Physicians’ Controversies Towards Fertility Preservation in Young Patients with Gynecological Cancer: An MITO Survey
Simple Summary
Abstract
1. Introduction
2. Material and Methods
Characteristics of the Survey
3. Results
3.1. Knowledge About Fertility Preservation Techniques
3.2. Artificial Reproduction Techniques
3.3. Fertility Preservation in Borderline Ovarian Tumors or Malignant Ovarian Tumors
3.4. Endometrial Cancer
3.5. Cervical Cancer
3.6. Genetic Issues
4. Discussion
4.1. Fertility Preservation Techniques
4.2. Borderline Ovarian Tumors
4.3. Endometrial Cancer
4.4. Cervical Cancer
4.5. Genetic Issues
4.6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOT | Borderline Ovarian Tumor |
| BRCA | BReast CAncer gene |
| BRIP1 | BReast CAncer 1 Interacting Protein 1 |
| ESGE | European Society for Gynaecological Endoscopy |
| ESGO | European Society of Gynaecological Oncology |
| ESHRE | European Society of Human Reproduction and Embryology |
| FP | Fertility Preservation |
| GnRHa | Gonadotropin-Releasing Hormone Analog |
| MITO | Multicenter Italian Trials in Ovarian Cancer and Gynecologic Malignancies |
| RAD51C | Radiation-Sensitive Protein 51C |
| RAD51D | Radiation-Sensitive Protein 51D |
| REI | Reproductive Endocrinology and Infertility |
| SPSS | Statistical Package for the Social Sciences |
| VUS | Variant of Unknown Significance |
References
- Scott, A.R.; Stoltzfus, K.C.; Tchelebi, L.T.; Trifiletti, D.M.; Lehrer, E.J.; Rao, P.; Bleyer, A.; Zaorsky, N.G. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973–2015. JAMA Netw. Open 2020, 3, e2027738. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana di Oncologia Medica. I Numeri del Cancro in Italia 2020; Rapporto AIOM–AIRTUM; Associazione Italiana di Oncologia Medica: Milan, Italy, 2020. [Google Scholar]
- Astolfi, P.; Zonta, L.A. Delayed maternity and risk at delivery. Paediatr. Perinat. Epidemiol. 2002, 16, 67–72. [Google Scholar] [CrossRef]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Pappo, A.S.; Acquazzino, M.; Allen-Rhoades, W.A.; Barnett, M.; Borinstein, S.C.; Casey, R.; Choo, S.; Chugh, R.; Dinner, S.; et al. Adolescent and Young Adult (AYA) Oncology, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023, 21, 851–880. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Hagege, E.; Sokteang, S.; Ayoubi, J.M.; de Ziegler, D. Fertility preservation counseling: Old indications, novel perspectives. Fertil. Steril. 2024, 121, 553–554. [Google Scholar] [CrossRef]
- Morice, P.; Scambia, G.; Abu-Rustum, N.R.; Acien, M.; Arena, A.; Brucker, S.; Cheong, Y.; Collinet, P.; Fanfani, F.; Filippi, F.; et al. Fertility-sparing treatment and follow-up in patients with cervical cancer, ovarian cancer, and borderline ovarian tumours: Guidelines from ESGO, ESHRE, and ESGE. Lancet Oncol. 2024, 25, e602–e610. [Google Scholar] [CrossRef]
- Practice Committee of the Oncofertility Consortium. Installing oncofertility programs for common cancers in optimum resource settings (Repro-Can-OPEN Study Part II): A committee opinion. J. Assist. Reprod. Genet. 2021, 38, 163–176. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Oktay, K. Fertility preservation during cancer treatment: Clinical guidelines. Cancer Manag. Res. 2014, 6, 105–117. [Google Scholar] [CrossRef]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Benoit, J.; Foong, S.; Saumet, J.; Korkidakis, A.; Marr, K.; McQuillan, S.; Todd, N. Fertility preservation in patients undergoing gonadotoxic treatments: A Canadian Fertility and Andrology Society clinical practice guideline. Reprod. Biomed. Online 2024, 48, 103767. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M. Fertility Preservation in Women. N. Engl. J. Med. 2018, 378, 400–401. [Google Scholar]
- Duffin, K.; Howie, R.; Kelsey, T.W.; Wallace, H.B.; Anderson, R.A. Long-term follow-up to assess criteria for ovarian tissue cryopreservation for fertility preservation in young women and girls with cancer. Hum. Reprod. 2023, 38, 1076–1085. [Google Scholar] [CrossRef]
- Poirot, C.; Fortin, A.; Lacorte, J.M.; Akakpo, J.P.; Genestie, C.; Vernant, J.P.; Brice, P.; Morice, P.; Leblanc, T.; Gabarre, J.; et al. Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum. Reprod. 2019, 34, 1083–1094. [Google Scholar] [CrossRef]
- Khattak, H.; Malhas, R.; Craciunas, L.; Afifi, Y.; Amorim, C.A.; Fishel, S.; Silber, S.; Gook, D.; Demeestere, I.; Bystrova, O.; et al. Correction to: Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: A systematic review and individual patient data meta-analysis. Hum. Reprod. Update 2022, 28, 455. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, R.; Cervini, L.; Taccagni, G.; Papaleo, E.; Pagliardini, L.; Bergamini, A.; Ferrari, S.; Mangili, G.; Candiani, M. A prospective, observational study of chemotherapy-induced ovarian damage on follicular reserve and maturation. Arch. Gynecol. Obstet. 2022, 306, 1723–1729. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Feng, J.; Ma, W.; Qiu, L.; Wang, D.; Yang, Z. Current status of fertility preservation procedures in gynecologic oncology: From a Chinese perspective. J. Assist. Reprod. Genet. 2025, 42, 635–645. [Google Scholar] [CrossRef]
- Geoffron, S.; Lier, A.; de Kermadec, E.; Sermondade, N.; Varinot, J.; Thomassin-Naggara, I.; Bendifallah, S.; Daraï, E.; Chabbert-Buffet, N.; Kolanska, K. Fertility preservation in women with malignant and borderline ovarian tumors: Experience of the French ESGO-certified center and pregnancy-associated cancer network (CALG). Gynecol. Oncol. 2021, 161, 817–824. [Google Scholar] [CrossRef]
- Mazzon, I.; Masciullo, V.; Scambia, G.; Ferrandina, G.; Corrado, G. Long-term survival of young endometrial cancer patients desiring fertility preservation treated with hysteroscopic resection followed by hormone therapy (NEMO technique). Int. J. Gynaecol. Obstet. 2020, 151, 305–307. [Google Scholar] [CrossRef]
- Tsonis, O.; Kopeika, J. Fertility preservation in patients with gynaecologic malignancy: Response to ovarian stimulation and long-term outcomes. Eur. J. Obs. Gynecol. Reprod. Biol. 2023, 290, 93–100. [Google Scholar] [CrossRef]
- Gallo, A.; Di Spiezio Sardo, A.; Conforti, A.; Iorio, G.G.; Zizolfi, B.; Buonfantino, C.; De Angelis, M.C.; Strina, I.; Marrone, V.; Bifulco, G.; et al. Assessing ovarian stimulation with letrozole and levonorgestrel intrauterine system after combined fertility-sparing approach for atypical endometrial lesions: A retrospective case-control study. Reprod. Biomed. Online 2023, 48, 103750. [Google Scholar]
- Rodolakis, A.; Scambia, G.; Planchamp, F.; Acien, M.; Di Spiezio Sardo, A.; Farrugia, M.; Grynberg, M.; Pakiz, M.; Pavlakis, K.; Vermeulen, N.; et al. ESGO/ESHRE/ESGE Guidelines for the fertility-sparing treatment of patients with endometrial carcinoma. Hum. Reprod. Open 2023, 2023, hoac057. [Google Scholar] [CrossRef]
- Cibula, D.; Rosaria Raspollini, M.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Radiother. Oncol. 2023, 184, 109682. [Google Scholar]
- Söderström-Anttila, V.; Wennerholm, U.B.; Loft, A.; Pinborg, A.; Aittomäki, K.; Romundstad, L.B.; Bergh, C. Surrogacy: Outcomes for surrogate mothers, children and the resulting families-a systematic review. Hum. Reprod. Update 2016, 22, 260–276. [Google Scholar] [CrossRef]
- Jadva, V.; Blake, L.; Casey, P.; Golombok, S. Surrogacy families 10 years on: Relationship with the surrogate, decisions over disclosure and children’s understanding of their surrogacy origins. Hum. Reprod. 2012, 27, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.; Covens, A. Fertility Preservation in Cervical Cancer-Treatment Strategies and Indications. Curr. Oncol. 2024, 31, 296–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomao, F.; Corrado, G.; Peccatori, F.A.; Boveri, S.; Preti, E.P.; Colombo, N.; Landoni, F. Fertility-Sparing Options in Young Women with Cervical Cancer. Curr. Treat. Options Oncol. 2016, 17, 5. [Google Scholar] [CrossRef]
- Bizzarri, N.; Pavone, M.; Loverro, M.; Querleu, D.; Fagotti, A.; Scambia, G. Ovarian preservation in gynecologic oncology: Current indications and techniques. Curr. Opin. Oncol. 2023, 35, 401–411. [Google Scholar] [CrossRef]
- Chaudhri, E.N.; Salman, A.; Awartani, K.; Khan, Z.; Hashmi, S.K. Ovarian Tissue Cryopreservation versus Other Fertility Techniques for Chemoradiation-Induced Premature Ovarian Insufficiency in Women: A Systematic Review and Future Directions. Life 2024, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Marchetti, C.; Trozzi, R.; Scambia, G.; Fagotti, A. Fertility preservation in patients with BRCA mutations or Lynch syndrome. Int. J. Gynecol. Cancer 2021, 31, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M.; Raanani, H.; Feldman, B.; Srebnik, N.; Dereck-Haim, S.; Manela, D.; Brenghausen, M.; Geva-Lerner, L.; Friedman, E.; Levi-Lahad, E.; et al. BRCA mutation carriers show normal ovarian response in in vitro fertilization cycles. Fertil. Steril. 2015, 104, 1162–1167. [Google Scholar] [CrossRef] [PubMed]

| Respondents | N = 56 | % |
|---|---|---|
| Age (years) | ||
| <40 | 21 | 37.5 |
| 40–50 | 16 | 28.6 |
| >50 | 19 | 33.9 |
| Gender | ||
| Male | 20 | 35.7 |
| Female | 36 | 64.3 |
| Religion | ||
| Catholic | 49 | 87.5 |
| Protestant | 0 | 0.0 |
| Muslim | 0 | 0.0 |
| Hindu | 1 | 1.8 |
| Jewish | 0 | 0.0 |
| Atheist/none | 4 | 7.1 |
| Prefer not to answer | 2 | 3.6 |
| Region of practice | ||
| Northern Italy | 30 | 53.6 |
| Central Italy | 12 | 21.4 |
| Southern Italy | 10 | 17.9 |
| Italian islands (i.e., Sicily, Sardinia) | 4 | 7.1 |
| Specialty | ||
| Gynecology | 33 | 58.9 |
| Medical oncology | 19 | 33.9 |
| Radiation oncology | 2 | 3.6 |
| Fertility specialist | 2 | 3.6 |
| Practice environment—1 | ||
| Specialized cancer center | 18 | 32.1 |
| Academic general hospital | 20 | 35.7 |
| Non-academic general hospital | 17 | 30.4 |
| Other | 1 | 1.8 |
| Practice environment—2 | ||
| Public | 44 | 78.6 |
| Private | 5 | 8.9 |
| Both | 7 | 12.5 |
| Working in a Gynecologic Oncology Unit | ||
| No | 22 | 39.3 |
| Yes | 34 | 60.7 |
| Years of clinical experience | ||
| <5 years | 12 | 21.4 |
| 5–10 years | 10 | 17.9 |
| 11–19 years | 15 | 26.8 |
| 20–29 years | 13 | 23.2 |
| >30 years | 6 | 10.7 |
| Respondents | N = 56 | % |
|---|---|---|
| Have you ever consulted some (inter)national guidelines on fertility preservation in patients with cancer and cancer survivors? | ||
| No, I am not aware of available guidelines on this topic | 3 | 5.4 |
| No, but I know where to find these guidelines, if needed | 2 | 3.6 |
| Yes | 51 | 91.1 |
| How would you describe your knowledge of the use of GnRH analogs in patients with gynecological cancer? | ||
| Not at all knowledgeable | 3 | 5.4 |
| Aware, but not very knowledgeable | 7 | 12.5 |
| Knowledgeable | 30 | 53.6 |
| Very knowledgeable | 16 | 28.6 |
| How would you describe your knowledge of oocyte cryopreservation in patients with gynecological cancer? | ||
| Not at all knowledgeable | 2 | 3.6 |
| Aware, but not very knowledgeable | 10 | 17.9 |
| Knowledgeable | 25 | 44.6 |
| Very knowledgeable | 19 | 33.9 |
| How would you describe your knowledge of ovarian tissue cryopreservation in patients with gynecological cancer? | ||
| Not at all knowledgeable | 0 | 0.0 |
| Aware, but not very knowledgeable | 14 | 25.0 |
| Knowledgeable | 27 | 48.2 |
| Very knowledgeable | 15 | 26.8 |
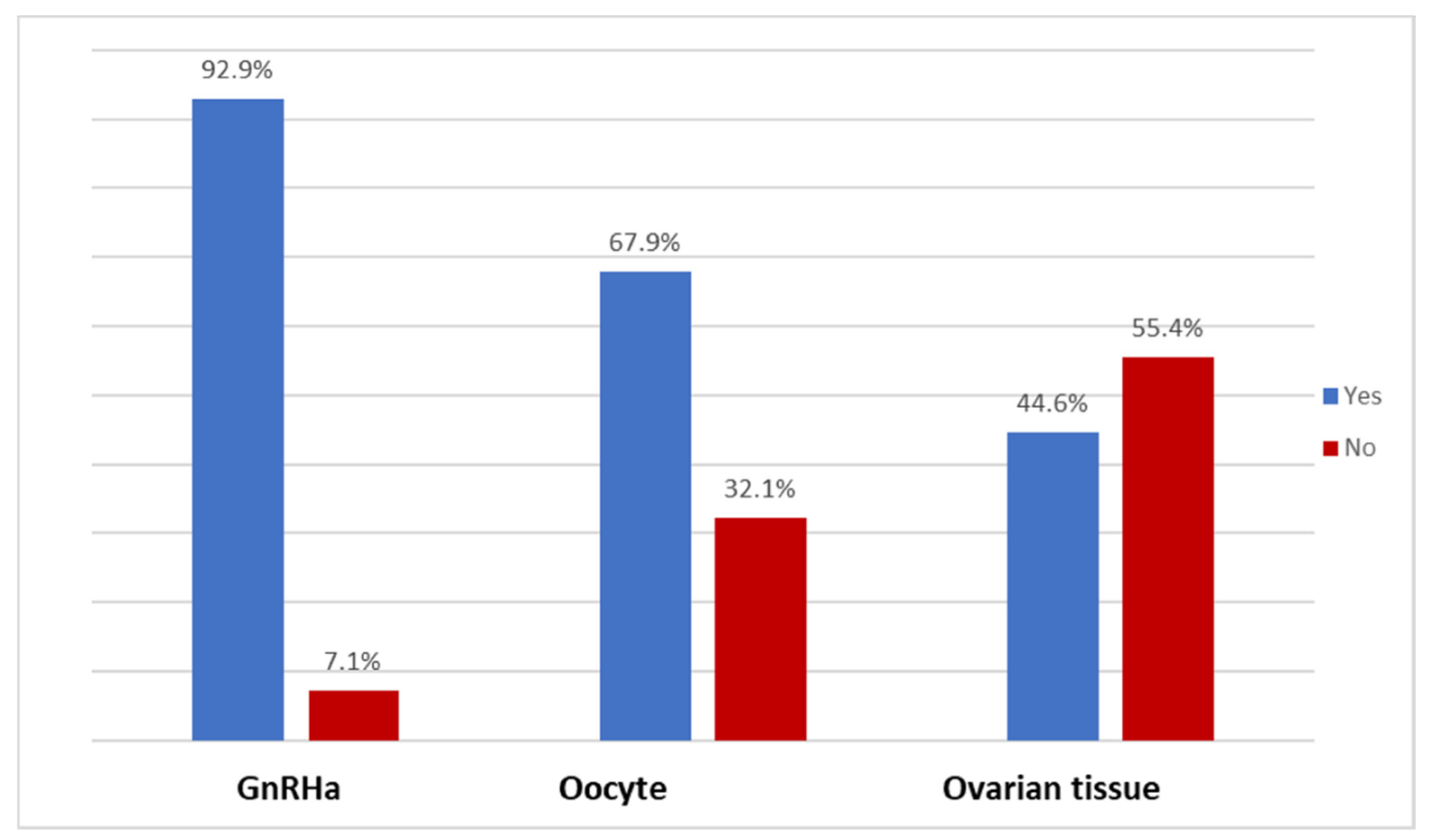
| Is ovarian suppression with GnRH analogs during chemotherapy available in your setting? | ||
| No | 4 | 7.1 |
| Yes | 52 | 92.9 |
| Is oocyte cryopreservation available in your setting? | ||
| No | 18 | 32.1 |
| Yes | 38 | 67.9 |
| Is ovarian tissue cryopreservation available in your setting? | ||
| No | 31 | 55.4 |
| Yes | 25 | 44.6 |
| To what extent do you think each of the following suggestions that may improve female oncofertility care might be of value in your setting? | ||
| Increment of patient awareness | ||
| Useless | 1 | 1.8 |
| Not valuable | 1 | 1.8 |
| Neutral | 5 | 8.9 |
| Valuable | 25 | 44.6 |
| Highly valuable | 24 | 42.9 |
| Development and provision of patient information materials (e.g., decision aids, leaflets) | ||
| Useless | 2 | 3.6 |
| Not valuable | 7 | 12.5 |
| Neutral | 0 | 0.0 |
| Valuable | 27 | 48.2 |
| Highly valuable | 20 | 35.7 |
| Education of professionals | ||
| Useless | 1 | 1.8 |
| Not valuable | 0 | 0.0 |
| Neutral | 1 | 1.8 |
| Valuable | 15 | 26.8 |
| Highly valuable | 39 | 69.6 |
| Feedback to professionals on their performance | ||
| Useless | 1 | 1.8 |
| Not valuable | 0 | 0.0 |
| Neutral | 5 | 8.9 |
| Valuable | 29 | 51.8 |
| Highly valuable | 21 | 37.5 |
| Role of specialized nurses | ||
| Useless | 1 | 1.8 |
| Not valuable | 2 | 3.6 |
| Neutral | 10 | 17.9 |
| Valuable | 21 | 37.5 |
| Highly valuable | 22 | 39.3 |
| Fertility as a standard item at the multidisciplinary tumor board | ||
| Useless | 1 | 1.8 |
| Not valuable | 0 | 0.0 |
| Neutral | 2 | 3.6 |
| Valuable | 24 | 42.9 |
| Highly valuable | 29 | 51.8 |
| Standard consultations with a gynecologist for all female cancer patients of reproductive age | ||
| Useless | 1 | 1.8 |
| Not valuable | 2 | 3.6 |
| Neutral | 4 | 7.1 |
| Valuable | 15 | 26.8 |
| Highly valuable | 34 | 60.7 |
| Reminders in medical records | ||
| Useless | 1 | 1.8 |
| Not valuable | 0 | 0.0 |
| Neutral | 7 | 12.5 |
| Valuable | 24 | 42.9 |
| Highly valuable | 24 | 42.9 |
| Agreement between healthcare departments on who is responsible for fertility discussions | ||
| Useless | 1 | 1.8 |
| Not valuable | 0 | 0.0 |
| Neutral | 4 | 7.1 |
| Valuable | 20 | 35.7 |
| Highly valuable | 31 | 55.4 |
| Improved referral to fertility centers | ||
| Useless | 1 | 1.8 |
| Not valuable | 1 | 1.8 |
| Neutral | 2 | 3.6 |
| Valuable | 16 | 28.6 |
| Highly valuable | 36 | 64.3 |
| Respondents | N = 56 | % |
|---|---|---|
| Borderline ovarian tumors and ovarian cancer | ||
| Ovarian stimulation with subsequent oocyte cryopreservation could be considered in patients who underwent surgery because of borderline ovarian tumor | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 8 | 14.3 |
| Neutral | 10 | 17.9 |
| Agree | 27 | 48.2 |
| Strongly agree | 11 | 19.6 |
| Ovarian tissue cryopreservation of the normally appearing contralateral ovary in patients who underwent unilateral surgery because of borderline ovarian tumor should be considered | ||
| Strongly disagree | 2 | 3.6 |
| Disagree | 12 | 21.4 |
| Neutral | 12 | 21.4 |
| Agree | 19 | 33.9 |
| Strongly agree | 11 | 19.6 |
| Oocyte cryopreservation should be proposed to women diagnosed with malignant ovarian germ cell tumors | ||
| Strongly disagree | 2 | 3.6 |
| Disagree | 8 | 14.3 |
| Neutral | 13 | 23.2 |
| Agree | 27 | 48.2 |
| Strongly agree | 6 | 10.7 |
| Patients diagnosed with granulosa cell tumors with concomitant atypical endometrial hyperplasia should be withheld from fertility preservation | ||
| Strongly disagree | 2 | 3.6 |
| Disagree | 21 | 37.5 |
| Neutral | 12 | 21.4 |
| Agree | 19 | 33.9 |
| Strongly agree | 2 | 3.6 |
| Endometrial cancer | ||
| Conservative management can be considered in patients with grade 2 endometrial cancers without myometrial invasion | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 15 | 26.8 |
| Neutral | 10 | 17.9 |
| Agree | 28 | 50.0 |
| Strongly agree | 3 | 5.4 |
| Oral progesterone and progesterone intrauterine devices are equally effective as a form of conservative management in patients with endometrial cancers | ||
| Strongly disagree | 4 | 7.1 |
| Disagree | 18 | 32.1 |
| Neutral | 13 | 23.2 |
| Agree | 19 | 33.9 |
| Strongly agree | 2 | 3.6 |
| Patients with low-risk endometrial cancer who had complete histological response following progesterone therapy and who have not yet become spontaneously | ||
| pregnant six months after progesterone treatment should be offered artificial reproductive technologies (e.g., in vitro fertilization) to increase fecundity | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 2 | 3.6 |
| Neutral | 8 | 14.3 |
| Agree | 34 | 60.7 |
| Strongly agree | 11 | 19.6 |
| Reinstatement of progesterone treatment should be considered in patients with recurrent endometrial cancer who had complete response | ||
| following initial progesterone treatment and who have not yet fulfilled their childbearing wishes | ||
| Strongly disagree | 4 | 7.1 |
| Disagree | 13 | 23.2 |
| Neutral | 12 | 21.4 |
| Agree | 27 | 48.2 |
| Strongly agree | 0 | 0.0 |
| Hysterectomy should always be performed following childbearing in patients with former endometrial cancer diagnosis, irrespective of histological response status | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 9 | 16.1 |
| Neutral | 5 | 8.9 |
| Agree | 32 | 57.1 |
| Strongly agree | 9 | 16.1 |
| Cervical cancer | ||
| Frozen sections of sentinel lymph nodes could be considered as an intra-operative decision tool for fertility preservation in early-stage cervical cancers | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 12 | 21.4 |
| Neutral | 7 | 12.5 |
| Agree | 31 | 55.4 |
| Strongly agree | 5 | 8.9 |
| Neoadjuvant chemotherapy in patients with cervical cancers larger than 2 cm should be exclusively administered to those who participate in clinical trials | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 12 | 21.4 |
| Neutral | 5 | 8.9 |
| Agree | 29 | 51.8 |
| Strongly agree | 10 | 17.9 |
| Ovarian transposition and ovarian tissue autotransplantation should not be considered safe in patients with cervical adenocarcinoma | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 18 | 32.1 |
| Neutral | 12 | 21.4 |
| Agree | 23 | 41.1 |
| Strongly agree | 2 | 3.6 |
| Ovarian transposition and ovarian tissue autotransplantation should be considered safe in patients with early-stage cervical cancer | ||
| showing lymph-vascular space invasion (LVSI) | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 12 | 21.4 |
| Neutral | 15 | 26.8 |
| Agree | 27 | 48.2 |
| Strongly agree | 2 | 3.6 |
| Oocyte cryopreservation and/or ovarian transposition should be considered in cervical cancer patients in whom adjuvant radiotherapy following | ||
| hysterectomy is advised based on final pathology results to preserve the option of genetic motherhood using a surrogate mother | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 6 | 10.7 |
| Neutral | 18 | 32.1 |
| Agree | 26 | 46.4 |
| Strongly agree | 5 | 8.9 |
| Uterine transplantation should be further explored as an option to preserve motherhood in patients who underwent radical hysterectomy | ||
| because of early-stage cervical cancer | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 15 | 26.8 |
| Neutral | 20 | 35.7 |
| Agree | 16 | 28.6 |
| Strongly agree | 4 | 7.1 |
| Respondents | N = 56 | % |
|---|---|---|
| Artificial reproductive technologies | ||
| Ovarian tissue cryopreservation and autotransplantation should exclusively be performed in highly specialized referral centers | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 3 | 5.4 |
| Neutral | 0 | 0.0 |
| Agree | 17 | 30.4 |
| Strongly agree | 35 | 62.5 |
| Ovarian tissue cryopreservation should only be performed in patients who have not yet been exposed to potentially gonadotoxic chemotherapeutic regimens | ||
| Strongly disagree | 5 | 8.9 |
| Disagree | 9 | 16.1 |
| Neutral | 11 | 19.6 |
| Agree | 25 | 44.6 |
| Strongly agree | 6 | 10.7 |
| Ovarian tissue cryopreservation should be limited to patients younger than 35 years of age to increase the potential for ovarian function restoration | ||
| upon autotransplantation of freeze–thawed ovarian tissue fragments | ||
| Strongly disagree | 3 | 5.4 |
| Disagree | 12 | 21.4 |
| Neutral | 10 | 17.9 |
| Agree | 27 | 48.2 |
| Strongly agree | 4 | 7.1 |
| Ovarian suppression with GnRH analogs should only be offered to patients in whom the cryopreservation of either oocytes or ovarian tissue is not deemed feasible | ||
| Strongly disagree | 6 | 10.7 |
| Disagree | 14 | 25.0 |
| Neutral | 11 | 19.6 |
| Agree | 20 | 35.7 |
| Strongly agree | 5 | 8.9 |
| Genetic screening and genetic mutation carriers | ||
| Patients younger than 40 years of age at the time of primary diagnosis of endometrial cancer should be referred to a clinical geneticist, | ||
| irrespective of mismatch repair status | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 12 | 21.4 |
| Neutral | 8 | 14.3 |
| Agree | 23 | 41.1 |
| Strongly agree | 12 | 21.4 |
| In patients with endometrial atypical hyperplasia or endometrial cancer who carry gene mutations associated with Lynch syndrome, | ||
| progesterone treatment should not be prescribed | ||
| Strongly disagree | 2 | 3.6 |
| Disagree | 18 | 32.1 |
| Neutral | 21 | 37.5 |
| Agree | 14 | 25.0 |
| Strongly agree | 1 | 1.8 |
| Since BRCA genetic mutation carriers may have a diminished ovarian reserve, fertility preservation should always be offered | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 4 | 7.1 |
| Neutral | 13 | 23.2 |
| Agree | 29 | 51.8 |
| Strongly agree | 10 | 17.9 |
| Preimplantation genetic testing should be discussed with patients who carry pathogenic BRCA mutation variants | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 7 | 12.5 |
| Neutral | 7 | 12.5 |
| Agree | 31 | 55.4 |
| Strongly agree | 11 | 19.6 |
| Preimplantation genetic testing should be discussed with Lynch syndrome carriers in whom endometrial aberrations have not yet occurred | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 9 | 16.1 |
| Neutral | 9 | 16.1 |
| Agree | 33 | 58.9 |
| Strongly agree | 5 | 8.9 |
| Preimplantation genetic testing should be discussed with patients who carry genetic variants in homologous recombination genes | ||
| other than BRCA1 or BRCA2 (e.g., RAD51C, RAD51D, BRIP1, etc.) | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 9 | 16.1 |
| Neutral | 11 | 19.6 |
| Agree | 28 | 50.0 |
| Strongly agree | 8 | 14.3 |
| Preimplantation genetic testing should be discussed with patients who carry BRCA genetic variants of unknown significance (VUSs) | ||
| Strongly disagree | 5 | 8.9 |
| Disagree | 16 | 28.6 |
| Neutral | 13 | 23.2 |
| Agree | 17 | 30.4 |
| Strongly agree | 5 | 8.9 |
| Ovarian tissue transplantation can be considered safe in BRCA genetic mutation carriers, as long as ovarian transplants are placed back | ||
| in the remaining ovary rather than the peritoneum | ||
| Strongly disagree | 6 | 10.7 |
| Disagree | 23 | 41.1 |
| Neutral | 15 | 26.8 |
| Agree | 12 | 21.4 |
| Strongly agree | 0 | 0.0 |
| Respondents | N = 56 | % |
|---|---|---|
| Artificial reproductive technologies | ||
| Ovarian tissue cryopreservation and autotransplantation should exclusively be performed in highly specialized referral centers | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 3 | 5.4 |
| Neutral | 0 | 0.0 |
| Agree | 17 | 30.4 |
| Strongly agree | 35 | 62.5 |
| Ovarian tissue cryopreservation should only be performed in patients who have not yet been exposed to potentially gonadotoxic chemotherapeutic regimens | ||
| Strongly disagree | 5 | 8.9 |
| Disagree | 9 | 16.1 |
| Neutral | 11 | 19.6 |
| Agree | 25 | 44.6 |
| Strongly agree | 6 | 10.7 |
| Ovarian tissue cryopreservation should be limited to patients younger than 35 years of age to increase the potential for ovarian function restoration upon autotransplantation of freeze–thawed ovarian tissue fragments | ||
| Strongly disagree | 3 | 5.4 |
| Disagree | 12 | 21.4 |
| Neutral | 10 | 17.9 |
| Agree | 27 | 48.2 |
| Strongly agree | 4 | 7.1 |
| Ovarian suppression with GnRH analogs should only be offered to patients in whom cryopreservation of either oocytes or ovarian tissue is not deemed feasible | ||
| Strongly disagree | 6 | 10.7 |
| Disagree | 14 | 25.0 |
| Neutral | 11 | 19.6 |
| Agree | 20 | 35.7 |
| Strongly agree | 5 | 8.9 |
| Genetic screening and genetic mutation carriers | ||
| Patients younger than 40 years of age at the time of primary diagnosis of endometrial cancer should be referred to a clinical geneticist, irrespective of mismatch repair status | ||
| Strongly disagree | 1 | 1.8 |
| Disagree | 12 | 21.4 |
| Neutral | 8 | 14..3 |
| Agree | 23 | 41.1 |
| Strongly agree | 12 | 21.4 |
| In patients with endometrial atypical hyperplasia or endometrial cancer who carry gene mutations associated with Lynch syndrome, progesterone treatment should not be prescribed | ||
| Strongly disagree | 2 | 3.6 |
| Disagree | 18 | 32.1 |
| Neutral | 21 | 37.5 |
| Agree | 14 | 25.0 |
| Strongly agree | 1 | 1.8 |
| Since BRCA genetic mutation carriers may have a diminished ovarian reserve, fertility preservation should always be offered | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 4 | 7.1 |
| Neutral | 13 | 23.2 |
| Agree | 29 | 51.8 |
| Strongly agree | 10 | 17.9 |
| Preimplantation genetic testing should be discussed with patents who carry pathogenic BRCA mutation variants | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 7 | 12.5 |
| Neutral | 7 | 12.5 |
| Agree | 31 | 55.4 |
| Strongly agree | 11 | 19.6 |
| Preimplantation genetic testing should be discussed with Lynch syndrome carriers in whom endometrial aberrations have not yet occurred | ||
| Strongly disagree | 0 | 0.0 |
| Disagree | 9 | 16.1 |
| Neutral | 9 | 16.1 |
| Agree | 33 | 58.9 |
| Strongly agree | 5 | 8.9 |
| Preimplantation genetic testing should be discussed with patients who carry genetic variants in homologous recombination genes other than BRCA1 or BRCA2 (e.g., RAD51C, RAD51D, BRIP1, etc.) | ||
| Strongly disagree | C | 0.0 |
| Disagree | 9 | 16.1 |
| Neutral | 11 | 19.6 |
| Agree | 23 | 50.0 |
| Strongly agree | 14.3 | |
| Preimplantation genetic testing should be discussed with patients who carry BRCA genetic variants of unknown significance (VUSs) | ||
| Strongly disagree | 5 | 8.9 |
| Disagree | 16 | 28.6 |
| Neutral | 13 | 23.2. |
| Agree | 17 | 30.4 |
| Strongly agree | 5 | 8.9 |
| Ovarian tissue transplantation can be considered safe in BRCA genetic mutation carriers, as long as ovarian transplants are placed back the remaining ovary rather than the peritoneum | ||
| Strongly disagree | 6 | 10.7 |
| Disagree | 23 | 41.1 |
| Neutral | 15 | 26.8 |
| Agree | 12 | 21.4 |
| Strongly agree | 0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrado, G.; Peters, I.; Silvestris, E.; Cioffi, R.; Iacobelli, M.; Mancini, E.; Vizza, R.; Thiella, S.; Cormio, G.; Pignata, S.; et al. Physicians’ Controversies Towards Fertility Preservation in Young Patients with Gynecological Cancer: An MITO Survey. Curr. Oncol. 2025, 32, 527. https://doi.org/10.3390/curroncol32090527
Corrado G, Peters I, Silvestris E, Cioffi R, Iacobelli M, Mancini E, Vizza R, Thiella S, Cormio G, Pignata S, et al. Physicians’ Controversies Towards Fertility Preservation in Young Patients with Gynecological Cancer: An MITO Survey. Current Oncology. 2025; 32(9):527. https://doi.org/10.3390/curroncol32090527
Chicago/Turabian StyleCorrado, Giacomo, Inge Peters, Erica Silvestris, Raffaella Cioffi, Marcello Iacobelli, Emanuela Mancini, Riccardo Vizza, Sofia Thiella, Gennaro Cormio, Sandro Pignata, and et al. 2025. "Physicians’ Controversies Towards Fertility Preservation in Young Patients with Gynecological Cancer: An MITO Survey" Current Oncology 32, no. 9: 527. https://doi.org/10.3390/curroncol32090527
APA StyleCorrado, G., Peters, I., Silvestris, E., Cioffi, R., Iacobelli, M., Mancini, E., Vizza, R., Thiella, S., Cormio, G., Pignata, S., & Mangili, G. (2025). Physicians’ Controversies Towards Fertility Preservation in Young Patients with Gynecological Cancer: An MITO Survey. Current Oncology, 32(9), 527. https://doi.org/10.3390/curroncol32090527









