We were interested to read the article by Pang et al. describing a retrospective review of 176 patients with lung metastases from a variety of primary tumours who were treated with either RFA or SABR at the Royal Marsden Hospital over 8 years [1]. It clearly showed how the different modalities were used for different tumours and suggested that SABR might in some circumstances be less effective than RFA at providing even local control. It also documents not insignificant adverse sequelae from both SBRT and RFA.
However, this study did not, nor could not, address the fundamental question as to whether removing or ablating lung metastases is actually effective in improving the patients’ most important clinical outcomes—overall survival (OS) and quality of life. In the Introduction Section they state that there is ‘a growing body of evidence for its survival benefit’. We have recently published a review in BMJ that shows, despite enthusiastic advocates worldwide, how insubstantial this evidence is [2].
In support of the view that SBRT might improve overall survival in patients with lung metastases they cite publications describing the long-term outcomes of two Phase II randomised trials [3,4] (Palma et al., Gomez et al.). Both were small studies including only 99 and 49 patients respectively. As we have pointed out before, in the Palma trial the two arms were imbalanced in favour of the intervention arm, which included patients with lower-stage and better-prognosis tumours [5]. The hazard ratio for OS was 0.57 but with very wide 95% confidence intervals. The Gomez et al. trial included only patients with non-small cell lung cancer. The primary outcome was progression-free survival, an unreliable outcome in ablation trials, as the identified sites of metastatic disease are those most likely to progress in the untreated arm. The trial was stopped early, and in the review of OS, only 28 patients were at risk at 2 years and 8 at 4 years—tiny numbers. Although there appeared to be a survival difference favouring intervention, it was not statistically significant and highly liable to chance variation. Overall, this is very poor evidence of effectiveness to justify the widespread uptake of this intervention. To our knowledge there is no good evidence supporting the use of RFA in this situation.
They cite the PulMiCC trial, of which we were co-investigators, but they only cite the initial publication. Further publications analysing a larger group of randomised patients [6] and the observational registration study in which the RCT was nested [7] lead us to believe that the major determinant of OS was patient selection rather than the intervention. Even with the small numbers randomised we have shown that a significant survival benefit is very unlikely (Figure 1).
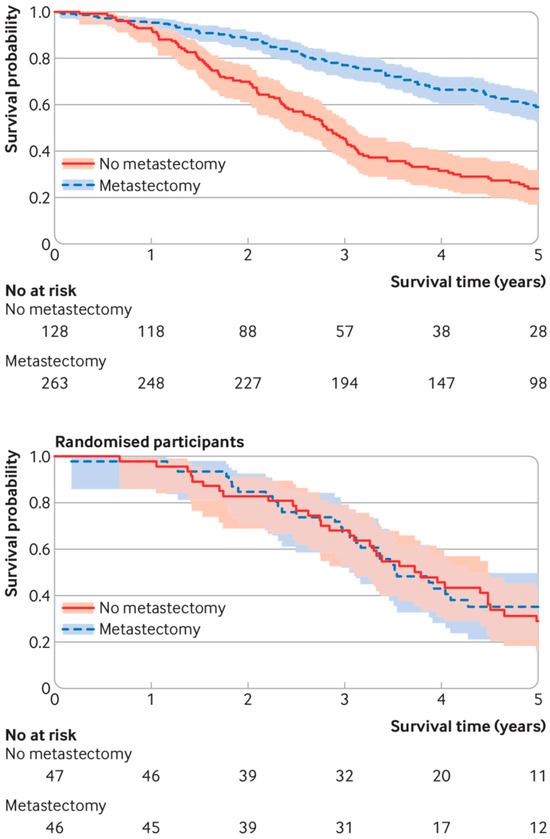
Figure 1.
Survival curves for patients in the PulMiCC study. Of 484 patients with colorectal lung metastases with baseline and follow-up data collected to trial standards, 263 were selected by the clinical teams for metastasectomy and 128 were selected to NOT have metastasectomy (upper panel). Those selected for surgery had fewer metastases; the majority did not have raised carcinoembryonic antigen; and they had a better cancer stage, less liver involvement, better performance status, better lung function, and were younger. Their survival results were similar to many observational studies. In the nested controlled trial (lower panel), there was good balance for all known factors in the randomly assigned arms. There was no hint of a difference in survival.
It is time that the ‘routine’ use of SBRT and RFA for the treatment of asymptomatic metastases was paused, allowing reliable randomised trial evidence of clinical effectiveness to be generated [2].
Funding
The research was funded by Cancer Research UK funding Grant No. C7678/A11393.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Pang, J.W.S.; Tong, D.; Fotiadis, N.; Satchwell, L.; Rajan, Z.; Emarah, M.; Taylor, H.; Bashir, U.; Ap Dafydd, D.; McCall, J.; et al. Ablative Techniques for Lung Metastases: Patient Selection and Outcomes Following Treatment with Stereotactic Radiotherapy or Radiofrequency Ablation. Curr. Oncol. 2025, 32, 303. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, F.; Fallowfield, L.; Treasure, E.; Ahmad, I.; Zheng, Y.; Treasure, T. Removal or ablation of asymptomatic lung metastases should be reconsidered. BMJ 2023, 383, e073042. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R.; Jr Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients with Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, F.; Treasure, T. Points to consider regarding the SABR-COMET trial. Lancet 2020, 395, e19. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Edwards, J.; Tsang, D.; Dunning, J.; Shackcloth, M.; Batchelor, T.; Coonar, A.; Hasan, J.; Davidson, B.; Marchbank, A.; et al. Pulmonary Metastasectomy in Colorectal Cancer: Updated analysis of 93 randomized patients-control survival is much better than previously assumed. Color. Dis. 2020, 22, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; Farewell, V.; Macbeth, F.; Batchelor, T.; Milosevic, M.; King, J.; Zheng, Y.; Leonard, P.; Williams, N.R.; Brew-Graves, C.; et al. The Pulmonary Metastasectomy in Colorectal Cancer cohort study: Analysis of case selection, risk factors and survival in a prospective observational study of 512 patients. Color. Dis. 2021, 23, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).