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets. Molecules 2010, 15, 3517–3555. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective Potency of Some Spice Herbs, a Literature Review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef]
- Chang, R.; Ho, Y. Introductory Chapter: Concept of Neuroprotection—A New Perspective. In Neuroprotection; Chang, R.C., Ho, Y., Eds.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–19. [Google Scholar]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid. Based Complement. Alternat. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef] [PubMed]
- Wie, M.-B. Protective Effects of Opuntia ficus-indica and Saururus Chinensis on Free Radical-Induced Neuronal Injury in Mouse Cortical Cell Cultures. Yakhak. Hoeji. 2000, 44, 613–619. [Google Scholar]
- Dok-Go, H.; Lee, K.H.; Kim, H.J.; Lee, E.H.; Lee, J.; Song, Y.S.; Lee, Y.-H.; Jin, C.; Lee, Y.S.; Cho, J. Neuroprotective Effects of Antioxidative Flavonoids, Quercetin, (+)-Dihydroquercetin and Quercetin 3-Methyl Ether, Isolated from Opuntia ficus-indica Var. Saboten. Brain Res. 2003, 965, 130–136. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, S.-M.; Ha, H.-J.; Moon, C.-J.; Shin, T.-K.; Kim, J.-M.; Lee, N.-H.; Kim, H.-C.; Jang, K.-J.; Wie, M.-B. Opuntia ficus-indica Attenuates Neuronal Injury in in Vitro and in Vivo Models of Cerebral Ischemia. J. Ethnopharmacol. 2006, 104, 257–262. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.Y.; Yoon, J.H.; Lim, H.J.; Kim, T.H.; Jin, C.; Kwak, W.-J.; Han, C.-K.; Ryu, J.-H. Inhibition of Nitric Oxide Synthase Expression in Activated Microglia and Peroxynitrite Scavenging Activity by Opuntia ficus indica Var. Saboten. Phytother Res. 2006, 20, 742–747. [Google Scholar] [CrossRef]
- Cho, J.-S.; Han, C.-K.; Lee, Y.-S.; Jin, C.-B. Neuroprotective and Antioxidant Effects of the Butanol Fraction Prepared from Opuntia ficus-indica Var. Saboten. Biomol Ther. 2007, 15, 205–211. [Google Scholar] [CrossRef][Green Version]
- Huang, X.; Li, Q.; Zhang, Y.; Lü, Q.; Guo, L.; Huang, L.; He, Z. Neuroprotective Effects of Cactus Polysaccharide on Oxygen and Glucose Deprivation Induced Damage in Rat Brain Slices. Cell Mol. Neurobiol. 2008, 28, 559–568. [Google Scholar] [CrossRef]
- Huang, X.; Li, Q.; Li, H.; Guo, L. Neuroprotective and Antioxidative Effect of Cactus Polysaccharides in Vivo and in Vitro. Cell Mol. Neurobiol. 2009, 29, 1211–1221. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, B.; Huang, X.; Zhan, J.; Zhao, Y.; Zhou, M.; Guo, L. Purification and Neuroprotective Effects of Polysaccharides from Opuntia Milpa Alta in Cultured Cortical Neurons. Int. J. Biol. Macromol. 2011, 49, 681–687. [Google Scholar] [CrossRef]
- Briffa, M.; Ghio, S.; Neuner, J.; Gauci, A.J.; Cacciottolo, R.; Marchal, C.; Caruana, M.; Cullin, C.; Vassallo, N.; Cauchi, R.J. Extracts from Two Ubiquitous Mediterranean Plants Ameliorate Cellular and Animal Models of Neurodegenerative Proteinopathies. Neurosci. Lett. 2017, 638, 12–20. [Google Scholar] [CrossRef]
- Gambino, G.; Allegra, M.; Sardo, P.; Attanzio, A.; Tesoriere, L.; Livrea, M.A.; Ferraro, G.; Carletti, F. Brain Distribution and Modulation of Neuronal Excitability by Indicaxanthin from Opuntia ficus indica Administered at Nutritionally-Relevant Amounts. Front. Aging Neurosci. 2018, 10, 133. [Google Scholar] [CrossRef]
- Tutone, M.; Virzì, A.; Almerico, A.M. Reverse Screening on Indicaxanthin from Opuntia ficus-indica as Natural Chemoactive and Chemopreventive Agent. J. Theor. Biol. 2018, 455, 147–160. [Google Scholar] [CrossRef]
- Kim, J.K.; Lim, M.L. Anti-Inflammatory and Neuroprotective Effects of Opuntia ficus-indica Var. Saboten Alone or Combined with Vitamin C. J. Korean Soc. Food Sci. Nutr. 2019, 48, 613–621. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS Profiling of Secondary Metabolites from Opuntia ficus-indica Cladode, Peel and Fruit Pulp Extracts and Their Antioxidant, Neuroprotective Effect in Rats with Aluminum Chloride Induced Neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Jouini, M.; Horchani, M.; Zardi-Bergaoui, A.; Znati, M.; Romdhane, A.; Krisa, S.; Waffo-Téguo, P.; Ben, J. Phytochemical Analysis, Neuroprotective, Anticholinesterase, Cytotoxic and Catalase Potentials of Opuntia microdasys Var. Rufida and Opuntia Leptocaulis. Chem. Africa. 2021, 4, 285–298. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Davies, J.; Randall, S.; Skinner, G.R. Antiviral Properties of Extract of Opuntia Streptacantha. Antivir. Res. 1996, 30, 75–85. [Google Scholar] [CrossRef]
- Bouslama, L.; Hayashi, K.; Lee, J.-B.; Ghorbel, A.; Hayashi, T. Potent Virucidal Effect of Pheophorbide a and Pyropheophorbide a on Enveloped Viruses. J. Nat. Med. 2011, 65, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Rasoulpour, R.; Izadpanah, K.; Afsharifar, A. Opuntin B, the Antiviral Protein Isolated from Prickly Pear (Opuntia ficus-indica (L.) Miller) Cladode Exhibits Ribonuclease Activity. Microb. Pathog. 2020, 140, 103929. [Google Scholar] [CrossRef] [PubMed]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef]
- Karunaratne, D.N.; Pamunuwa, G. Natural Antimicrobials, Their Sources and Food Safety. In Food Additives; IntechOpen: Rijeka, Croatia, 2017; pp. 87–102. ISBN 978-953-51-3489-3. [Google Scholar]
- Nascimento, G.; Locatelli, J.; Freitas, C.; Silva, G. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents. 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) Plant Compounds, Biological Activities and Prospects—A Comprehensive Review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, Chemical, and Biochemical Characterization of Cladodes from Prickly Pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Sánchez, E.; García, S.; Heredia, N. Extracts of Edible and Medicinal Plants Damage Membranes of Vibrio Cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Javeed, A.; Ashraf, M.; Riaz, A.; Mukhtar, M.; Afzal, S.; Altaf, R. Polarity-Based Solvents Extraction of Opuntia dillenii and Zingiber officinale for In Vitro Antimicrobial Activities. Int. J. Food Prop. 2010, 16, 114–124. [Google Scholar] [CrossRef][Green Version]
- Jacobo-Salcedo, M.D.R.; Alonso-Castro, A.J.; Salazar-Olivo, L.A.; Carranza-Alvarez, C.; González-Espíndola, L.A.; Domínguez, F.; Maciel-Torres, S.P.; García-Lujan, C.; González-Martínez, M.D.R.; Gómez-Sánchez, M.; et al. Antimicrobial and Cytotoxic Effects of Mexican Medicinal Plants. Nat. Prod. Commun. 2011, 6, 1925–1928. [Google Scholar] [CrossRef]
- Castillo, S.L.; Heredia, N.; Contreras, J.F.; García, S. Extracts of Edible and Medicinal Plants in Inhibition of Growth, Adherence, and Cytotoxin Production of Campylobacter jejuni and Campylobacter coli. J. Food Sci. 2011, 76, M421–M426. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.A.; Ibrahim, S.A. Antimicrobial Activity of Xoconostle Pears (Opuntia matudae) against Escherichia coli O157:H7 in Laboratory Medium. Int. J. Microbiol. 2012, 2012, 368472. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Khemakhem, B.; Yangui, T.; Attia, H. Variation in Chemical Composition and Biological Activities of Two Species of Opuntia Flowers at Four Stages of Flowering. Ind. Crop. Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Ennouri, M.; Ammar, I.; Khemakhem, B.; Attia, H. Chemical Composition and Antibacterial Activity of Opuntia ficus-indica f. inermis (Cactus Pear) Flowers. J. Med. Food. 2014, 17, 908–914. [Google Scholar] [CrossRef]
- Ratnaweera, P.B.; de Silva, E.D.; Williams, D.E.; Andersen, R.J. Antimicrobial Activities of Endophytic Fungi Obtained from the Arid Zone Invasive Plant Opuntia dillenii and the Isolation of Equisetin, from Endophytic Fusarium sp. BMC Complement. Altern. Med. 2015, 15, 220. [Google Scholar] [CrossRef]
- Ammar, I.; Bardaa, S.; Mzid, M.; Sahnoun, Z.; Rebaii, T.; Attia, H.; Ennouri, M. Antioxidant, Antibacterial and in Vivo Dermal Wound Healing Effects of Opuntia Flower Extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar] [CrossRef]
- Gopi, D.; Kanimozhi, K.; Kavitha, L. Opuntia ficus indica Peel Derived Pectin Mediated Hydroxyapatite Nanoparticles: Synthesis, Spectral Characterization, Biological and Antimicrobial Activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 135–143. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivas Morales, C.; Castillo, S.; Leos-Rivas, C.; García-Becerra, L.; Ortiz Martínez, D.M. Antibacterial and Antibiofilm Activity of Methanolic Plant Extracts against Nosocomial Microorganisms. Evid. Based Complement. Alternat. Med. 2016, 2016, 1572697. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Cerna-Cortes, J.F.; Rangel-Vargas, E.; Torres-Vitela, M.R.; Villarruel-López, A.; Gutiérrez-Alcántara, E.J.; Castro-Rosas, J. Presence of Multidrug-Resistant Shiga Toxin-Producing Escherichia Coli, Enteropathogenic E. coli and Enterotoxigenic E. coli, on Raw Nopalitos (Opuntia ficus-indica L.) and in Nopalitos Salads from Local Retail Markets in Mexico. Foodborne Pathog. Dis. 2016, 13, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Chahdoura, H.; Barreira, J.C.M.; Fernández-Ruiz, V.; Morales, P.; Calhelha, R.C.; Flamini, G.; Soković, M.; Ferreira, I.C.F.R.; Achour, L. Bioactivity, Proximate, Mineral and Volatile Profiles along the Flowering Stages of Opuntia microdasys (Lehm.): Defining Potential Applications. Food Funct. 2016, 7, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, E.; Cariño-Cortés, R.; Cruz-Cansino, N.D.S.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Montañez-Izquierdo, V.Y.; Hernández-Herrero, M.M.; Filardo-Kerstupp, T. Antioxidant and Antimicrobial Properties of Cactus Pear (Opuntia) Seed Oils. J. Food Qual. 2017, 2017, e3075907. [Google Scholar] [CrossRef]
- Koubaa, M.; Mhemdi, H.; Barba, F.J.; Angelotti, A.; Bouaziz, F.; Chaabouni, S.E.; Vorobiev, E. Seed Oil Extraction from Red Prickly Pear Using Hexane and Supercritical CO2: Assessment of Phenolic Compound Composition, Antioxidant and Antibacterial Activities. J. Sci. Food Agric. 2017, 97, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Arbia, L.; Chikhi-Chorfi, N.; Betatache, I.; Pham-Huy, C.; Zenia, S.; Mameri, N.; Drouiche, N.; Lounici, H. Antimicrobial Activity of Aqueous Extracts from Four Plants on Bacterial Isolates from Periodontitis Patients. Environ. Sci. Pollut. Res. Int. 2017, 24, 13394–13404. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldapa, C.A.; Gutiérrez-Alcántara, E.J.; Torres-Vitela, M.R.; Rangel-Vargas, E.; Villarruel-López, A.; Castro-Rosas, J. Prevalence and Behavior of Multidrug-Resistant Salmonella Strains on Raw Whole and Cut Nopalitos (Opuntia ficus-indica L.) and on Nopalitos Salads. J. Sci. Food Agric. 2017, 97, 4117–4123. [Google Scholar] [CrossRef]
- Welegerima, G.; Zemene, A.; Tilahun, Y. Phytochemical composition and antibacterial activity of Opuntia ficus-indica cladodes extracts. J. Med. Plants Stud. 2018, 6, 243–246. [Google Scholar]
- Palmeri, R.; Parafati, L.; Restuccia, C.; Fallico, B. Application of Prickly Pear Fruit Extract to Improve Domestic Shelf Life, Quality and Microbial Safety of Sliced Beef. Food Chem. Toxicol. 2018, 118, 355–360. [Google Scholar] [CrossRef]
- Khémiri, I.; Essghaier Hédi, B.; Sadfi Zouaoui, N.; Ben Gdara, N.; Bitri, L. The Antimicrobial and Wound Healing Potential of Opuntia ficus indica L. Inermis Extracted Oil from Tunisia. Evid. Based Complement. Alternat. Med. 2019, 2019, 9148782. [Google Scholar] [CrossRef]
- Maema, L.P.; Potgieter, M.; Masevhe, N.A.; Samie, A. Antimicrobial Activity of Selected Plants against Fungal Species Isolated from South African AIDS Patients and Their Antigonococcal Activity. J. Complement. Integr. Med. 2020, 17, 20190087. [Google Scholar] [CrossRef]
- Elkady, W.M.; Bishr, M.M.; Abdel-Aziz, M.M.; Salama, O.M. Identification and Isolation of Anti-Pneumonia Bioactive Compounds from Opuntia ficus-indica Fruit Waste Peels. Food Funct. 2020, 11, 5275–5283. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.A.; Lorenzo, J.M.; Trindade, M.A. Fruit and Agro-Industrial Waste Extracts as Potential Antimicrobials in Meat Products: A Brief Review. Foods 2021, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Surup, F.; Minh Thi Tran, T.; Pfütze, S.; Budde, J.; Moussa-Ayoub, T.E.; Rohn, S.; Jerz, G. Opuntisines, 14-Membered Cyclopeptide Alkaloids from Fruits of Opuntia stricta Var. Dillenii Isolated by High-Performance Countercurrent Chromatography. Food Chem. 2021, 334, 127552. [Google Scholar] [CrossRef] [PubMed]
- Pourmajed, R.; Jabbari Amiri, M.; Karami, P.; Khaledi, A. Antimicrobial Effect of Opuntia ficus-indica Extract on Escherichia coli Isolated from Patients with Urinary Tract Infection. Iran J. Public Health 2021, 50, 634–636. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin Wound Healing and Phytomedicine: A Review. Skin Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Monforte, M.T.; Galluzzo, M.; Miceli, N.; Tripodo, M.M. Effect of Opuntia ficus-indica (L.) Mill. Cladodes in the Wound-Healing Process. J. Prof. Assoc. Cact. Dev. 2003, 5, 1–16. [Google Scholar]
- Trombetta, D.; Puglia, C.; Perri, D.; Licata, A.; Pergolizzi, S.; Lauriano, E.R.; De Pasquale, A.; Saija, A.; Bonina, F.P. Effect of Polysaccharides from Opuntia ficus-indica (L.) Cladodes on the Healing of Dermal Wounds in the Rat. Phytomedicine 2006, 13, 352–358. [Google Scholar] [CrossRef]
- Nakahara, T.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Uchi, H.; Yan, X.; Hachisuka, J.; Chiba, T.; Esaki, H.; et al. Antioxidant Opuntia ficus-indica Extract Activates AHR-NRF2 Signaling and Upregulates Filaggrin and Loricrin Expression in Human Keratinocytes. J. Med. Food. 2015, 18, 1143–1149. [Google Scholar] [CrossRef]
- Deters, A.M.; Meyer, U.; Stintzing, F.C. Time-Dependent Bioactivity of Preparations from Cactus Pear (Opuntia ficus indica) and Ice Plant (Mesembryanthemum Crystallinum) on Human Skin Fibroblasts and Keratinocytes. J. Ethnopharmacol. 2012, 142, 438–444. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The Polysaccharide and Low Molecular Weight Components of Opuntia ficus indica Cladodes: Structure and Skin Repairing Properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Choi, H.-S.; Hong, Y.H.; Jung, E.Y.; Suh, H.J. Cactus Cladodes (Opuntia humifusa) Extract Minimizes the Effects of UV Irradiation on Keratinocytes and Hairless Mice. Pharm. Biol. 2017, 55, 1032–1040. [Google Scholar] [CrossRef]
- Fang, Q.; Huang, C.; You, C.; Ma, S. Opuntia Extract Reduces Scar Formation in Rabbit Ear Model: A Randomized Controlled Study. Int. J. Low Extrem. Wounds. 2015, 14, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Bardaa, S.; Chabchoub, N.; Jridi, M.; Moalla, D.; Mseddi, M.; Rebai, T.; Sahnoun, Z. The Effect of Natural Extracts on Laser Burn Wound Healing. J. Surg. Res. 2016, 201, 464–472. [Google Scholar] [CrossRef]
- Bassino, E.; Gasparri, F.; Munaron, L. Natural Dietary Antioxidants Containing Flavonoids Modulate Keratinocytes Physiology: In Vitro Tri-Culture Models. J. Ethnopharmacol. 2019, 238, 111844. [Google Scholar] [CrossRef] [PubMed]
- Kandan, P.V.; Balupillai, A.; Kanimozhi, G.; Khan, H.A.; Alhomida, A.S.; Prasad, N.R. Opuntiol Prevents Photoaging of Mouse Skin via Blocking Inflammatory Responses and Collagen Degradation. Oxid. Med. Cell Longev. 2020, 2020, 5275178. [Google Scholar] [CrossRef]
- Moon, J.Y.; Ngoc, L.T.N.; Chae, M.; Tran, V.V.; Lee, Y.C. Effects of Microwave-Assisted Opuntia humifusa Extract in Inhibiting the Impacts of Particulate Matter on Human Keratinocyte Skin Cell. Antioxidants 2020, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Koshak, A.E.; Algandaby, M.M.; Mujallid, M.I.; Abdel-Naim, A.B.; Alhakamy, N.A.; Fahmy, U.A.; Alfarsi, A.; Badr-Eldin, S.M.; Neamatallah, T.; Nasrullah, M.Z.; et al. Wound Healing Activity of Opuntia ficus-indica Fixed Oil Formulated in a Self-Nanoemulsifying Formulation. Int. J. Nanomedicine. 2021, 16, 3889–3905. [Google Scholar] [CrossRef]
- Catanzano, O.; Gomez d’Ayala, G.; D’Agostino, A.; Di Lorenzo, F.; Schiraldi, C.; Malinconico, M.; Lanzetta, R.; Bonina, F.; Laurienzo, P. PEG-Crosslinked-Chitosan Hydrogel Films for in Situ Delivery of Opuntia ficus-indica Extract. Carbohydr. Polym. 2021, 264, 117987. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, M.; Azmat, A.; Ahmad, S.I.; Faizi, Z.; Abidi, L.; Faizi, S. Hypotensive Activity, Toxicology and Histopathology of Opuntioside-I and Methanolic Extract of Opuntia dillenii. Biol. Pharm. Bull. 2005, 28, 1844–1851. [Google Scholar] [CrossRef]
- Boukeloua, A.; Belkhiri, A.; Djerrou, Z.; Bahri, L.; Boulebda, N.; Hamdi Pacha, Y. Acute Toxicity of Opuntia ficus indica and Pistacia lentiscus Seed Oils in Mice. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 607–611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osorio-Esquivel, O.; Ortiz-Moreno, A.; Garduño-Siciliano, L.; Alvarez, V.B.; Hernández-Navarro, M.D. Antihyperlipidemic Effect of Methanolic Extract from Opuntia joconostle Seeds in Mice Fed a Hypercholesterolemic Diet. Plant Foods Hum. Nutr. 2012, 67, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Berraaouan, A.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Sindic, M.; Aziz, M.; Bnouham, M. Evaluation of Antidiabetic Properties of Cactus Pear Seed Oil in Rats. Pharm. Biol. 2014, 52, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Tsafantakis, N.; Katsanou, E.S.; Kyriakopoulou, K.; Psarou, E.-C.; Raptaki, I.; Skaltsounis, A.L.; Audebert, M.; Machera, K.A.; Fokialakis, N. Comparative UHPLC-HRMS Profiling, Toxicological Assessment, and Protection Against H2O2-Induced Genotoxicity of Different Parts of Opuntia ficus indica. J. Med. Food. 2019, 22, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Lim, M.K.; Lee, S.H.; Rahman, M.M.; Lim, Y.-H. An Oral Toxicity Test in Rats and a Genotoxicity Study of Extracts from the Stems of Opuntia ficus-indica Var. Saboten. BMC Complement. Altern. Med. 2019, 19, 31. [Google Scholar] [CrossRef]
- Bouhrim, M.; Ouassou, H.; Boutahiri, S.; Daoudi, N.E.; Mechchate, H.; Gressier, B.; Eto, B.; Imtara, H.; A Alotaibi, A.; Al-Zharani, M.; et al. Opuntia dillenii (Ker Gawl.) Haw., Seeds Oil Antidiabetic Potential Using In Vivo, In Vitro, In Situ, and Ex Vivo Approaches to Reveal Its Underlying Mechanism of Action. Molecules 2021, 26, 1677. [Google Scholar] [CrossRef]
- Bouhrim, M.; Boutahiri, S.; Kharchoufa, L.; Mechchate, H.; Mohamed Al Kamaly, O.; Berraaouan, A.; Eto, B.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; et al. Acute and Subacute Toxicity and Cytotoxicity of Opuntia dillenii (Ker-Gawl) Haw. Seed Oil and Its Impact on the Isolated Rat Diaphragm Glucose Absorption. Molecules 2021, 26, 2172. [Google Scholar] [CrossRef]
- Siddiqui, F.; Farooq, A.D.; Kabir, N.; Fatima, N.; Abidi, L.; Faizi, S. Toxicological Assessment of Opuntia dillenii (Ker Gawl.) Haw. Cladode Methanol Extract, Fractions and Its Alpha Pyrones: Opuntiol and Opuntioside. J. Ethnopharmacol. 2021, 280, 114409. [Google Scholar] [CrossRef]
- Torres-Ponce, R.L.; Morales-Corral1, D.; Ballinas-Casarrubias, M.L.; Nevárez-Moorillón, G.V. Nopal: Semi-desert plant with applications in pharmaceuticals, food and animal nutrition. Rev. Mex. Cienc. Agr. 2015, 6, 1129–1142. [Google Scholar]
- Stintzing, F.C.; Carl, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Ortiz-González, A.; González-Pérez, P.P.; Cárdenas-García, M.; Hernández-Linares, M.G. In silico Prediction on the PI3K/AKT/mTOR Pathway of the Antiproliferative Effect of O. joconostle in Breast Cancer Models. Cancer Inform. 2022, 21, 1–17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).