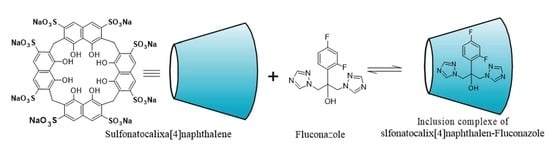
Insight into the Inclusion Complexation of Fluconazole with Sulfonatocalix[4]naphthalene in Aqueous Solution, Solid-State, and Its Antimycotic Activity
Abstract
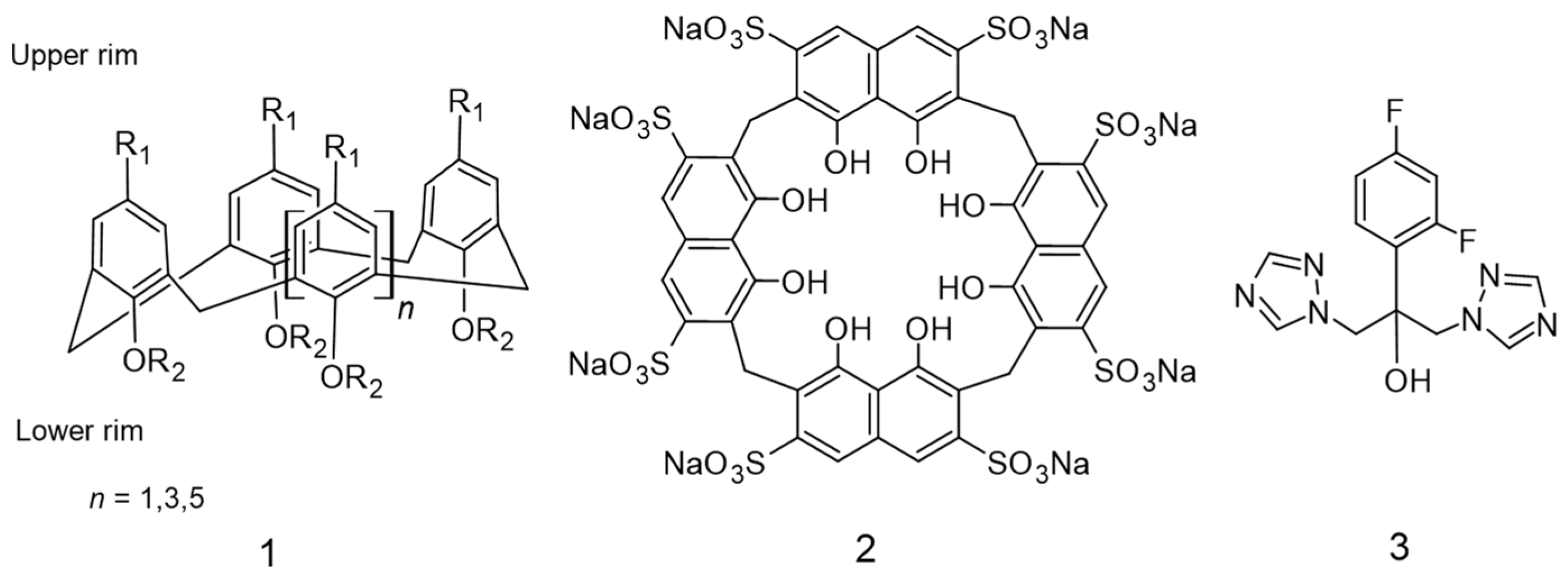
:1. Introduction
2. Results and Discussion
2.1. Phase Solubility Test
2.2. Physicochemical Characterization for the Conformation of Inclusion Complex
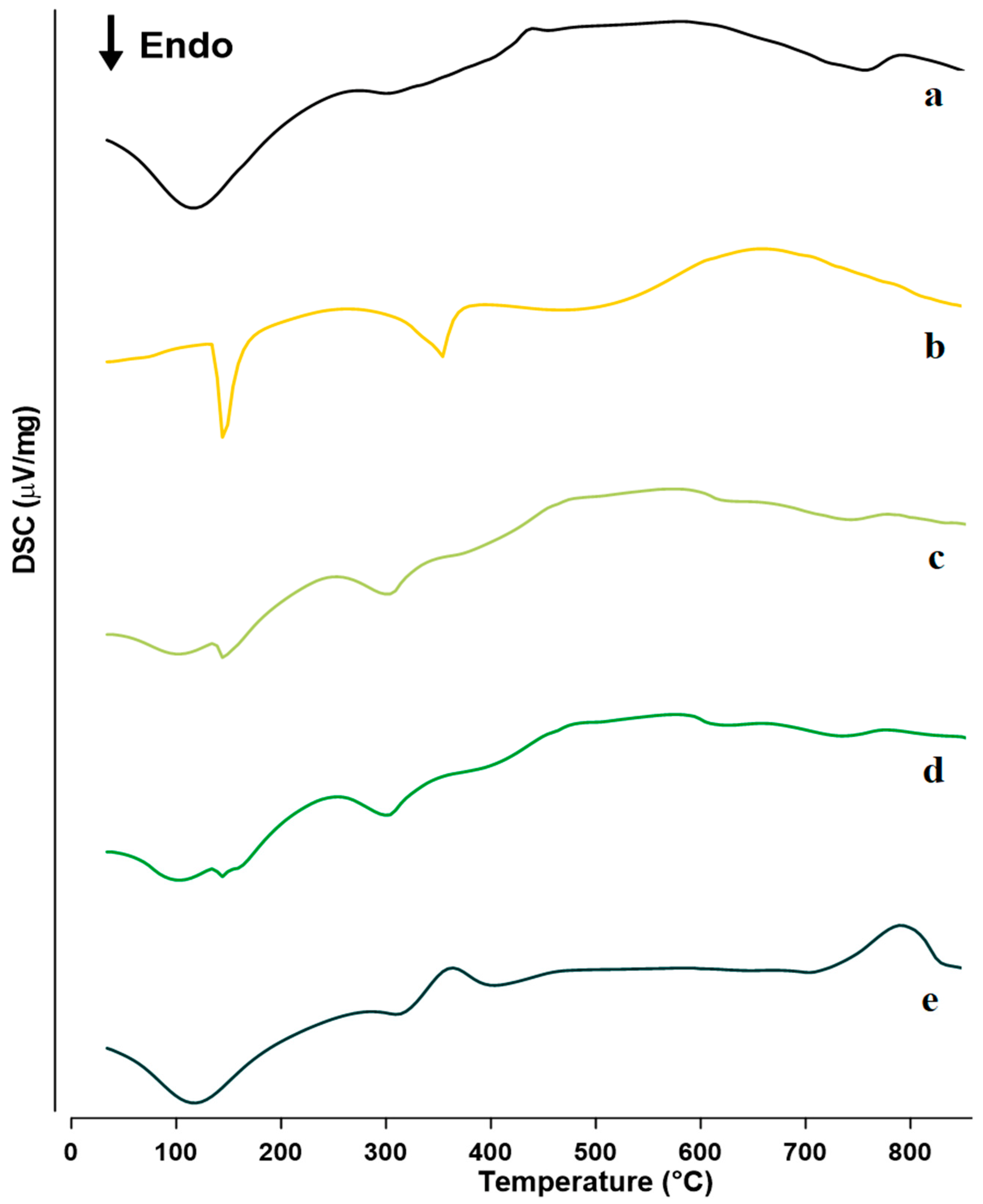
2.2.1. Differential Scanning Calorimetry (DSC)
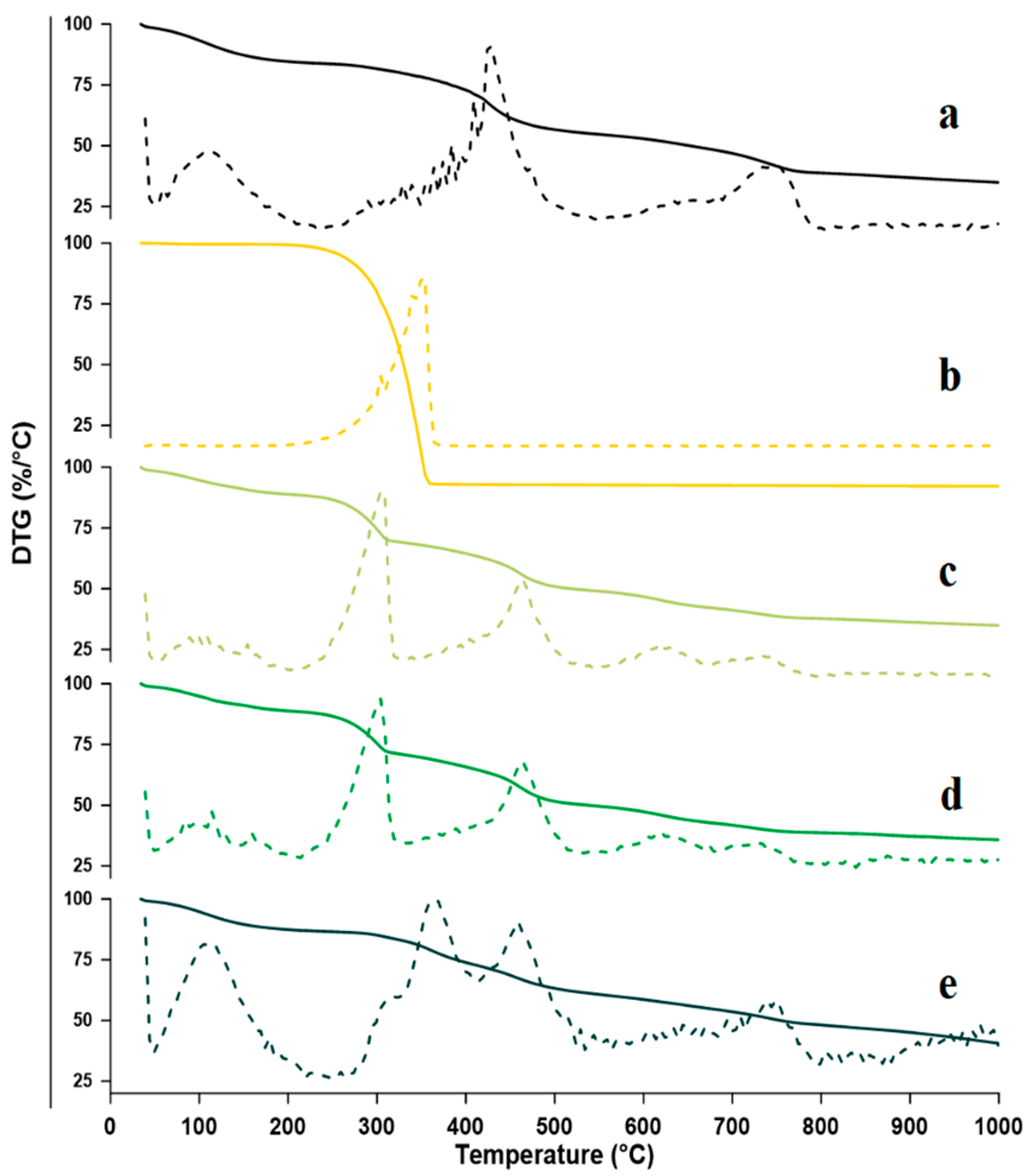
2.2.2. Thermogravimetric Analysis (TGA)
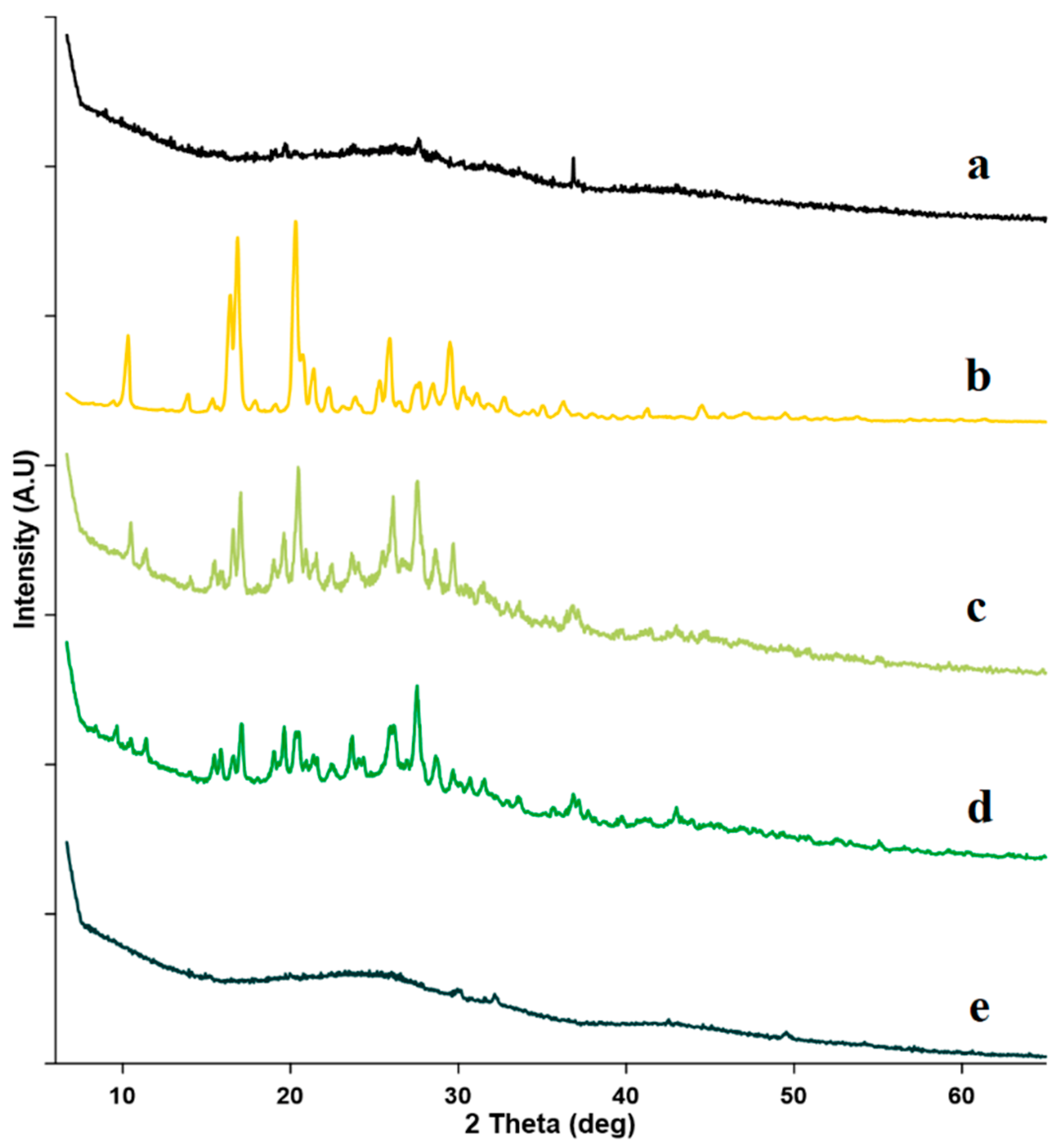
2.2.3. Powder X-ray Diffractometry (PXRD)
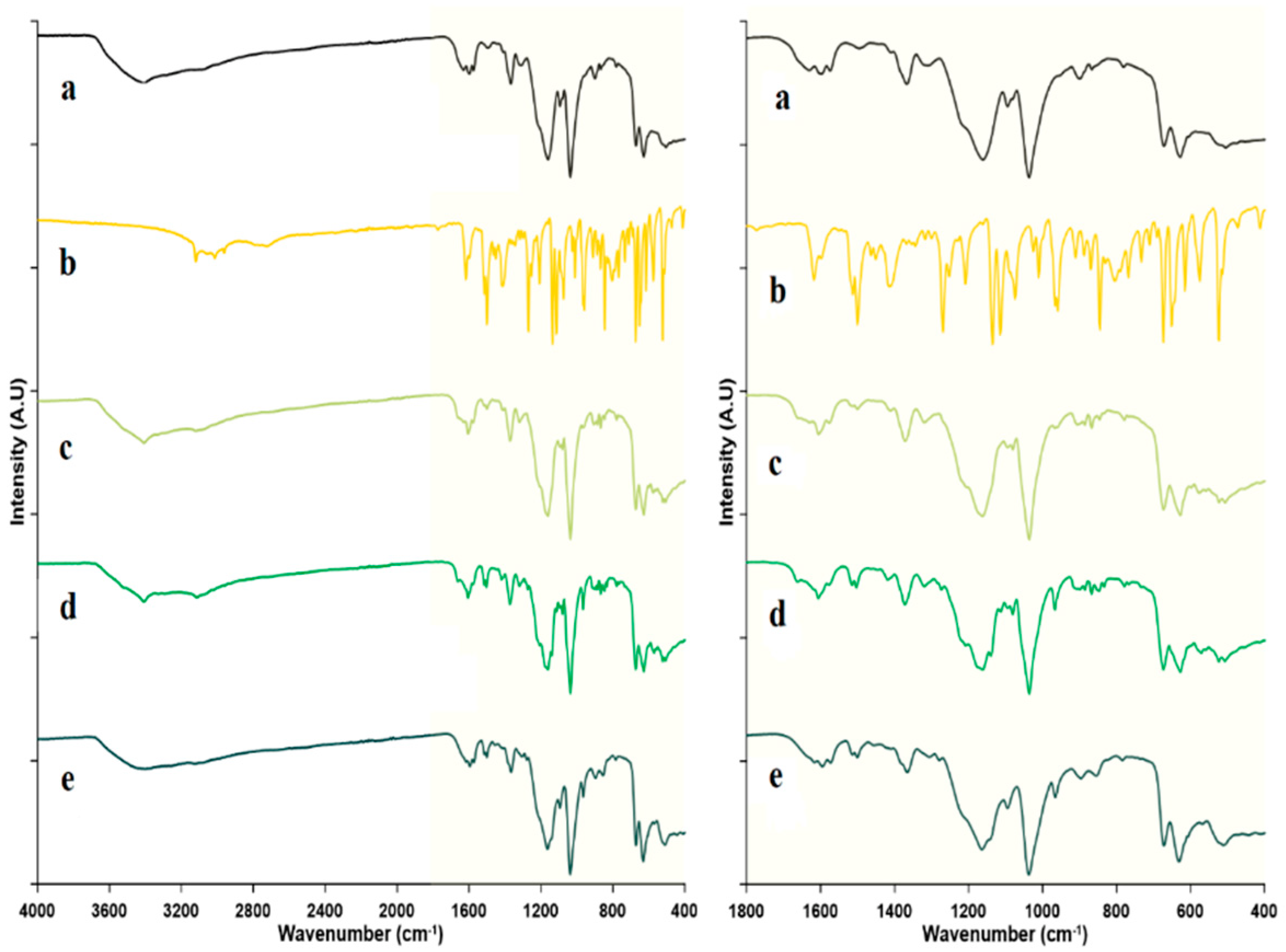
2.2.4. Fourier Transform Infrared Spectroscopy
2.3. Dissolution Test
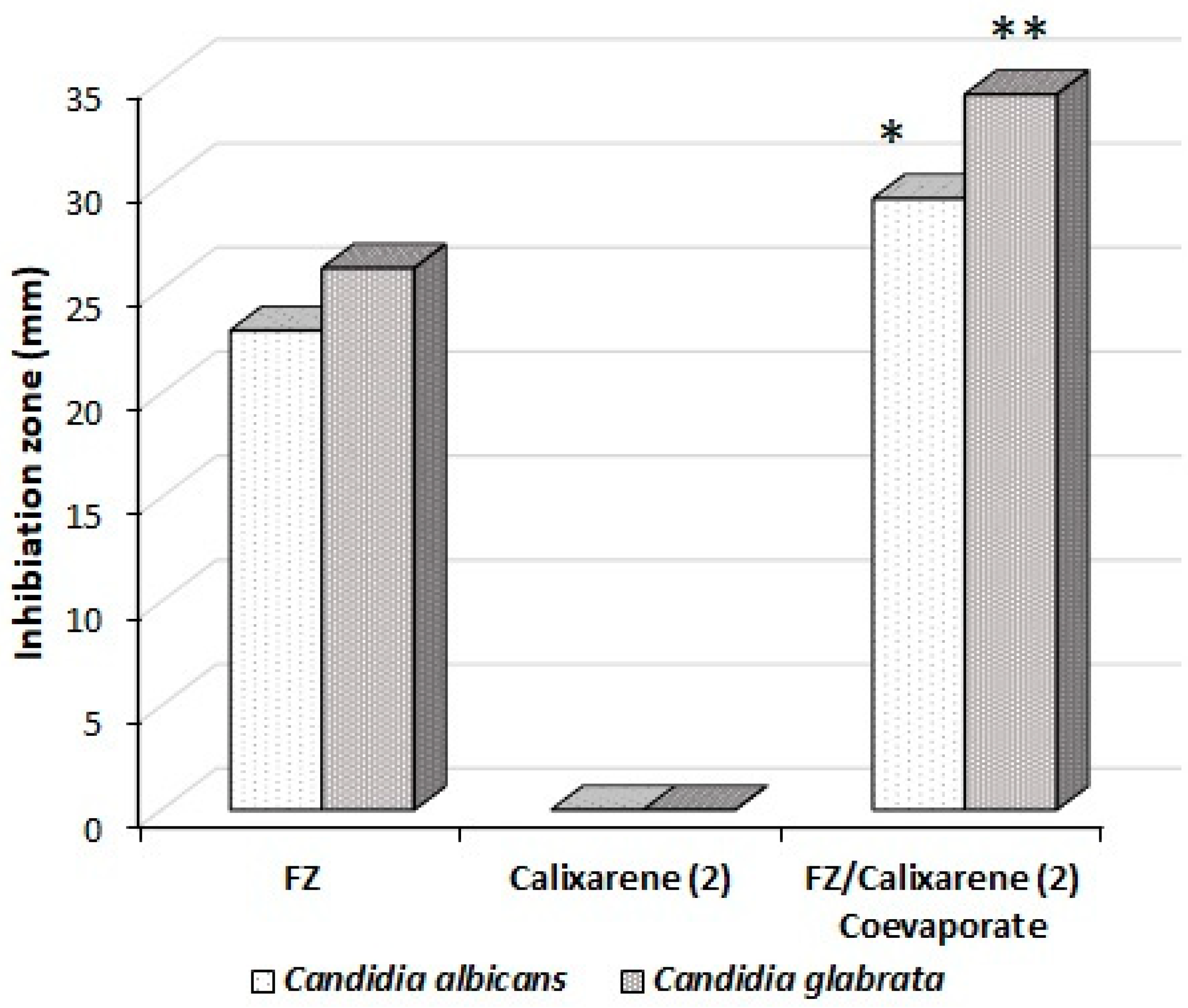
2.4. Antimycotic Activity
3. Materials and Methods
3.1. Chemicals
3.2. Phase Solubility Test
3.3. Preparation and Confirmation of Inclusion Complexes
3.3.1. Preparation of Inclusion Complexes
3.3.2. Confirmation of Inclusion Complex
Differential Scanning Calorimetry (DSC)
Thermogravimetric Analysis (TGA), Derivative Thermogravity (DTG)
Powder X-ray Diffraction (PXRD)
Fourier Transform Infrared Spectroscopy (FT-IR)
3.4. Dissolution Test
3.5. Antimycotic Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Caldera, F.; Cecone, C.; López-nicolás, J.M.; Trotta, F. Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals 2020, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.B.; Duarte, F.Í.C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin-Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bülbül, E.O.; Kalliopi, E.; Okur, N.Ü.; Siafaka, P.I. An Update on Cyclodextrins as Drug Vehicles for Antimicrobial Applications. J. Pharm. Technol. 2020, 1, 18–25. [Google Scholar] [CrossRef]
- Ramesh, K.; Anugrah, D.S.B.; Lim, K.T. Supramolecular Poly(N-Acryloylmorpholine)-b-Poly(D,L-Lactide) Pseudo-Block Copolymer via Host-Guest Interaction for Drug Delivery. React. Funct. Polym. 2018, 131, 12–21. [Google Scholar] [CrossRef]
- Hsiung, E.; Celebioglu, A.; Chowdhury, R.; Kilic, M.E.; Durgun, E.; Altier, C.; Uyar, T. Antibacterial Nanofibers of Pullulan/Tetracycline-Cyclodextrin Inclusion Complexes for Fast-Disintegrating Oral Drug Delivery. J. Colloid Interface Sci. 2022, 610, 321–333. [Google Scholar] [CrossRef]
- Kost, B.; Brzezinski, M.; Socka, M.; Basko, M.; Biela, T. Biocompatible Polymers Combined with Cyclodextrins: Fascinating Materials for Drug Delivery Applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef]
- Ramesh, K.; Balavigneswaran, C.K.; Siboro, S.A.P.; Muthuvijayan, V.; Lim, K.T. Synthesis of Cyclodextrin-Derived Star Poly(N-Vinylpyrrolidone)/Poly(Lactic-Co-Glycolide) Supramolecular Micelles via Host-Guest Interaction for Delivery of Doxorubicin. Polymer 2021, 214, 123243. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-Cyclodextrin (HP-β-CD): A Toxicology Review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Ukhatskaya, E.V.; Kurkov, S.V.; Matthews, S.E.; Loftsson, T. Encapsulation of Drug Molecules into Calix[n]Arene Nanobaskets. Role of Aminocalix[n]Arenes in Biopharmaceutical Field. J. Pharm. Sci. 2013, 102, 3485–3512. [Google Scholar] [CrossRef]
- Guo, D.S.; Liu, Y. Supramolecular Chemistry of p -Sulfonatocalix[n]Arenes and Its Biological Applications. Acc. Chem. Res. 2014, 47, 1925–1934. [Google Scholar] [CrossRef]
- Bayrakcı, M.; Ertul, Ş.; Yılmaz, M. Solubilizing Effect of the P-Phosphonate Calix[n]Arenes towards Poorly Soluble Drug Molecules Such as Nifedipine, Niclosamide and Furosemide. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 415–423. [Google Scholar] [CrossRef]
- Da Silva, E.; Lazar, A.N.; Coleman, A.W. Biopharmaceutical Applications of Calixarenes. J. Drug Deliv. Sci. Technol. 2004, 14, 3–20. [Google Scholar] [CrossRef]
- Shahgaldian, P.; Da Silva, E.; Coleman, A.W. A First Approach to the Study of Calixarene Solid Lipid Nanoparticle (SLN) Toxicity. J. Incl. Phenom. Macrocycl. Chem. 2003, 46, 175–177. [Google Scholar] [CrossRef]
- Da Silva, E.; Shahgaldian, P.; Coleman, A.W. Haemolytic Properties of Some Water-Soluble Para-Sulphonato-Calix-[n]- Arenes. Int. J. Pharm. 2004, 273, 57–62. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Iqbal, M. P-Tert-butylcalix[4]arene. Org. Synth. 1990, 68, 234. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Dhawan, B.; Leonis, M.; Stewart, D. P-Tert-butylcalix[6]arene. Org. Synth. 1990, 68, 238. [Google Scholar] [CrossRef]
- Saberman, M.N. Host-Guest Complexation of Oxaliplatin and Para-Sulfonatocalix[n]Arenes for Potential Use in Cancer Therapy. Molecules 2020, 25, 5926. [Google Scholar] [CrossRef]
- Basilotta, R.; Mannino, D.; Filippone, A.; Casili, G.; Prestifilippo, A.; Colarossi, L.; Raciti, G.; Esposito, E.; Campolo, M. Role of Calixarene in Chemotherapy Delivery Strategies. Molecules 2021, 26, 3963. [Google Scholar] [CrossRef]
- Moussa, Y.E.; Ong, Y.Q.E.; Perry, J.D.; Cheng, Z.; Kayser, V.; Cruz, E.; Kim, R.R.; Sciortino, N.; Wheate, N.J. Demonstration of In Vitro Host-Guest Complex Formation and Safety of Para-Sulfonatocalix[8]Arene as a Delivery Vehicle for Two Antibiotic Drugs. J. Pharm. Sci. 2018, 107, 3105–3111. [Google Scholar] [CrossRef]
- Jarange, A.B.; Patil, S.V.; Malkhede, D.D.; Deodhar, S.M.; Nandre, V.S.; Athare, S.V.; Kodam, K.M.; Gejji, S.P. P-Sulfonatocalixarene versus p-Thiasulfonatocalixarene: Encapsulation of Tenofovir Disoproxil Fumarate and Implications to ESI-MS, HPLC, NMR, DFT and Anti-MRSA Activities. J. Incl. Phenom. Macrocycl. Chem. 2021, 99, 43–59. [Google Scholar] [CrossRef]
- Bayrakc, M.; Ertul, Ş.; Yilmaz, M. Phase Solubility Studies of Poorly Soluble Drug Molecules by Using O-Phosphorylated Calixarenes as Drug-Solubilizing Agents. J. Chem. Eng. Data 2012, 57, 233–239. [Google Scholar] [CrossRef]
- Poh, B.-L.; Lim, C.S. Complexations of Amines with Water-Soluble Cyclotetrachromotropylen. Tetrahedron Lett. 1990, 46, 6387–6390. [Google Scholar]
- Poh, B.-L.; Tan, C.M. Cyclotetrachromotropylene Plays Host to α-, β-, and γ-Cyclodextrin in Water. Tetrahedron Lett. 1994, 35, 6387–6390. [Google Scholar] [CrossRef]
- Poh, B.-L.; Lim, C.S.; Khoo, K.S. A Water-Soluble Cyclic Tetramer from Reacting Chromotropic Acid with Formaldehyde. Tetrahedron Lett. 1989, 30, 1005–1008. [Google Scholar] [CrossRef]
- Poh, B.-L.; Tan, C.M. Contribution of Guest-Host CH-π Interaction to the Stability of Complexes Formed from Cyclotetrachromotropylene as Host and Alcohols and Sugars as Guests in Water. Tetrahedron 1993, 49, 9581–9592. [Google Scholar] [CrossRef]
- Al Hujran, T.A.; Magharbeh, M.K.; Al-Gharabli, S.; Haddadin, R.R.; Al Soub, M.N.; Tawfeek, H.M. Studying the Complex Formation of Sulfonatocalix[4]Naphthalene and Meloxicam towards Enhancing Its Solubility and Dissolution Performance. Pharmaceutics 2021, 13, 994. [Google Scholar] [CrossRef]
- Corrêa, J.C.R.; Salgado, H.R.N. Review of Fluconazole Properties and Analytical Methods for Its Determination. Crit. Rev. Anal. Chem. 2011, 41, 124–132. [Google Scholar] [CrossRef]
- Rewak-soroczynska, J.; Sobierajska, P.; Targonska, S.; Piecuch, A.; Grosman, L.; Rachuna, J.; Wasik, S.; Arabski, M.; Ogorek, R.; Wiglusz, R.J. New Approach to Antifungal Activity of Fluconazole Incorporated into the Porous 6-anhydro-α-l-galacto-β-d-galactan Structures Modified with Nanohydroxyapatite for Chronic-wound Treatments—in Vitro Evaluation. Int. J. Mol. Sci. 2021, 22, 3112. [Google Scholar] [CrossRef]
- Mangrule, V.; Pore, Y.; Disouza, J. Synthesis and Physicochemical Studies of Fluconazole Ionic Liquids. J. Appl. Pharm. Sci. 2017, 7, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Cyr, T.D.; Dawson, B.A.; Neville, G.A.; Shurvell, H.F. Spectral Characterization of Fluconazole. J. Pharm. Biomed. Anal. 1996, 14, 247–255. [Google Scholar] [CrossRef]
- Abranches, P.A.S.; Varejão, E.V.V.; Da Silva, C.M.; De Fátima, A.; Magalhães, T.F.F.; Da Silva, D.L.; De Resende-Stoianoff, M.A.; Reis, F.S.; Nascimento, J.S.; De Almeida, W.B.; et al. Complexes of Fluconazole with Sodium P-Sulfonatocalix[n]Arenes: Characterization, Solubility and Antifungal Activity. RSC Adv. 2015, 5, 44317–44325. [Google Scholar] [CrossRef]
- Longley, N.; Muzoora, C.; Taseera, K.; Mwesigye, J.; Rwebembera, J.; Chakera, A.; Wall, E.; Andia, I.; Jaffar, S.; Harrison, T.S. Dose Response Effect of High-Dose Fluconazole for HIV-Associated Cryptococcal Meningitis in Southwestern Uganda. Clin. Infect. Dis. 2008, 47, 1556–1561. [Google Scholar] [CrossRef] [Green Version]
- Halpern, A.B.; Lyman, G.H.; Walsh, T.J.; Kontoyiannis, D.P.; Walter, R.B. Primary Antifungal Prophylaxis during Curative-Intent Therapy for Acute Myeloid Leukemia. Blood 2015, 126, 2790–2797. [Google Scholar] [CrossRef] [Green Version]
- Starzl, T.E. Fluconazole Therapy in Transplant Recipients Receiving FK506. Transplantation 1994, 57, 1521–1523. [Google Scholar] [CrossRef] [Green Version]
- Abdul Ahad, H.; Sreeramulu, J.; Padmaja, B.S.; Reddy, M.N.; Prakash, P.G. Preparation of Fluconazole -Cyclodextrin Complex Ocuserts: In Vitro and In Vivo Evaluation. ISRN Pharm. 2011, 2011, 237501. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Ahmed, M.; Ahmed, S.; Ali, S.I.; Perveen, S.; Mumtaz, M.; Haider, S.M.; Nazim, U. Fluconazole and Its Interaction with Metal (II) Complexes: SEM, Spectroscopic and Antifungal Studies. Pak. J. Pharm. Sci. 2017, 30, 187–194. [Google Scholar]
- Ali, S.I.; Lei, Z.N.; Ali, M.; Kojima, K.; Ahmed, M.; Peng, R.; Yang, D.H.; Haider, S.M.; Ayatollahi, S.A.; Chen, Z.S. Metal (II) Complexes of Fluconazole: Thermal, Xrd and Cytotoxicity Studies. Iran. J. Pharm. Res. 2020, 19, 171–182. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Kumar, G. NMR and Molecular Modelling Studies on the Interaction of Fluconazole with β-Cyclodextrin. Chem. Cent. J. 2009, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Iftode, A.; Racoviceanu, R.; Susan, R.; Marti, D.; Pinzaru, I.; Lazau, R.; Susan, M.; Gheorghisor, A.; Soica, C.; Trandafirescu, C. Fluconazole-Beta-Cyclodextrin Inclusion Complexes. Preparation and Characterization in Solid State. Rev. Chim. 2020, 71, 325–334. [Google Scholar] [CrossRef]
- Charoo, N.; Cristofoletti, R.; Graham, A.; Lartey, P.; Abrahamsson, B.; Groot, D.W.; Kopp, S.; Langguth, P.; Polli, J.; Shah, V.P.; et al. Biowaiver Monograph for Immediate-Release Solid Oral Dosage Forms: Fluconazole. J. Pharm. Sci. 2014, 103, 3843–3858. [Google Scholar] [CrossRef]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Hassan, Y.A.; Fathalla, D. Transfersomal Gel Nanocarriers for Enhancement the Permeation of Lornoxicam. J. Drug Deliv. Sci. Technol. 2020, 56, 101540. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid Vesicles: A Versatile Drug Delivery Platform for Dermal and Transdermal Applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef]
- Vlaia, L.; Coneac, G.; Muţ, A.M.; Olariu, I.; Vlaia, V.; Anghel, D.F.; Maxim, M.E.; Dobrescu, A.; Hîrjău, M.; Lupuleasa, D. Topical Biocompatible Fluconazole-Loaded Microemulsions Based on Essential Oils and Sucrose Esters: Formulation Design Based on Pseudo-Ternary Phase Diagrams and Physicochemical Characterization. Processes 2021, 9, 144. [Google Scholar] [CrossRef]
- Elmotasem, H.; Awad, G.E.A. A Stepwise Optimization Strategy to Formulate in Situ Gelling Formulations Comprising Fluconazole-Hydroxypropyl-Beta-Cyclodextrin Complex Loaded Niosomal Vesicles and Eudragit Nanoparticles for Enhanced Antifungal Activity and Prolonged Ocular Delivery. Asian J. Pharm. Sci. 2020, 15, 617–636. [Google Scholar] [CrossRef]
- Alizadeh, M.N.; Shayanfar, A.; Jouyban, A. Solubilization of Drugs Using Sodium Lauryl Sulfate: Experimental Data and Modeling. J. Mol. Liq. 2018, 268, 410–414. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Sia Heng, P.W. Overview of Milling Techniques for Improving the Solubility of Poorly Water-Soluble Drugs. Asian J. Pharm. Sci. 2014, 10, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Weyna, D.R.; Cheney, M.L.; Shan, N.; Hanna, M.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Improving Solubility and Pharmacokinetics of Meloxicam via Multiple-Component Crystal Formation. Mol. Pharm. 2012, 9, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Charumanee, S.; Titwan, A.; Sirithunyalug, J.; Okonogi, S.; Wolschann, P.; Viernstein, H. Enhancement of Solubility and Dissolution of Meloxicam by Cyclodextrin Complexation Materials. Starch Update 2005, 357–363. [Google Scholar]
- Yurtdaş, G.; Demirel, M.; Genç, L. Inclusion Complexes of Fluconazole with β-Cyclodextrin: Physicochemical Characterization and in Vitro Evaluation of Its Formulation. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 429–435. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Andersson, S.B.E.; Fagerberg, J.H.; Ragnarsson, G.; Lindahl, A. Is the Full Potential of the Biopharmaceutics Classification System Reached? Eur. J. Pharm. Sci. 2014, 57, 224–231. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhou, Y.; Guan, S.; Zhang, L. Inclusion Complexes of Fluconazole with β-Cyclodextrin and 2-Hydroxypropyl-β-Cyclodextrin in Aqueous Solution: Preparation, Characterization and a Structural Insight. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 209–217. [Google Scholar] [CrossRef]
- Orgován, G.; Kelemen, H.; Noszál, B. Protonation and β-Cyclodextrin Complex Formation Equilibria of Fluconazole. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 189–196. [Google Scholar] [CrossRef]
- Mader, W.J.; Higuchi, T. Phase Solubility Analysis. CRC Crit. Rev. Anal. Chem. 1970, 1, 193–215. [Google Scholar] [CrossRef]
- Zayed, M.A.; Abdallh, M.A. Structure Investigation of Solid Ion Pairs of Three Imidazole Drugs-Rose Bengal Using Different Spectroscopic Techniques and Their Biological Activities. Egypt. J. Chem. 2019, 62, 2143–2162. [Google Scholar] [CrossRef]
- Hassan, A.F.; Helmy, S.A.; Donia, A. MCM-41 for Meloxicam Dissolution Improvement: J. Braz. Chem. Soc. 2015, 26, 1367–1378. [Google Scholar]
- Yang, W.; De Villiers, M.M. The Solubilization of the Poorly Water Soluble Drug Nifedipine by Water Soluble 4-Sulphonic Calix[n]Arenes. Eur. J. Pharm. Biopharm. 2004, 58, 629–636. [Google Scholar] [CrossRef]
- Yang, W.; de Villiers, M.M. Aqueous Solubilization of Furosemide by Supramolecular Complexation with 4-Sulphonic Calix[n]Arenes. J. Pharm. Pharmacol. 2004, 56, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Auti, S.D.; Jadhav, S.L.; Gadhave, M.V. Dissolution Method Devolopment of Fluconazole in Fluconazole Tablets Dossage Form. PharmaTutor 2015, 3, 29–35. [Google Scholar]
- Newman, A.; Zografi, G. Commentary: Considerations in the Measurement of Glass Transition Temperatures of Pharmaceutical Amorphous Solids. AAPS PharmSciTech 2020, 21, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Sol, V.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Garnero, C.; Aloisio, C.; Longhi, M. Ibuprofen-Maltodextrin Interaction: Study of Enantiomeric Recognition and Complex Characterization. Pharmacol. Pharm. 2013, 4, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Hancock, B.C.; Zografi, G. Characteristics and Significance of the Amorphous State in Pharmaceutical Systems. J. Pharm. Sci. 1997, 86, 1. [Google Scholar] [CrossRef]
- Doile, M.M.; Fortunato, K.A.; Schmücker, I.C.; Schucko, S.K.; Silva, M.A.S.; Rodrigues, P.O. Physicochemical Properties and Dissolution Studies of Dexamethasone Acetate-β-Cyclodextrin Inclusion Complexes Produced by Different Methods. AAPS PharmSciTech 2008, 9, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef]
- Phadnis, N.V.; Cavatur, R.K.; Suryanarayanan, R. Identification of Drugs in Pharmaceutical Dosage Forms by X-Ray Powder Diffractometry. J. Pharm. Biomed. Anal. 1997, 15, 929–943. [Google Scholar] [CrossRef]
- Etman, M.; Shekedef, M.; Nada, A.; Ismail, A. In Vitro and In Vivo Evaluation of Tablets Containing Meloxicam-PEG 6000 Ball-Milled Co-Ground Mixture. J. Appl. Pharm. Sci. 2017, 7, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Tawfeek, H.M.; Saleem, I.Y.; Roberts, M. Dissolution Enhancement and Formulation of Rapid-Release Lornoxicam Mini-Tablets. J. Pharm. Sci. 2014, 103, 2470–2483. [Google Scholar] [CrossRef] [PubMed]
- Kumara, P.; Mohanb, C.; Uma Shankara, M.K.S.; Gulatia, M. Physiochemical Characterization and Release Rate Studies of Solid Dispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran. J. Pharm. Res. 2011, 10, 685–694. [Google Scholar] [CrossRef]
- Abou-Taleb, A.E.; Abdel-Rhman, A.A.; Samy, E.M.; Tawfeek, H.M. Interaction of Rofecoxib With WITH B-cyclodextrin and hp-β-cyclodextrin in aquoues solution and in solid state. Bull. Pharm. Sci. Assiut Univ. 2006, 29, 236–252. [Google Scholar] [CrossRef] [Green Version]
- Brusnikina, M.; Silyukov, O.; Chislov, M.; Volkova, T.; Proshin, A.; Mazur, A.; Tolstoy, P.; Terekhova, I. Effect of Cyclodextrin Complexation on Solubility of Novel Anti-Alzheimer 1,2,4-Thiadiazole Derivative. J. Therm. Anal. Calorim. 2017, 130, 443–450. [Google Scholar] [CrossRef]
- Vadher, A.H.; Parikh, J.R.; Parikh, R.H.; Solanki, A.B. Preparation and Characterization of Co-Grinded Mixtures of Aceclofenac and Neusilin US2 for Dissolution Enhancement of Aceclofenac. AAPS PharmSciTech 2009, 10, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Abidi, S.S.A.; Garg, U.; Azim, Y.; Alam, M.; Gupta, A.K.; Pradeep, C.P.; Azum, N.; Asiri, A.M. Spectroscopic, Structural, DFT and Molecular Docking Studies on Novel Cocrystal Salt Hydrate of Chromotropic Acid and Its Antibiofilm Activity. Arab. J. Sci. Eng. 2021, 46, 353–364. [Google Scholar] [CrossRef]
- Alkhamis, K.A.; Obaidat, A.A.; Nuseirat, A.F. Solid-State Characterization of Fluconazole. Pharm. Dev. Technol. 2002, 7, 491–503. [Google Scholar] [CrossRef]
- Chokshi, R.J.; Zia, H.; Sandhu, H.K.; Shah, N.H.; Malick, W.A. Improving the Dissolution Rate of Poorly Water Soluble Drug by Solid Dispersion and Solid Solution—Pros and Cons. Drug Deliv. 2007, 14, 33–45. [Google Scholar] [CrossRef]
- Khan, C.A.; Rhodes, C.T. The Concept of Dissolution Efficiency. J. Pharm. Pharmac. 1975, 27, 48–49. [Google Scholar] [CrossRef]
- Ali, H.R.H.; Saleem, I.Y.; Tawfeek, H.M. Insight into Inclusion Complexation of Indomethacin Nicotinamide Cocrystals. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 179–188. [Google Scholar] [CrossRef]
- Kirkpatrick, W.R.; Turner, T.M.; Fothergill, A.W.; McCarthy, D.I.; Redding, S.W.; Rinaldi, M.G.; Patterson, T.F. Fluconazole Disk Diffusion Susceptibility Testing of Candida Species. J. Clin. Microbiol. 1998, 36, 3429–3432. [Google Scholar] [CrossRef] [Green Version]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Williams, L. Wilkins. In Antibiotics in Laboratory Medicine, 5th ed.; Lorian, V., Ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
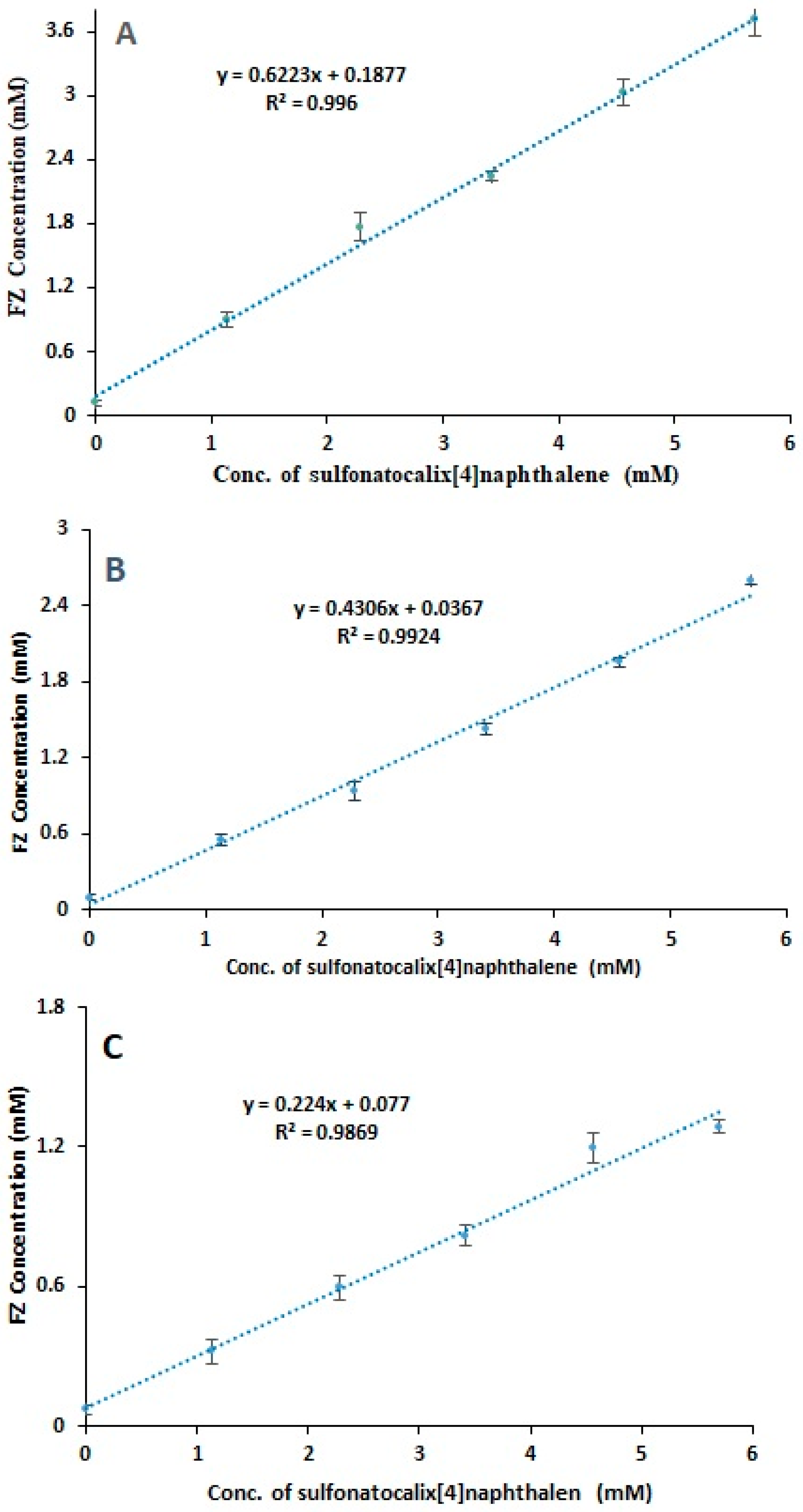


| Medium Type | Phase Solubility Diagram Type | Stability Constant ± S.D. (M−1) | Increasing of Solubility (St/So) |
|---|---|---|---|
| 0.1N HCl pH 1.2 | AL | 2861.112 ± 0.095 | 31.103 |
| Phosphate buffer of pH 7.4 | AL | 1881. 830 ± 0.012 | 25.241 |
| Distilled water | AL | 501.266 ± 0.011 | 18.500 |
| Methods | ED25 ± S.D., n = 3 | Fold Increase | RDR10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 1.2 | pH 7.4 | D.W | pH 1.2 | pH 7.4 | D.W | pH 1.2 | pH 7.4 | D.W | |
| CO | 32.02 ± 0.57 | 30.64 ± 0.63 | 23.95 ± 0.71 | 4.30 | 4.25 | 3.41 | 6.18 | 4.64 | 3.54 |
| KN | 29.18 ± 0.50 | 27.99 ± 0.56 | 21.88 ± 0.64 | 3.92 | 3.88 | 3.11 | 5.66 | 4.26 | 3.28 |
| PM | 25.95 ± 0.72 | 24.23 ± 0.51 | 17.99 ± 0.64 | 3.48 | 3.36 | 2.56 | 4.95 | 3.54 | 2.67 |
| FZ powder | 7.45 ± 0.64 | 7.20 ± 0.62 | 7.02 ± 0.70 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hujran, T.A.; Magharbeh, M.K.; Habashneh, A.Y.; Al-Dmour, R.S.; Aboelela, A.; Tawfeek, H.M. Insight into the Inclusion Complexation of Fluconazole with Sulfonatocalix[4]naphthalene in Aqueous Solution, Solid-State, and Its Antimycotic Activity. Molecules 2022, 27, 4425. https://doi.org/10.3390/molecules27144425
Al Hujran TA, Magharbeh MK, Habashneh AY, Al-Dmour RS, Aboelela A, Tawfeek HM. Insight into the Inclusion Complexation of Fluconazole with Sulfonatocalix[4]naphthalene in Aqueous Solution, Solid-State, and Its Antimycotic Activity. Molecules. 2022; 27(14):4425. https://doi.org/10.3390/molecules27144425
Chicago/Turabian StyleAl Hujran, Tayel A., Mousa K. Magharbeh, Almeqdad Y. Habashneh, Rasha S. Al-Dmour, Ashraf Aboelela, and Hesham M. Tawfeek. 2022. "Insight into the Inclusion Complexation of Fluconazole with Sulfonatocalix[4]naphthalene in Aqueous Solution, Solid-State, and Its Antimycotic Activity" Molecules 27, no. 14: 4425. https://doi.org/10.3390/molecules27144425
APA StyleAl Hujran, T. A., Magharbeh, M. K., Habashneh, A. Y., Al-Dmour, R. S., Aboelela, A., & Tawfeek, H. M. (2022). Insight into the Inclusion Complexation of Fluconazole with Sulfonatocalix[4]naphthalene in Aqueous Solution, Solid-State, and Its Antimycotic Activity. Molecules, 27(14), 4425. https://doi.org/10.3390/molecules27144425