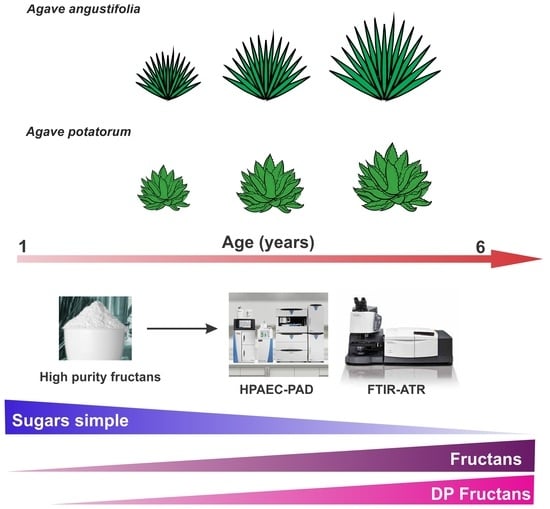
Agave Fructans in Oaxaca’s Emblematic Specimens: Agave angustifolia Haw. and Agave potatorum Zucc.
Abstract
:1. Introduction
2. Results
2.1. Morphology Analysis
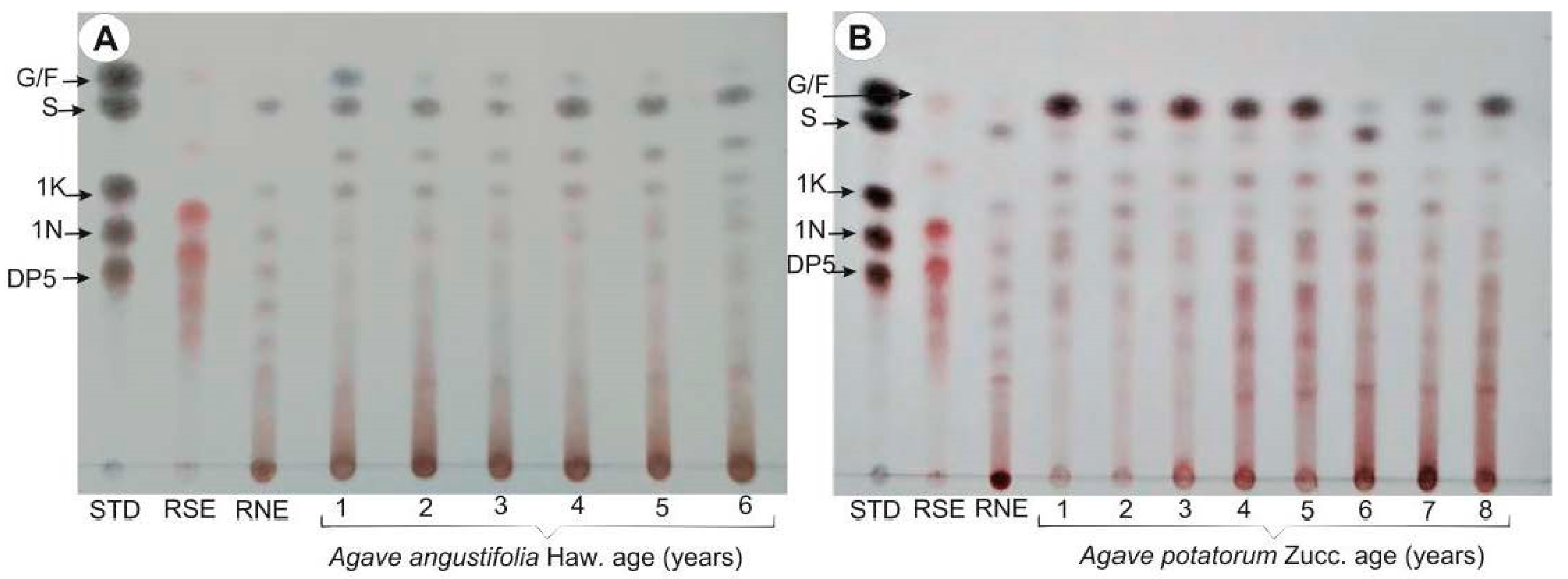
2.2. Thin Layer Chromatography (TLC) Profiles of Fructans in A. angustifolia and A. potatorum Plants of Different Ages
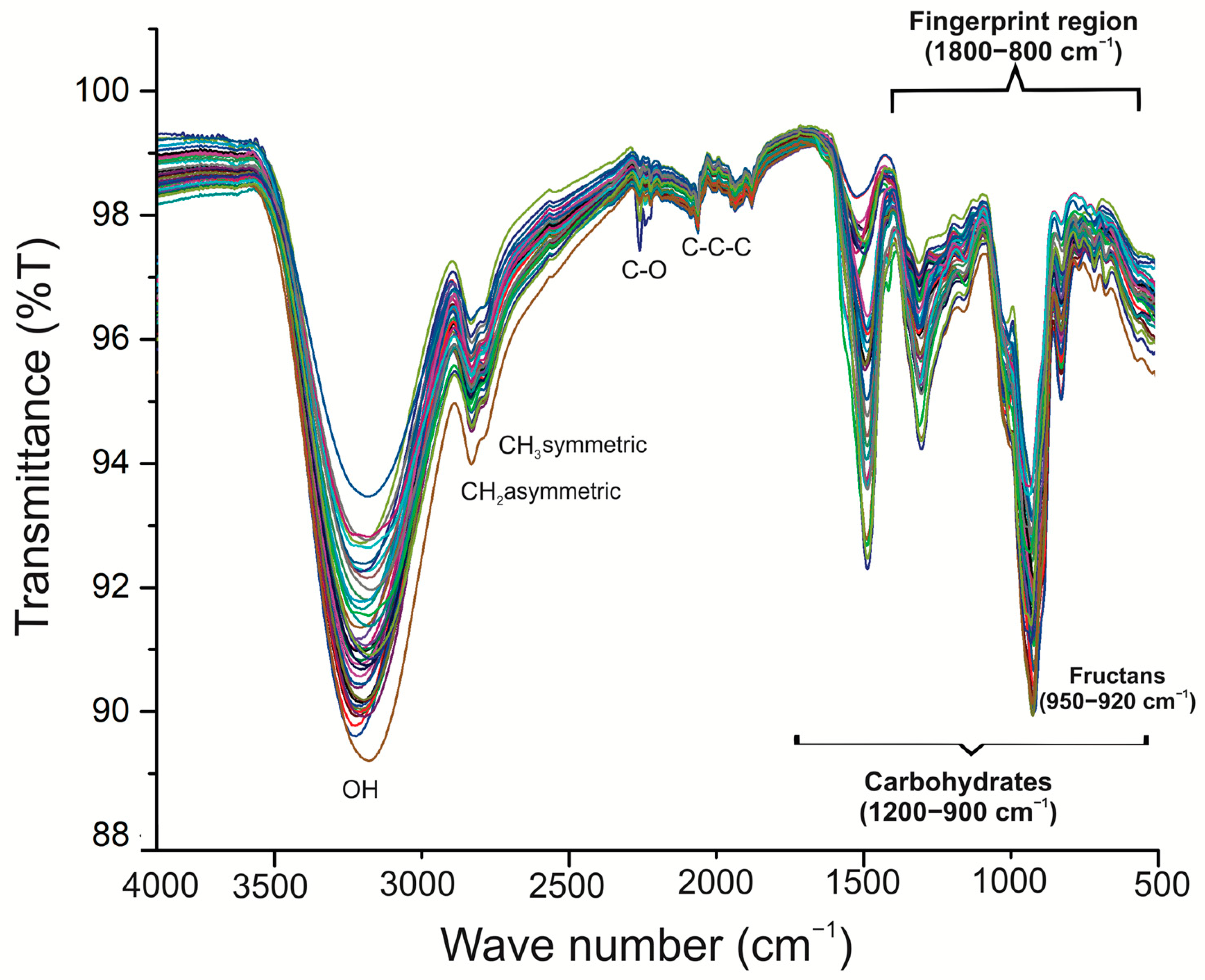
2.3. Fourier-Transform Infrared (FT-IR) Spectroscopy of Fructans in A. angustifolia and A. potatorum Plants of Different Ages
2.4. Fructan Content According to Plant Age in A. angustifolia Haw. and A. potatorum Zucc.
2.5. Identification of Fructans Using HPAEC-PAD of A. angustifolia and A. potatorum Plants of Different Ages
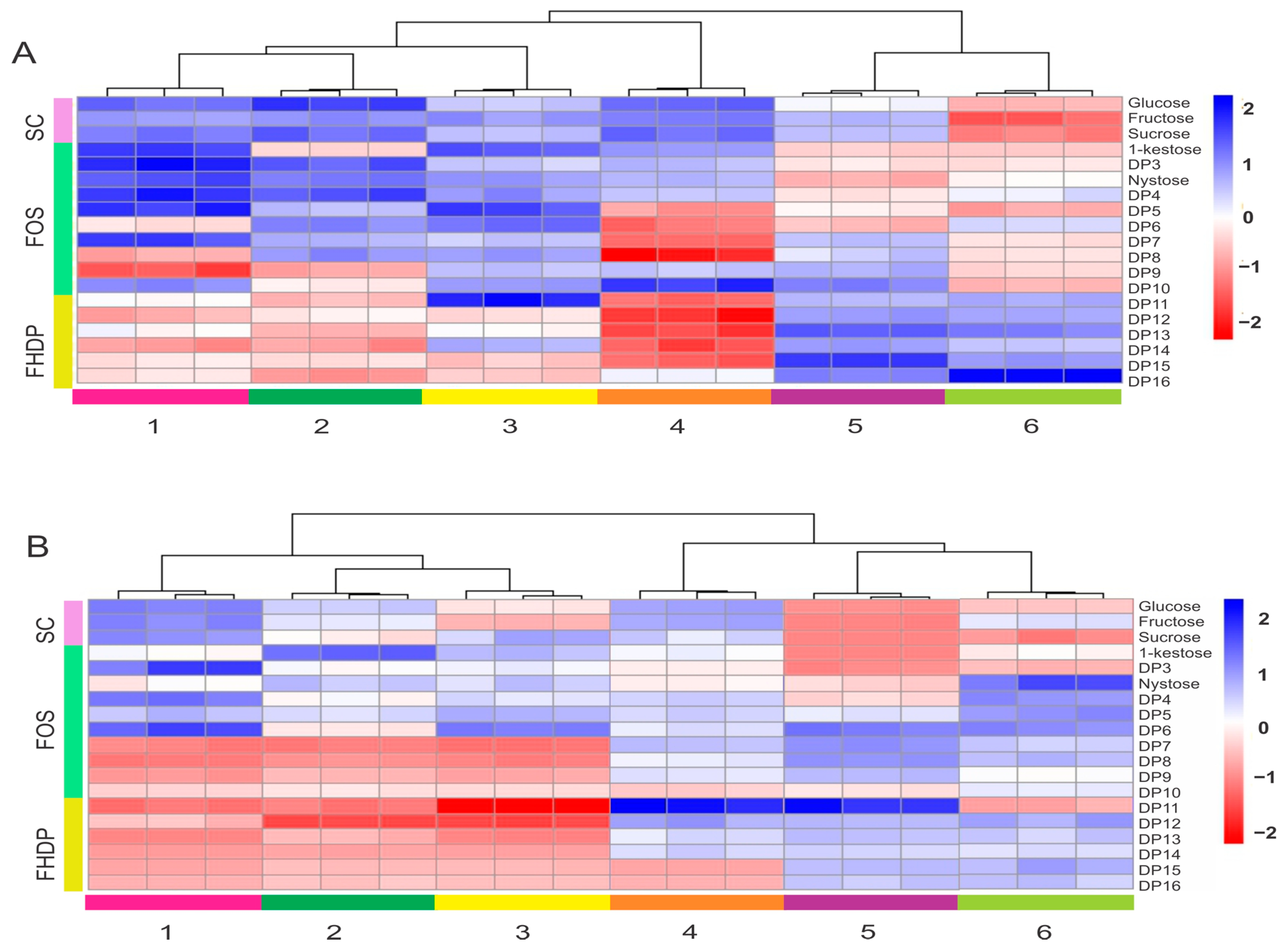
2.6. Carbohydrate Profiling Analysis of Agave Plants at Different Ages
3. Discussion
3.1. Morphological Parameters
3.2. Thin Layer Chromatography (TLC)
3.3. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.4. Enzymatic Assay and HPAEC-PAD
3.5. HPAEC-PAD and Heatmaps
4. Materials and Methods
4.1. Plant Material
4.2. Fructan Extraction
4.3. Thin Layer Chromatography (TLC)
4.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
4.5. Enzymatic Determination of Total Fructan Content in Extracts of A. angustifolia Haw. and A. potatorum Zucc. Plants
4.6. Fructan Determination
4.7. Data Processing and Multivariate Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendry, G.A.F. Evolutionary origins and natural functions of fructans—A climatological, biogeographic and mechanistic appraisal. New Phytol. 1993, 123, 3–14. [Google Scholar] [CrossRef]
- Matros, A.; Peukert, M.; Lahnstein, J.; Seiffert, U.; Burton, R. Determination of fructans in plants: Current analytical means for extraction, detection, and quantification. Annu. Plant Rev. Online 2019, 2, 117–156. [Google Scholar]
- Van Laere, A.; Van Den Ende, W. Inulin metabolism in dicots: Chicory as a model system. Plant Cell Environ. 2002, 25, 803–813. [Google Scholar] [CrossRef]
- Van Den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247–257. [Google Scholar] [PubMed] [Green Version]
- López, M.G.; Mancilla-Margalli, N.A.; Mendoza-Díaz, G. Molecular structures of fructans from Agave tequilana Weber var. azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-Margalli, N.A.; López, M.G. Water-Soluble carbohydrates and fructan structure patterns from Agave and Dasylirion Species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.G. Agaves of Continental North America; University of Arizona Press: Tucson, AZ, USA, 2004. [Google Scholar]
- García Mendoza, A. Agavaceae. flora del valle de Tehuacán-Cuicatlán. Inst. Biol. Univ. Nac. Autónoma México México City México 2011, 88, 1–95. [Google Scholar]
- Colunga-García Marín, P.; Zizumbo-Villarreal, D.; Martínez-Torres, J. Tradiciones en el aprovechamiento de los Agaves Mexicanos: Una aportación a la protección legal y conservación de su diversidad biológica y cultural. In En lo Ancestral Hay Futuro: Del Tequila, los Mezcales y Otros Agaves; Colunga-García, M., Larqué, S.A., Zizumbo-Villarreal, D., Eds.; CICY-CONACYT-CONABIO-INE: Mérida, México, 2007; pp. 229–248. [Google Scholar]
- Mellado-Mojica, E.; López, M.G. Identification, classification, and discrimination of agave syrups from natural sweeteners by infrared spectroscopy and HPAEC-PAD. Food Chem. 2015, 167, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Andrews, H.; Urías-Silvas, J.E.; Morales-Hernández, N. The role of agave fructans in health and food applications: A review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Soto-Castro, D.; Pérez-Herrera, A.; García-Sánchez, E.; Santiago-García, P.A. Identification and quantification of bioactive compounds in Agave potatorum Zucc. leaves at different stages of development and a preliminary biological assay. Waste Biomass Valorization 2021, 12, 4537–4547. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Ortiz-Hernández, Y.D. Microscopical observations of crystal deposits on the epidermis of leaves of Agave potatorum. Micron 2022, 154, 103201–103210. [Google Scholar] [CrossRef] [PubMed]
- Huazano-García, A.; Shin, H.; López, M.G. Modulation of gut microbiota of overweight mice by agavins and their association with body weight loss. Nutrients 2017, 9, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; López, M.G. Evaluation of Agave angustifolia fructans as fat replacer in the cookies manufacture. LWT 2017, 77, 100–109. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martinez, F.M.; Dzul-Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Fructans (agavins) from Agave angustifolia and Agave potatorum as fat replacement in yogurt: Effects on physicochemical, rheological, and sensory properties. LWT 2021, 140, 110846–110857. [Google Scholar] [CrossRef]
- Velázquez-Martínez, J.R.; González-Cervantes, R.M.; Hernández-Gallegos, M.A.; Mendiola, R.C.; Aparicio, A.R.J.; Ocampo, M.L.A. Prebiotic potential of Agave angustifolia Haw. fructans with different degrees of polymerization. Molecules 2014, 19, 12660–12675. [Google Scholar] [CrossRef] [Green Version]
- Hong, T.; Yin, J.; Nie, S.; Xie, M. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. J. Food Chem. 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kliks, M.M.; Jun, S.; Jackson, M.; Li, Q.X. Rapid analysis of glucose, fructose, sucrose, and maltose in honeys from different geographic regions using fourier transform infrared spectroscopy and multivariate Analysis. J. Food Sci. 2010, 75, C208–C214. [Google Scholar] [CrossRef]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Feasibility study on the use of attenuated total reflectance MIR spectroscopy to measure the fructan content in barley. Anal. Methods 2014, 6, 7710–7715. [Google Scholar] [CrossRef]
- Hölemann, A.; Seeberger, P.H. Carbohydrate diversity: Synthesis of glycoconjugates and complex carbohydrates. Curr. Opin. Biotechnol. 2004, 15, 615–622. [Google Scholar] [CrossRef]
- Álvarez-Ríos, G.D.; Pacheco-Torres, F.; Figueredo-Urbina, C.J.; Casas, A. Management, morphological and genetic diversity of domesticated agaves in Michoacán, México. J. Ethnobiol. Ethnomed. 2020, 16, 3–21. [Google Scholar] [CrossRef]
- Figueredo-Urbina, C.J.; Casas, A.; Torres-García, I. Morphological and genetic divergence between Agave inaequidens, A. cupreata and the domesticated A. hookeri. Analysis of their evolutionary relationships. PLoS ONE 2017, 12, e0187260. [Google Scholar]
- Rodríguez-Garay, B.; Lomelí-Sención, J.A.; Tapia-Campos, E.; Gutiérrez-Mora, A.; García-Galindo, J.; Rodríguez-Domínguez, J.M.; Urbina-López, D.; Vicente-Ramírez, I. Morphological and molecular diversity of Agave tequilana Weber var. Azul and Agave angustifolia Haw. var. Lineño. Ind. Crops Prod. 2009, 29, 220–228. [Google Scholar] [CrossRef]
- Figueredo-Urbina, C.J.; Álvarez-Ríos, G.D.; García-Montes, M.A.; Octavio-Aguilar, P. Morphological and genetic diversity of traditional varieties of agave in Hidalgo State, Mexico. PLoS ONE 2021, 16, e0254376. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernández, Y.D.; Gutiérrez-Hernández, G.F.; Corzo-Ríos, L.J.; García-Ramírez, E.; Martínez-Tomás, S.H. Varietal and germinative characterization of Agave potatorum (Asparagaceae) seeds with different origins. Bot. Sci. 2018, 96, 628–639. [Google Scholar] [CrossRef] [Green Version]
- Ghebregzabher, M.; Rufini, S.; Monaldi, B.; Lato, M. Thin-layer chromatography of carbohydrates. J. Chromatogr. A 1976, 127, 133–162. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Achour, L.; Paineau, D.; Riottot, M.; Attar, A.; Bornet, F. Four-week short chain fructo-oligosaccharides ingestion leads to increasing fecal bifidobacteria and cholesterol excretion in healthy elderly volunteers. Nutr. J. 2007, 6, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerreiro, I.; Enes, P.; Oliva-Teles, A. Effects of short-chain fructooligosaccharides (scFOS) and rearing temperature on growth performance and hepatic intermediary metabolism in gilthead sea bream (Sparus aurata) juveniles. Fish Physiol. Biochem. 2015, 41, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Krähmer, A.; Böttcher, C.; Gudi, G.; Stürtz, M.; Schulz, H. Application of ATR-FTIR spectroscopy for profiling of non-structural carbohydrates in onion (Allium cepa L.) bulbs. Food Chem. 2021, 360, 129978–129986. [Google Scholar] [CrossRef]
- Kacuráuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Aldrete-Herrera, P.I.; López, M.G.; Medina-Torres, L.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-Ávila, M.; Ortiz-Basurto, R.I. Physicochemical composition and apparent degree of polymerization of fructans in five wild Agave varieties: Potential industrial use. Foods 2019, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, A.V.; Simpson, J. The sweet taste of adapting to the desert: Fructan metabolism in Agave species. Front. Plant Sci. 2020, 11, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellado-Mojica, E.; López, M.G. Fructan metabolism in A. tequilana Weber Blue variety along its developmental cycle in the field. J. Agric. Food Chem. 2012, 60, 11704–11713. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Mojica, E.; González de la Vara, L.; López, M.G. Fructan active enzymes (FAZY) activities and biosynthesis of fructooligosaccharides in the vacuoles of Agave tequilana Weber Blue variety plants of different age. Planta 2017, 245, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, N. Food bIochemical study on fructans and related synthesis enzymes. J. Appl. Glycosci. 2008, 55, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Tarkowski, L.P.; Van den Ende, W. Cold tolerance triggered by soluble sugars: A multifaceted countermeasure. Front. Plant Sci. 2015, 6, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Versluys, M.; Tarkowski, L.P.; Van den Ende, W. Fructans as DAMPs or MAMPs: Evolutionary prospects, cross-tolerance, and multistress resistance potential. Front. Plant Sci. 2017, 7, 2061–2068. [Google Scholar] [CrossRef] [Green Version]
- Arrizon, J.; Morel, S.; Gschaedler, A.; Monsan, P. Comparison of the water-soluble carbohydrate composition and fructan structures of Agave tequilana plants of different ages. Food Chem. 2010, 122, 123–130. [Google Scholar] [CrossRef]
- Verspreet, J.; Cimini, S.; Vergauwen, R.; Dornez, E.; Locato, V.; Le Roy, K.; De Gara, L.; Van den Ende, W.; Delcour, J.A.; Courtin, C.M. Fructan metabolism in developing wheat (Triticum aestivum L.) kernels. Plant Cell Physiol. 2013, 54, 2047–2057. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M. Fructan structure and metabolism in overwintering plants. Plants 2021, 10, 933. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Loyola-Vargas, V.M.; Santiago-García, P.A. Interaction between fructan metabolism and plant growth regulators. Planta 2022, 255, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Torres-García, I.; Casas, A.; Delgado-Lemus, A.; Rangel-Landa, S. Aprovechamiento, demografía y establecimiento de Agave potatorum en el Valle de Tehuacán, México: Aportes ecológicos y etnobiológicos para su manejo sustentable. Zonas Arid. 2013, 15, 92–109. [Google Scholar]
- Kanaya, K.i.; Chiba, S.; Shimomura, T. Thin-layer chromatography of linear oligosaccharides. Null 1978, 42, 1947–1948. [Google Scholar]
- Anderson, K.; Li, S.C.; Li, Y.T. Diphenylamine–aniline–phosphoric acid reagent, a versatile spray reagent for revealing glycoconjugates on thin-layer chromatography plates. Anal. Biochem. 2000, 287, 337–339. [Google Scholar] [CrossRef] [PubMed]




| Age (Years) | A. angustifolia (mg/g) | A. potatorum (mg/g) |
|---|---|---|
| 1 | 174.80 ± 2.29 | 115.65 ± 2.89 |
| 2 | 252.87 ± 3.02 | 128.67 ± 2.06 |
| 3 | 321.61 ± 2.18 | 251.65 ± 2.43 |
| 4 | 434.50 ± 3.62 | 344.60 ± 3.32 |
| 5 | 564.84 ± 3.97 | 464.84 ± 3.97 |
| 6 | 605.11 ± 3.71 | 502.11 ± 3.71 |
| Age (Years) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Carbohydrate Contents * | ||||||
| Glucose | 9.95 ± 1.64 | 8.31 ± 0.71 | 5.77 ± 1.53 | 5.12 ± 1.07 | 5.43 ± 0.78 | 2.85 ± 0.90 |
| Fructose | 25.63 ± 0.68 | 14.61 ± 3.10 | 11.41 ± 0.92 | 6.85 ± 0.91 | 3.98 ± 0.68 | 2.71 ± 0.85 |
| Sucrose | 42.65 ± 1.76 | 35.74 ± 2.23 | 35.06 ± 1.86 | 32.31 ± 1.27 | 26.95 ± 1.38 | 22.76 ± 2.20 |
| Fructooligosaccharide Contents * | ||||||
| 1-kestose (1K) | 17.01 ± 2.39 | 15.08 ± 1.61 | 15.55 ± 2.84 | 8.12 ± 0.87 | 1.42 ± 0.13 | 2.24 ±0.07 |
| 1-nystose (1N) | 10.66 ± 1.39 | 10.54 ± 0.91 | 10.00 ± 0.48 | 8.51 ± 0.16 | 7.81 ± 0.32 | 6.66 ± 1.56 |
| 1-kestopentaose (DP5) | 8.92 ± 1.03 | 6.62 ± 1.13 | 3.21 ± 0.28 | 0.92 ± 0.04 | 0.38 ± 0.06 | 1.31 ± 0.05 |
| Age (Years) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Carbohydrate Contents * | ||||||
| Glucose | 40.38 ± 2.04 | 35.00 ± 1.82 | 31.55 ± 1.41 | 28.40 ± 1.86 | 22.72 ± 1.79 | 26.10 ± 0.17 |
| Fructose | 46.49 ± 1.84 | 38.32 ± 1.26 | 37.22 ± 1.60 | 37.22 ± 2.90 | 16.96 ± 2.86 | 25.33 ± 0.23 |
| Sucrose | 30.00 ± 2.71 | 19.26 ± 1.27 | 15.94 ± 3.18 | 8.25 ± 1.05 | 7.00 ± 1.72 | 6.29 ± 0.35 |
| Fructooligosaccharide Contents * | ||||||
| 1-kestose (1K) | 29.57 ± 1.05 | 24.26 ± 1.83 | 25.09 ± 1.37 | 10.43 ± 1.79 | 8.27 ± 0.33 | 8.64 ± 1.28 |
| 1-nystose (1N) | 16.38 ± 2.11 | 16.20 ± 0.68 | 13.99 ± 3.06 | 10.06 ± 1.90 | 10.64 ± 0.36 | 9.77 ± 0.33 |
| 1-kestopentaose (DP5) | 8.79 ± 1.99 | 6.47 ± 0.93 | 5.41 ± 0.08 | 4.55 ± 0.28 | 3.55 ± 0.28 | 2.73 ± 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-López, R.E.; Santiago-García, P.A.; López, M.G. Agave Fructans in Oaxaca’s Emblematic Specimens: Agave angustifolia Haw. and Agave potatorum Zucc. Plants 2022, 11, 1834. https://doi.org/10.3390/plants11141834
Márquez-López RE, Santiago-García PA, López MG. Agave Fructans in Oaxaca’s Emblematic Specimens: Agave angustifolia Haw. and Agave potatorum Zucc. Plants. 2022; 11(14):1834. https://doi.org/10.3390/plants11141834
Chicago/Turabian StyleMárquez-López, Ruth E., Patricia Araceli Santiago-García, and Mercedes G. López. 2022. "Agave Fructans in Oaxaca’s Emblematic Specimens: Agave angustifolia Haw. and Agave potatorum Zucc." Plants 11, no. 14: 1834. https://doi.org/10.3390/plants11141834
APA StyleMárquez-López, R. E., Santiago-García, P. A., & López, M. G. (2022). Agave Fructans in Oaxaca’s Emblematic Specimens: Agave angustifolia Haw. and Agave potatorum Zucc. Plants, 11(14), 1834. https://doi.org/10.3390/plants11141834