Melanoma in Primary Care: A Narrative Review of Training Interventions and the Role of Telemedicine in Medical Education
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Educational interventions and topics addressed;
- Training methodologies (e.g., delivery format, in-person, e-learning, literature-based feedback strategies, patient interaction, interactivity and course duration);
- Outcomes of face-to-face training programs;
- Outcomes of e-learning-based training programs.
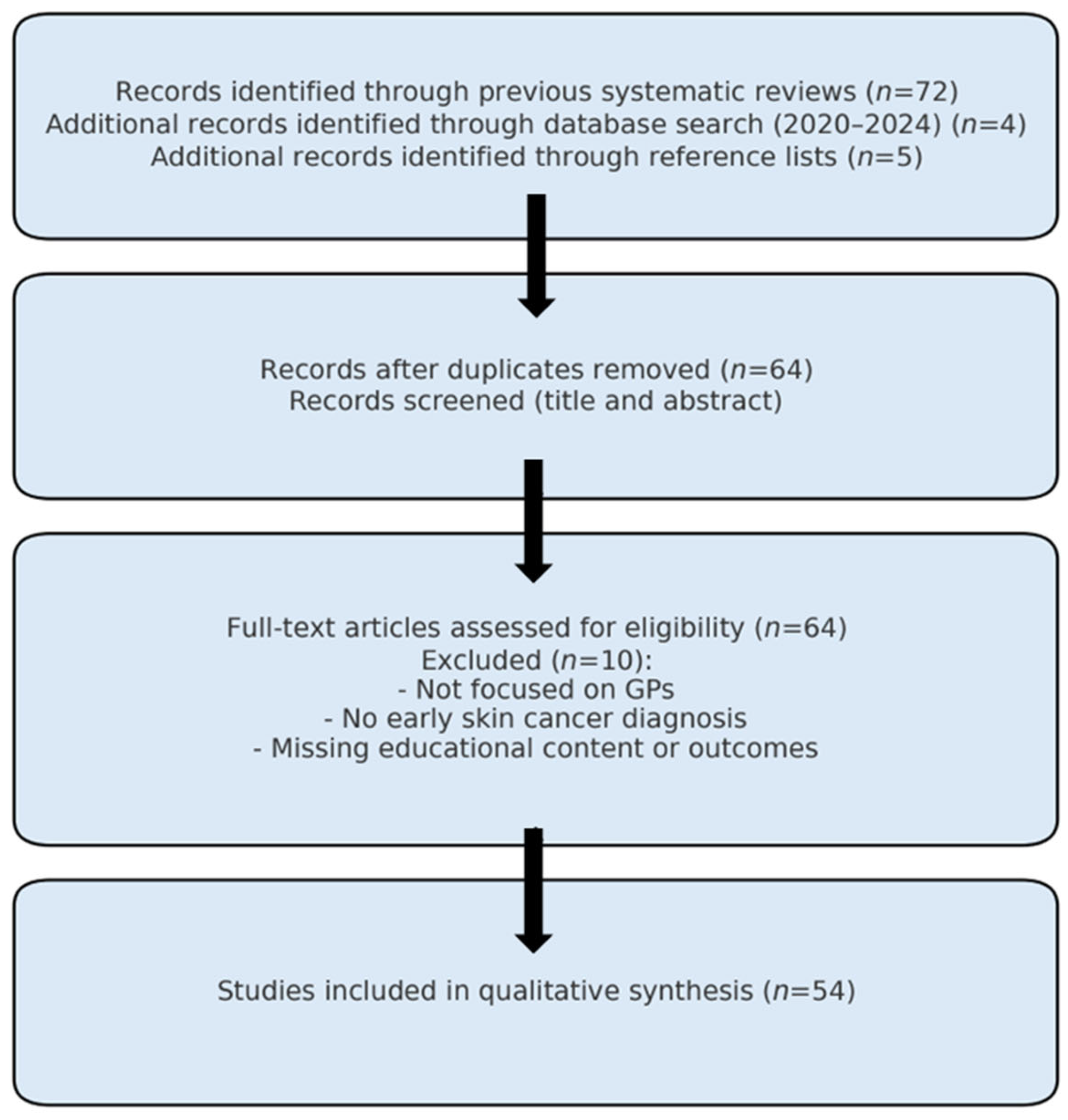
3. Results
3.1. Educational Interventions and Topics Addressed
- A reduction in the number of benign lesions unnecessarily referred to dermatologists;
- An improved benign-to-malignant ratio among excised lesions;
- Measurable improvements in the diagnostic accuracy and clinical decision-making of trained GPs;
- Demonstrated effectiveness even among practitioners with limited prior experience, especially when reinforced by refresher courses;
- A favorable cost-effectiveness profile, with positive implications for both healthcare systems and patient mobility.
3.2. Training Delivery Methods and Implementation Strategies
3.2.1. Frontal Learning Courses: Comparison of Results
3.2.2. E-Learning Courses: Comparison of Results
- Synchronous Training: This modality involves real-time interaction between participants and instructors through platforms like webinars and virtual classrooms. It enables immediate feedback but requires precise scheduling and coordination.
- Asynchronous Training: Participants learn independently at their own pace, accessing materials on demand. This flexible format promotes self-directed learning but lacks real-time interaction and immediate instructor feedback.
- Blended Learning: Combining synchronous and asynchronous elements, this approach balances flexibility with engagement. It requires careful planning to allocate content appropriately between self-paced and live sessions, optimizing interaction and knowledge transfer.
- Blended learning: refers to a combination of synchronous and asynchronous e-learning activities conducted entirely online.
- Hybrid learning models: combine digital (online) components with in-person (face-to-face) sessions, such as workshops or clinical case discussions.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GPs | General Practitioners |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
References
- Perez, M.; Abisaad, J.A.; Rojas, K.D.; Marchetti, M.A.; Jaimes, N. Skin cancer: Primary, secondary, and tertiary prevention. Part I. J. Am. Acad. Dermatol. 2022, 87, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Najmi, M.; Duke, T.; Grabell, D.A.; Koshelev, M.V.; Nelson, K.C. Skin Cancer Education Interventions for Primary Care Providers: A Scoping Review. J. Gen. Intern. Med. 2022, 37, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Gonna, N.; Tran, T.; Bassett, R.L.; Farris, D.P.; Nelson, K.C. Sensitivity and Specificity for Skin Cancer Diagnosis in Primary Care Providers: A Systematic Literature Review and Meta-analysis of Educational Interventions and Diagnostic Algorithms. J. Cancer Educ. 2022, 37, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Anders, M.P.; Fengler, S.; Volkmer, B.; Greinert, R.; Breitbart, E.W. Nationwide skin cancer screening in Germany: Evaluation of the training program. Int. J. Dermatol. 2017, 56, 1046–1051. [Google Scholar] [CrossRef]
- Badertscher, N.; Rosemann, T.; Tandjung, R.; Braun, R.P. minSKIN does a multifaceted intervention improve the competence in the diagnosis of skin cancer by general practitioners? Study protocol for a randomised controlled trial. Trials 2011, 12, 165. [Google Scholar] [CrossRef]
- Badertscher, N.; Braun, R.P.; Held, U.; Kofmehl, R.; Senn, O.; Hofbauer, G.F.; O Rossi, P.; Wensing, M.J.; Rosemann, T.; Tandjung, R. Diagnostic competence of Swiss general practitioners in skin cancer. Swiss Med. Wkly. 2013, 143, w13834. [Google Scholar] [CrossRef]
- Badertscher, N.; Tandjung, R.; Senn, O.; Kofmehl, R.; Held, U.; Rosemann, T.; Hofbauer, G.; Wensing, M.; Rossi, P.; Braun, R. A multifaceted intervention: No increase in general practitioners’ competence to diagnose skin cancer (minSKIN)—randomized controlled trial. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 1493–1499. [Google Scholar] [CrossRef]
- Brochez, L.; Verhaeghe, E.; Bleyen, L.; Naeyaert, J.-M. Diagnostic ability of general practitioners and dermatologists in discriminating pigmented skin lesions. J. Am. Acad. Dermatol. 2001, 44, 979–986. [Google Scholar] [CrossRef]
- Carli, P.; De Giorgi, V.; Crocetti, E.; Caldini, L.; Ressel, C.; Giannotti, B. Diagnostic and referral accuracy of family doctors in melanoma screening: Effect of a short formal training. Eur. J. Cancer Prev. 2005, 14, 51–55. [Google Scholar] [CrossRef]
- Grange, F.; Woronoff, A.; Bera, R.; Colomb, M.; Lavole, B.; Fournier, E.; Arnold, F.; Barbe, C. Efficacy of a general practitioner training campaign for early detection of melanoma in France. Br. J. Dermatol. 2014, 170, 123–129. [Google Scholar] [CrossRef]
- Harris, J.M., Jr.; Salasche, S.J.; Harris, R.B. Using the Internet to teach melanoma management guidelines to primary care physicians. J. Eval. Clin. Pract. 1999, 5, 199–211. [Google Scholar] [CrossRef]
- Peuvrel, L.; Quereux, G.; Jumbou, O.; Sassolas, B.; Lequeux, Y.; Dreno, B. Impact of a campaign to train general practitioners in screening for melanoma. Eur. J. Cancer Prev. 2009, 18, 225–229. [Google Scholar] [CrossRef]
- Stanganelli, I.; Magi, S.; Bucchi, L.; Crocetti, E.; Mancini, S.; Vattiato, R.; Falcinelli, S.; Re, P.; Melandri, D.; Brusasco, M.; et al. Evaluation of a training course for general practitioners within the melanoma multimedia education program of the Italian melanoma intergroup: Study protocol. Dermatol. Rep. 2024, 16, 9919. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, F.; Falcini, F.; Magi, S.; Bucchi, L.; Mancini, S.; Vattiato, R.; Crocetti, E.; Falcinelli, S.; Feliciani, C.; Lombardo, M.; et al. Preliminary analysis of the melanoma multimedia educational program for general practitioners on behalf of the Italian melanoma intergroup. Dermatol. Rep. 2024, 16, 9920. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Puig, S.; Zalaudek, I.; Sera, F.; Corona, R.; Alsina, M.; Barbato, F.; Carrera, C.; Ferrara, G.; Guilabert, A.; et al. Dermoscopy Improves Accuracy of Primary Care Physicians to Triage Lesions Suggestive of Skin Cancer. J. Clin. Oncol. 2006, 24, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, A.; Paoli, J. Effects of a 1-Day Training Course in Dermoscopy Among General Practitioners. Dermatol. Pract. Concept. 2019, 9, 195–199. [Google Scholar] [CrossRef]
- Bedlow, A.J.; Cliff, S.; Melia, J.; Moss, S.M.; Seyan, R.; Harland, C.C. Impact of skin cancer education on general practitioners’ diagnostic skills. Clin. Exp. Dermatol. 2000, 25, 115–118. [Google Scholar] [CrossRef]
- Beecher, S.M.; Keogh, C.; Healy, C. Dedicated general practitioner education sessions can improve diagnostic capabilities and may have a positive effect on referral patterns for common skin lesions. Ir. J. Med Sci. 2018, 187, 959–963. [Google Scholar] [CrossRef]
- Bourne, P.; Rosendahl, C.; Keir, J.; Cameron, A. BLINCK—A diagnostic algorithm for skin cancer diagnosis combining clinical features with dermatoscopy findings. Dermatol. Pract. Concept. 2012, 2, 55–61. [Google Scholar] [CrossRef]
- Burton, R.C.; Howe, C.; Adamson, L.; Reid, A.L.; Hersey, P.; Watson, A.; Watt, G.; Relic, J.; Holt, D.; Thursfield, V.; et al. General practitioner screening for melanoma: Sensitivity, specificity, and effect of training. J. Med. Screen. 1998, 5, 156–161. [Google Scholar] [CrossRef]
- De Gannes, G.C.; Ip, J.L.; Martinka, M.; Crawford, R.I.; Rivers, J.K. Early detection of skin cancer by family physicians: A pilot project. J. Cutan. Med. Surg. 2004, 8, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Del Mar, C.B.; Green, A.C. Aid to diagnosis of melanoma in primary medical care. BMJ 1995, 310, 492–495. [Google Scholar] [CrossRef][Green Version]
- Dolan, N.C.; Ng, J.S.; Martin, G.J.; Robinson, J.K.; Rademaker, A.W. Effectiveness of a skin cancer control educational intervention for internal medicine housestaff and attending physicians. J. Gen. Intern. Med. 1997, 12, 531–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dolianitis, C.; Kelly, J.; Wolfe, R.; Simpson, P. Comparative Performance of 4 Dermoscopic Algorithms by Nonexperts for the Diagnosis of Melanocytic Lesions. Arch. Dermatol. 2005, 141, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.F.; da Costa-Pereira, A.; Del-Marmol, V.; Correia, O. Are General Physicians Prepared for Struggling Skin Cancer?—Cross-Sectional Study. J. Cancer Educ. 2016, 33, 321–324. [Google Scholar] [CrossRef]
- Eide, M.J.; Asgari, M.M.; Fletcher, S.W.; Geller, A.C.; Halpern, A.C.; Shaikh, W.R.; Li, L.; Alexander, G.L.; Altschuler, A.; Dusza, S.W.; et al. Effects on Skills and Practice from a Web-Based Skin Cancer Course for Primary Care Providers. J. Am. Board Fam. Med. 2013, 26, 648–657. [Google Scholar] [CrossRef]
- English, D.R.; Burton, R.C.; del Mar, C.B.; Donovan, R.J.; Ireland, P.D.; Emery, G. Evaluation of aid to diagnosis of pigmented skin lesions in general practice: Controlled trial randomised by practice. BMJ 2003, 327, 375. [Google Scholar] [CrossRef][Green Version]
- Friche, P.; Moulis, L.; Du Thanh, A.; Dereure, O.; Duflos, C.; Carbonnel, F. Training Family Medicine Residents in Dermoscopy Using an e-Learning Course: Pilot Interventional Study. JMIR Form. Res. 2024, 8, e56005. [Google Scholar] [CrossRef]
- Gerbert, B.; Bronstone, A.; Wolff, M.; Maurer, T.; Berger, T.; Pantilat, S.; McPhee, S.J. Improving primary care residents’ proficiency in the diagnosis of skin cancer. J. Gen. Intern. Med. 1998, 13, 91–97. [Google Scholar] [CrossRef]
- Gerbert, B.; Bronstone, A.; Maurer, T.; Berger, T.; McPhee, S.J.; Caspers, N. The effectiveness of an Internet-based tutorial in improving primary care physicians’ skin cancer triage skills. J. Cancer Educ. 2002, 17, 7–11. [Google Scholar] [CrossRef]
- Girgis, A.; Sanson-Fisher, R.W.; Howe, C.; Raffan, B. A skin cancer training programme: Evaluation of a postgraduate training for family doctors. Med. Educ. 1995, 29, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, L.; Silvestri, A.; Brandi, C.; Nisi, G.; Brafa, A.; Calabrò, M.; Campa, A.; D’Aniello, C. Digital epiluminescence dermoscopy for pigmented cutaneous lesions, primary care physicians, and telediagnosis: A useful tool? J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Harwood, C.; Rolph, J.; Pottinger, E.; Mcgregor, J.; Goad, N.; Proby, C. Is an online skin cancer toolkit an effective way to educate primary care physicians about skin cancer diagnosis and referral? J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Harkemanne, E.; Goublomme, N.; Sawadogo, K.; Tromme, I. Early Melanoma Detection in Primary Care: Clinical Recognition of Melanoma is Not Enough, One Must Also Learn the Basics. J. Cancer Educ. 2020, 37, 898–904. [Google Scholar] [CrossRef]
- Harris, J.M., Jr.; Salasche, S.J.; Harris, R.B. Can Internet-based continuing medical education improve physicians’ skin cancer knowledge and skills? J. Gen. Intern. Med. 2001, 16, 50–56. [Google Scholar] [CrossRef][Green Version]
- Koelink, C.; Vermeulen, K.; Kollen, B.; de Bock, G.; Dekker, J.; Jonkman, M.; van der Heide, W. Diagnostic accuracy and cost-effectiveness of dermoscopy in primary care: A cluster randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 1442–1449. [Google Scholar] [CrossRef]
- Markova, A.; Weinstock, M.A.; Risica, P.; Kirtania, U.; Shaikh, W.R.; Ombao, H.; Chambers, C.V.; Kabongo, M.L.; Kallail, K.J.; Post, D. Effect of a Web-Based Curriculum on Primary Care Practice: Basic Skin Cancer Triage Trial. Fam. Med. 2013, 45, 558–568. [Google Scholar]
- Marra, E.; van Rijsingen, M.; Alkemade, J.; Groenewoud, J.; Hueskes, K.; Bijvank, C.N.; van de Laar, F.; Lubeek, S. The effect of a dermato-oncological training programme on the diagnostic skills and quality of referrals for suspicious skin lesions by general practitioners*. Br. J. Dermatol. 2020, 184, 538–544. [Google Scholar] [CrossRef]
- Menzies, S.; Emery, J.; Staples, M.; Davies, S.; McAvoy, B.; Fletcher, J.; Shahid, K.; Reid, G.; Avramidis, M.; Ward, A.; et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: A sequential intervention trial. Br. J. Dermatol. 2009, 161, 1270–1277. [Google Scholar] [CrossRef]
- Mikkilineni, R.; Weinstock, M.A.; Goldstein, M.G.; Dube, C.E.; Rossi, J.S. Impact of the basic skin cancer triage curriculum on providers’ skin cancer control practices. J. Gen. Intern. Med. 2001, 16, 302–307. [Google Scholar] [CrossRef]
- Mikkilineni, R.; Weinstock, M.A.; Goldstein, M.G.; Dube, C.E.; Rossi, J.S. The Impact of the Basic Skin Cancer Triage Curriculum on Providers’ Skills, Confidence, and Knowledge in Skin Cancer Control. Prev. Med. 2002, 34, 144–152. [Google Scholar] [CrossRef]
- Moscarella, E.; Lallas, A.; Longo, C.; Alfano, R.; Argenziano, G. Five-point checklist for skin cancer detection in primary care. Ital. J. Dermatol. Venereol. 2019, 154, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Nervil, G.G.; Ternov, N.K.; Vestergaard, T.; Sølvsten, H.; Chakera, A.H.; Tolsgaard, M.G.; Hölmich, L.R. Improving Skin Cancer Diagnostics Through a Mobile App With a Large Interactive Image Repository: Randomized Controlled Trial. JMIR Dermatol. 2023, 6, e48357. [Google Scholar] [CrossRef] [PubMed]
- Raasch, B.A.; Hays, R.; Buettner, P.G. An educational intervention to improve diagnosis and management of suspicious skin lesions. J. Contin. Educ. Health Prof. 2000, 20, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Rivet, C.; Motamedi, F.; Burns, J.; Archibald, D. A structured curriculum and procedure clinic to help family medicine residents diagnose and treat skin cancer. Can. Med. Educ. J. 2021, 12, 108–111. [Google Scholar] [CrossRef]
- Robinson, J.K.; MacLean, M.; Reavy, R.; Turrisi, R.; Mallett, K.; Martin, G.J. Dermoscopy of Concerning Pigmented Lesions and Primary Care Providers’ Referrals at Intervals After Randomized Trial of Mastery Learning. J. Gen. Intern. Med. 2018, 33, 799–800. [Google Scholar] [CrossRef]
- Robinson, J.K.; Jain, N.; Marghoob, A.A.; McGaghie, W.; MacLean, M.; Gerami, P.; Hultgren, B.; Turrisi, R.; Mallett, K.; Martin, G.J. A Randomized Trial on the Efficacy of Mastery Learning for Primary Care Provider Melanoma Opportunistic Screening Skills and Practice. J. Gen. Intern. Med. 2018, 33, 855–862. [Google Scholar] [CrossRef]
- Rogers, T.; Marino, M.L.; Dusza, S.W.; Bajaj, S.; Usatine, R.P.; Marchetti, M.A.; Marghoob, A.A. A Clinical Aid for Detecting Skin Cancer: The Triage Amalgamated Dermoscopic Algorithm (TADA). J. Am. Board Fam. Med. 2016, 29, 694–701. [Google Scholar] [CrossRef]
- Sawyers, E.A.; Wigle, D.T.; Marghoob, A.A.; Blum, A. Dermoscopy Training Effect on Diagnostic Accuracy of Skin Lesions in Canadian Family Medicine Physicians Using the Triage Amalgamated Dermoscopic Algorithm. Dermatol. Pract. Concept. 2020, 10, e2020035. [Google Scholar] [CrossRef]
- Secker, L.; Buis, P.; Bergman, W.; Kukutsch, N. Effect of a Dermoscopy Training Course on the Accuracy of Primary Care Physicians in Diagnosing Pigmented Lesions. Acta Derm. Venereol. 2017, 97, 263–265. [Google Scholar] [CrossRef]
- Seiverling, E.V.; Ahrns, H.T.; Greene, A.; Butt, M.; Yélamos, O.; Dusza, S.W.; Marghoob, A.A. Teaching Benign Skin Lesions as a Strategy to Improve the Triage Amalgamated Dermoscopic Algorithm (TADA). J. Am. Board Fam. Med. 2019, 32, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Shariff, Z.; Roshan, A.; Williams, A.; Platt, A. 2-Week wait referrals in suspected skin cancer: Does an instructional module for general practitioners improve diagnostic accuracy? Surgeon 2010, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Swetter, S.M.; Chang, J.; Shaub, A.R.; Weinstock, M.A.; Lewis, E.T.; Asch, S.M. Primary Care–Based Skin Cancer Screening in a Veterans Affairs Health Care System. JAMA Dermatol. 2017, 153, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Boyle, C. Evaluation of skin cancer seminar for general practitioners: Changes in knowledge, diagnostic and procedural skills, beliefs, and self-reported practices. J. Contin. Educ. Health Prof. 1995, 15, 217–226. [Google Scholar] [CrossRef]
- Weinstock, M.A.; Ferris, L.K.; Saul, M.I.; Geller, A.C.; Risica, P.M.; Siegel, J.A.; Solano, F.X.; Kirkwood, J.M. Downstream consequences of melanoma screening in a community practice setting: First results. Cancer 2016, 122, 3152–3156. [Google Scholar] [CrossRef]
- Westerhoff, K.; Mccarthy, W.; Menzies, S. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br. J. Dermatol. 2000, 143, 1016–1020. [Google Scholar] [CrossRef]
- Youl, P.H.; Raasch, B.A.; Janda, M.; Aitken, J.F. The effect of an educational programme to improve the skills of general practitioners in diagnosing melanocytic/pigmented lesions. Clin. Exp. Dermatol. 2007, 32, 365–370. [Google Scholar] [CrossRef][Green Version]
- De Bedout, V.; Williams, N.; Muñoz, A.; Londoño, A.; Munera, M.; Naranjo, N.; Rodriguez, L.; Toro, A.; Miao, F.; Koru-Sengul, T.; et al. Skin Cancer and Dermoscopy Training for Primary Care Physicians: A Pilot Study. Dermatol. Pract. Concept. 2021, 11, e2021145. [Google Scholar] [CrossRef]
- Pagnanelli, G.; Soyer, H.; Argenziano, G.; Talamini, R.; Barbati, R.; Bianchi, L.; Campione, E.; Carboni, I.; Carrozzo, A.; Chimenti, M.; et al. Diagnosis of pigmented skin lesions by dermoscopy: Web-based training improves diagnostic performance of non-experts. Br. J. Dermatol. 2003, 148, 698–702. [Google Scholar] [CrossRef]
- Zararsız, G.E.; Taştan, S.I.Y.; Gürbulak, E.Ç.; Erakcaoğlu, A.; Işıkhan, S.Y.; Demirbaş, A.; Ertaş, R.; Eroğlu, İ.; Korkmaz, S.; Elmas, Ö.F. Diagnosis melanoma with artificial intelligence systems: A meta-analysis study and systematic review. J. Eur. Acad. Dermatol. Venereol. 2025. [Google Scholar] [CrossRef]
- Harkemanne, E.; Baeck, M.; Tromme, I. Training general practitioners in melanoma diagnosis: A scoping review of the literature. BMJ Open 2021, 11, e043926. [Google Scholar] [CrossRef] [PubMed]
- Herschorn, A. Dermoscopy for melanoma detection in family practice. Can. Fam. Physician 2012, 58, 740–745. [Google Scholar] [PubMed]
- Delungahawatta, T.; Dunne, S.S.; Hyde, S.; Halpenny, L.; McGrath, D.; O’rEgan, A.; Dunne, C.P. Advances in e-learning in undergraduate clinical medicine: A systematic review. BMC Med Educ. 2022, 22, 711. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Nelson, E.; Wetter, N. Medical students’ online learning technology needs. Clin. Teach. 2014, 11, 15–19. [Google Scholar] [CrossRef]
- Feng, J.; Chang, Y.; Chang, H.; Erdley, W.S.; Lin, C.; Chang, Y. Systematic Review of Effectiveness of Situated E-Learning on Medical and Nursing Education. Worldviews Evid. Based Nurs. 2013, 10, 174–183. [Google Scholar] [CrossRef]
- Vaona, A.; Banzi, R.; Kwag, K.H.; Rigon, G.; Cereda, D.; Pecoraro, V.; Tramacere, I.; Moja, L. E-learning for health professionals. Cochrane Database Syst. Rev. 2018, 1, CD011736. [Google Scholar] [CrossRef]
- Posada, E.L.; Lauck, K.C.; Tran, T.; Krause, K.J.; Nelson, K.C. Educational Interventions to Support Primary Care Provider Performance of Diagnostic Skin Cancer Examinations: A Systematic Literature Review. J. Cancer Educ. 2022, 37, 1579–1588. [Google Scholar] [CrossRef]
- Salinas, M.P.; Sepúlveda, J.; Hidalgo, L.; Peirano, D.; Morel, M.; Uribe, P.; Rotemberg, V.; Briones, J.; Mery, D.; Navarrete-Dechent, C. A systematic review and meta-analysis of artificial intelligence versus clinicians for skin cancer diagnosis. npj Digit. Med. 2024, 7, 125. [Google Scholar] [CrossRef]
- Sinclair, P.; Kable, A.; Levett-Jones, T. The effectiveness of internet-based e-learning on clinician behavior and patient outcomes: A systematic review protocol. JBI Evid. Synth. 2015, 13, 52–64. [Google Scholar] [CrossRef]
- Harrington, E.; Clyne, B.; Wesseling, N.; Sandhu, H.; Armstrong, L.; Bennett, H.; Fahey, T. Diagnosing malignant melanoma in ambulatory care: A systematic review of clinical prediction rules. BMJ Open 2017, 7, e014096. [Google Scholar] [CrossRef]
| Epidemiology | Melanoma Incidence and Mortality Trends Risk Factors |
|---|---|
| Pigmented skin lesions | Basic principles for the recognition of melanoma and benign pigmented lesions |
| Non pigmented skin lesions | Basic principles for the recognition of squamous cell carcinoma, basal cell carcinoma and benign non-pigmented lesions |
| Dermoscopy | Basics of dermoscopy aimed at recognizing suspicious lesions for skin cancers, in particular melanoma |
| Algorithm | Teaching of a clinical and/or dermatoscopic algorithm (new or pre-existing) to aid the identification of suspicious skin lesions |
| Management | In-depth study of the integrated care pathway (ICP) of melanoma and skin cancers |
| Counseling | In-depth study of prevention strategies such as photoprotection and self-examination of the skin |
| Author | Epidemiology | Pigmented Skin Lesions | Non Pigmented Skin Lesions | Dermoscopy | Algorithm | Management | Counseling |
|---|---|---|---|---|---|---|---|
| Anders et al. (2017) [4] | X | X | X | X | X | ||
| Argenziano et al. (2006) [15] | X | X | X | X | |||
| Augustsson et al. (2019) [16] | X | X | X | X | |||
| Badertscher et al. (2011) [5] | X | X | X | X | X | ||
| Badertscher et al. (2013) [6] | X | X | X | X | X | ||
| Badertscher et al. (2015) [7] | X | X | X | X | X | ||
| Bedlow et al. (2001) [17] | X | X | |||||
| Beecher et al. (2018) [18] | X | X | X | ||||
| Bourne et al. (2012) [19] | X | X | X | X | |||
| Brochez et al. (2001) [8] | X | X | X | ||||
| Burton et al. (1998) [20] | X | X | X | ||||
| Carli et al. (2005) [9] | ꓫ | ꓫ | ꓫ | ꓫ | |||
| De Gannes et al. (2004) [21] | X | X | X | X | X | ||
| Del Mar et al. (1995) [22] | X | X | X | ||||
| Dolan et al. (1997) [23] | X | X | X | X | |||
| Dolianitis et al. (2005) [24] | X | X | X | ||||
| Duarte et al. (2018) [25] | ꓫ | ꓫ | ꓫ | ꓫ | |||
| Eide et al. (2012) [26] | X | X | X | X | X | X | X |
| English et al. (2003) [27] | X | X | X | ||||
| Friche et al. (2024) [28] | X | X | X | ||||
| Gerbert et al. (1998) [29] | X | X | X | X | X | ||
| Gerbert et al. (2002) [30] | X | X | X | X | X | ||
| Girgis et al. (1995) [31] | X | X | X | ||||
| Grange et al. (2014) [10] | X | X | X | X | |||
| Grimaldi et al. (2009) [32] | X | X | X | X | |||
| Gulati et al. (2015) [33] | X | X | X | ||||
| Harkemanne et al. (2020) [34] | X | X | X | ||||
| Harris et al. (1999) [11] Harris et al. (2001) [35] | X | X | X | X | X | ||
| Koelink et al. (2014) [36] | X | X | X | X | X | ||
| Markova et al. (2013) [37] | X | X | X | X | X | X | |
| Marra et al. (2021) [38] | X | X | X | X | X | ||
| Menzies et al. (2009) [39] | X | X | |||||
| Mikkilineni et al. (2001) [40] | X | X | X | X | X | X | |
| Mikkilineni et al. (2002) [41] | X | X | X | X | X | X | |
| Moscarella et al. (2017) [42] | X | X | X | X | X | ||
| Nervil et al. (2013) [43] | X | X | X | ||||
| Peuvrel et al. (2009) [12] | X | X | X | X | X | ||
| Raasch et al. (2000) [44] | X | X | X | ||||
| Rivet et al. (2018) [45] | X | X | X | X | X | ||
| Robinson et al. (2018) [46] | X | X | X | X | X | ||
| Robinson et al. (2018) [47] | X | X | X | X | X | ||
| Rogers et al. (2016) [48] | X | X | |||||
| Sawyers et al. (2020) [49] | X | X | |||||
| Secker et al. (2017) [50] | ꓫ | ꓫ | ꓫ | ||||
| Seiverling et al. (2019) [51] | X | X | X | X | |||
| Shariff et al. (2010) [52] | X | X | |||||
| Swetter et al. (2017) [53] | X | X | X | X | X | X | |
| Ward et al. (1995) [54] | X | X | X | X | |||
| Weinstock et al. (2016) [55] | X | X | X | X | X | X | X |
| Westerhoff et al. (2000) [56] | X | X | X | ||||
| Youl et al. (2007) [57] | X | X | X | X | X | ||
| Stanganelli et al. (2024), Zamagni et al.(2024) [13,14] | X | X | X | X | X | X | X |
| De Bedout et al. (2021) [58] | X | X | X | X | |||
| Pagnanelli et al. (2003) [59] | X | X | X | X |
| Author | Country (N° GPs *) | Live | Literature | E-Learning | Feedback | Interaction with Patient | Interactivity | Modality ** | Days *** |
|---|---|---|---|---|---|---|---|---|---|
| Anders et al. [4] | DE (573) | X | S | N.A | |||||
| Argenziano et al. (2006) [5] | IT, ES (73) | ꓫ | S | Sing | |||||
| Augustsson et al. (2019) [6] | SE (56) | X | X | S | Sing | ||||
| Badertscher et al. (2011) [7] | CH (60) | X | X | X | S | S.M. | |||
| Badertscher et al. (2013) [8] | CH (78) | X | X | X | S | S.M. | |||
| Badertscher et al. (2015) [9] | CH (78) | X | X | X | S | S.M. | |||
| Bedlow et al. (2001) [10] | UK (17) | X | X | S | Sing | ||||
| Beecher et al. (2018) [11] | IE (23) | X | S | Sing | |||||
| Bourne et al. (2012) [12] | AU (4) | X | X | S | N.S. | ||||
| Brochez et al. (2001) [13] | BE (160) | X | X | S | Sing | ||||
| Burton et al. (1998) [14] | AU (143) | X | X | A | S.M. | ||||
| Carli et al. (2005) [15] | IT (41) | X | S | Sing. | |||||
| De Gannes et al. (2004) [16] | CA (52) | X | S | Sing. | |||||
| Del Mar et al. (1995) [17] | AU (93) | X | X | A | S.M. | ||||
| Dolan et al. (1997) [18] | US (82) | X | S | Sing. | |||||
| Dolianitis et al. (2005) [19] | AU (35) | X | X | X | A | Sing. | |||
| Duarte et al. (2018) [20] | PT (11) | X | X | S | Sing. | ||||
| Eide et al. (2012) [21] | US (54) | X | X | A | Sing. | ||||
| English et al. (2003) [22] | AU (468) | X | X | A | S.M. | ||||
| Friche et al. (2024) [23] | FR (134) | X | X | A | S.M. | ||||
| Gerbert et al. (1998) [24] | USA (77) | X | X | X | X | X | A | S.M. | |
| Gerbert et al. (2002) [25] | USA (46) | X | X | X | X | X | A | S.M. | |
| Girgis et al. (1995) [26] | AU (41) | X | X | A | 3 S. | ||||
| Grange et al. (2014) [27] | FR (398) | X | X | X | X | S | Sing. | ||
| Grimaldi et al. (2009) [28] | IT (13) | X | X | X | X | X | S | S.M. | |
| Gulati et al. (2015) [29] | UK (967) | X | X | A | S.M. | ||||
| Harkemanne et al. (2020) [30] | BE (56) | X | S | Sing. | |||||
| Robinson (1999) Harris et al. (2001) [31,32] | UK (354) | X | X | X | A | S.M. | |||
| Koelink et al. (2014) [33] | NL (53) | X | S | S.M. | |||||
| Markova et al. (2013) [34] | US (57) | X | S | Sing. | |||||
| Marra et al. (2021) [35] | NL (185) | X | X | A | S.M. | ||||
| Menzies et al. (2009) [36] | AU (63) | X | X | X | X | A | S.M. | ||
| Mikkilineni et al. (2001) [37] | US (22) | X | X | X | S | Sing. | |||
| Mikkilineni et al. (2002) [38] | US (28) | X | X | X | S | Sing. | |||
| Moscarella et al. (2017) [39] | IT (N.S.) | X | X | N.A. | N.A. | ||||
| Nervil et al. (2013) [40] | DK (115) | X | X | A | N.A. | ||||
| Peuvrel et al. (2009) [41] | FR (210) | X | X | S | Sing. | ||||
| Raasch et al. (2000) [42] | AU (46) | X | X | A | S.M. | ||||
| Rivet et al. (2018) [43] | CA (25) | X | X | X | X | X | X | A | S.M. |
| Robinson et al. (2018) [44] | US (89) | X | X | X | A | S.M. | |||
| Robinson et al. (2018) [45] | US (44) | X | X | X | A | S.M. | |||
| Rogers et al. (2016) [46] | US (41) | X | S | Sing. | |||||
| Sawyers et al. (2020) [47] | CA (33) | X | S | Sing. | |||||
| Secker et al. (2017) [48] | NL (309) | X | X | X | A | Sing. | |||
| Seiverling et al. (2019) [49] | US (59) | X | X | S | Sing. | ||||
| Shariff et al. (2010) [50] | UK (94) | X | X | A | N.S. | ||||
| Swetter et al. (2017) [51] | US (6) | X | X | A | Sing. | ||||
| Ward et al. (1995) [52] | AU (N.S.) | X | X | S | Sing. | ||||
| Weinstock et al. (2016) [53] | US (108) | X | X | A | Sing. | ||||
| Westerhoff et al. (2000) [54] | AU (74) | X | X | S | Sing. | ||||
| Youl et al. (2007) [55] | AU (16) | X | X | A | S.M. | ||||
| Stanganelli et al. (2024), Zamagni et al. (2024) [56,57] | IT (298) | X | X | A | N.A. | ||||
| De Bedout et al. (2021) [58] | CO (21) | X | X | X | S | S.M. | |||
| Pagnanelli et al. (2003) [59] | IT (16) | X | X | N.A. | N.A. |
| Article | N° GPs | Pre-Test | Post-Test | Post-Test (2) | Se | Sp | Accuracy | VPP | VPN |
|---|---|---|---|---|---|---|---|---|---|
| Girgis et al., 1995, CH [31] | 59 | 41 | 41 | - | - | - | - | - | - |
| Burton et al., 1998, AU [20] | 74 | 63 | 63 | - | - | - | - | - | - |
| Raasch et al., 2000, AU [44] | 46 | 46 | 46 | - | 72.2%/ 77.1% | 44.7%/ 37.3% | - | - | - |
| Westerhoff et al., 2000, UK [56] | 74 | 74 | 74 | - | 54.6%/62.7% | - | - | - | - |
| Bedlow et al., 2001, UK [17] | 23 | 17 | 17 | - | 63%/76% | 55%/62% | 56%/56% | - | - |
| Brochez et al., 2001, BE [8] | 1956 | 160 | 146 | - | 72%/84% | 71%/70% | 49%/56% | 61%/ 63% | 80%/87% |
| English et al., 2003, AU [27] | 1221 | 468 | - | - | - | - | - | - | - |
| Carli et al., 2005, IT [9] | 41 | 41 | 41 | - | 46.8%/76.2% | 55%/73.1% | - | - | |
| Argenziano et al., 2006, IT [15] | 88 | 73 | 73 | - | 54.1%/79.2% | 71.3%/71.8% | - | - | 11.3%/ 95.8% |
| Youl et al., 2007, AU [57] | 16 | 16 | 16 | - | 62.3%/64.5% | - | - | - | - |
| Peuvrel et al., 2009, FR [12] | 210 | 87 | 87 | - | - | - | - | - | - |
| Shariff et al., 2010, UK [52] | 94 | 94 | - | - | - | - | 23.5%/21.2% | - | - |
| Badertscher et al., 2015, CH [7] | 1000 | 78 | 78 | 74 | - | - | 63.8%/77.7%/66.6% | - | |
| Koelink et al., 2014, NL [36] | 281 | 53 | 53 | - | - | - | - | - | - |
| Anders et al., 2017, DE [4] | 573 | 573 | 573 | - | - | - | 62%/77.2% | - | - |
| Duarte et al., 2018, PT [25] | 11 | - | - | - | - | - | - | - | - |
| Seiverling et al., 2019, US [51] | 59 | 59 | 59 | - | 62.5%/88.1% | 90.3%/87.8% | 59.7%/78.3% | - | - |
| De Bedout et al., 2021, CO [58] | 21 | 21 | 19 | - | 62.9%/86.5% | 54.7%/75.7% | - | - | - |
| Harkemanne et al., 2020, BE [34] | 56 | 56 | 56 | - | - | - | - | - | - |
| Synchronous Training | Asynchronous Training | Blended Learning | |
|---|---|---|---|
| Simultaneous presence of teachers and students | X | Combination of synchronous and asynchronous training elements (variable according to the structure of the training project) | |
| Possibility to ask questions to the teacher | X | X (Chat e forum) | |
| Possibility to do exercises | X (in real time) | X | |
| Feedback from faculty and attendees | X (immediate) | X (Chat e forum) | |
| Management of individual learning times | X | ||
| Possibility to carry out training wherever you prefer | X | X | |
| Ability to train whenever you want | X | ||
| Possibility to review the training materials | X | ||
| Training defined by a medium-long duration time program (weeks/months/year) | X |
| Article | N° GPs | Pre-Test | Post-Test | Post-Test (2) | Se | Sp | Accuracy | VPP | VPN |
|---|---|---|---|---|---|---|---|---|---|
| Gerbert et al., 1998, US [29] * | 77 | 65 | 52 | - | - | - | 43%/56% | - | - |
| Harris et al., 2001, UK [35] | 691 | 354 | 354 | - | 91%/95% | 72%/92% | 52%/85% | - | - |
| Mikkilineni et al., 2001, US [40] | 28 | 22 | 22 | - | - | - | - | - | - |
| Pagnanelli et al., 2003, IT [59] | 16 | 16 | 16 | - | for each different method | ||||
| Dolianitis et al. 2005, AU [24] | 61 | 61 | - | comparison of different algorithms | |||||
| Menzies et al., 2009, AU [39] * | 102 | 63 | 63 | - | 23.1%/69.2% | 90.6%/92.8% | - | 18.8%/47.4% | 93.3%/97.9% |
| Eide et al., 2013, US [26] | 54 | 54 | 54 | 54 (6 months) | 39.4%/ 48.8%/ 44% | 44.8%/56.7%/46% | 41.5%/51.9%/44.8% | - | - |
| Markova et al., 2013, US [37] | 57 GP + 3341 patients | 57 | 57 (1−2 months) | 57 (12 months) | - | - | - | - | - |
| Gulati et al., 2015, UK [33] | 8163 | 1002 | 1007 | - | - | - | - | - | - |
| Weinstock et al., 2016, US [55] | 618 GP + 16,472 patients | - | 8 months | - | - | - | - | - | - |
| Secker et al., 2017, NL [50] * | 309 | 293 | 293 | - | For each category of lesion | - | - | ||
| Robinson et al., 2018, US [46,47] | 181 | 90 | 89 | - | For each category of lesion | - | - | ||
| Marra et al., 2021, NL [38] * | 194 | - | - | - | - | 70.30% | - | - | |
| Nervil et al., 2023, DK [43] | 279 | 115 | 115 | - | 67.1%/73.7% | - | 42.5%/53% | - | - |
| Friche et al., 2024, FR [28] | 134 | 89 | 63 | - | - | - | - | - | - |
| Stanganelli et al (2024), Zamagni et al. (2024) [13,14] | 1320 | 298 | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanganelli, I.; Mora, E.; Cantagalli, D.; Magi, S.; Mazzoni, L.; Medri, M.; Massone, C.; Melandri, D.; Zamagni, F.; Zanna, I.; et al. Melanoma in Primary Care: A Narrative Review of Training Interventions and the Role of Telemedicine in Medical Education. Curr. Oncol. 2025, 32, 522. https://doi.org/10.3390/curroncol32090522
Stanganelli I, Mora E, Cantagalli D, Magi S, Mazzoni L, Medri M, Massone C, Melandri D, Zamagni F, Zanna I, et al. Melanoma in Primary Care: A Narrative Review of Training Interventions and the Role of Telemedicine in Medical Education. Current Oncology. 2025; 32(9):522. https://doi.org/10.3390/curroncol32090522
Chicago/Turabian StyleStanganelli, Ignazio, Edoardo Mora, Debora Cantagalli, Serena Magi, Laura Mazzoni, Matelda Medri, Cesare Massone, Davide Melandri, Federica Zamagni, Ines Zanna, and et al. 2025. "Melanoma in Primary Care: A Narrative Review of Training Interventions and the Role of Telemedicine in Medical Education" Current Oncology 32, no. 9: 522. https://doi.org/10.3390/curroncol32090522
APA StyleStanganelli, I., Mora, E., Cantagalli, D., Magi, S., Mazzoni, L., Medri, M., Massone, C., Melandri, D., Zamagni, F., Zanna, I., Pistore, G., Caini, S., Amato, S., De Giorgi, V., Quaglino, P., Pizzichetta, M. A., Tripepi, G. L., Ravaglia, G., & Spagnolini, S. (2025). Melanoma in Primary Care: A Narrative Review of Training Interventions and the Role of Telemedicine in Medical Education. Current Oncology, 32(9), 522. https://doi.org/10.3390/curroncol32090522










