Simple Summary
Cancer treatment in children, such as chemotherapy and radiation, can cause serious permanent hearing loss by damaging the external, middle, and/or the inner ear. Several factors influence how much the hearing is affected at the level of the inner ear, including the child’s age, the total treatment dose, genetic background, and the use of other therapies. The damage can also extend beyond the ear, affecting nearby bones and even the brain areas involved in hearing. While a new medication may help reduce some of the harmful effects of cisplatin-based chemotherapy, it is not suitable for all children, and its benefits are still limited. Some children may benefit from hearing devices such as cochlear implants or bone-anchored hearing aids. However, choosing the right time and type of rehabilitation is complex, especially since these devices can interfere with the MRI scans needed to monitor cancer. More research is needed to guide personalized decisions that balance hearing care with cancer surveillance. This review highlights the urgent need for better strategies to protect hearing during treatment, monitor changes early, and plan effective individualized hearing rehabilitation. It also emphasizes the importance of close collaboration between healthcare providers to support each child’s hearing and overall development.
Abstract
Childhood cancer treatments, including chemotherapy, radiation therapy, and combined modalities, pose significant risks to auditory function due to their ototoxic effects. Cisplatin, a chemotherapeutic agent commonly used in pediatric oncology, causes dose-dependent irreversible sensorineural hearing loss by damaging the inner ear structures, primarily through the generation of reactive oxygen species and the activation of apoptotic pathways. Radiation therapy exacerbates these effects, contributing to both sensorineural and conductive hearing loss via mechanisms such as vascular injury, inflammation, and fibrosis. The severity of hearing loss is influenced by the treatment timing, the cumulative dose, patient age, genetics, and concurrent therapies. The damaging effects of chemotherapy and radiation extend beyond the cochlea, involving the surrounding temporal bone as well as multiple levels of the auditory pathway. While pediatric patients may be candidates for bone-anchored hearing devices or cochlear implants, the need for serial imaging and the potential for implant-related MRI artifacts can complicate the timing of hearing rehabilitation. Moreover, the impact on the subcortical and cortical auditory structures may further influence the rehabilitation outcomes. This scoping review lays the foundation for future clinical and research efforts focused on the development of comprehensive pediatric guidelines for hearing preservation, monitoring, and rehabilitation, while also fostering multidisciplinary collaboration.
1. Introduction
The continuous progress in innovative therapies, leveraging state-of-the-art technologies, has significantly enhanced the survival rate among the pediatric oncological population [1,2], consequently prompting a shift in focus towards addressing both short- and long-term conditions associated with the disease [3,4,5]. Among these concerns, hearing loss is receiving growing attention. Though not a life-threatening danger, it is a barrier to proper language development [6], cognitive development [7], executive function [8], communication skills, education and social integration [9,10], and overall life quality [11,12,13]. As a result, it is now one of the focus areas for multiple national and international health organizations [14,15,16]. Children with malignancies often face complex factors contributing to hearing impairment, potentially exacerbated by additive effects [17,18].
Interdisciplinary collaboration is essential in managing pediatric cancer patients [19,20]. Advances in multidisciplinary care, preventive strategies, and therapies have significantly improved short- and long-term hearing outcomes [21]. However, oncologic therapies frequently involve the temporal bone, resulting in structural and functional alterations of the auditory system. These changes affect the inner ear and multiple levels of the auditory system [22,23], while also inducing inflammation, infection, and disruption of the temporal bone microarchitecture [24].
This review aims to highlight the multifaceted challenges faced by clinical teams when considering hearing rehabilitation in pediatric oncologic patients and provides a foundation for future research.
2. Treatment Modalities for Childhood Cancers and the Pathophysiology of Their Impact on Hearing (Figure 1)
2.1. Overview of Ototoxic Treatments in Pediatric Oncology
The most common cancer types affecting children and adolescents include leukemias, brain tumors, and lymphomas [25]. Treatment typically involves surgery, chemotherapy, radiation therapy (RT), or a combination of these modalities [18]. Chemotherapy-induced ototoxicity primarily results in sensorineural hearing loss [17,26], whereas radiation-related ototoxicity may manifest as a sensorineural component, a conductive component, or a mixed presentation [27,28,29].
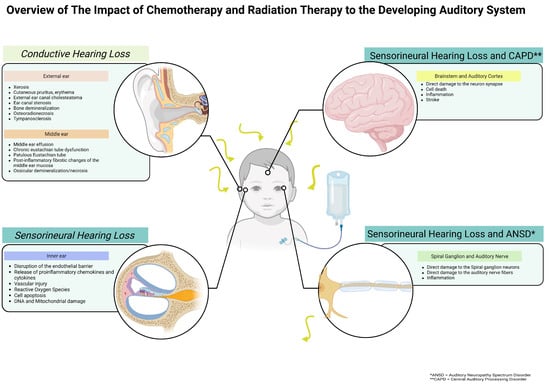
Figure 1.
Schematic overview of the impact of chemotherapy and radiation therapy on the developing auditory system.
2.2. Cisplatin-Induced Ototoxicity
Among the chemotherapy agents, platinum-based compounds such as cisplatin are frequently employed [30]. In the cochlea, cisplatin undergoes structural transformation in response to low chloride concentrations, resulting in intracellular accumulation and impaired efflux [31,32]. Cisplatin ototoxicity involves multiple mechanisms that induce sustained cellular stress, primarily through the generation of reactive oxygen species (ROS) [33,34] along with a reduction in antioxidant defenses and increased intracellular calcium levels [35,36] among other pathways [37]. Its dose-dependent cytotoxicity primarily targets outer hair cells, spiral ganglion neurons, and the stria vascularis [32,38,39], following a basal-to-apical gradient, with the basal outer hair cells being most vulnerable [40], likely due to the rapid accumulation of high levels of cisplatin in the basal turn scala tympani, with delayed elimination relative to serum [41]. Tonotopic variations in transport mechanisms, such as the copper transporter (CTR1), organic cation transporter 2 (OCT2) and mechanoelectrical transduction (MET) channel complex [42], result in increased cisplatin uptake in basal hair cells, reflecting variations in transporter expression and activity along the cochlea [43]. Additional factors contributing to vulnerability include disrupted calcium homeostasis and lower glutathione levels in basal outer hair cells, heightening their susceptibility to oxidative stress [44,45]. The risk and severity of cisplatin-induced hearing loss are influenced by multiple factors, including the patient’s age (particularly under 5 years) [46,47], cumulative dose (typically 400–500 mg/m2 in children) [48], nutritional status, duration of treatment, method of infusion, number of chemotherapy cycles, renal function, and concurrent radiation therapy. This type of ototoxicity typically presents as irreversible, sensorineural, and bilateral, primarily impacting high frequencies [49]. Onset may occur within hours to days after cisplatin administration, with hearing loss potentially progressing over time [50,51], as cisplatin is retained in the cochlea for months to years in both mouse and human cochlea as shown in temporal bone studies [41]. Tinnitus is a commonly reported accompanying symptom [52]. This prolonged retention contributes to progressive cochlear damage by sustaining oxidative stress and inflammation, while impairing mitochondrial function and DNA repair [53,54]. These processes collectively lead to the progressive and irreversible loss of inner ear cells. Interindividual variability in susceptibility is partly genetic. Variants in genes such as thiopurine methyltransferase (TPMT), which influence drug metabolism, have been linked to an increased risk of cisplatin-induced ototoxicity [55].
The impact of chemotherapy, and cisplatin in particular, extends beyond the inner ear. For instance, macrophages and fibroblasts—critical to the wound healing process—are similarly susceptible to its cytotoxic effects, as are cancer cells [56].
2.3. Radiation Therapy and Effects on the Inner Ear
The combination of cisplatin and RT is a common therapeutic approach for pediatric cancers [57], enhancing survival but causing ototoxicity, which manifests as hearing loss and tinnitus [49]. Hearing-related side effects are often bilateral, increase with the cumulative dose, tend to worsen over time, and are typically permanent. In contrast to chemotherapy-induced sensorineural damage, radiation therapy can cause conductive hearing loss due to structural and functional changes within the outer and middle ear. These changes may include narrowing of the ear canal, thickening of the eardrum, and impaired function of the middle ear or Eustachian tube, commonly resulting in middle ear effusion [58,59,60]. Among RT-related causes, otitis media with effusion (OME) is the most frequently observed contributor to conductive hearing loss [24,61]. Nevertheless, additional complications, such as chronic middle ear infections, persistent tympanic membrane perforation, post-inflammatory fibrosis within the middle ear, and ossicular demineralization, can also contribute [24]. The radiation-induced effects encompass a complex cascade, involving reactive oxygen species (ROS) production, vascular injury, chronic hypoxia, inflammatory response, and myofibroblast activation leading to fibrosis [62]. Vascular injury is a pivotal element in radiation damage, initiating coagulation pathways, vascular occlusion, tissue ischemia, and chronic hypoxia. This hypoxic state perpetuates ROS production, exacerbating the cellular damage. At the microscopic level, radiation disrupts the endothelial barrier, increasing the vascular permeability, releasing proinflammatory chemokines and cytokines, and facilitating immune cell migration [63,64]. These acute effects, akin to other off-target radiation effects, may lead to long-term vascular dysfunction if unaddressed [64]. Endothelial injury is increasingly attributed to cancer treatment, directly impacting the vasculature by inducing endothelial cell apoptosis and senescence and altering normal vascular homeostasis, contributing to a systemic chronic inflammatory state. Non-irradiated tissues are influenced by signals from nearby radiated cells through the bystander effect [65]. Early radiation-induced damage to the ear is visible on a macroscopic level as swelling, inflammation, and scaling of the tissues in the outer, middle, and inner ear [66]. When the external auditory canal is exposed to radiation, there is increased vulnerability to infections of the soft tissues in that area [67]. Within the inner ear level, the stria vascularis is pivotal in mediating inflammation by recruiting macrophages to the damaged area [68,69]. The pediatric population, especially those patients receiving treatment for malignant conditions, face increased risks for chronic OME due to factors such as upper airway infections and eustachian tube alterations [58], leading to significant morbidity. Managing chronic otitis media with effusion in oncologic patients and addressing long-term sequelae through corrective surgery demands a thorough understanding of the complex underlying pathophysiology to optimize the timing while balancing the benefits and risks [70,71]. While temporal bone osteoradionecrosis is a long-term complication observed in the adult population [72], the oncologic population may present with altered temporal bone function at the microstructural level. Ionizing radiation disrupts intracellular homeostasis by directly damaging the DNA and indirectly generating free radicals from water molecules.
2.4. Risk Mitigation and Prevention
Recently, proton beam radiotherapy (PBT) has seen exponential growth, especially in managing childhood cancer. PBT, available at select institutions, is globally acknowledged for use in pediatric brain, head, and neck and adult skull base malignancies. It has the potential for superior outcomes in terms of local control while maintaining acceptable levels of toxicity, when compared to photon therapy, in settings where high doses are required at sites adjacent to sensitive normal tissues [73]. Where dose escalation is not required, PBT demonstrates a superior ability to reduce the doses to normal tissues, thereby potentially mitigating both acute and late toxicities [73,74,75,76].
Recent studies suggest that children are more vulnerable to hearing loss than adults, with a correlation between the average radiation dose received by the cochlea and the risk of auditory impairment [28]. Nonetheless, data in children confirming the suggested cochlear radiation limit of 30 to 45 Gy, along with a clearer understanding of how cisplatin and radiation therapy interact to worsen hearing loss, are still scarce [77,78].
Hearing loss induced by chemotherapy and RT may be further exacerbated by the potential additive effect of a bone marrow transplant [26], heightening the risk for patients undergoing multimodal therapy with combined cisplatin and RT administration [27,47,79,80], Individuals with central nervous system (CNS) tumors face heightened risks of hearing loss from additional factors, including exposure to the neurotoxic vinca-alkaloid vincristine, cerebrospinal fluid-shunt implants, and brain surgeries involving the auditory system [81,82,83]. The effects of the therapies may also extend to neural structures at the nerve level or higher, including cortical areas [84,85], emphasizing that the prevention of ototoxic hearing loss requires the implementation of primary, secondary, and tertiary strategies [86]. Primary prevention aims to avert the onset of hearing loss, often through modifying treatments or exploring alternative pharmaceutical approaches [87]. Pharmacologic otoprotectants show promise in clinical trials; however, challenges remain regarding regulatory approval and global accessibility [88,89,90]. Sodium thiosulfate, is currently FDA-approved to reduce the risk of cisplatin-related SNHL in children aged 1 month to 18 years with localized non-metastatic solid tumors [91]. Despite this, 30% to 40% of children receiving cisplatin still develop SNHL, even when treated with sodium thiosulfate [92].
Ultimately, while auditory symptoms may represent a common clinical endpoint of cancer therapy, the underlying mechanisms vary based on the treatment type and the specific tissues affected. A deeper understanding of these complex pathophysiological processes is essential to minimize long-term auditory complications in childhood cancer survivors.
3. Effects of Oncologic Therapies on the Temporal Bone: Insights into Bone Mineral Density and Candidate Biomarkers of Temporal Bone Metabolisms in Pediatric Patients
Early research has identified decreased bone mineral density (BMD) in the spine and long bones of pediatric survivors who underwent cranial RT for conditions such as medulloblastoma [93], as well as acute lymphoblastic leukemia (ALL) [94], despite these skeletal sites not being directly in the high-dose radiation field [95,96]. The reported incidence of osteopenia in childhood cancer survivors ranges from 33% to 51% [97], with cranial irradiation during childhood consistently identified as the strongest predictor of low BMD in adulthood [98]. Similar findings have been observed in other long-term survivor cohorts [99]. However, conventional BMD assessments, such as lumbar spine BMD, femoral BMD, and total body BMD do not effectively capture the localized damage to the temporal bone. Currently, no standardized method exists for directly evaluating the mineralization of the temporal bone. Similarly, indirect assessment using computed tomography (CT) is limited by concerns over radiation exposure, which restricts the routine use of CT imaging in pediatric populations [100].
In the context of surgical strategies involving implantable devices, the quality of bone and, particularly, its mineral density and microarchitecture, is a critical factor influencing surgical outcomes and long-term device integration [101,102]. While the literature supporting this relationship is well-established in the field of dental implants [103,104], corresponding data on hearing implants would benefit from additional research [24,105,106,107]. Nonetheless, the principles of osseointegration and bone health are likely to be similar. Given the current gap in research and the limited access to direct evaluation methods for temporal bone mineralization in children, circulating biomarkers of bone metabolism present a promising alternative for assessing bone health [108]. These biomarkers offer valuable insights into various aspects of bone turnover, including formation, resorption, and the dynamic balance between the two. They are well-established proxies, used in clinics, for bone cell activity [109,110,111,112].
Other therapies commonly included in pediatric cancer treatment regimens, such as cisplatin and corticosteroids are well-known potent inducers of osteopenia [113,114,115]. Consequently, the combined effects of the tumor itself, radiotherapy, and these pharmacologic agents may synergistically contribute to compromised bone health [116].
Enhanced detection of alterations in bone mineralization and microarchitecture, at both molecular and structural levels, hold significant implications for hearing rehabilitation strategies. Biomarkers capable of identifying early changes in the temporal bone quality could enable timely intervention and help anticipate potential challenges related to the placement and long-term stability of surgical hearing devices.
4. Types of Hearing Implants and Considerations for Implant Osseointegration (Figure 2)
Early and individualized hearing rehabilitation is best achieved through a coordinated effort between oncologists, radiation therapists, audiologists, and otolaryngologists. Rehabilitation options may include standard hearing aids, CROS and bi-CROS devices, bone conduction systems, including osseointegrated implants, as well as non-surgical alternatives such as bone conduction devices on soft bands, eyeglass-mounted aids, adhesives, or cochlear implants when indicated.
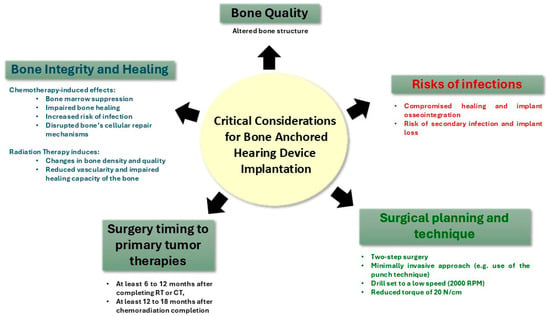
Figure 2.
The effects of therapeutic interventions on the temporal bone and its implications for the placement of bone-anchored hearing devices.
4.1. Bone Conduction Hearing Devices: Indications and Options
When planning hearing rehabilitation with osseointegrated implants in pediatric oncology patients, surgical challenges and compromised bone quality due to cancer treatments must be carefully considered [107]. Medications such as cyclosporine A, methotrexate, cisplatin, proton pump inhibitors, anticonvulsants, selective serotonin reuptake inhibitors, warfarin, and specific types of heparins might impede the osseointegration process [117]. Cisplatin, in particular, has been shown to hinder bone regeneration around implants in animal models, compromising the titanium integration of dental implants [118,119]. However, long-term outcomes suggest that implant survival remains unaffected when placement occurs approximately 10.5 months after chemotherapy [120].
Bone conduction [121] hearing devices (BCHD) are effective for conductive or mixed hearing loss and single-sided deafness, where sound is transmitted to the functioning contralateral cochlea [122,123]. These devices are available in both surgical (osseointegrated) and non-surgical forms, including soft bands, metal head bands, eyeglasses, or adhesive. While non-surgical devices are suitable for younger children, they offer reduced sound transmission, especially at higher frequencies, due to soft tissue attenuation [124]. Additionally, children over age six often prefer a surgical option due to peer-related cosmetic concerns [125]. BCHDs are particularly beneficial in cases of a conductive component ≥30 dB or when traditional hearing aids are not tolerated.
Surgically implanted BCHDs are either percutaneous or transcutaneous [126]. Percutaneous devices establish a direct coupling between the transducer and the skull bone via a percutaneous abutment, eliminating skin or soft tissue attenuation. In contrast, transcutaneous devices, whether passive or active, transmit vibrations either directly through an implanted subcutaneous portion or via transcutaneous electromagnetic signals, with passive systems exhibiting some degree of soft tissue attenuation [127]. The FDA has approved the surgical implantation of BCHDs in children aged 5 and older [128].
As part of pre-surgical planning, CT imaging of the temporal bone is primarily used to assess bone thickness and guide optimal implant placement [129,130]. However, its ability to evaluate bone quality is limited. Cone-beam CT has shown promise in the pre-surgical assessment of bone quality for dental implants [131,132]; however, its utility in the context of BCHD has yet to be clearly established [133,134]. Ideally, techniques such as microCT could provide high-resolution insights into bone architecture [135,136], but their use in vivo for temporal bone evaluation is not feasible. Consequently, intraoperative evaluation remains essential. The recent introduction of dual-energy CT, which enables assessment of bone mineral density by analyzing calcium and other mineral content [137,138], offers a promising advancement. This technique may hold future value in preoperative planning for BCHD placement.
When considering a percutaneous device, a two-stage minimally invasive punch technique is advised based on our pediatric experience to minimize tissue trauma and improve osseointegration. The literature also supports low-speed drilling and a torque of 20 N/cm to minimize tissue trauma, preserve bone quality, and enhance osseointegration [24]. While these devices produce fewer magnetic resonance imaging (MRI) artifacts, they carry risks such as osseointegration failure, implant extrusion, and soft tissue complications, including adverse skin reactions and soft tissue infections, observed in up to 84% of patients [127].
Transcutaneous BCHDs can be passive or active systems. Passive transcutaneous systems aim to overcome the limitations of percutaneous implants. While they avoid skin-penetrating components and reduce cosmetic concerns [139,140], they may lead to sound attenuation [141]. Conversely, active transcutaneous devices aim to optimize the advantages of both percutaneous and passive transcutaneous implants, avoiding complications related to skin and soft tissue while minimizing sound attenuation through soft tissue [127]. In single-sided deafness, modern active transcutaneous devices improve high-frequency hearing and speech understanding in noisy environments [142]. Although these implants have shown benefits in the general pediatric population, their use among pediatric oncology patients remains limited. Several factors may contribute to this, one of which is the significant MRI artifact they produce, potentially interfering with imaging-based surveillance of the brain [143].
4.2. Cochlear Implants in Pediatric Oncology
In pediatric patients experiencing severe to profound SNHL and who derive limited improvement from conventional hearing aids, cochlear implantation is recommended as the next-line intervention to help reduce further challenges related to their hearing impairment [11]. Any level of hearing loss has the potential to hinder language development, verbal proficiency, and reasoning skills [144]. A prevailing concern in the general population is the prevalence of OME, a common middle ear infection in childhood. Nevertheless, studies in typical cases suggest that cochlear implantation is generally safe in children with OME, implying that deferring the implantation process may be unnecessary [145]. While one might argue that oncology patients, facing an elevated risk of eustachian tube dysfunction and alterations in the middle ear mucosa and function, encounter additional challenges, recent reports indicate that these factors do not seem to impede eligibility for cochlear implantation in the pediatric oncologic population [146]. Furthermore, recent studies endorse electric–acoustic stimulation, utilizing two technologies—a cochlear implant plus acoustic amplification—to cover the full range of hearing, as a viable treatment option for this specific population [147].
When planning surgery, several factors require attention: radiation-induced inflammation and fibrosis in the middle ear can hinder dissection, while radiation-related demineralization calls for extra caution during drilling of the facial recess. Scar tissue and cochlear sclerosis can make electrode insertion difficult [24]. Additionally, the risk of wound dehiscence and infection is increased [56]. While MRI alone may suffice in the general population [148] combined CT and MRI offer complementary insights essential for surgical planning in the pediatric oncologic population. CT delineated bony anatomy; MRI detects early tissue changes such as early fibrosis, and this dual-modality approach is standard in our oncologic cohort.
4.3. MRI-Related Considerations for Hearing Implants
Advances in cochlear implant technology now allow many patients to safely undergo MRI, provided specific safety guidelines are followed [149]. Similar observations apply to bone conduction implants, wherein the magnet remains securely in place, signifying a noteworthy enhancement [150]. This development opens the possibility of considering these implants for use in the pediatric oncology population, where serial follow-up MRI is needed. The use of implanted hearing devices is increasing, and many patients with these devices need MRI for clinical reasons. However, both cochlear and transcutaneous bone conduction implants create MRI artifacts due to the internal magnet and differences in magnetic susceptibility between the implants and adjacent soft tissues [151]. MRI compatibility with otological implants has both clinical and practical implications. In the context of cochlear implantation for oncologic recipients, individuals with primary brain tumors undergoing MRI follow-up present a specific challenge owing to the proximity of the field of interest to the cochlear implant, compounded by the smaller anatomy of the pediatric skull [151,152]. Given that CI shadow artifacts can extend approximately 5 to 6 cm, optimizing the MRI sequences is essential. Diffusion-weighted imaging sequences tend to exhibit greater susceptibility to artifact generation compared to other MRI sequences. Fast spin echo and turbo spin echo demonstrate markedly reduced artifact burden, and advanced fat suppression techniques show improvement over traditional fat saturation sequences [151]. Previous studies have shown that the artifact size depends on the implant characteristics, MRI magnet strength, and patient-specific factors [143,151]. However, further research is needed.
Percutaneous BCHDs produce smaller artifacts compared to transcutaneous devices; however, they still produce artifacts near the titanium implant measuring 15.1 to 17.4 mm [152]. Transcutaneous implants generate larger and more variable artifacts depending on the device [143].
5. Impact of Chemotherapy, Radiotherapy, and Chemoradiation on the Auditory System
Despite the fact that a significant number of children are eligible for implanted hearing devices, relatively few proceed with implantation [153]. A frequently overlooked factor is the impact of chemotherapy and radiation on the auditory system. The number of pediatric CI recipients remains limited, and patients show inconsistent preoperative hearing aid use. Chemotherapy-induced ototoxicity primarily targets cochlear hair cells, but the damage extends beyond the cochlea to the spiral ganglion [154], auditory nerve, and central processing areas, potentially affecting cortical structures both structurally and functionally [41,155,156]. Recent investigations of the function of the brainstem auditory pathways of children with acute lymphoid leukemia submitted to chemotherapy revealed abnormal brainstem and central auditory evoked potentials in some children, with a predominance of impaired auditory pathways in the lower brainstem [22,157]. These results suggest that changes observed in the brainstem- and central auditory-evoked potentials are due to the neurotoxicity of certain drugs, rather than disease progression, since examination of the children’s cerebrospinal fluid were negative for neoplastic cells, indicating that the disease had not infiltrated into the central nervous system. Similarly, the presence of ABR abnormalities in children treated with the chemotherapeutic drug methotrexate suggests a neurotoxic effect of chemotherapy on the central auditory system. Although the mechanism(s) by which methotrexate causes neurotoxicity is(are) not fully understood, its potential neurotoxicity in the central auditory nervous system must be considered [22,23].
Similarly, while animal and human studies on irradiated ears showed damage to various cochlear structures (membranous labyrinth, hair cells, and stria vascularis), they also revealed atrophy of the spiral ganglion cells and the cochlear nerve with radiation alone or in combination with chemotherapy [66,158,159].
Although it has been suggested that the cochlea is more sensitive to the effects of radiation than the brain or auditory nerves, radiation-induced ear pathology is not uncommon and can affect all parts of the auditory system [160,161,162,163].
Although neurons may exhibit some degree of resistance to radiation effects owing to their low mitotic activity, the surrounding neural tissue remains vulnerable due to radiation-induced damage to connective tissue cells that contribute to myelin production and other supportive functions [164]. Evaluations of cranial irradiation on the hippocampus and cortical neurons revealed dose-dependent damage to the synapses similar to changes found in neurodegenerative diseases [165,166,167]. Therefore, irradiation-induced damage to neural components in the auditory system should not be overlooked. Studies using animal models have demonstrated that high doses of radiation led to the destruction of eight nerve fibers [160]. Reports of abnormal auditory brainstem responses in patients diagnosed with radiation induced SNHL have been made [168,169], as have auditory brainstem responses in patients after radiation-therapy for nasopharyngeal carcinoma [170]. Additionally, evidence indicates that damage to the retrocochlear pathways may go undetected in some patients, implying that the true impact of radiation therapy on hearing could be underestimated [171]. Furthermore, a recent animal study suggests that cochlear ribbon synapses may also be a subcellular target of radiation-induced hearing loss, potentially disrupting neural synchrony throughout the auditory pathways [172].
In summary, all patients with post-irradiation SNHL should be evaluated by MRI involving the inner ear as well as the auditory pathways and include auditory-evoked potential testing (both at the brainstem and central level) to objectively evaluate the function of the acoustic nerve and the central auditory pathways.
6. Potential Future Directions for Research
Future research endeavors may follow several promising avenues, spanning prevention, early detection, and optimization of rehabilitation strategies related to the auditory system’s chemo- and/or RT-induced injuries. A critical area of investigation involves the development of advanced monitoring strategies through the identification and validation of inner ear-specific [173,174] biomarkers, detectable in peripheral blood samples [175]. While initial progress has been made in this domain, the relationship between cochlear and auditory nerve injury and systemic bone metabolism remains largely unexplored. Further research is needed to elucidate how radiation-induced inner ear damage is reflected in circulating bone turnover markers. Preliminary research indicates that children treated with cranial radiation therapy for diseases such as acute lymphoblastic leukemia and medulloblastoma may experience reduction in bone mineral density, particularly in the spine and long bone [93,94]. Building on these findings, future research could explore potential correlations between hearing loss and bone turnover markers. Such potential biomarkers coupled with inner-ear specific markers could provide a powerful tool for early diagnosis and longitudinal monitoring of cochlear degeneration and radiation-associated auditory dysfunction.
In addition to molecular biomarkers, functional monitoring should incorporate electrophysiological testing to assess the integrity of the auditory pathway. Techniques such as otoacoustic emissions, auditory brainstem response testing, middle latency responses, and cortical auditory evoked potentials, particularly when elicited using varied and complex stimuli [155], can detect early changes in auditory processing and speech perception [176], expanding the early detection of changes in hearing and speech processing [177].
Keeping in mind the ongoing maturation of the key components of the auditory system in children [178,179,180], longitudinal evaluation of neuroplastic changes at both subcortical and cortical levels remains essential. In this context, developing protocols involving electrophysiological functional markers as exemplified in the general literature [181] would offer valuable tools for assessing auditory function across multiple levels of the auditory pathway [156,178,182]. These markers not only would provide valuable insight into localizing the site of impairment and identifying early signs of damage but also would serve as objective indicators of functional recovery and the effectiveness of interventions such as amplification or implanted hearing devices [183,184].
Different avenues remain to be explored in the prevention of hearing loss. While significant progress has been made, particularly with the FDA approval of sodium thiosulfate (STS) [91] as an otoprotective agent in the pediatric oncologic population, indications are still limited. This is largely due to concerns about the potential effect of STS on the antitumor efficacy of cisplatin. Although evidence suggests that delayed administration of STS does not compromise the cisplatin’s oncologic effectiveness [185,186], current protocols rely primarily on intravenous delivery of STS [187]. A new era in otoprotection is now emerging within the otolaryngology community, with growing interest in targeted inner ear drug delivery [87]. The development of new vectors and alternative delivery routes [188,189] has opened promising avenues for localized therapy, potentially enhancing therapeutic precision while minimizing systemic toxicity [190].
Understanding these alterations along the auditory pathway could significantly inform both prognostic modeling and support the development of personalized rehabilitation protocols. However, further progress is needed to address imaging artifacts caused by the magnetic component of implanted hearing devices [151,191].
Advancing our understanding of auditory pathway alterations will be crucial for improving early detection, guiding personalized rehabilitation strategies, and refining preventive approaches, ultimately paving the way for more effective individualized care in the pediatric oncologic population.
7. Conclusions
Childhood cancer treatments occur during a critical window of hearing development, where the ototoxic effects of chemotherapy and radiation can cause irreversible damage to multiple levels of the auditory system. Hearing rehabilitation is further complicated by the timing of the primary tumor treatment, compromised bone quality affecting implant integration, and the need for ongoing imaging surveillance that can be hindered by device-related artifacts. These challenges underscore the need for comprehensive standardized guidelines to guide monitoring, prevention, and tailored rehabilitation strategies in pediatric oncologic patients.
Author Contributions
The authors included each contributed substantially to this research per the guidelines of the International Committee of Medical Journal Editors (ICMJE); Conceptualization: G.C. and C.R.; Methodology: G.C. and C.R.; Literature review: G.C., L.L., C.R. and T.M.; Original Draft Preparation: G.C. and C.R.; Review and Editing: G.C., L.L., C.R., T.M., J.K.B. and T.E.M.; Figures: G.C. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work did not receive support from any grant.
Conflicts of Interest
The authors declare no financial disclosures or conflicts of interest related to the research described in this manuscript.
Abbreviations
| QoL | quality of life |
| RT | radiation therapy |
| OME | otitis media with effusion |
| CNS | central nervous system |
| ALL | acute lymphoblastic leukemia |
| BMD | bone mineral density |
| LS | lumbar spine |
| DXA | dual energy x-ray absorptiometry |
| CS | corticosteroids |
| PBT | proton beam radiotherapy |
| ROS | reactive oxygen species |
| CI | cochlear implant |
| BCHD | bone conduction hearing devices |
| MRI | magnetic resonance imaging |
References
- CDC; United States Cancer Statistics (USCS). United States Cancer Statistics. 10 June 2025. Available online: https://www.cdc.gov/united-states-cancer-statistics/index.html (accessed on 21 July 2025).
- National Childhood Cancer Registry Explorer (NCCR*Explorer). Available online: https://nccrexplorer.ccdi.cancer.gov/ (accessed on 4 February 2024).
- Freedman, J.L.; Beeler, D.M.; Bowers, A.; Bradford, N.; Cheung, Y.T.; Davies, M.; Dupuis, L.L.; Elgarten, C.W.; Jones, T.M.; Jubelirer, T.; et al. Supportive Care in Pediatric Oncology: Opportunities and Future Directions. Cancers 2023, 15, 5549. [Google Scholar] [CrossRef] [PubMed]
- Strebel, S.; Baust, K.; Grabow, D.; Byrne, J.; Langer, T.; Am Zehnhoff-Dinnesen, A.; Kuonen, R.; Weiss, A.; Kepak, T.; Kruseova, J.; et al. Auditory complications among childhood cancer survivors and health-related quality of life: A PanCareLIFE study. J. Cancer Surviv. 2023, 19, 162–173. [Google Scholar] [CrossRef]
- Late Effects of Treatment for Childhood Cancer (PDQ®)—NCI. 23 April 2004. Available online: https://www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq (accessed on 28 June 2025).
- Yoshinaga-Itano, C.; Sedey, A.L.; Coulter, D.K.; Mehl, A.L. Language of early- and later-identified children with hearing loss. Pediatrics 1998, 102, 1161–1171. [Google Scholar] [CrossRef]
- Bass, J.K.; Liu, W.; Banerjee, P.; Brinkman, T.M.; Mulrooney, D.A.; Gajjar, A.; Pappo, A.S.; Merchant, T.E.; Armstrong, G.T.; Srivastava, D.; et al. Association of Hearing Impairment With Neurocognition in Survivors of Childhood Cancer. JAMA Oncol. 2020, 6, 1363–1371. [Google Scholar] [CrossRef]
- Hall, M.L.; Eigsti, I.-M.; Bortfeld, H.; Lillo-Martin, D. Executive Function in Deaf Children: Auditory Access and Language Access. J. Speech Lang. Hear. Res. 2018, 61, 1970–1988. [Google Scholar] [CrossRef]
- de Jong, T.J.; van der Schroeff, M.P.; Stapersma, L.; Vroegop, J.L. A systematic review on the impact of auditory functioning and language proficiency on psychosocial difficulties in children and adolescents with hearing loss. Int. J. Audiol. 2024, 63, 675–685. [Google Scholar] [CrossRef]
- Lieu, J.E.C. Unilateral hearing loss in children: Speech-language and school performance. B-ENT 2013, (Suppl. 21), 107–115. [Google Scholar] [PubMed] [PubMed Central]
- Lieu, J.E.C.; Kenna, M.; Anne, S.; Davidson, L. Hearing Loss in Children: A Review. JAMA 2020, 324, 2195–2205. [Google Scholar] [CrossRef]
- Fellinger, J.; Holzinger, D.; Pollard, R. Mental health of deaf people. Lancet 2012, 379, 1037–1044. [Google Scholar] [CrossRef]
- Phillips, O.R.; Baguley, D.M.; Pearson, S.E.; Akeroyd, M.A. The long-term impacts of hearing loss, tinnitus and poor balance on the quality of life of people living with and beyond cancer after platinum-based chemotherapy: A literature review. J. Cancer Surviv. 2023, 17, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Neumann, K.J.; Saunders, J.E. The global burden of disabling hearing impairment: A call to action. Bull. World Health Organ. 2014, 92, 367–373. [Google Scholar] [CrossRef] [PubMed]
- WHO. Deafness and Hearing Loss. February 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 8 June 2025).
- CDC. Treatment and Intervention for Hearing Loss. Hearing Loss in Children; 16 May 2024. Available online: https://www.cdc.gov/hearing-loss-children/treatment/index.html (accessed on 30 June 2025).
- Keilty, D.; Khandwala, M.; Liu, Z.A.; Papaioannou, V.; Bouffet, E.; Hodgson, D.; Yee, R.; Cushing, S.; Laperriere, N.; Ahmed, S.; et al. Hearing Loss After Radiation and Chemotherapy for CNS and Head-and-Neck Tumors in Children. J. Clin. Oncol. 2021, 39, 3813–3821. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Childhood Cancers and Disability. Childhood Cancer and Functional Impacts Across the Care Continuum; Aiuppa, L., Cartaxo, T., Spicer, C.M., Volberding, P.A., Eds.; National Academies Press (US): Washington, DC, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK569409/ (accessed on 8 June 2025).
- Lamb, B.W.; Taylor, C.; Lamb, J.N.; Strickland, S.L.; Vincent, C.; Green, J.S.A.; Sevdalis, N. Facilitators and barriers to teamworking and patient centeredness in multidisciplinary cancer teams: Findings of a national study. Ann. Surg. Oncol. 2013, 20, 1408–1416. [Google Scholar] [CrossRef]
- Graetz, D.E.; Chen, Y.; Devidas, M.; Antillon-Klussmann, F.; Fu, L.; Quintero, K.; Fuentes-Alabi, S.L.; Gassant, P.Y.; Kaye, E.C.; Baker, J.N.; et al. Interdisciplinary care of pediatric oncology patients: A survey of clinicians in Central America and the Caribbean. Pediatr. Blood Cancer 2023, 70, e30244. [Google Scholar] [CrossRef]
- Helms, L.; Guimera, A.E.; Janeway, K.A.; Bailey, K.M. Innovations in Cancer Treatment of Children. Pediatrics 2023, 152, e2023061539. [Google Scholar] [CrossRef]
- Leite, R.A.; Vosgrau, J.S.; Neto, L.C.; Santos, N.P.; Matas, S.L.d.A.; Filho, V.O.; Matas, C.G. Brainstem auditory pathway of children with acute lymphoid leukemia on chemotherapy with methotrexate. Arq. Neuro-Psiquiatr. 2020, 78, 63–69. [Google Scholar] [CrossRef]
- Shuper, A.; Stark, B.; Kornreich, L.; Cohen, I.J.; Avrahami, G.; Yaniv, I. Methotrexate-related neurotoxicity in the treatment of childhood acute lymphoblastic leukemia. Isr. Med. Assoc. J. 2002, 4, 1050–1053. [Google Scholar] [PubMed]
- Nader, M.-E.; Gidley, P.W. Challenges of Hearing Rehabilitation after Radiation and Chemotherapy. J. Neurol. Surg. B Skull Base 2019, 80, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Cancer in Children and Adolescents—NCI. 29 August 2024. Available online: https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet (accessed on 15 July 2025).
- Cohen-Cutler, S.; Wong, K.; Mena, V.; Sianto, K.; Wright, M.A.; Olch, A.; Orgel, E. Hearing Loss Risk in Pediatric Patients Treated with Cranial Irradiation and Cisplatin-Based Chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1488–1495. [Google Scholar] [CrossRef]
- Hua, C.; Bass, J.K.; Khan, R.; Kun, L.E.; Merchant, T.E. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 892–899. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, H.; An, F.; Zhao, A.; Wu, J.; Wang, M.; Luo, J. The relevance of ototoxicity induced by radiotherapy. Radiat. Oncol. 2023, 18, 95. [Google Scholar] [CrossRef]
- Murphy, B.; Jackson, A.; Bass, J.K.; Tsang, D.S.; Ronckers, C.M.; Kremer, L.; Baliga, S.; Olch, A.; Zureick, A.H.; Jee, K.-W.; et al. Modeling the Risk of Hearing Loss From Radiation Therapy in Childhood Cancer Survivors: A PENTEC Comprehensive Review. Int. J. Radiat. Oncol. Biol. Phys. 2023, 119, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Types of Cancer Treatment—NCI. 31 July 2017. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 21 January 2024).
- Ad, K.; Hj, P. Mode of DNA binding of cis-platinum(II) antitumor drugs: A base sequence-dependent mechanism is proposed. Cancer Treat. Rep. 1979, 63, 1445–1452. [Google Scholar]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Xue, D.-F.; Pan, S.-T.; Huang, G.; Qiu, J.-X. ROS enhances the cytotoxicity of cisplatin by inducing apoptosis and autophagy in tongue squamous cell carcinoma cells. Int. J. Biochem. Cell Biol. 2020, 122, 105732. [Google Scholar] [CrossRef]
- Mukherjea, D.; Jajoo, S.; Whitworth, C.; Bunch, J.R.; Turner, J.G.; Rybak, L.P.; Ramkumar, V. Short Interfering RNA against Transient Receptor Potential Vanilloid 1 Attenuates Cisplatin-Induced Hearing Loss in the Rat. J. Neurosci. 2008, 28, 13056–13065. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Paken, J.; Govender, C.D.; Pillay, M.; Sewram, V. A Review of Cisplatin-Associated Ototoxicity. Semin. Hear. 2019, 40, 108–121. [Google Scholar] [CrossRef]
- Chirtes, F.; Albu, S. Prevention and Restoration of Hearing Loss Associated with the Use of Cisplatin. BioMed Res. Int. 2014, 2014, 925485. [Google Scholar] [CrossRef]
- Callejo, A.; Sedó-Cabezón, L.; Domènech Juan, I.; Llorens, J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef]
- Prayuenyong, P.; Baguley, D.M.; Kros, C.J.; Steyger, P.S. Preferential Cochleotoxicity of Cisplatin. Front. Neurosci. 2021, 15, 695268. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Whitworth, C.A.; Mukherjea, D.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear. Res. 2007, 226, 157–167. [Google Scholar] [CrossRef]
- Maruyama, A.; Kawashima, Y.; Fukunaga, Y.; Makabe, A.; Nishio, A.; Tsutsumi, T. Susceptibility of mouse cochlear hair cells to cisplatin ototoxicity largely depends on sensory mechanoelectrical transduction channels both Ex Vivo and In Vivo. Hear. Res. 2024, 447, 109013. [Google Scholar] [CrossRef]
- Beurg, M.; Cui, R.; Goldring, A.C.; Ebrahim, S.; Fettiplace, R.; Kachar, B. Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat. Commun. 2018, 9, 2185. [Google Scholar] [CrossRef]
- Sha, S.-H.; Taylor, R.; Forge, A.; Schacht, J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res. 2001, 155, 1–8. [Google Scholar] [CrossRef]
- Engel, J.; Braig, C.; Rüttiger, L.; Kuhn, S.; Zimmermann, U.; Blin, N.; Sausbier, M.; Kalbacher, H.; Münkner, S.; Rohbock, K.; et al. Two classes of outer hair cells along the tonotopic axis of the cochlea. Neuroscience 2006, 143, 837–849. [Google Scholar] [CrossRef]
- Moke, D.J.; Luo, C.; Millstein, J.; Knight, K.R.; Rassekh, S.R.; Brooks, B.; Ross, C.J.D.; Wright, M.; Mena, V.; Rushing, T.; et al. Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: A multi-institutional North American cohort study. Lancet Child. Adolesc. Health 2021, 5, 274–283. [Google Scholar] [CrossRef]
- Zuur, C.L.; Simis, Y.J.; Lansdaal, P.E.; Hart, A.A.; Rasch, C.R.; Schornagel, J.H.; Dreschler, W.A.; Balm, A.J. Risk factors of ototoxicity after cisplatin-based chemo-irradiation in patients with locally advanced head-and-neck cancer: A multivariate analysis. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Biro, K.; Noszek, L.; Prekopp, P.; Nagyiványi, K.; Géczi, L.; Gaudi, I.; Bodrogi, I. Characteristics and risk factors of cisplatin-induced ototoxicity in testicular cancer patients detected by distortion product otoacoustic emission. Oncology 2006, 70, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hearing Loss in Cancer Patients. AAO-HNS Bulletin, 15 March 2024. Available online: https://bulletin.entnet.org/clinical-patient-care/article/22889518/hearing-loss-in-cancer-patients (accessed on 26 July 2025).
- Bertolini, P.; Lassalle, M.; Mercier, G.; Raquin, M.A.; Izzi, G.; Corradini, N.; Hartmann, O. Platinum compound-related ototoxicity in children: Long-term follow-up reveals continuous worsening of hearing loss. J. Pediatr. Hematol. Oncol. 2004, 26, 649–655. [Google Scholar] [CrossRef]
- Knight, K.R.G.; Kraemer, D.F.; Neuwelt, E.A. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 2005, 23, 8588–8596. [Google Scholar] [CrossRef]
- Chattaraj, A.; Syed, M.P.; Low, C.A.; Owonikoko, T.K. Cisplatin-Induced Ototoxicity: A Concise Review of the Burden, Prevention, and Interception Strategies. JCO Oncol. Pract. 2023, 19, 278–283. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Vlajkovic, S.M. Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions. Int. J. Mol. Sci. 2023, 24, 16545. [Google Scholar] [CrossRef]
- Lee, D.S.; Schrader, A.; Warchol, M.; Sheets, L. Cisplatin exposure acutely disrupts mitochondrial bioenergetics in the zebrafish lateral-line organ. Hear. Res. 2022, 426, 108513. [Google Scholar] [CrossRef]
- Hagleitner, M.M.; Coenen, M.J.H.; Patino-Garcia, A.; de Bont, E.S.J.M.; Gonzalez-Neira, A.; Vos, H.I.; van Leeuwen, F.N.; Gelderblom, H.; Hoogerbrugge, P.M.; Guchelaar, H.-J.; et al. Influence of Genetic Variants in TPMT and COMT Associated with Cisplatin Induced Hearing Loss in Patients with Cancer: Two New Cohorts and a Meta-Analysis Reveal Significant Heterogeneity between Cohorts. PLoS ONE 2014, 9, e115869. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, M.; Zieliński, J.; Wardowska, A.; Pikuła, M. Wound healing complications in oncological patients: Perspectives for cellular therapy. Adv. Dermatol. Allergol. 2019, 36, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Desoize, B.; Madoulet, C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Nakai, Y.; Esaki, Y.; Ikeoka, H.; Koshimo, H.; Onoyama, Y. Acute effects of irradiation on middle ear mucosa. Ann. Otol. Rhinol. Laryngol. 1988, 97, 173–178. [Google Scholar] [CrossRef]
- Elwany, S. Delayed ultrastructural radiation induced changes in the human mesotympanic middle ear mucosa. J. Laryngol. Otol. 1985, 99, 343–353. [Google Scholar] [CrossRef]
- Magnuson, K.; Franzén, L.; Henriksson, R.; Gustafsson, H.; Hellström, S. Structural changes in the middle ear tissues of the rat after fractionated irradiation. Eur. Arch. Otorhinolaryngol. 1993, 250, 92–96. [Google Scholar] [CrossRef]
- Walker, G.V.; Ahmed, S.; Allen, P.; Gidley, P.W.; Woo, S.Y.; DeMonte, F.; Chang, E.L.; Mahajan, A. Radiation-induced middle ear and mastoid opacification in skull base tumors treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e819–e823. [Google Scholar] [CrossRef]
- Wang, B.; Wei, J.; Meng, L.; Wang, H.; Qu, C.; Chen, X.; Xin, Y.; Jiang, X. Advances in pathogenic mechanisms and management of radiation-induced fibrosis. Biomed. Pharmacother. 2020, 121, 109560. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.-I.; Krishnan, S. Radiation-Induced Endothelial Vascular Injury: A Review of Possible Mechanisms. JACC Basic. Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Fardid, R.; Hadadi, G.; Fardid, M. The mechanisms of radiation-induced bystander effect. J. Biomed. Phys. Eng. 2014, 4, 163–172. [Google Scholar] [PubMed]
- Jereczek-Fossa, B.A.; Zarowski, A.; Milani, F.; Orecchia, R. Radiotherapy-induced ear toxicity. Cancer Treat. Rev. 2003, 29, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Million, R.R.; Parsons, J.T.; Mendenhall, W.M. Effect of radiation on normal tissues in the head and neck. Bone, cartilage, and soft tissue. Front. Radiat. Ther. Oncol. 1989, 23, 221–237; discussion 251–254. [Google Scholar]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef]
- Shi, X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010, 342, 21–30. [Google Scholar] [CrossRef]
- Schwarz, Y.; Manogaran, M.; Daniel, S.J. Ventilation tubes in middle ear effusion post-nasopharyngeal carcinoma radiation: To insert or not? Laryngoscope 2016, 126, 2649–2651. [Google Scholar] [CrossRef]
- Richard, C.; Baker, E.; Wood, J. Special Considerations for Tympanoplasty Type I in the Oncological Pediatric Population: A Case-Control Study. Front. Surg. 2022, 9, 844810. [Google Scholar] [CrossRef]
- Morrissey, D.; Grigg, R. Incidence of osteoradionecrosis of the temporal bone. ANZ J. Surg. 2011, 81, 876–879. [Google Scholar] [CrossRef]
- Merchant, T.E. Clinical controversies: Proton therapy for pediatric tumors. Semin. Radiat. Oncol. 2013, 23, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Gaito, S.; Hwang, E.J.; France, A.; Aznar, M.C.; Burnet, N.; Crellin, A.; Holtzman, A.L.; Indelicato, D.J.; Timmerman, B.; Whitfield, G.A.; et al. Outcomes of Patients Treated in the UK Proton Overseas Programme: Central Nervous System Group. Clin. Oncol. 2023, 35, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.; Tsang, D.; Ng, A.; Laperriere, N.; Hodgson, D.C. Monte Carlo-driven predictions of neurocognitive and hearing impairments following proton and photon radiotherapy for pediatric brain-tumor patients. J. Neurooncol. 2017, 135, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Hoehn, M.E.; Khan, R.B.; Sabin, N.D.; Klimo, P.; Boop, F.A.; Wu, S.; Li, Y.; Burghen, E.A.; Jurbergs, N.; et al. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): A single-arm, phase 2 study. Lancet Oncol. 2023, 24, 523–534. [Google Scholar] [CrossRef]
- Paulino, A.C.; Lobo, M.; Teh, B.S.; Okcu, M.F.; South, M.; Butler, E.B.; Su, J.; Chintagumpala, M. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1445–1450. [Google Scholar] [CrossRef]
- Scoccianti, S.; Detti, B.; Gadda, D.; Greto, D.; Furfaro, I.; Meacci, F.; Simontacchi, G.; Di Brina, L.; Bonomo, P.; Giacomelli, I.; et al. Organs at risk in the brain and their dose-constraints in adults and in children: A radiation oncologist’s guide for delineation in everyday practice. Radiother. Oncol. 2015, 114, 230–238. [Google Scholar] [CrossRef]
- Grewal, S.; Merchant, T.; Reymond, R.; McInerney, M.; Hodge, C.; Shearer, P. Auditory late effects of childhood cancer therapy: A report from the Children’s Oncology Group. Pediatrics 2010, 125, e938–e950. [Google Scholar] [CrossRef]
- Packer, R.J.; Gurney, J.G.; Punyko, J.A.; Donaldson, S.S.; Inskip, P.D.; Stovall, M.; Yasui, Y.; Mertens, A.C.; Sklar, C.A.; Nicholson, H.S.; et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood cancer survivor study. J. Clin. Oncol. 2003, 21, 3255–3261. [Google Scholar] [CrossRef] [PubMed]
- Wells, E.M.; Ullrich, N.J.; Seidel, K.; Leisenring, W.; Sklar, C.A.; Armstrong, G.T.; Diller, L.; King, A.; Krull, K.R.; Neglia, J.P.; et al. Longitudinal assessment of late-onset neurologic conditions in survivors of childhood central nervous system tumors: A Childhood Cancer Survivor Study report. Neuro Oncol. 2018, 20, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, D.J.; Knight, K.; Marquez, C.; Kraemer, D.F.; Bardo, D.M.E.; Neuwelt, E.A. Cerebrospinal fluid shunting and hearing loss in patients treated for medulloblastoma: Clinical article. J. Neurosurg. Pediatr. 2012, 9, 421–427. [Google Scholar] [CrossRef]
- Landier, W. Ototoxicity and cancer therapy. Cancer 2016, 122, 1647–1658. [Google Scholar] [CrossRef]
- Azzam, P.; Mroueh, M.; Francis, M.; Daher, A.A.; Zeidan, Y.H. Radiation-induced neuropathies in head and neck cancer: Prevention and treatment modalities. Ecancermedicalscience 2020, 14, 1133. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S.R.; Stence, N.; Hemenway, M.; Hankinson, T.C.; Foreman, N.; Liu, A.K. Cerebral radiation necrosis in pediatric patients. Pediatr. Hematol. Oncol. 2015, 32, 78–83. [Google Scholar] [CrossRef]
- Dillard, L.K.; Martinez, R.X.; Perez, L.L.; Fullerton, A.M.; Chadha, S.; McMahon, C.M. Prevalence of aminoglycoside-induced hearing loss in drug-resistant tuberculosis patients: A systematic review. J. Infect. 2021, 83, 27–36. [Google Scholar] [CrossRef]
- Streefkerk, N.; Masroor, A.; Geller, J.I.; van Grotel, M.; Ansari, M.; Bouffet, E.; Bleyer, A.; Fresnau, B.; Sullivan, M.; Huitema, A.D.R.; et al. Local application of sodium thiosulfate as an otoprotectant for cisplatin-exposed patients—A narrative literature review to explore the potential benefit for children with cancer. EJC Paediatr. Oncol. 2025, 5, 100211. [Google Scholar] [CrossRef]
- van As, J.W.; van den Berg, H.; van Dalen, E.C. Medical interventions for the prevention of platinum-induced hearing loss in children with cancer. Cochrane Database Syst. Rev. 2019, 5, CD009219. [Google Scholar] [CrossRef]
- Freyer, D.R.; Brock, P.R.; Chang, K.W.; Dupuis, L.L.; Epelman, S.; Knight, K.; Mills, D.; Phillips, R.; Potter, E.; Risby, D.; et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: A clinical practice guideline. Lancet Child Adolesc. Health 2020, 4, 141–150. [Google Scholar] [CrossRef]
- Freyer, D.R.; Brock, P.; Knight, K.; Reaman, G.; Cabral, S.; Robinson, P.D.; Sung, L. Interventions for cisplatin-induced hearing loss in children and adolescents with cancer. Lancet Child Adolesc. Health 2019, 3, 578–584. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. FDA Approves Sodium Thiosulfate to Reduce the Risk of Ototoxicity Associated with Cisplatin in Pediatric Patients with Localized, Non-Metastatic Solid Tumors. FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sodium-thiosulfate-reduce-risk-ototoxicity-associated-cisplatin-pediatric-patients (accessed on 24 February 2024).
- Window KSSO in a New Window O in a New Window O in a New. FDA Approval Marks New Era for Preventing Cisplatin Induced Hearing Loss. Children’s Hospital Los Angeles. Available online: https://www.chla.org/blog/experts/research-and-breakthroughs/fda-approval-marks-new-era-preventing-cisplatin-induced-hearing (accessed on 27 July 2025).
- Mithal, N.P.; Almond, M.K.; Evans, K.; Hoskin, P.J. Reduced bone mineral density in long-term survivors of medulloblastoma. Br. J. Radiol. 1993, 66, 814–816. [Google Scholar] [CrossRef]
- Warner, J.T.; Evans, W.D.; Webb, D.K.H.; Bell, W.; Gregory, J.W. Relative Osteopenia after Treatment for Acute Lymphoblastic Leukemia. Pediatr. Res. 1999, 45, 544–551. [Google Scholar] [CrossRef]
- Petraroli, M.; D’Alessio, E.; Ausili, E.; Barini, A.; Caradonna, P.; Riccardi, R.; Caldarelli, M.; Rossodivita, A. Bone mineral density in survivors of childhood brain tumours. Childs Nerv. Syst. 2007, 23, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Odame, I.; Duckworth, J.; Talsma, D.; Beaumont, L.; Furlong, W.; Webber, C.; Barr, R. Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr. Blood Cancer 2006, 46, 357–362. [Google Scholar] [CrossRef]
- Pietilä, S.; Sievänen, H.; Ala-Houhala, M.; Koivisto, A.-M.; Liisa Lenko, H.; Mäkipernaa, A. Bone mineral density is reduced in brain tumour patients treated in childhood. Acta Paediatr. 2006, 95, 1291–1297. [Google Scholar] [CrossRef]
- van Atteveld, J.E.; Pluijm, S.M.F.; Ness, K.K.; Hudson, M.M.; Chemaitilly, W.; Kaste, S.C.; Robison, L.L.; Neggers, S.J.C.M.M.; Yasui, Y.; van den Heuvel-Eibrink, M.M.; et al. Prediction of Low and Very Low Bone Mineral Density Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2019, 37, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Remes, T.M.; Arikoski, P.M.; Lähteenmäki, P.M.; Arola, M.O.; Pokka, T.M.-L.; Riikonen, V.P.; Sirkiä, K.H.; Rantala, H.M.J.; Harila-Saari, A.H.; Ojaniemi, M.K. Bone mineral density is compromised in very long-term survivors of irradiated childhood brain tumor. Acta Oncol. 2018, 57, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Smoll, N.R.; Brady, Z.; Scurrah, K.J.; Lee, C.; Berrington de González, A.; Mathews, J.D. Computed tomography scan radiation and brain cancer incidence. Neuro Oncol. 2023, 25, 1368–1376. [Google Scholar] [CrossRef]
- Xiao, Y.; Lv, L.; Xu, Z.; Zhou, L.; Lin, Y.; Lin, Y.; Guo, J.; Chen, J.; Ou, Y.; Lin, L.; et al. Correlation between peri-implant bone mineral density and primary implant stability based on artificial intelligence classification. Sci. Rep. 2024, 14, 3009. [Google Scholar] [CrossRef]
- Anderson, K.D.; Ko, F.C.; Fullam, S.; Virdi, A.S.; Wimmer, M.A.; Sumner, D.R.; Ross, R.D. The Relative Contribution of Bone Microarchitecture and Matrix Composition to Implant Fixation Strength in Rats. J. Orthop. Res. 2022, 40, 862–870. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Abu Alfaraj, T.; Al-Madani, S.; Alqahtani, N.S.; Almohammadi, A.A.; Alqahtani, A.M.; AlQabbani, H.S.; Bajunaid, M.K.; Alharthy, B.A.; Aljalfan, N. Optimizing Osseointegration in Dental Implantology: A Cross-Disciplinary Review of Current and Emerging Strategies. Cureus 2023, 15, e47943. [Google Scholar] [CrossRef]
- Kiringoda, R.; Lustig, L.R. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol. Neurotol. 2013, 34, 790–794. [Google Scholar] [CrossRef]
- Larsson, A.; Wigren, S.; Andersson, M.; Ekeroth, G.; Flynn, M.; Nannmark, U. Histologic evaluation of soft tissue integration of experimental abutments for bone anchored hearing implants using surgery without soft tissue reduction. Otol. Neurotol. 2012, 33, 1445–1451. [Google Scholar] [CrossRef]
- Wilkie, M.D.; Lightbody, K.A.; Salamat, A.A.; Chakravarthy, K.M.; Luff, D.A.; Temple, R.H. Stability and survival of bone-anchored hearing aid implant systems in post-irradiated patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 1371–1376. [Google Scholar] [CrossRef]
- Seibel, M.J. Biochemical Markers of Bone Turnover Part I: Biochemistry and Variability. Clin. Biochem. Rev. 2005, 26, 97–122. [Google Scholar]
- Yang, L.; Grey, V. Pediatric reference intervals for bone markers. Clin. Biochem. 2006, 39, 561–568. [Google Scholar] [CrossRef]
- Rauchenzauner, M.; Schmid, A.; Heinz-Erian, P.; Kapelari, K.; Falkensammer, G.; Griesmacher, A.; Finkenstedt, G.; Högler, W. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J. Clin. Endocrinol. Metab. 2007, 92, 443–449. [Google Scholar] [CrossRef]
- Eapen, E.; Grey, V.; Don-Wauchope, A.; Atkinson, S.A. Bone Health in Childhood: Usefulness of Biochemical Biomarkers. eJIFCC 2008, 19, 123–136. [Google Scholar]
- Zhang, Y.; Zhang, J.; Huang, X.; Yu, X.; Li, Y.; Yu, F.; Zhou, W. Variation of Bone Turnover Markers in Childhood and Adolescence. Int. J. Clin. Pract. 2023, 2023, e5537182. [Google Scholar] [CrossRef]
- Hartmann, K.; Koenen, M.; Schauer, S.; Wittig-Blaich, S.; Ahmad, M.; Baschant, U.; Tuckermann, J.P. Molecular Actions of Glucocorticoids in Cartilage and Bone During Health, Disease, and Steroid Therapy. Physiol. Rev. 2016, 96, 409–447. [Google Scholar] [CrossRef]
- Ehrhart, N.; Eurell, J.A.C.; Tommasini, M.; Constable, P.D.; Johnson, A.L.; Feretti, A. Effect of cisplatin on bone transport osteogenesis in dogs. Am. J. Vet. Res. 2002, 63, 703–711. [Google Scholar] [CrossRef]
- Frost, H.M. On Our Age-Related Bone Loss: Insights from a New Paradigm. J. Bone Miner. Res. 1997, 12, 1539–1546. [Google Scholar] [CrossRef]
- Yao, Z.; Murali, B.; Ren, Q.; Luo, X.; Faget, D.V.; Cole, T.; Ricci, B.; Thotala, D.; Monahan, J.; van Deursen, J.M.; et al. Therapy-Induced Senescence Drives Bone Loss. Cancer Res. 2020, 80, 1171–1182. [Google Scholar] [CrossRef]
- Zidrou, C.; Kapetanou, A.; Rizou, S. The effect of drugs on implant osseointegration- A narrative review. Injury 2023, 54, 110888. [Google Scholar] [CrossRef]
- Al-Mahalawy, H.; Marei, H.F.; Abuohashish, H.; Alhawaj, H.; Alrefaee, M.; Al-Jandan, B. Effects of cisplatin chemotherapy on the osseointegration of titanium implants. J. Craniomaxillofac Surg. 2016, 44, 337–346. [Google Scholar] [CrossRef]
- Matheus, H.R.; Ervolino, E.; Faleiros, P.L.; Novaes, V.C.N.; Theodoro, L.H.; Garcia, V.G.; de Almeida, J.M. Cisplatin chemotherapy impairs the peri-implant bone repair around titanium implants: An in vivo study in rats. J. Clin. Periodontol. 2018, 45, 241–252. [Google Scholar] [CrossRef]
- Kovács, A.F. Influence of chemotherapy on endosteal implant survival and success in oral cancer patients. Int. J. Oral. Maxillofac. Surg. 2001, 30, 144–147. [Google Scholar] [CrossRef]
- Stenfelt, S.; Goode, R.L. Bone-conducted sound: Physiological and clinical aspects. Otol. Neurotol. 2005, 26, 1245–1261. [Google Scholar] [CrossRef]
- van Zyl, C.; Rogers, C.; Kuschke, S. Outcomes and device use in children with bone-conduction hearing devices in South Africa. S. Afr. J. Commun. Disord. 2024, 71, 1005. [Google Scholar] [CrossRef]
- Doshi, J.; Banga, R.; Child, A.; Lawrence, R.; Reid, A.; Proops, D.; McDermott, A.-L. Quality-of-life outcomes after bone-anchored hearing device surgery in children with single-sided sensorineural deafness. Otol. Neurotol. 2013, 34, 100–103. [Google Scholar] [CrossRef]
- Röösli, C.; Dobrev, I.; Pfiffner, F. Transcranial attenuation in bone conduction stimulation. Hear. Res. 2022, 419, 108318. [Google Scholar] [CrossRef]
- Wheeler, L.R.; Tharpe, A.M. Young Children’s Attitudes Toward Peers Who Wear Hearing Aids. Am. J. Audiol. 2020, 29, 110–119. [Google Scholar] [CrossRef]
- Brinkman, D.; Hill, R.; Hone, S.; Kieran, S. Bone-anchored hearing aids: Percutaneous versus transcutaneous attachments—A health economics comparison in paediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2023, 175, 111773. [Google Scholar] [CrossRef]
- Casazza, G.C.; Kesser, B.W. Modern Advances in Bone Conduction–Hearing Devices. Curr. Otorhinolaryngol. Rep. 2022, 10, 370–376. [Google Scholar] [CrossRef]
- Šikolová, S.; Urík, M.; Hošnová, D.; Kruntorád, V.; Bartoš, M.; Motyka, O.; Jabandžiev, P. Two Bonebridge bone conduction hearing implant generations: Audiological benefit and quality of hearing in children. Eur. Arch. Otorhinolaryngol. 2022, 279, 3387–3398. [Google Scholar] [CrossRef]
- Casselman, J.W.; Ars, B.; Van de Heyning, P.; Koekelkoren, E. Preoperative computed tomography in patients requiring a bone-anchored hearing aid. Eur. Arch. Otorhinolaryngol. 1995, 252, 401–404. [Google Scholar] [CrossRef]
- Takumi, Y.; Suzuki, N.; Moteki, H.; Kobayashi, K.; Usami, S. Pre-Baha operation three dimensional computed tomography with markers for determining optimal implant site. Laryngoscope 2008, 118, 1824–1826. [Google Scholar] [CrossRef]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. CBCT-based bone quality assessment: Are Hounsfield units applicable? Dentomaxillofac Radiol. 2015, 44, 20140238. [Google Scholar] [CrossRef]
- Al-Jamal, M.F.J.; Al-Jumaily, H.A. Can the Bone Density Estimated by CBCT Predict the Primary Stability of Dental Implants? A New Measurement Protocol. J. Craniofac Surg. 2021, 32, e171–e174. [Google Scholar] [CrossRef]
- Calon, T.G.A.; Johansson, M.L.; van den Burg, E.L.; Janssen, A.M.L.; van Hoof, M.; Stokroos, R.J. The Use of Cone Beam Computed Tomography in Assessing the Insertion of Bone Conduction Hearing Implants. Front. Surg. 2017, 4, 38. [Google Scholar] [CrossRef]
- Vautrin, A.; Thierrin, R.; Wili, P.; Klingler, S.; Chappuis, V.; Varga, P.; Zysset, P. Prediction of Dental Implants Primary Stability With Cone Beam Computed Tomography-Based Homogenized Finite Element Analysis. Clin. Implant. Dent. Relat. Res. 2025, 27, e70016. [Google Scholar] [CrossRef]
- Richard, C.; Courbon, G.; Laroche, N.; Prades, J.M.; Vico, L.; Malaval, L. Inner ear ossification and mineralization kinetics in human embryonic development—Microtomographic and histomorphological study. Sci. Rep. 2017, 7, 4825. [Google Scholar] [CrossRef]
- Richard, C.; Laroche, N.; Malaval, L.; Dumollard, J.M.; Martin, C.; Peoch, M.; Vico, L.; Prades, J.M. New insight into the bony labyrinth: A microcomputed tomography study. Auris Nasus Larynx 2010, 37, 155–161. [Google Scholar] [CrossRef]
- Choi, K.Y.; Lee, S.-W.; In, Y.; Kim, M.S.; Kim, Y.D.; Lee, S.-Y.; Lee, J.-W.; Koh, I.J. Dual-Energy CT-Based Bone Mineral Density Has Practical Value for Osteoporosis Screening around the Knee. Medicina 2022, 58, 1085. [Google Scholar] [CrossRef]
- Wesarg, S.; Kirschner, M.; Becker, M.; Erdt, M.; Kafchitsas, K.; Khan, M.F. Dual-energy CT-based assessment of the trabecular bone in vertebrae. Methods Inf. Med. 2012, 51, 398–405. [Google Scholar] [CrossRef]
- Gerdes, T.; Salcher, R.B.; Schwab, B.; Lenarz, T.; Maier, H. Comparison of Audiological Results Between a Transcutaneous and a Percutaneous Bone Conduction Instrument in Conductive Hearing Loss. Otol. Neurotol. 2016, 37, 685–691. [Google Scholar] [CrossRef]
- Shapiro, S.; Ramadan, J.; Cassis, A. BAHA Skin Complications in the Pediatric Population: Systematic Review with Meta-analysis. Otol. Neurotol. 2018, 39, 865–873. [Google Scholar] [CrossRef]
- den Besten, C.A.; Monksfield, P.; Bosman, A.; Skarzynski, P.H.; Green, K.; Runge, C.; Wigren, S.; Blechert, J.I.; Flynn, M.C.; Mylanus, E.A.M.; et al. Audiological and clinical outcomes of a transcutaneous bone conduction hearing implant: Six-month results from a multicentre study. Clin. Otolaryngol. 2019, 44, 144–157. [Google Scholar] [CrossRef]
- Willenborg, K.; Avallone, E.; Maier, H.; Lenarz, T.; Busch, S. A New Active Osseointegrated Implant System in Patients with Single-Sided Deafness. Audiol. Neurotol. 2022, 27, 83–92. [Google Scholar] [CrossRef]
- Nassiri, A.M.; Messina, S.A.; Benson, J.C.; Lane, J.I.; McGee, K.P.; Trzasko, J.D.; Carlson, M.L. Magnetic Resonance Imaging Artifact Associated With Transcutaneous Bone Conduction Implants: Cholesteatoma and Vestibular Schwannoma Surveillance. Otolaryngol. Head Neck Surg. 2024, 170, 187–194. [Google Scholar] [CrossRef]
- Gurney, J.G.; Tersak, J.M.; Ness, K.K.; Landier, W.; Matthay, K.K.; Schmidt, M.L. Children’s Oncology Group Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children’s Oncology Group. Pediatrics 2007, 120, e1229–e1236. [Google Scholar] [CrossRef]
- Alzhrani, F.; Alahmari, M.S.; Al Jabr, I.K.; Garadat, S.N.; Hagr, A.A. Cochlear Implantation in Children with Otitis Media. Indian. J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. 2), 1266–1271. [Google Scholar] [CrossRef]
- Generous, A. Giving Survivors of Childhood Cancer a Chance at Hearing. St. Jude Research. Available online: https://www.stjude.org/research/progress/2025/giving-survivors-of-childhood-cancer-chance-at-hearing.html (accessed on 27 July 2025).
- Ryu, N.-G.; Moon, I.J.; Chang, Y.S.; Kim, B.K.; Chung, W.-H.; Cho, Y.-S.; Hong, S.H. Cochlear Implantation for Profound Hearing Loss After Multimodal Treatment for Neuroblastoma in Children. Clin. Exp. Otorhinolaryngol. 2015, 8, 329–334. [Google Scholar] [CrossRef]
- Bharadwaja, S.; Patnaik, U.; Sahoo, L.; Raghavan, D.; Mathur, Y.; Badal, S.; Srivastava, K. Role of Pre-Operative High-Resolution Computed Tomography for Surgical Planning in Patients Undergoing Cochlear Implantation—An Observational Study. Indian. J. Otolaryngol. Head Neck Surg. 2024, 76, 1630–1636. [Google Scholar] [CrossRef]
- Health Class C for Device and Regulation. Cochlear Implants and MRI Safety. FDA. Available online: https://www.fda.gov/medical-devices/cochlear-implants/cochlear-implants-and-mri-safety (accessed on 27 July 2025).
- The Current State of Implantable Hearing Devices and MRI Compatibility. AAO-HNS Bulletin, 15 March 2024. Available online: https://bulletin.entnet.org/clinical-patient-care/article/22889513/the-current-state-of-implantable-hearing-devices-and-mri-compatibility (accessed on 27 July 2025).
- Berry, J.M.; Tansey, J.B.; Wu, L.; Choudhri, A.; Yawn, R.J.; MacDonald, C.B.; Richard, C. A Systematic Review of Cochlear Implant-Related Magnetic Resonance Imaging Artifact: Implications for Clinical Imaging. Otol. Neurotol. 2024, 45, 204–214. [Google Scholar] [CrossRef]
- Arndt, S.; Kromeier, J.; Berlis, A.; Maier, W.; Laszig, R.; Aschendorff, A. Imaging procedures after bone-anchored hearing aid implantation. Laryngoscope 2007, 117, 1815–1818. [Google Scholar] [CrossRef]
- Bass, J.K.; Warren, S.E.; Dillard, S.G.; Peeples, A.L.; Li, K.; Jones, S.; Hua, C.-H.; Xie, L.; Liu, S.; Ness, K.K.; et al. Eligibility for Cochlear Implant Candidacy Evaluation in Childhood Cancer Survivors. JAMA Oncol. 2025, 11, 794–796. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Y.; Yang, H.; Lai, C.; Yu, J.; Sun, Y. High doses of radiation cause cochlear immunological stress and sensorineural hearing loss. Heliyon 2024, 10, e37223. [Google Scholar] [CrossRef]
- Rybak, L.P. Neurochemistry of the peripheral and central auditory system after ototoxic drug exposure: Implications for tinnitus. Int. Tinnitus J. 2005, 11, 23–30. [Google Scholar]
- Firoozabad, L.A.; Cheraghi, S.; Farahani, S.; Nikoofar, A.; Rezaeijo, S.M.; Bakhshandeh, M.; Paydar, R. Prediction of auditory brain stem responses damage in patients with head-and-neck cancers receiving radiotherapy using the functional assays of normal tissue complication probability models. J. Cancer Res. Ther. 2024, 20, 802. [Google Scholar] [CrossRef]
- Vosgrau, J.S.; Silva, L.A.F.; Filho, V.O.; Matas, C.G. A longitudinal study of the peripheral and central auditory pathways in individuals with acute lymphoid leukemia. Clinics 2023, 78, 100234. [Google Scholar] [CrossRef]
- Leach, W. Irradiation of the ear. J. Laryngol. Otol. 1965, 79, 870–880. [Google Scholar] [CrossRef]
- Hoistad, D.L.; Ondrey, F.G.; Mutlu, C.; Schachern, P.A.; Paparella, M.M.; Adams, G.L. Histopathology of human temporal bone after cis-platinum, radiation, or both. Otolaryngol. Head Neck Surg. 1998, 118, 825–832. [Google Scholar] [CrossRef]
- Bohne, B.A.; Marks, J.E.; Glasgow, G.P. Delayed effects of ionizing radiation on the ear. Laryngoscope 1985, 95, 818–828. [Google Scholar] [CrossRef]
- Gamble, J.E.; Peterson, E.A.; Chandler, J.R. Radiation Effects on the Inner Ear. Arch. Otolaryngol. 1968, 88, 156–161. [Google Scholar] [CrossRef]
- Tokimoto, T.; Kanagawa, K. Effects of X-Ray irradiation on hearing in guinea pigs. Acta Oto-Laryngol. 1985, 100, 266–272. [Google Scholar] [CrossRef]
- Winther, F.Ø. X-Ray irradiation of the inner ear of the guinea pig. Early degenerative changes in the cochlea. Acta Oto-Laryngol. 1969, 68, 98–117. [Google Scholar] [CrossRef]
- Rubin, P. The Franz Buschke lecture: Late effects of chemotherapy and radiation therapy: A new hypothesis. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 5–34. [Google Scholar] [CrossRef]
- Gaidamakin, N.A.; Ushakov, I.B. State of synapses of the cortex of cerebral hemispheres on gamma-irradiation. Neurosci. Behav. Physiol. 1989, 19, 483–488. [Google Scholar] [CrossRef]
- Parihar, V.K.; Limoli, C.L. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc. Natl. Acad. Sci. USA 2013, 110, 12822–12827. [Google Scholar] [CrossRef]
- Mujica-Mota, M.A.; Lehnert, S.; Devic, S.; Gasbarrino, K.; Daniel, S.J. Mechanisms of radiation-induced sensorineural hearing loss and radioprotection. Hear. Res. 2014, 312, 60–68. [Google Scholar] [CrossRef]
- Grau, C.; Moller, K.; Overgaard, M.; Overgaard, J.; Elbrond, O. Sensori-neural hearing loss in patients treated with irradiation for nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 723–728. [Google Scholar] [CrossRef]
- Grau, C.; Møler, K.; Overgaard, M.; Overgaard, J.; Elbønd, O. Auditory brain stem responses in patients after radiation therapy for nasopharyngeal carcinoma. Cancer 1992, 70, 2396–2401. [Google Scholar] [CrossRef]
- Kroczka, S.; Stepien, K.; Witek-Motyl, I.; Kwiecinska, K.; Kapusta, E.; Biedron, A.; Skorek, P.; Skoczen, S. Clinical utility of complex assessment with evoked potentials in acute lymphoblastic leukemia survivors: Comparison of various treatment protocols. BMC Cancer 2021, 21, 150. [Google Scholar] [CrossRef]
- Anteunis, L.J.; Wanders, S.L.; Hendriks, J.J.; Langendijk, J.A.; Manni, J.J.; de Jong, J.M. A prospective longitudinal study on radiation-induced hearing loss. Am. J. Surg. 1994, 168, 408–411. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Y.; Guo, R.; Yang, B.; Shi, L.; Sun, J.; Guo, W.; Gong, S.; Jiang, X.; Liu, K. Head and neck radiotherapy causes significant disruptions of cochlear ribbon synapses and consequent sensorineural hearing loss. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 173, 207–214. [Google Scholar] [CrossRef]
- Deans, M.R.; Peterson, J.M.; Wong, G.W. Mammalian Otolin: A Multimeric Glycoprotein Specific to the Inner Ear that Interacts with Otoconial Matrix Protein Otoconin-90 and Cerebellin-1. PLoS ONE 2010, 5, e12765. [Google Scholar] [CrossRef]
- Doğan, M.; Şahin, M.; Kurtulmuş, Y. Otolin-1, as a Potential Marker for Inner Ear Trauma after Mastoidectomy. J. Int. Adv. Otol. 2019, 15, 200–203. [Google Scholar] [CrossRef]
- Gomaa, N.A.; Jimoh, Z.; Campbell, S.; Zenke, J.K.; Szczepek, A.J. Biomarkers for Inner Ear Disorders: Scoping Review on the Role of Biomarkers in Hearing and Balance Disorders. Diagnostics 2020, 11, 42. [Google Scholar] [CrossRef]
- Richard, C.; Jeanvoine, A.; Stark, A.R.; Hague, K.; Kjeldsen, C.; Maitre, N.L. Randomized Trial to Increase Speech Sound Differentiation in Infants Born Preterm. J. Pediatr. 2022, 241, 103–108.e3. [Google Scholar] [CrossRef]
- Morlet, T.; Valania, J.; Walter, C.; Morini, G.; O’Reilly, R.C.; Parkes, W.; Pritchett, C. Cortical Auditory Evoked Potential Testing in Children With Auditory Neuropathy Spectrum Disorder. Am. J. Audiol. 2023, 33, 171–182. [Google Scholar] [CrossRef]
- Ponton, C.W.; Eggermont, J.J.; Kwong, B.; Don, M. Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000, 111, 220–236. [Google Scholar] [CrossRef]
- Fisch, L. Integrated development and maturation of the hearing system. A critical review article. Br. J. Audiol. 1983, 17, 137–154. [Google Scholar] [CrossRef]
- Litovsky, R. Development of the auditory system. Handb. Clin. Neurol. 2015, 129, 55–72. [Google Scholar] [CrossRef]
- Rüttiger, L.; Zimmermann, U.; Knipper, M. Biomarkers for Hearing Dysfunction: Facts and Outlook. ORL J. Otorhinolaryngol. Relat. Spec. 2017, 79, 93–111. [Google Scholar] [CrossRef]
- Agung, K.; Purdy, S.C.; McMahon, C.M.; Newall, P. The use of cortical auditory evoked potentials to evaluate neural encoding of speech sounds in adults. J. Am. Acad. Audiol. 2006, 17, 559–572. [Google Scholar] [CrossRef]
- Mostafa, I.Z.; Shabana, M.I.; El Shennawy, A.M.; Weheiba, H.M. Assessing the applications of cortical auditory evoked potentials as a biomarker in children with hearing aids. Egypt. J. Otolaryngol. 2014, 30, 38–42. [Google Scholar] [CrossRef]
- Bellier, L.; Veuillet, E.; Vesson, J.-F.; Bouchet, P.; Caclin, A.; Thai-Van, H. Speech Auditory Brainstem Response through hearing aid stimulation. Hear. Res. 2015, 325, 49–54. [Google Scholar] [CrossRef]
- Muldoon, L.L.; Pagel, M.A.; Kroll, R.A.; Brummett, R.E.; Doolittle, N.D.; Zuhowski, E.G.; Egorin, M.J.; Neuwelt, E.A. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin. Cancer Res. 2000, 6, 309–315. [Google Scholar]
- Harned, T.M.; Kalous, O.; Neuwelt, A.; Loera, J.; Ji, L.; Iovine, P.; Sposto, R.; Neuwelt, E.A.; Reynolds, C.P. Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin. Cancer Res. 2008, 14, 533–540. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef]
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2019, 5, 122–131. [Google Scholar] [CrossRef]
- Liu, S.S.; Yang, R. Inner Ear Drug Delivery for Sensorineural Hearing Loss: Current Challenges and Opportunities. Front. Neurosci. 2022, 16, 867453. [Google Scholar] [CrossRef]
- Vitry, S.; Mendia, C.; Maudoux, A.; El-Amraoui, A. Advancing precision ear medicine: Leveraging animal models for disease insights and therapeutic innovations. Mamm. Genome 2025, 36, 417–443. [Google Scholar] [CrossRef]
- Ondrejová, B.; Rajťúková, V.; Šavrtková, K.; Galajdová, A.; Živčák, J.; Hudák, R. Analysis of MRI Artifacts Induced by Cranial Implants in Phantom Models. Healthcare 2025, 13, 803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).