Real-World Utilization of Palbociclib as First-Line Treatment for Canadian HR+/HER2− Women with Metastatic Breast Cancer: Results from PALCAN Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
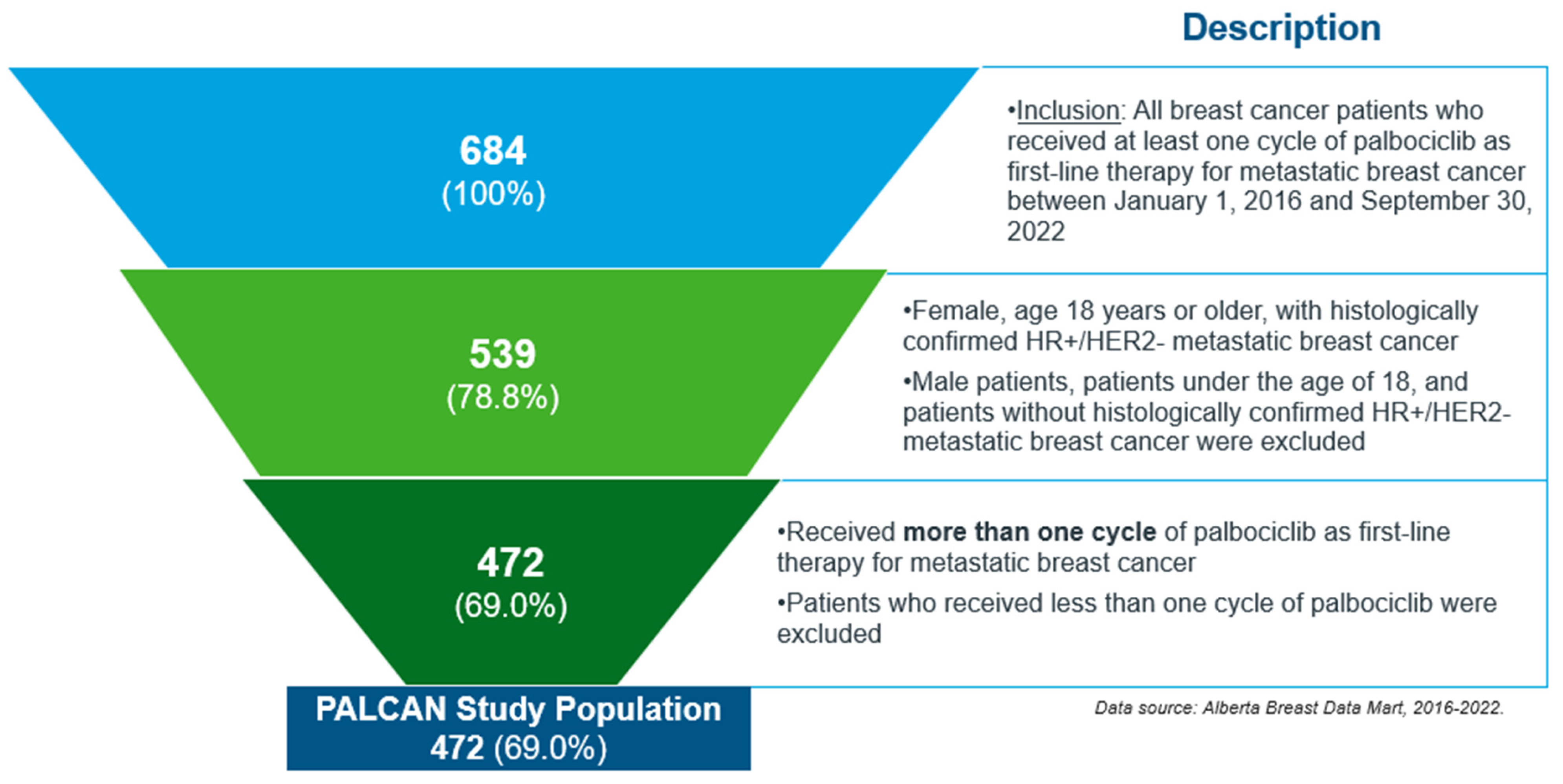
Study Population and Cohort
2.2. Data Sources
2.3. Outcomes, Variables, and Covariates
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Treatment with Palbociclib and Accompanying Therapy
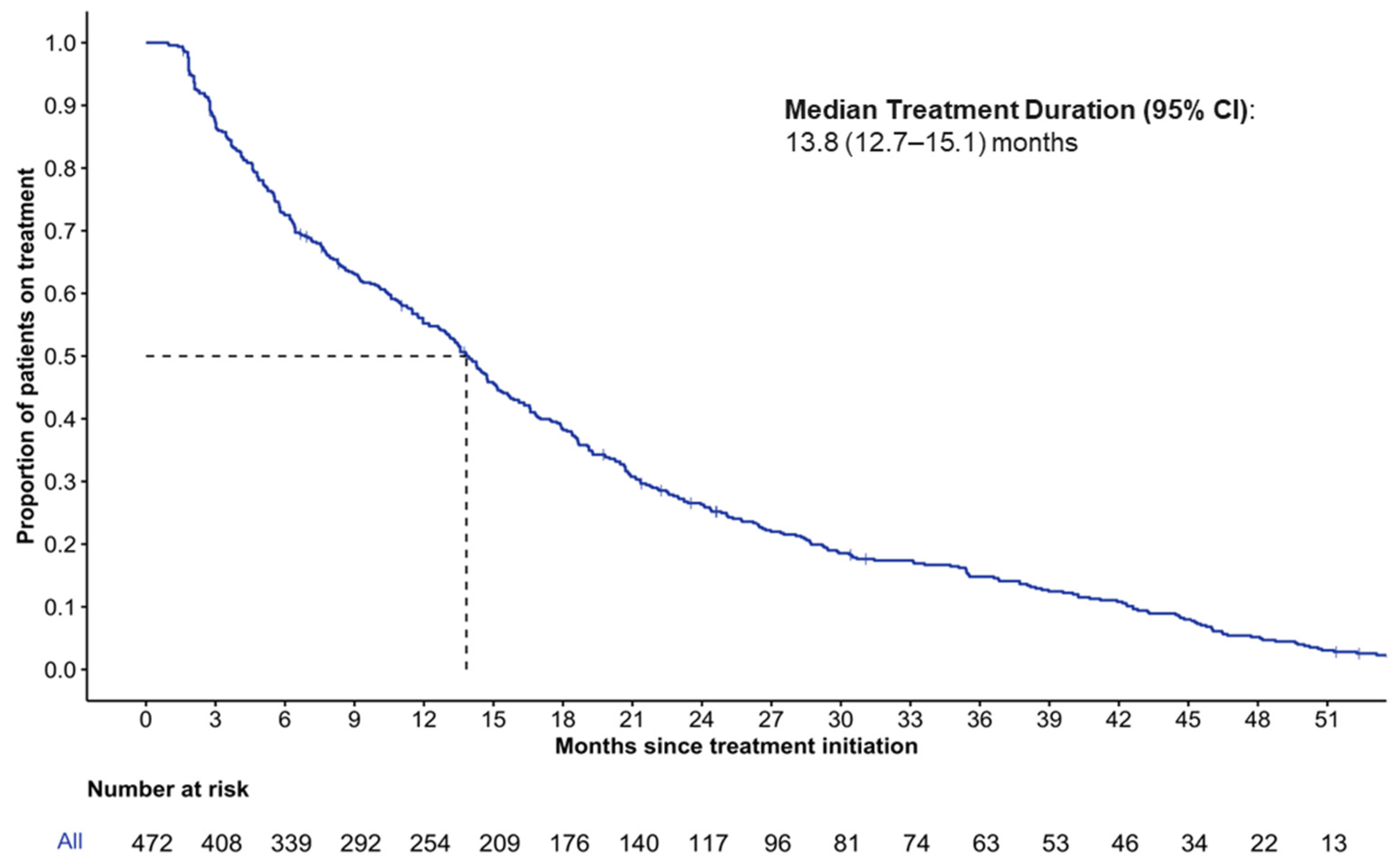
3.3. Duration of Treatment
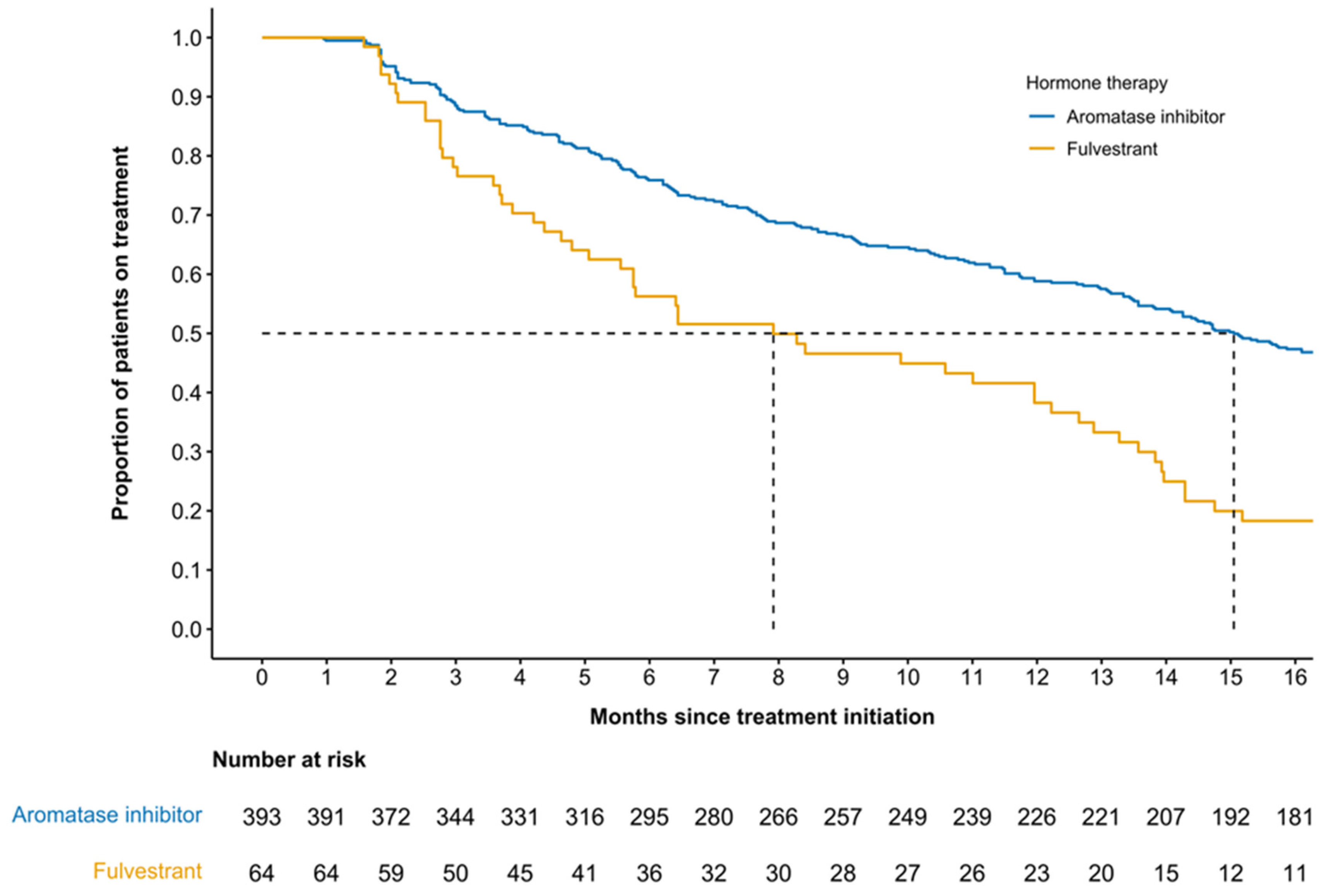
3.4. Duration of Treatment by Accompanying Therapy
3.5. Duration of Treatment by Inferred Menopausal Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2025. Available online: https://cancer.ca/Canadian-Cancer-Statistics-2025-EN (accessed on 7 December 2025).
- Canadian Cancer Survivor Network. Statistics and Research in Metastatic Breast Cancer. Available online: https://survivornet.ca/cancer-type/breast-cancer/metastatic-breast-cancer/statistics-and-research-in-metastatic-breast-cancer/ (accessed on 24 April 2025).
- Seung, S.J.; Traore, A.N.; Pourmirza, B.; Fathers, K.E.; Coombes, M.; Jerzak, K.J. A population-based analysis of breast cancer incidence and survival by subtype in Ontario women. Curr. Oncol. 2020, 27, e191–e198. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Society. Survival Statistics for Breast Cancer. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast/prognosis-and-survival/survival-statistics (accessed on 24 April 2025).
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2020, 11, 632079. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Bethesda MD SEER Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 24 April 2025).
- Noone, A.M.; Cronin, K.A.; Altekruse, S.F.; Howlader, N.; Lewis, D.R.; Petkov, V.I.; Penberthy, L. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiol. Biomark. Prev. 2017, 26, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Metastatic Breast Cancer. Available online: http://www.bccancer.bc.ca/books/breast/management/metastatic-breast-cancer (accessed on 24 April 2025).
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 24 April 2025).
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Ibrance (Palbociclib). Product Monograph. Pfizer Canada ULC. Available online: https://www.pfizer.ca/en/our-products/ibrance-palbociclib (accessed on 25 April 2025).
- Finn, R.S.; Rugo, H.S.; Gelmon, K.A.; Cristofanilli, M.; Colleoni, M.; Loi, S.; Schnell, P.; Lu, D.R.; Theall, K.P.; Mori, A.; et al. Long-Term Pooled Safety Analysis of Palbociclib in Combination with Endocrine Therapy for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Updated Analysis with up to 5 Years of Follow-Up. Oncologist 2021, 26, e749–e755. [Google Scholar] [CrossRef] [PubMed]
- Amaro, C.P.; Batra, A.; Lupichuk, S. First-Line Treatment with a Cyclin-Dependent Kinase 4/6 Inhibitor Plus an Aromatase Inhibitor for Metastatic Breast Cancer in Alberta. Curr. Oncol. 2021, 28, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Moulson, R.; Feugère, G.; Moreira-Lucas, T.S.; Dequen, F.; Weiss, J.; Smith, J.; Brezden-Masley, C. Real-World Treatment Patterns and Clinical Outcomes among Patients Receiving CDK4/6 Inhibitors for Metastatic Breast Cancer in a Canadian Setting Using AI-Extracted Data. Curr. Oncol. 2024, 31, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Patt, D.; Liu, X.; Li, B.; McRoy, L.; Layman, R.M.; Brufsky, A. Real-World Treatment Patterns and Outcomes of Palbociclib Plus an Aromatase Inhibitor for Metastatic Breast Cancer: Flatiron Database Analysis. Clin. Breast Cancer 2022, 22, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.S.; Mazursky, O.F.; Shalev, H.; Apter, L.; Chodick, G.; Siegelmann-Danieli, N. Real-world outcomes of patients with metastatic endocrine-responsive breast cancer receiving palbociclib-based combinations. Future Oncol. 2023, 19, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Mycock, K.; Zhan, L.; Taylor-Stokes, G.; Milligan, G.; Mitra, D. Real-World Palbociclib Use in HR+/HER2- Advanced Breast Cancer in Canada: The IRIS Study. Curr. Oncol. 2021, 28, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Mycock, K.; Zhan, L.; Hart, K.; Taylor-Stokes, G.; Milligan, G.; Atkinson, C.; Mitra, D. Real-world treatment of patients with palbociclib for HR+/HER2-advanced/metastatic breast cancer: The Europe IRIS study. Future Oncol. 2022, 18, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.; Mitra, D.; Mycock, K.; Taylor-Stokes, G.; Milligan, G.; Zhan, L.; Iyer, S. Real-World Treatment Patterns and Clinical Outcomes in Patients Receiving Palbociclib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced or Metastatic Breast Cancer in Argentina: The IRIS Study. J. Glob. Oncol. 2019, 5, JGO1800239. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.Q.; Saha, P.; Peiffer, D.S.; Chen, N.; Shubeck, S.P.; Yarlagadda, S.R.; Nanda, R.; Huo, D.; Howard, F.M. Adjuvant Chemotherapy Use for Hormone Receptor–Positive, ERBB2-Negative Breast Cancer After RxPONDER Trial. JAMA Netw. Open 2025, 8, e2549109. [Google Scholar] [CrossRef]
- Gelmon, K.; Walshe, J.M.; Mahtani, R.; Joy, A.A.; Karuturi, M.; Neven, P.; Lu, D.R.; Kim, S.; Schnell, P.; Bananis, E.; et al. Efficacy and safety of palbociclib in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with preexisting conditions: A post hoc analysis of PALOMA-2. Breast 2021, 59, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Nickel, B.; Ngo, P.; McFadden, K.; Meagan, B.; Marinovick, M.L.; Houssami, N. A systematic review of the impact of the COVID-19 pandemic on breast cancer screening and diagnosis—The Breast. Breast 2024, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Borges, F.; Alves da Costa, F.; Ramos, A.; Ramos, C.; Bernardo, C.; Brito, C.; Mayer-da-Silva, A.; Furtado, C.; Ferreira, A.R.; Martins-Branco, D.; et al. Real-world effectiveness of palbociclib plus fulvestrant in advanced breast cancer: Results from a population-based cohort study. Breast 2022, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 20, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Fasching, P.A.; Fehm, T.; Gerber, B.; Jackisch, C.; Loibl, S.; Schmidt, M.; Stickeler, E.; Wöckel, A.; Janni, W.; et al. AGO Algorithms for the Treatment of Breast Cancer: Update 2021. Geburtshilfe Frauenheilkd 2021, 81, 1101–1111. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Shin, J.; Ahn, J.S.; Park, Y.H.; Im, Y.-H. Real World Experience of Second-Line Treatment Strategies after Palbociclib and Letrozole: Overall Survival in Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. Cancers 2023, 15, 3431. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall, n (%) (n = 472) | Aromatase Inhibitor, n (%) (n = 393) | Fulvestrant n (%) (n = 64) |
|---|---|---|---|
| Age at index date, years | |||
| Mean ± SD | 64 ± 12 | 64 ± 12 | 66 ± 14 |
| Median (IQR) | 64 (27–95) | 64 (27–90) | 67 (36–95) |
| <50 years, n (%) | 62 (13.1%) | 45 (11.5%) | 12 (18.8%) |
| 50–70 years, n (%) | 259 (54.9%) | 228 (58.0%) | 25 (39.1%) |
| >70 years, n (%) | 151 (32.0%) | 120 (30.5%) | 27 (42.2%) |
| Inferred menopausal status, n (%) | |||
| Assumed pre-menopausal (age < 50 years) | 62 (13.1%) | 45 (11.5%) | 12 (18.8%) |
| Assumed post-menopausal (age ≥ 50 years) | 410 (86.9%) | 348 (88.5%) | 52 (81.3%) |
| Hormone receptor status, n (%) | |||
| ER+ only | 59–67 * | 43–51 * | 4–12 * |
| PR+ only | <10 * | <10 * | <10 * |
| Both ER+ and PR+ | 404 (85.6%) | 341 (86.8%) | 51 (79.7%) |
| HER2 IHC score, n (%) | |||
| 0 | 57 (28.4%) | 48 (29.4%) | <10 * |
| 1 | 77 (38.3%) | 63 (38.7%) | 11 (39.3%) |
| 2+ (ISH/FISH-negative) | 67 (33.3%) | 52 (31.9%) | 13 (46.4%) |
| Unknown | 271 | 230 | 31–39 * |
| Histopathology, n (%) | |||
| Ductal | 369 (78.2%) | 307 (78.1%) | 49 (76.6%) |
| Lobular | 48 (10.2%) | 1–47 * | <10 * |
| Other | 55 (11.7%) | 1–54 * | <10 * |
| Metastatic presentation, n (%) | |||
| De novo | 214 (45.3%) | 186 (47.3%) | 28 (28.1%) |
| Prior early breast cancer | 258 (54.7%) | 207 (52.7%) | 46 (71.9%) |
| Metastatic disease distribution, n (%) | |||
| Non-visceral | 112 (52.3%) | 95–103 * | 9–17 * |
| Visceral | 102 (47.7%) | 91–101 * | <10 * |
| Unknown ** | 258 | 207 | 46 |
| Number of metastatic sites, n (%) | |||
| Unknown ** | 258 (54.7%) | 207 (52.7%) | 46 (71.9%) |
| 1 | 100 (21.2%) | 83–91 * | 9–17 * |
| 2+ | 114 (24.2%) | 105–113 * | <10 * |
| Charlson comorbidity index, n (%) | |||
| 0 | 316 (66.9%) | 266 (67.7%) | 42 (65.6%) |
| 1 | 116 (24.6%) | 99 (25.3%) | 12 (18.8%) |
| 2+ or missing | 40 (8.5%) | 28 (7.0%) | 10 (15.6%) |
| Vascular/cardiac ***, n (%) | 29 (6.1%) | 20 (5.1%) | <10 * |
| Gastrointestinal ***, n (%) | 23 (4.9%) | 15 (3.8%) | <10 * |
| Musculoskeletal ***, n (%) | 13 (2.8%) | 11 (2.8%) | <10 * |
| Metabolic ***, n (%) | 48 (10.2%) | 39 (9.9%) | <10 * |
| Median follow-up time, months (range) | 22.8 (0.7–88.2) | 26.3 (0.7–88.2) | 16.4 (1.2–35.0) |
| Year of palbociclib initiation | |||
| 2016–2017 | 38 (8.1%) | 29–37 * | <10 * |
| 2018 | 103 (21.8%) | 94–101 * | <10 * |
| 2019 | 93 (19.7%) | 84–92 * | <10 * |
| 2020 | 84 (17.8%) | 65 (16.5%) | 18 (28.1%) |
| 2021 | 103 (21.8%) | 71 (18.1%) | 27 (42.2%) |
| 2022 | 51 (10.8%) | 40 (10.2%) | 11 (17.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Rayson, D.; Bertin, J.; Lemelin, M.; Tong, M.; Ng, R.; Ding, P.; Cheung, W.Y.; Sharma, A.; On, P.V.; Feugère, G.; et al. Real-World Utilization of Palbociclib as First-Line Treatment for Canadian HR+/HER2− Women with Metastatic Breast Cancer: Results from PALCAN Study. Curr. Oncol. 2026, 33, 81. https://doi.org/10.3390/curroncol33020081
Rayson D, Bertin J, Lemelin M, Tong M, Ng R, Ding P, Cheung WY, Sharma A, On PV, Feugère G, et al. Real-World Utilization of Palbociclib as First-Line Treatment for Canadian HR+/HER2− Women with Metastatic Breast Cancer: Results from PALCAN Study. Current Oncology. 2026; 33(2):81. https://doi.org/10.3390/curroncol33020081
Chicago/Turabian StyleRayson, Daniel, Jonathan Bertin, Maxim Lemelin, Madeline Tong, Ryan Ng, Philip Ding, Winson Y. Cheung, Arushi Sharma, Phu Vinh On, Guillaume Feugère, and et al. 2026. "Real-World Utilization of Palbociclib as First-Line Treatment for Canadian HR+/HER2− Women with Metastatic Breast Cancer: Results from PALCAN Study" Current Oncology 33, no. 2: 81. https://doi.org/10.3390/curroncol33020081
APA StyleRayson, D., Bertin, J., Lemelin, M., Tong, M., Ng, R., Ding, P., Cheung, W. Y., Sharma, A., On, P. V., Feugère, G., & Lupichuk, S. (2026). Real-World Utilization of Palbociclib as First-Line Treatment for Canadian HR+/HER2− Women with Metastatic Breast Cancer: Results from PALCAN Study. Current Oncology, 33(2), 81. https://doi.org/10.3390/curroncol33020081





