Cytotoxicity of Esculetin Compared with Vinblastine and Paclitaxel in PC-3 Prostate Cancer Cells †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments
2.2. Metabolic Activity
2.3. Cytotoxicity
2.4. Cell Cycle Analyses
2.5. Apoptosis Analyses by Annexin-V-FITC Cytometry Assay
2.6. Statistical Analysis
3. Results
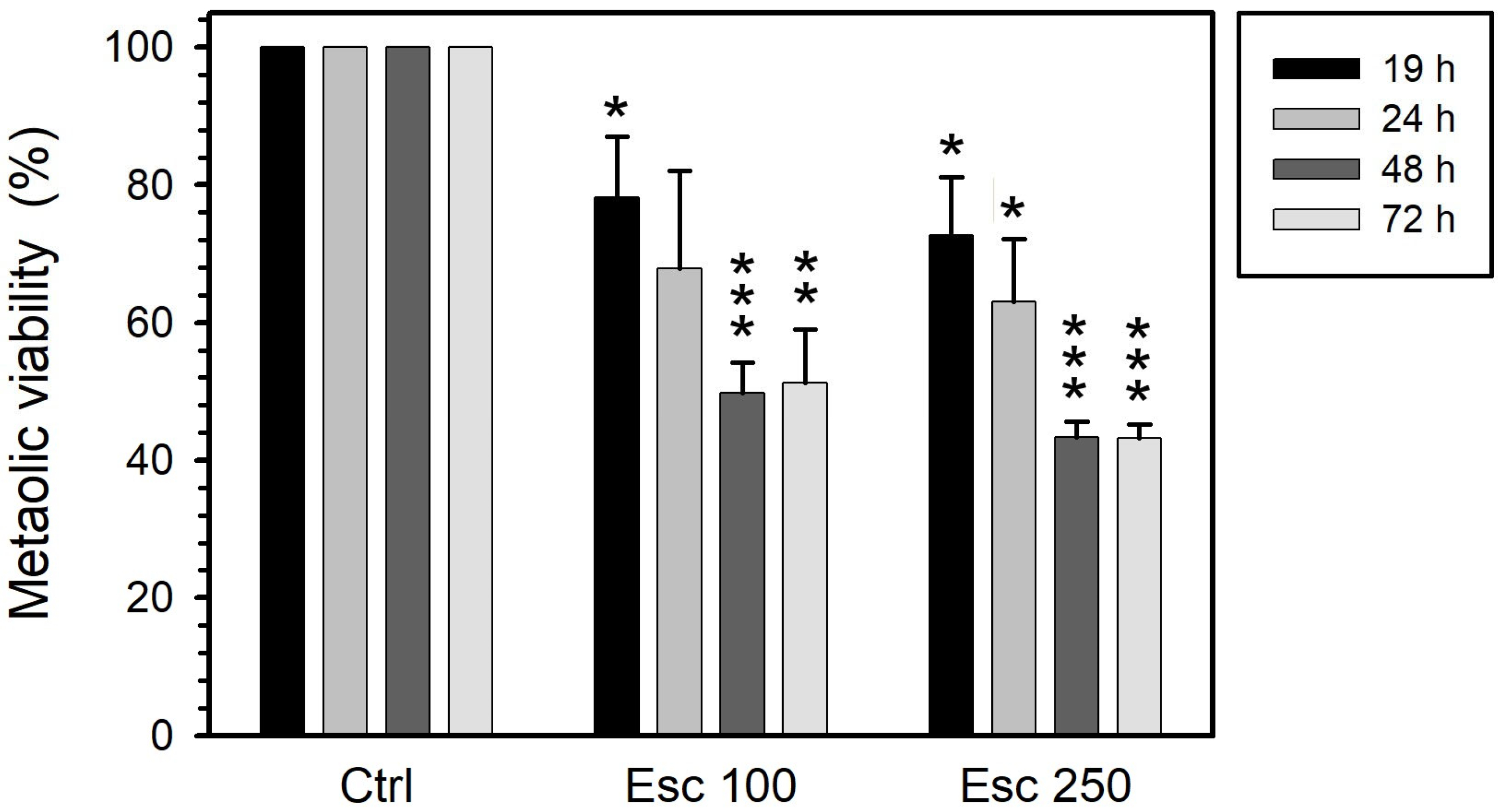
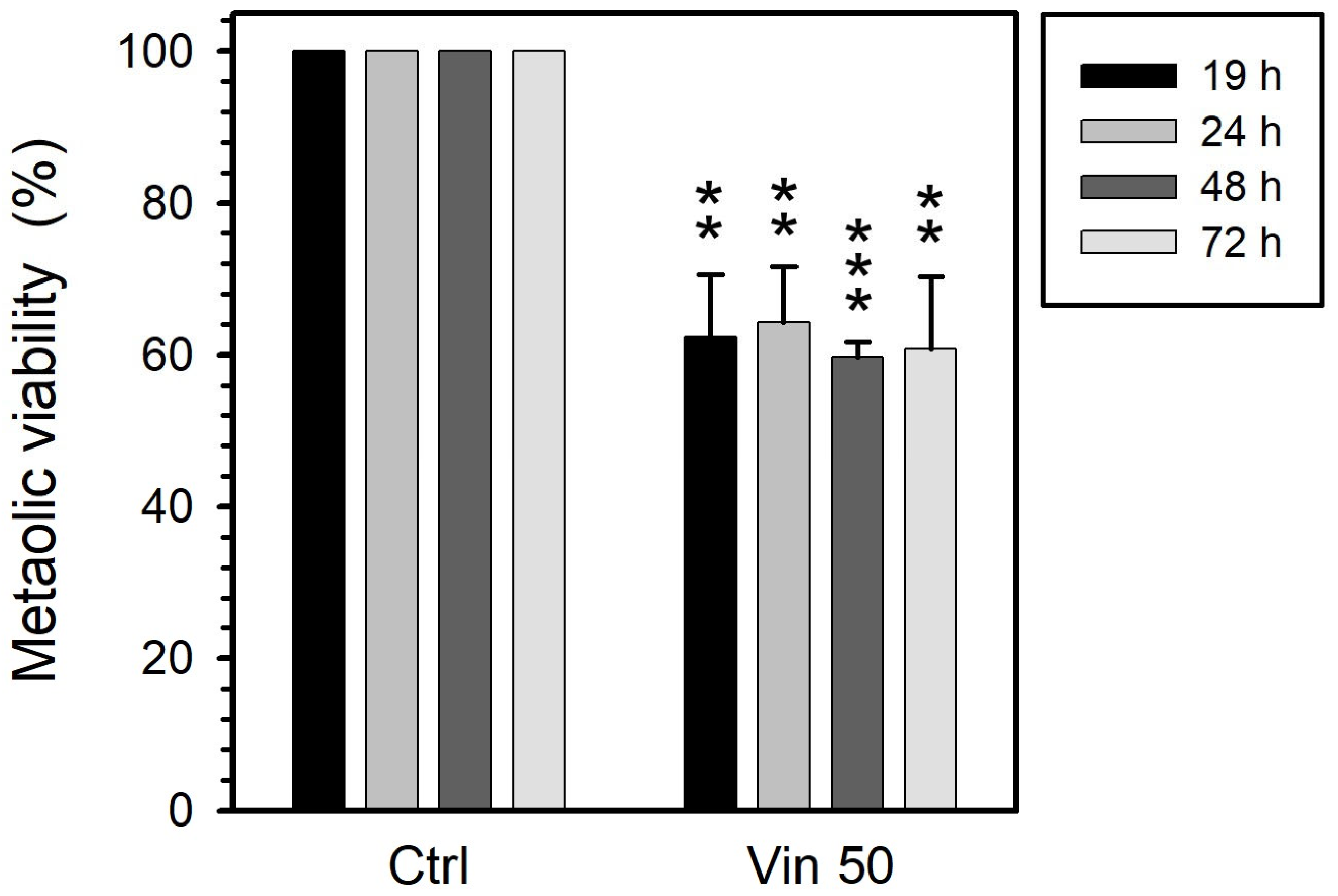
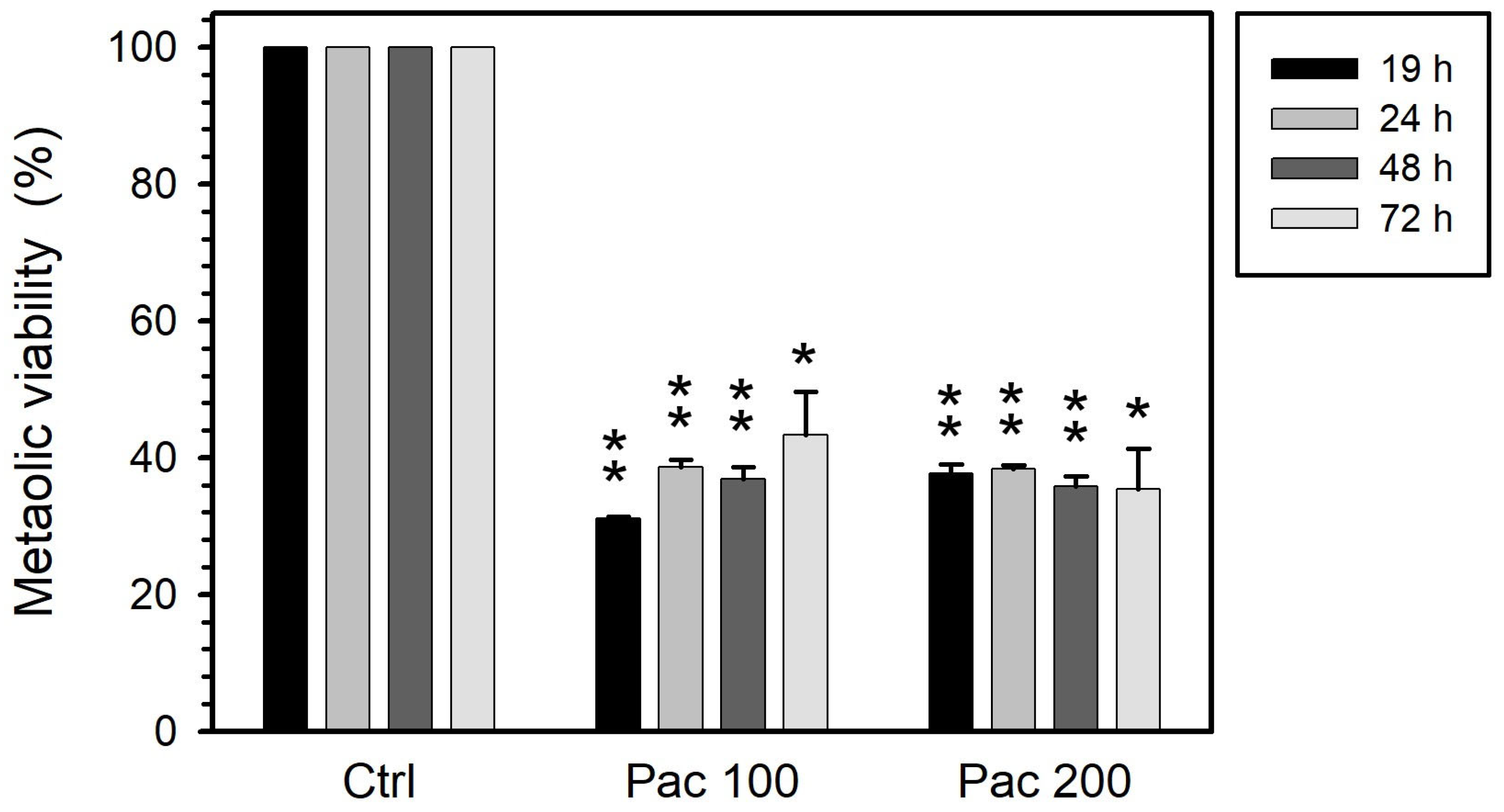
3.1. Metabolic Viability
3.2. Cell Viability
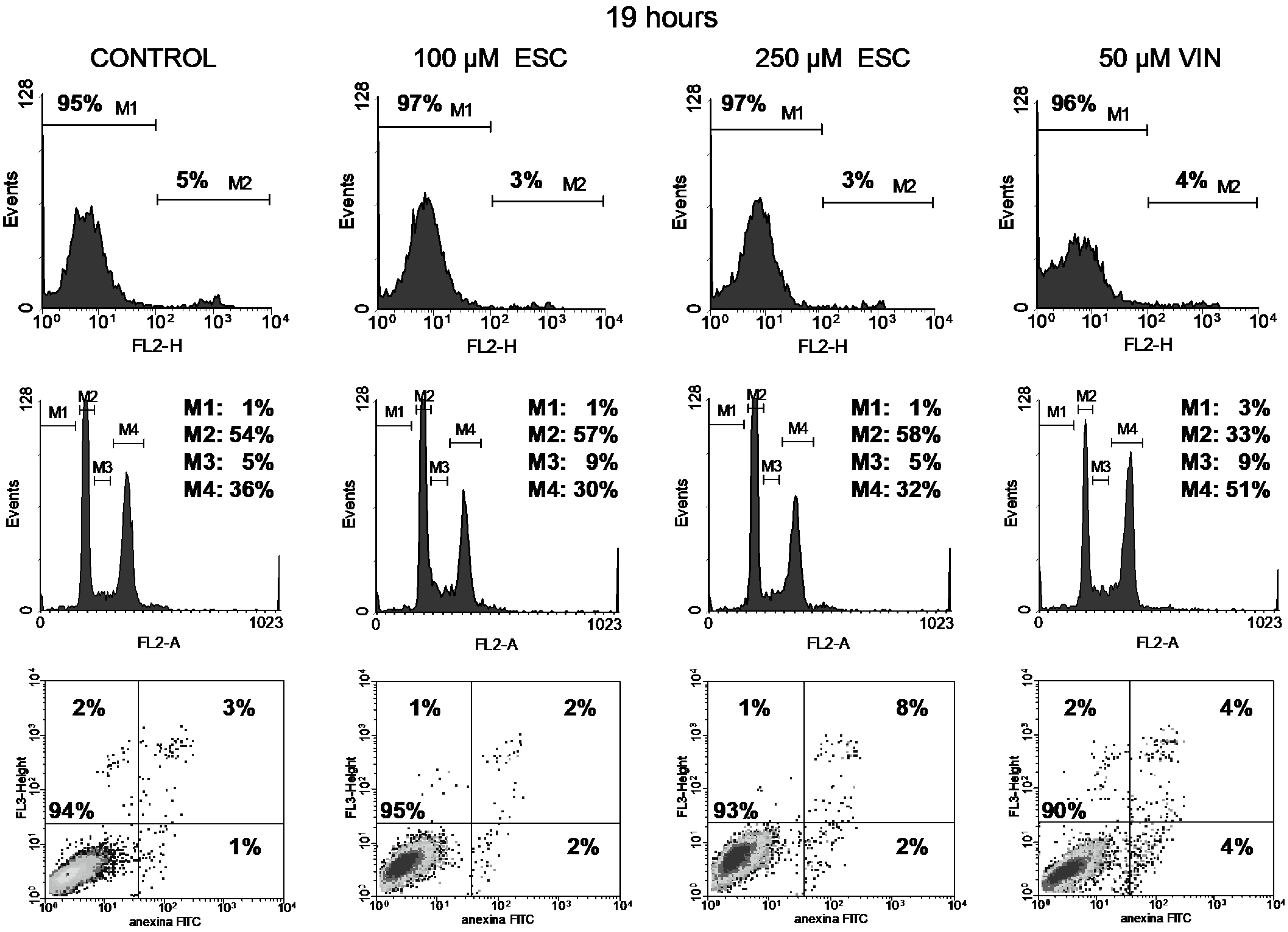
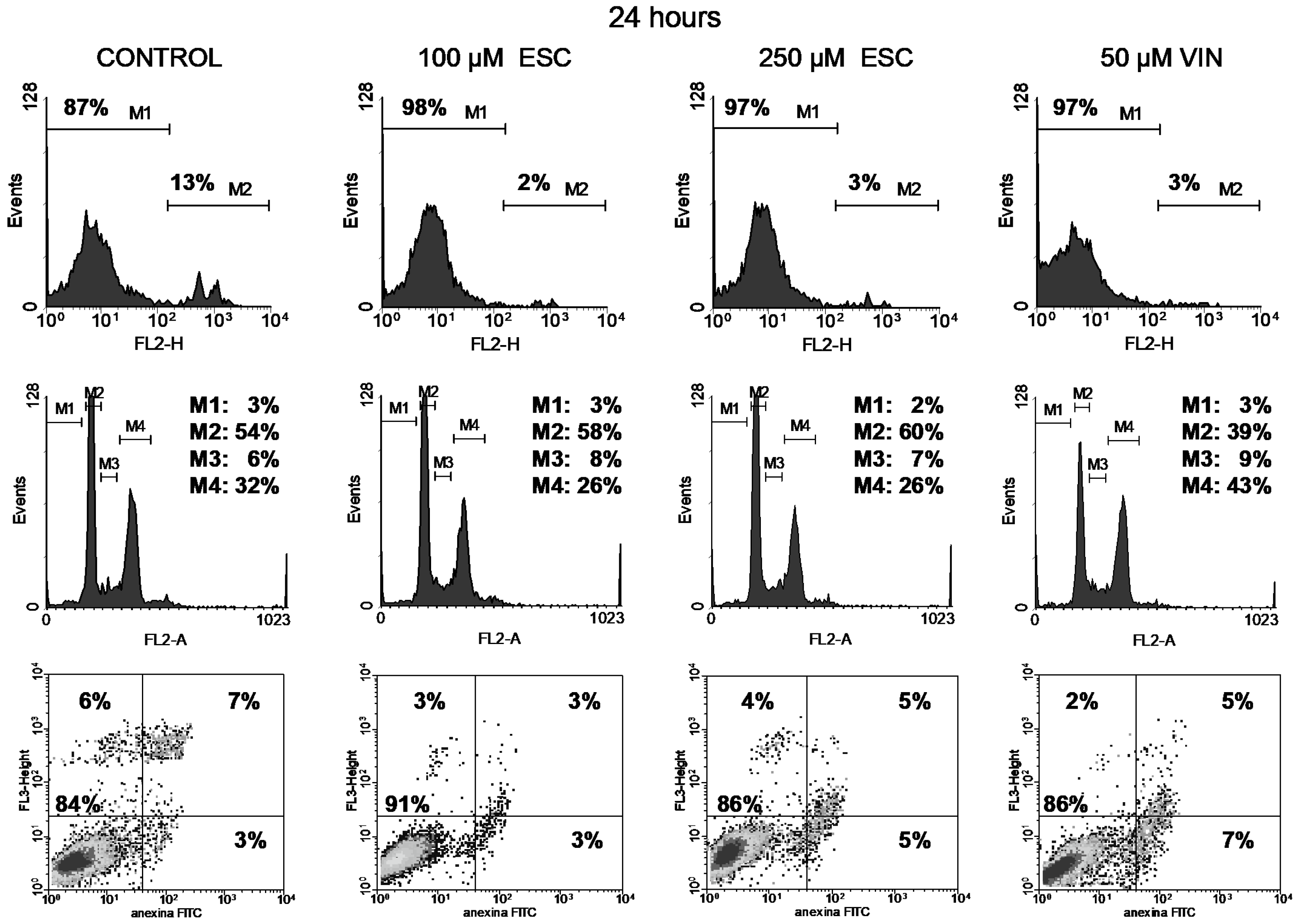
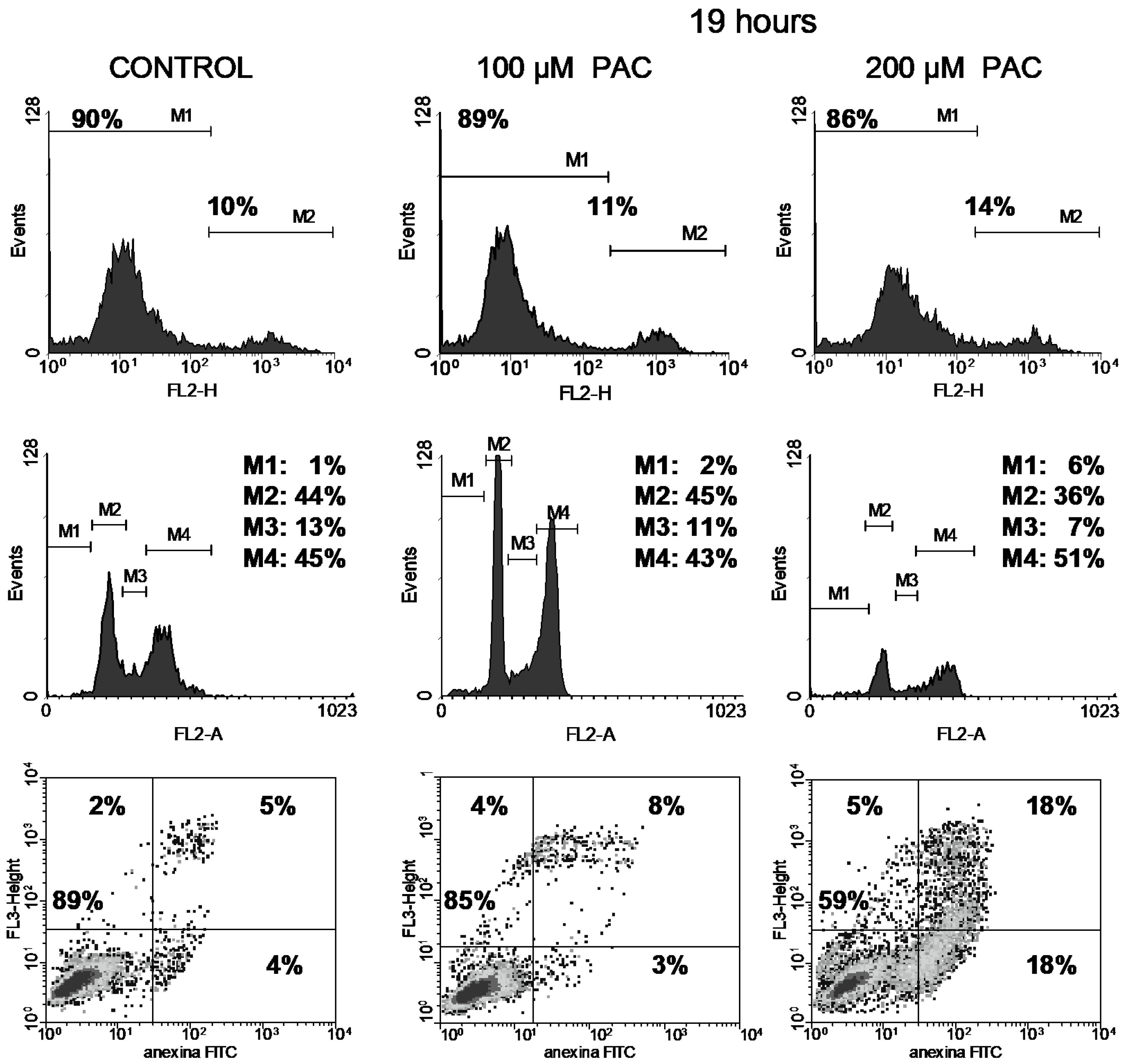
3.3. Cell Cycle
3.4. Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stavridi, F.; Karapanagiotou, E.M.; Syrigos, K.N. Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat. Rev. 2010, 36, 122–130. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, G.; Getzenberg, R.H.; Veltri, R.W. The upregulation of PI3K/Akt and MAP kinase pathways is associated with resistance of microtubule-targeting drugs in prostate cancer. J. Cell. Biochem. 2015, 116, 1341–1349. [Google Scholar] [CrossRef]
- Chuu, C.P.; Kokontis, J.M.; Hiipakka, R.A.; Liao, S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J. Biomead. Sci. 2007, 14, 543–553. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Liao, P.C.; Lieu, C.H. Cell cycle specific induction of apoptosis and necrosis by paclitaxel in the leukemic U937 cells. Life Sci. 2005, 76, 1623–1639. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxel-induced cell death: Where the cell cycle and apoptosis come together. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, F.; Budman, D.R. Review: Tubulin function, action of antitubulin drugs, and new drug development. Cancer Investig. 2005, 23, 264–273. [Google Scholar] [CrossRef]
- Bergstralh, D.T.; Ting, J.P. Microtubule stabilizing agents: Their molecular signaling consequences and the potential for enhancement by drug combination. Cancer Treat. Rev. 2006, 32, 166–179. [Google Scholar] [CrossRef]
- Ganansia-Leymarie, V.; Bischoff, P.; Bergerat, J.P.; Holl, V. Signal transduction pathways of taxanes-induced apoptosis. Curr. Med. Chem. Anticancer Agents 2003, 3, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Z. Indole alkaloids with potential anticancer activity. Curr. Top. Med. Chem. 2020, 20, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Hridoy, M.; Gorapi, M.Z.H.; Noor, S.; Chowdhury, N.S.; Rahman, M.M.; Muscari, I.; Masia, F.; Adorisio, S.; Delfino, D.V.; Mazid, M.A. Putative anticancer compounds from plant-derived endophytic fungi: A review. Molecules 2022, 27, 296. [Google Scholar] [CrossRef] [PubMed]
- Liao, V.W.Y.; Kumari, A.; Narlawar, R.; Vignarajan, S.; Hibbs, D.E.; Panda, D.; Groundwater, P.W. Tubulin-binding 3,5-bis(styryl)pyrazoles as lead compounds for the treatment of castration-resistant prostate cancer. Mol. Pharmacol. 2020, 97, 409–422. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Kaneko, T.; Tahara, S.; Takabayashi, F. Suppression of lipid hydroperoxide-induced oxidative damage to cellular DNA by esculetin. Biol. Pharm. Bull. 2023, 26, 840–844. [Google Scholar] [CrossRef]
- Kaneko, T.; Tahara, S.; Takabayashi, F. Inhibitory effect of natural coumarin compounds, esculetin and esculin, on oxidative DNA damage and formation of aberrant crypt foci and tumours induced by 1,2-dimethylhydrazine in rat colons. Biol. Pharm. Bull. 2007, 30, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, L.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef]
- Peng, X.M.; Damu, G.L.V.; Zhou, C.H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- Sekiya, K.; Okuda, H.; Arich, S. Selective inhibition of platelet lipoxygenase by esculetin. Biochim. Biophys. Acta 1982, 713, 68–72. [Google Scholar]
- Subramaniam, S.R.; Ellis, E.M. Esculetin-induced protection of human hepatoma HepG2 cells against hydrogen peroxide is associated with the Nrf2-dependent induction of the NAD(P)H:quinone oxidoreductase 1 gene. Toxicol. Appl. Pharmacol. 2011, 250, 130–136. [Google Scholar] [CrossRef]
- Yang, J.Y.; Della-Fera, M.A.; Hartzell, D.L.; Nelson-Dooley, C.; Hausman, D.B.; Baile, C.A. Esculetin induces apoptosis and inhibits adipogenesis in 3T3-L1 cells. Obesity 2006, 14, 1691–1699. [Google Scholar] [CrossRef]
- Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kwon, T.K.; Choi, B.T.; Lee, S.J.; Lee, W.H.; Choi, Y.H. Induction of apoptosis by esculetin in human leukemia U937 cells through activation of JNK and ERK. Toxicol. Appl. Pharmacol. 2008, 227, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jin, C.Y.; Kwon, H.J.; Hwang, H.J.; Kim, G.Y.; Choi, I.W.; Know, T.K.; Kim, B.W.; Kim, W.J.; Choi, Y.H. Induction of apoptosis by esculetin in human leukaemia U937 cells: Roles of Bcl-2 and extracellular-regulated kinase signalling. Toxicol. Vitr. 2010, 24, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.Y.; Tsai, Y.Y.; Wang, C.J.; Lin, W.L.; Tseng, T.H. Induction of apoptosis by esculetin in human leukemia cells. Eur. J. Pharmacol. 2001, 416, 25–32. [Google Scholar] [CrossRef]
- Rubio, V.; Calviño, E.; García-Pérez, A.I.; Herráez, A.; Diez, J.C. Human acute promyelocytic leukemia NB4 cells are sensitive to esculetin through induction of an apoptotic mechanism. Chem.-Biol. Interact. 2014, 220, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; García-Pérez, A.I.; Tejedor, M.C.; Herráez, A.; Diez, J.C. Esculetin neutralises cytotoxicity of t-BHP but not of H2O2 on human leukaemia NB4 cells. BioMed Res. Int. 2017, 2017, 9491045. [Google Scholar] [CrossRef]
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Tejedor, M.C.; Diez, J.C. Esculetin modulates cytotoxicity induced by oxidants in NB4 human leukemia cells. Exp. Toxicol. Pathol. 2017, 69, 700–712. [Google Scholar] [CrossRef]
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Diez, J.C. Different roles of Nrf2 and NFκB in the antioxidant imbalance produced by esculetin or quercetin on NB4 leukemia cells. Chem.-Biol. Interact. 2018, 294, 156–166. [Google Scholar] [CrossRef]
- Kuo, H.C.; Lee, H.J.; Hu, C.C.; Shun, H.I.; Tseng, T.H. Enhancement of esculetin on taxol-induced apoptosis in human hepatoma HepG2 cells. Toxicol. Appl. Pharmacol. 2006, 210, 55–62. [Google Scholar] [CrossRef]
- Barthomeuf, C.; Grassi, J.; Demeule, M.; Fournier, C.; Boivin, D.; Béliveau, R. Inhibition of P-glycoprotein transport function and reversion of MDR1 multidrug resistance by cnidiadin. Cancer Chemother. Pharmacol. 2005, 56, 173–181. [Google Scholar] [CrossRef]
- Makowska, K.; Estañ, M.C.; Gañán-Gómez, I.; Boyano-Adánez, M.C.; García-Pérez, A.I.; Sancho, P. Changes in mitochondrial function induced by dequalinium precede oxidative stress and apoptosis in the human prostate cancer cell line PC-3. Mol. Biol. 2014, 48, 359–370. [Google Scholar] [CrossRef]
- Shankar, S.; Chen, X.; Srivastava, R.K. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate 2005, 62, 165–186. [Google Scholar] [CrossRef]
- Morales-Cano, D.; Calviño, E.; Rubio, V.; Herráez, A.; Sancho, P.; Tejedor, M.C.; Diez, J.C. Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp. Toxicol. Pathol. 2013, 65, 1101–1108. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, P.; Hoffman, R.M. Early reporting of apoptosis by real-time imaging of cancer cells labeled with green fluorescent protein in the nucleus and red fluorescent protein in the cytoplasm. Anticancer Res. 2015, 35, 2539–2543. [Google Scholar]
- Pierre, M.E.; Manneh, R.; Hernández, A.; Rodríguez, J.; Fletcher, A.V.; Ramírez, H.M.; Niño, O.M.; Gómez, D.A.; Sanabria, D.; Contreras, F.; et al. Expert consensus: Profiling and management of advanced or metastatic epithelial ovarian cancer. Rev. Colomb. Obstet. Ginecol. 2024, 75, 4094. [Google Scholar] [CrossRef]
- Hitchen, N.; Waldron, N.R.; Deva, S.; Findlay, M.; Lawrence, B. Real-world outcomes of cisplatin, capecitabine, and gemcitabine with either epirubicin (PEXG) or docetaxel (PDXG) as first-line palliative treatment in metastatic or unresectable locally advanced pancreatic adenocarcinoma. Asia Pac. J. Clin. Oncol. 2023, 19, e231–e238. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Góralczyk, A.; Łuszczki, J.J. Synergy, additivity and antagonism between esculetin and six commonly used chemotherapeutics in various malignant melanoma cell lines—An isobolographic analysis. Molecules 2023, 28, 3889. [Google Scholar] [CrossRef] [PubMed]
- Turkekul, K.; Colpan, R.D.; Baykul, T.; Ozdemir, M.D.; Erdogan, S. Esculetin inhibits the survival of human prostate cancer cells by inducing apoptosis and arresting the cell cycle. J. Cancer Prev. 2018, 23, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, C.M.; Park, S.H.; Nam, M.J. Esculetin induces cell cycle arrest and apoptosis in human colon cancer LoVo cells. Environ. Toxicol. 2019, 34, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Chou, C.T.; Lin, Y.S.; Shieh, P.; Kuo, D.H.; Jan, C.R.; Liang, W.Z. Esculetin, a natural coumarin compound, evokes Ca2+ movement and activation of Ca2+-associated mitochondrial apoptotic pathways that involved cell cycle arrest in ZR-75-1 human breast cancer cells. Tumour Biol. 2016, 37, 4665–4678. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Moon, S.K.; Hyun, J.W.; Lee, W.H.; Choi, B.T.; Kwon, T.K.; Yoo, Y.H.; et al. Involvement of extracellular signal-related kinase signaling in esculetin induced G1 arrest of human leukemia U937 cells. Biomed. Pharmacother. 2008, 62, 723–729. [Google Scholar] [CrossRef]
- You, B.J.; Chen, L.Y.; Hsu, P.H.; Sung, P.H.; Hung, Y.C.; Lee, H.Z. Orlistat displays antitumour activity and enhances the efficacy of paclitaxel in human hepatoma Hep3B cells. Chem. Res. Toxicol. 2019, 32, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Puebla, L.; Diez, J.C. The antioxidants esculetin and quercetin modulate differently redox balance in human NB4 leukemia cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Liao, J.; Liao, H.; Zhang, M.; Yu, Y.; Cai, L.; Le, K.; Fu, W.; Qin, Y.; Hou, T.; Li, D.; et al. Identification of oral bioavailable coumarin derivatives as potential AR antagonists targeting prostate cancer. J. Med. Chem. 2024, 67, 19395–19416. [Google Scholar] [CrossRef]
- Pullaiah, C.P.; Nelson, V.K.; Rayapu, S.; Kumar, N.; Kedam, T. Exploring cardioprotective potential of esculetin against isoproterenol induced myocardial toxicity in rats: In vivo and in vitro evidence. BMC Pharmacol. Toxicol. 2021, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Srirangan, P.; Prince, S.E. Exploring the therapeutic potential of esculetin in mitigating cyclophosphamide-induced hepato-toxicity. Indian J. Clin. Biochem. 2025. [Google Scholar] [CrossRef]
- Köroğlu, Z.; Duygu Kizir, D.; Karaman, M.; Demir, Y.; Türkeş, C.; Beydemir, S. Protective effects of esculetin against doxorubicin-induced toxicity correlated with oxidative stress in rat liver: In vivo and in silico studies. Biochem. Mol. Toxicol. 2024, 38, e23702. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, J.; Hu, B.; Tu, X.; Yang, J.; Sun, W.; Duan, X. Esculetin inhibits liver cancer by targeting glucose-6-phosphate isomerase mediated glycolysis. Biomed. Pharmacother. 2025, 188, 118118. [Google Scholar] [CrossRef]
- Hossain, M.R.; Shova, F.T.Z.; Akter, M.; Shuvo, S.; Ahmed, N.; Akter, A.; Haque, M.; Salma, U.; Mogal, M.R.; Saha, H.R.; et al. Esculetin unveiled: Decoding its anti-tumor potential through molecular mechanisms—A comprehensive review. Cancer Rep. 2024, 7, e1948. [Google Scholar] [CrossRef]
- Liu, M.; Sheng, Y.; Guo, F.; Wu, J.; Huang, Y.; Yang, X.; Wang, M.; Zhang, S.; Li, P. Therapeutic potential of esculetin in various cancer types. Oncol. Lett. 2024, 28, 305. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Jeena Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Perween, A.; Momal, U.; Imran, M.; Hassan, M.H.U.; Naeem, H.; Mujtaba, A.; Hussain, M.; Alsagaby, S.A.; Abdulmonem, W.A.; et al. Recent perspectives on anticancer potential of coumarin against different human malignancies: An updated review. Food Sci. Nutr. 2024, 13, e4696. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pérez, A.I.; Rubio, V.; Herráez, A.; Puebla, L.; Diez, J.C. Cytotoxicity of Esculetin Compared with Vinblastine and Paclitaxel in PC-3 Prostate Cancer Cells. Curr. Oncol. 2025, 32, 526. https://doi.org/10.3390/curroncol32090526
García-Pérez AI, Rubio V, Herráez A, Puebla L, Diez JC. Cytotoxicity of Esculetin Compared with Vinblastine and Paclitaxel in PC-3 Prostate Cancer Cells. Current Oncology. 2025; 32(9):526. https://doi.org/10.3390/curroncol32090526
Chicago/Turabian StyleGarcía-Pérez, Ana I., Virginia Rubio, Angel Herráez, Lilian Puebla, and José C. Diez. 2025. "Cytotoxicity of Esculetin Compared with Vinblastine and Paclitaxel in PC-3 Prostate Cancer Cells" Current Oncology 32, no. 9: 526. https://doi.org/10.3390/curroncol32090526
APA StyleGarcía-Pérez, A. I., Rubio, V., Herráez, A., Puebla, L., & Diez, J. C. (2025). Cytotoxicity of Esculetin Compared with Vinblastine and Paclitaxel in PC-3 Prostate Cancer Cells. Current Oncology, 32(9), 526. https://doi.org/10.3390/curroncol32090526





