Simple Summary
Peritoneal carcinomatosis (PC) is a serious condition where cancer spreads throughout the lining of the abdominal cavity. It often occurs in late stages of cancers like ovarian, colon, or stomach cancer and is hard to treat. Patients with PC usually suffer from complications such as fluid buildup (ascites), bowel blockages, and poor nutrition, which severely impact quality of life and limit treatment options. This review focuses on a molecule called interleukin-8 (IL-8), which is part of the body’s immune system but also helps cancers grow and spread. IL-8 attracts immune cells like neutrophils that, instead of fighting the cancer, create an environment through its receptor CXCR1/CXCR2 signaling that helps tumors survive, invade new areas, and avoid immune attack. IL-8 also encourages inflammation, blood vessel growth to feed tumors, and scar tissue buildup that causes bowel obstruction. Blocking IL-8—either with specific antibodies or drugs that stop its action—has shown promise in early lab studies and clinical trials. These treatments may help reduce cancer spread, ease symptoms like ascites, and improve how well other cancer therapies work. Our review concludes that targeting IL-8 and its receptor CXCR1/CXCR2 is a promising new strategy for treating peritoneal carcinomatosis and could improve survival and quality of life for patients. More research is needed to confirm its benefits and develop combination treatments.
Abstract
Peritoneal carcinomatosis (PC) is a late-stage manifestation of abdominopelvic malignancies with poor prognosis and limited treatment options. Unique biochemical mechanisms within the peritoneal cavity play a key role in disease progression and resistance to therapy. Despite current therapies like systemic chemotherapy and cytoreductive surgery, patients frequently develop severe complications, including bowel obstruction, nutritional decline, and ascites, driving the need to address the pro-tumorigenic niche in the peritoneal cavity. The immune microenvironment in PC is marked by elevated proinflammatory mediators, such as IL-6 and IL-8, which skew the response toward innate rather than adaptive immune responses. IL-8 signaling, through its receptors CXCR1 and CXCR2, promotes neutrophil recruitment, chronic inflammation, angiogenesis, epithelial–mesenchymal transition, and immune evasion, making the IL-8/CXCR1/CXCR2 axis a potential therapeutic target in PC. Pre-clinical models provide evidence that IL-8 or CXCR1/CXCR2 blockade may be a valuable therapeutic strategy. IL-8 targeting agents such as monoclonal antibodies (BMS-986253) and small-molecule inhibitors (SX-682, AZD5069, navarixin) have shown efficacy in mitigating tumor growth and improving the efficacy of immune checkpoint inhibitors. Phase I and II trials have demonstrated encouraging safety profiles and preliminary efficacy when treating multiple abdominopelvic malignancies. In this review, we discuss the influence of the IL-8/CXCR1/CXCR2 axis within the peritoneal immune environment in PC and highlight recent work using IL-8 or CXCR1/CXCR2 blockade as a therapeutic strategy for PC. Continued research into the peritoneal immune microenvironment and the development of targeted therapies are essential for improving the management and prognosis of PC, potentially enhancing antitumor immunity and patient outcomes.
1. Introduction
Peritoneal carcinomatosis (PC) is a late manifestation of abdominopelvic malignancies characterized by metastasis within the peritoneal cavity [1,2]. Although any tumor type can theoretically metastasize to the peritoneal cavity, the most common sites of tumor origin are the digestive or gynecologic tracts. Less common, but equally difficult to manage, are tumors metastatic from extra-abdominal organs as well as rare primary neoplasms of the mesothelial lining itself (e.g., mesothelioma and primary peritoneal carcinomatosis). Considering all the various sites of tumor origin, it has been estimated that over 70,000 patients per year are affected by PC in the United States alone [3,4]. PC has a poor prognosis, and treatment strategies often rely on palliative chemotherapy and surgery. However, recent investigations are focusing on the peritoneal immune environment as a potential target for immunotherapy. Here, we review the role of interleukin-8 (IL-8) in PC progression and its potential as a target for PC treatment.
Recent studies have highlighted the unique biochemical environment of the peritoneal cavity in PC and demonstrated that this compartment significantly influences cancer progression and disease-related morbidity and mortality [5,6,7,8,9,10]. The peritoneal cavity is a sequestered immune environment, which differs in soluble and cellular immune elements relative to the systemic circulation [1,11]. Moreover, the peritoneal environment differs dramatically in carcinomatosis relative to the physiological state [1]. The cytokine milieu in malignant peritoneal fluid is skewed toward a maladaptive polarity that favors non-specific innate immunity and inflammation over adaptive cytotoxic antitumor immunity [1,2,12]. Driven by a proinflammatory secretome, the PC environment facilitates epithelial–mesenchymal transition (EMT) of and immune evasion by metastatic cancer cells [1,12]. EMT promotes motility and enables tumor cells to migrate from the primary site throughout the peritoneal cavity [1,2,12], whereas mesenchymal-to-epithelial transition (MET) drives adhesion and epithelialization. Tumor cells are often in a partial EMT state (pEMT), that enables rapid adaptation and metastatic seeding [13]. This mechanism leads to colonization and invasion of mesothelial surfaces, a hallmark of PC. These mechanisms contribute to the pathobiology of PC and are an area of intense ongoing research given the high demand for innovative treatment options in these advanced cancer settings [1,3].
We and others have reported on the soluble and cellular components of the peritoneal immune environment [1,14,15], and found significantly elevated proinflammatory mediators such as interleukin-6 (IL-6), interleukin-8 (IL-8), fibroblast growth factor 2 (FGF2), and monocyte chemoattractant protein-1 (MCP-1 or CCL2) in the peritoneal fluid of PC patients [1]. Concomitantly, malignant peritoneal fluid is characterized by low concentrations of adaptive immune markers, such as interferon-γ (IFNγ), interferon-α (IFNα), interleukin-1 (IL-2), and interleukin-10 (IL-10), suggesting that the immune landscape in PC favors innate immunity and suppresses tumor-specific immune responses. This dysregulation of the immune response is complex and involves the interplay of multiple cytokines, including IL-8, to promote the development of cancer. The proinflammatory cytokine milieu, including IL-6 and IL-8, contributes to PC’s immune evasion mechanisms [1] and is under investigation as a therapeutic target [16].
The current standard of care for PC often involves systemic chemotherapy and cytoreductive surgery in fit patients with amenable tumor biology. Although aggressive therapy leads to prolonged survival, or even remission in some individuals, most patients eventually progress and develop late complications of PC, including bowel obstruction and malignant ascites. In addition to having few treatment options, patients at this late stage generally experience malnutrition and cachexia, which further limits their eligibility for clinical trial enrollment [5,6,16,17]. Since the peritoneal cavity is sequestered by peritoneal mesothelial lining from systemic circulation, regional immunotherapy has gained traction as a potential adjunct to systemic therapy [16,18]. However, regional immunotherapy modalities have not achieved clinical utility, to date, in spite of investigation into monoclonal antibodies, cytokines, adoptive cellular therapies, and other options as candidate therapies [1,9,19,20,21]. However, the most opportune targets and practical combination of therapies remain to be defined, warranting more in-depth translational investigations.
Since both IL-6 and IL-8 are potent mediators of inflammation, their elevated presence in the peritoneal cavity suggests that they play a role in the maladaptive, chronic inflammation seen in PC [22]. IL-8, an important proinflammatory cytokine and a key mediator of neutrophil recruitment and degranulation, has also been found to have direct and indirect implications in pro-tumorigenic pathways [23,24,25]. Given this cytokine’s inflammatory and tumorigenic potential, it is hypothesized that treating PC patients using an IL-8 blockade would be beneficial [1,26,27]. Ongoing studies are exploring the potential of IL-6 blockade as a treatment for combating PC’s inflammatory and oncogenic effects [16,17] and may hold implications for IL-8 inhibition or synergistic benefit in combination blockade. This review summarizes the role of IL-8 in PC progression and its potential as a therapeutic target.
2. Physiologic Functions of IL-8
IL-8, also known as CXCL8, is an essential chemokine that orchestrates inflammatory and immune responses, but its significance extends beyond innate immunity to pathological conditions such as chronic inflammation and fibrosis. Identified in 1987 as a neutrophil-activating cytokine, it has since been recognized for its broader roles in immune cell recruitment, activation [28] and tumor EMT [25]. IL-8 belongs to the CXC chemokine family and acts primarily through two G-protein-coupled receptors, chemokine receptor type 1 (CXCR1) and chemokine receptor type 2 (CXCR2), which are abundantly expressed on neutrophils [28,29].
A diverse range of cells produce IL-8, including immune cells such as monocytes, macrophages, neutrophils, and T lymphocytes, as well as non-immune cells like endothelial cells, fibroblasts, keratinocytes, epithelial cells, hepatocytes, and tumor cells. IL-8 production is triggered by various stimuli, including bacterial endotoxins (e.g., lipopolysaccharides), cytokines (e.g., IL-1 and TNF-α), and cellular stress signals [30,31]. The specific stimuli and cell types influence the intensity and duration of IL-8 secretion. For example, phagocytes and endothelial cells produce IL-8 robustly in response to endotoxins, whereas mesenchymal cells are relatively insensitive to endotoxin-driven IL-8 secretion [30]. Production by neutrophils is most robust in response to opsonized particles, allowing for juxtacrine secretion, additional neutrophil recruitment, and amplification of the inflammatory cascade [30]. The synthesis of IL-8 is tightly regulated by signaling pathways such as the interleukin-1 (IL-1)–interleukin-1 receptor (IL-1R)–TNF receptor-associated factor 6 (TRAF6) pathway and the tumor necrosis factor alpha (TNF-α)–tumor necrosis factor receptor (TNFR)–TNF receptor-associated factor 2 (TRAF2) pathway, both of which converge on nuclear factor kappa B (NF-κB) activation [24,32], allowing for IL-8 gene transcription, protein synthesis and the release of the cytokine into the extracellular space where it exerts its biological effects.
Once secreted, IL-8 binds to its receptors, CXCR1 and CXCR2. These receptors are expressed on various immune cells—including neutrophils, monocytes, T cells, and natural killer (NK) cells—as well as non-immune cells like endothelial and epithelial cells [29,30]. CXCR1 is present on both CD8+ cytotoxic T cells and CD4+FoxP3+ regulatory T cells, while CXCR1 and CXCR2 are found on CD14+ monocytes, CD56dimCD16+ NK cells, and basophils [33]. Additionally, CXCR1/2 expression on neurons and mesenchymal cells plays a role in angiogenesis and pain regulation [24]. IL-8 binding to its receptors initiates G-protein signaling, leading to G-protein subunit dissociation. The β and γ subunits of the G-protein activate effectors like PI3Kγ, PLCβ, and small GTPases (Ras, Rac, Rho), while the Gαi2 subunit inhibits adenyl cyclase, reducing cAMP levels [24,30]. These cascades elevate intracellular calcium, activate protein kinase C, and drive processes such as actin polymerization, cell adhesion and migration. In neutrophils, this signaling enhances chemotaxis, granule exocytosis, respiratory burst- through generation of reactive oxygen species (ROS), and neutrophil extracellular trap (NET) formation, supporting pathogen clearance and tissue repair [28,30,32,34]. IL-8 directs neutrophils to sites of injury or infection where they adhere to endothelial cells, transmigrate into tissues, and release proteolytic enzymes like elastase and myeloperoxidase to degrade pathogens and damaged tissues. IL-8 strengthens neutrophil attachment to the vascular endothelium and promotes migration to inflamed tissues by further upregulating adhesion molecules such as CD11b/CD18 [28,30,32,34].
While IL-8-driven inflammation is crucial for host defense, sustained production can lead to maladaptive responses and detrimental outcomes. Persistent IL-8 signaling can result in chronic inflammation, characterized by continuous neutrophil recruitment and activation, which damages host tissues. This pathobiology is observed in chronic conditions such as rheumatoid arthritis, inflammatory bowel disease, and chronic obstructive pulmonary disease [29,31,35]. Chronic inflammation often progresses to fibrosis, marked by excessive deposition of collagen and extracellular matrix components that disrupt normal tissue architecture, as seen in pulmonary fibrosis, liver cirrhosis, and systemic sclerosis. IL-8 also directly activate fibroblasts, contributing to tissue remodeling by direct activation of M2-like tissue macrophages whose activity releases matrix metalloproteinases (MMP) involved in extracellular matrix degradation, chronic tissue remodeling and wound healing. Chronic inflammation within the peritoneal cavity drives maladaptive fibrosis development, adhesive scar development, and subsequent bowel obstructions. Further, in inflammatory conditions like peritonitis and endometriosis, IL-8 orchestrates key pathological mechanisms [36,37,38,39]. In endometriosis, IL-8-driven angiogenesis promotes the proliferation and maintenance of ectopic endometrial tissue, perpetuating inflammation and contributing to symptoms such as pelvic pain and infertility [29]. In severe cases, such as sepsis or extensive trauma, IL-8 contributes to systemic inflammatory response syndrome, characterized by a cytokine storm, capillary leakage, and multi-organ failure [29]. In addition to these pathological effects, chronic IL-8 signaling can drive metabolic dysregulation, deplete nutritional reserves, impair tissue repair, and accentuate the development of cachexia [40,41,42].
In Summary, IL-8 often acting in concert with signaling molecules like TNFα and transcription factors like NF-kβ, is a double-edged sword in inflammation, immunity, tissue remodeling, pain, and wound healing. While essential for neutrophil-mediated defense against infections, dysregulated of chronic IL-8 activity can lead to significant pathological consequences, including chronic inflammation, fibrosis, and systematic immune dysregulation. A deeper understanding of the molecular mechanisms governing IL-8 activity offers opportunities for targeted therapies to mitigate its harmful effects in inflammatory and neoplastic diseases.
3. IL-8 and Cancer Progression
IL-8, often in concert with other cytokines like TNFα, significantly influences various aspects of cancer biology across multiple cancer types, including melanoma, prostate, colon, breast, lung, and pancreatic cancers [43]. It exerts both direct oncogenic influence on tumor cells, as well as indirect effects on the tumor microenvironment (TME). Together, these effects promote increased proliferation, resistance to apoptosis, enhanced cell motility, and angiogenesis. IL-8 alters the local immune response, recruits neutrophils, and polarizes the environment towards innate immunity while suppressing adaptive, tumor-specific cytotoxic responses, resulting in a microenvironment that supports tumor survival and progression [1,23,44]. Reviews of these mechanisms are found in Table 1 and Figure 1.

Table 1.
Various tumor-promoting functions derived from IL-8 axis stimulation and their associated mechanism.
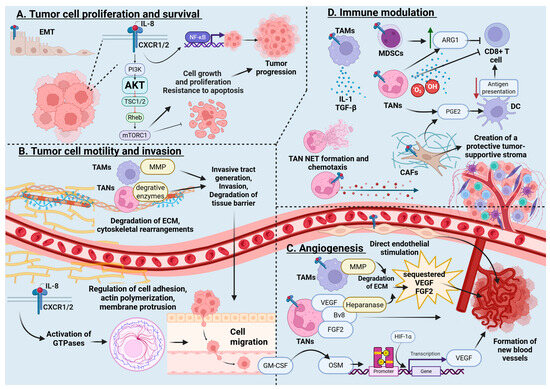
Figure 1.
Effects of IL-8 on tumor biology. IL-8 plays a multifaceted and potent role in the pathogenesis and progression of cancer and especially peritoneal carcinomatosis (PC). Through its capacity to induce epithelial–mesenchymal transition, recruit immunosuppressive cells, promote angiogenesis, remodel the extracellular matrix, and support tumor cell survival and dissemination, IL-8 acts as a central orchestrator of PC. Created in BioRender. Wagner, P., (2025) https://BioRender.com/wt0hsjd. Note: EMT, epithelial-to-mesenchymal transition; IL-8, interleukin-8; PI3K, Phosphoinositide 3-kinase; AKT, protein kinase B; TSC1/2, Tuberous Sclerosis Complex 1/2; Rheb, Ras homolog enriched in brain; mTORC, mammalian target of rapamycin complex 1; NF-kβ, Nuclear Factor kappa B; TAMs. Tumor-associated macrophages; MDSCs, myeloid-derived suppressor cells; TANs, tumor-associated neutrophils; ARG1, arginase 1; PGE2, prostaglandin E2; CAFs, cancer-associated fibroblasts; NETs, neutrophil extracellular traps; MMP, matrix metalloproteinase; ECM, extracellular matrix; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; OSM, oncostatin M; VEGF, Vascular Endothelial Growth Factor; FGF2, fibroblast growth factor 2; Bv8, prokineticin 2; IL-1β, interleukin-1 beta; TGF-β, Transforming Growth Factor beta; HIF-1α, hypoxia inducible factor-1 alpha.
IL-8 promotes tumor cell proliferation and resistance to apoptosis by activating signaling pathways such as protein kinase B (AKT pathway). Pasquier et al. demonstrated that IL-8 plays a pivotal role in creating a pro-metastatic niche following surgical peritoneal stress, promoting cancer cell survival and resistance to apoptosis [45]. IL-8 was found to be responsible for resistance to apoptosis through the activation of the AKT pathway and chemoresistance in the observed ovarian cancer cells, which persisted through the establishment of an autocrine IL-8 loop [45]. IL-8 activates upstream pathways culminating in NFκ-β signaling, a driver of tumor proliferation, inflammation, and signaling [46,47,48,49]. In colorectal cancer (CRC), estrogen-related receptor α (ERRα) has been shown to be overexpressed and increases the expression of IL-8 and in turn facilitates tumor cell proliferation and migration. ERRα inhibition by XCT-790 significantly reduced IL-8 levels and suppressed tumor growth, demonstrating the cytokine’s central role in CRC biology [50].
Through regulating cellular adhesion, actin polymerization, and cytoskeletal dynamics, IL-8 enhances tumor cell motility and invasion. This is mediated by downstream activation of small GTPases, which regulate cell adhesion, actin polymerization, membrane protrusion, and eventually cell migration [23]. IL-8 also activates tumor associated macrophages (TAMs), whose activity releases MMP and contributes to invasive track generation, tumor EMT and invasion. This allows for cancer cell motility that may disseminate and metastasize. IL-8 also drives cytoskeletal rearrangements that enhance chemotaxis, a critical step for invasion. Neutrophils and TAMs, recruited by IL-8, secrete enzymes and proteases that degrade tissue barriers, facilitating tumor invasion of mesothelial surfaces [51].
IL-8 also plays a central role in angiogenesis, a key process in tumor growth, by directly stimulating endothelial cells to form new blood vessels that supply tumors with oxygen and nutrients [23]. Neutrophils recruited by IL-8 enhance this effect by releasing proangiogenic factors such as vascular endothelial growth factor (VEGF) and Bv8, while tumor and TAM-secreted matrix metalloproteinase-9 (MMP-9) degrades the extracellular matrix (ECM), releasing sequestered VEGF to further promote vascularization [50,52,53]. In colorectal cancer models, IL-8-driven angiogenesis increases vascular permeability and disrupts endothelial junctions, facilitating tumor extravasation [50,52]. Granulocyte-macrophage colony-stimulating factor (GM-CSF) from tumor cells recruits myeloid and polymorphonuclear cells and induces neutrophils to release oncostatin M (OSM), activating the JAK-STAT pathway and further amplifying VEGF expression and effects [53]. FGF2, stored in neutrophils and the ECM, is also released via neutrophil-secreted heparanase, contributing further angiogenic support of tumor and metastasis [53].
IL-8 has also been shown to be pivotal in immune modulation within the TME, promoting immune evasion by recruiting and activating neutrophils, myeloid-derived suppressor cells (MDSCs), TAMs, and cancer associated fibroblasts (CAFs) [23,44]. Through PI3K/AKT signaling, IL-8 promotes MDSC-mediated arginase I (ARG1) expression, inhibiting CD8+ T-cell cytotoxicity, while TAMs secrete factors that drive immune invasion [25] and immune escape [23]. IL-8 also stimulates fibroblasts, modifying extracellular matrix collagen deposition and creating a protective tumor-supportive stroma [23]. Neutrophils recruited to the TME differentiate into tumor-associated neutrophils with immunosuppressive and tumor-promoting functions, releasing ROS, NET, and cytokines that suppress tumor-infiltrating lymphocytes and facilitate metastasis [53]. Similarly to MDSC, neutrophils produce ARG1, depleting L-arginine and impairing T-cell function, while prostaglandin E2 and lipid peroxidation further suppress antigen presentation by dendritic cells [53]. Tumor-derived factors like TNF-α, GM-CSF, and IL-6 enhance PD-L1 expression on neutrophils via JAK-STAT3 signaling, directly inhibiting T-cell-mediated antitumor responses [53]. In addition to immunosuppression, neutrophils contribute to chronic inflammation and genetic instability by releasing ROS and proinflammatory microRNA that damage DNA and enhance cancer cell proliferation. They also support metastasis by interacting with circulating tumor cells, facilitating adhesion to distant organs, and supplying metabolic resources [53]. In gastric cancer, tumor-derived IL-8 promotes lymph node metastasis by upregulating PD-1 expression on CD8+ T cells, weakening their cytotoxic function [44].
4. IL-8 as a Cancer Biomarker
Given the wide cellular range of production and activity of IL-8, the local and systemic concentration of this molecule has been evaluated as a potential biomarker for use in clinical oncology. Elevated IL-8 levels are rarely observed in the serum of healthy individuals [30] but are markedly increased in cancer patients, including PC patients, and correlate with advanced disease and reduced survival [55,56,57,58]. While the evidence strongly supports IL-8 as a biomarker in digestive and gynecologic malignancies, its role remains primarily correlative, and mechanistic insights behind this association are limited. We speculate that the prognostic value of IL-8 is likely a surrogate for the increased tumor-supporting environment or systemic inflammatory state created by IL-8 secretion. As circulating levels of IL-8 increase, it is likely that regional or local secretome levels of IL-8 increase in the TME as well [59], culminating in pro-tumorigenic effects by driving cellular proliferation, resistance to apoptosis, and tumor dissemination [59]. Elevated IL-8 levels also represent the systemic inflammatory state characteristic of patients with advanced malignancies, contributing to increased catabolic state, nutritional decompensation, and overall demise [41,42], which is more pronounced in PC. Further research is needed to interrogate whether IL-8 is a causal contributor to disease progression, or merely a correlate of advanced malignancy.
Given the strong connection between IL-8 and neutrophils, it is perhaps unsurprising that the peripheral blood neutrophil-to-lymphocyte ratio (NLR) has emerged as a significant prognostic biomarker in cancer. Elevated NLR reflects systemic inflammation, a hallmark of cancer progression and poor prognosis. In metastatic CRC, a high NLR (>5) is independently associated with poor prognosis, regardless of other prognostic factors and molecular alterations. This elevation correlates with increased levels of inflammatory and angiogenic cytokines, underscoring NLR’s role in cancer progression [60]. Pre-treatment NLR serves as a valuable prognostic tool, with higher levels predicting worse survival outcomes; however, the optimal NLR cut-off may vary based on cancer type and timing of assessment [61,62,63]. In gastrointestinal cancers, including those with carcinomatosis, meta-analyses have consistently linked elevated NLR to worse overall survival (OS), disease-free survival, and progression-free survival (PFS) across different tumor sites and stages [64]. NLR is often considered superior compared to other inflammatory markers such as the platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in terms of predicting cancer outcomes. In colorectal cancer, NLR demonstrates higher predictive reliability, better concordance, and a more substantial predictive capacity for survival and cancer-specific survival [64]. NLR’s predictive value is further enhanced when combined with other biomarkers. For example, integrating NLR with carcinoembryonic antigen (CEA) and C-reactive protein improved prognostic accuracy in CRC [65]. As further investigations shed light on the predictive and therapeutic outcome value of IL-8 levels as a biomarker, the stand-alone utility and in combination with other biomarkers, like NLR, will hopefully become standard in treatment algorithms to gauge prognosis and steer treatment options, especially for patients suffering from PC.
Beyond prognostication, IL-8 has shown to be a predictive marker for therapeutic outcomes in non-PC patients that may inform PC treatment. In multiple studies [66,67,68] of breast cancer, lower baseline serum IL-8 levels were associated with improved survival and reduced chemoresistance [69]. Similar findings were reported in patients with hepatocellular carcinoma, where baseline IL-8 levels have been identified as predictive biomarkers for treatment response and survival [26]. In the SORAMIC trial, Öcal et al. demonstrated that baseline IL-6 and IL-8 levels predict the response to sorafenib as well as predict overall survival, supporting the role of IL-8 as a potentially predictive biomarker for optimizing treatment in hepatocellular carcinoma [70]. Elevated serum IL-8 levels have also been associated with reduced efficacy of immunotherapy in cancer patients [71,72]. Furthermore, IL-8 contributes to an immunosuppressive TME, aiding immune evasion and therapy resistance [23,44,53]. Future research is much needed to determine the causal mechanisms of IL-8 in cancer pathobiology and treatment.
In summary, serum IL-8 and NLR are robust, cost-effective biomarkers that provide valuable prognostic insights across multiple cancer types and should be used to monitor and inform therapeutic decisions in PC. Further research is essential to elucidate the mechanistic role of IL-8 in oncogenesis and its predictive relevance in guiding therapy.
5. IL-8 as a Driver of Peritoneal Carcinomatosis
PC represents an advanced stage of malignancy characterized by widespread tumor dissemination within the peritoneal cavity. IL-8 facilitates PC progression by promoting EMT in tumor and mesothelial cells by modulating the TME [73]. Chronic IL-8 stimulation sustains a cycle of inflammation and tumor proliferation, exacerbating disease progression and complications such as malignant bowel obstruction and ascites that are both common in patients with PC (Figure 2). Here, we depict the mechanisms by which IL-8 contributes to PC and evaluate preclinical trials that targeted IL-8.
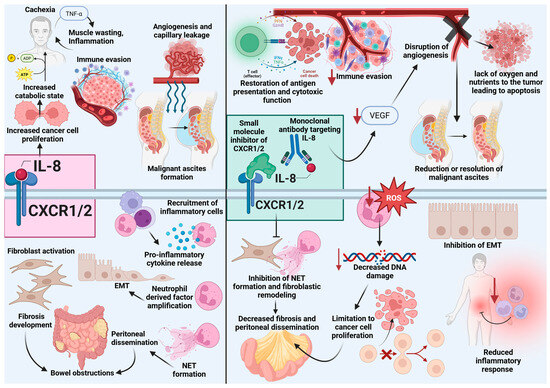
Figure 2.
Effects of IL-8 stimulation versus IL-8 inhibition in peritoneal carcinomatosis. On the left, IL-8 signaling through CXCR1/2 promotes tumor progression by enhancing cancer cell proliferation, immune evasion, angiogenesis, fibroblastic activation and subsequent fibrosis, epithelial-to-mesenchymal transition (EMT), neutrophil recruitment and NET formation, peritoneal dissemination, malignant ascites formation, and bowel obstructions. These processes are driven by proinflammatory cytokine release and proliferative transcription mediators, contributing to a catabolic and immunosuppressive state. On the right, inhibition of IL-8/CXCR1/2 signaling- via monoclonal antibodies or small molecule inhibitors- counteracts these effects by disrupting inflammatory feedback loops sustaining the TME, reduce neutrophil and MDSC recruitment, restoring antigen presentation, reducing immune evasion, inhibiting angiogenesis, resolving ascites, limiting ROS-mediated DNA damage, suppressing EMT and NET formation- limiting peritoneal dissemination, and decrease fibrosis. These changes result in reduced proliferation, improved immune responses, and attenuation of inflammation and tumor burden. Created in BioRender. Wagner, P., (2025) https://BioRender.com/vfqbh5f. Note: IL-8, interleukin-8; NET, neutrophil extracellular trap; EMT, epithelial-to-mesenchymal transition; DNA, deoxyribonucleic acid; TNFα, tumor necrosis factor alpha.
Several potential mechanisms account for the ability of IL-8 to drive EMT in PC [54]. IL-8 has been shown to promote tumor progression in several cancer subtypes, including ovarian, breast, non-small cell lung, esophageal, and mesothelial cancer [25,73,74]. Other neutrophil-derived factors, such as IL-17, transforming growth factor-β (TGF-β), and neutrophil elastase (NE), further amplify EMT and tumor progression [53]. In gastric cancer, exosomal high-mobility group box 1 (HMGB1) triggers neutrophil autophagy via the HMGB1/TLR4/NF-κB pathway, releasing pro-tumor factors like IL-1β and OSM that enhance EMT and migration [53]. Even after primary tumor removal, EMT and inflammation continue to contribute to cancer recurrence. Persistent inflammation likely transforms dormant occult cancer cells into invasive tumors through NE and MMP-9 release during NET formation. These enzymes degrade adhesion proteins, release ECM sequestered growth factors, activating integrins like α3β1 to drive recurrence [53]. Once carcinoma has disseminated throughout the peritoneal cavity, IL-8 binds to CXCR1 on tumor cells triggering the signaling cascade and amplifying aggressive tumor growth, metastasis, and fibrosis [46,47,48,75,76], ultimately leading to tumor growth, disease progression, peritoneal spread in PC.
IL-8 is also critical to modulating an immunosuppressive environment within the peritoneal cavity. By recruiting neutrophils and MDSC, IL-8 enhances immune evasion in PC by directly inhibiting cytotoxic T-cell responses and supporting maladaptive would healing and inflammation. As IL-8 levels are elevated in the secretome of peritoneal fluid in the setting of PC [1], activated neutrophils are continuously stimulated within the peritoneal cavity to release various proinflammatory cytokines, proteases, and ROS, remodeling the extracellular matrix, promoting angiogenesis, and amplifying a pro-tumorigenic microenvironment that supports tumor growth and further dissemination in PC [77]. The release of NETs traps freely disseminated tumor cells in peritoneal fluid, facilitating their adhesion and invasion into the peritoneal cavity [78,79]. Neutrophils sequestered at tumor foci also modulate the integrity of the endothelial barrier, making it more permeable and permissive to extravasation of tumor cells, facilitating tumor cell dissemination [51].
IL-8 also contributes to systemic complications of PC such as ascites, fibrosis, and malignant bowel obstructions. The accumulation of protein-rich ascitic fluid, due to increasing vascular permeability and upregulation of pro-angiogenic factors like VEGF, Bv8, and OSM [50,52,53] leads to patient discomfort and impaired organ function, particularly the heart and kidneys, due to significant volume shifts [80,81]. Persistent IL-8 signaling also promotes peritoneal fibrosis, a key factor in developing malignant bowel obstruction—a leading cause of morbidity in PC [82,83,84,85]. Chronic inflammation activates fibroblasts, producing excessive extracellular matrix deposition, peritoneal thickening, and structural rigidity [23]. Because of this maladaptive environment, surgical intervention often exacerbates adhesions and can lead to bowel resections, further compromising intestinal function. IL-8-induced capillary leakage also potentiates chemotherapy-related side effects [86], further challenging the management of affected patients. Frequent hospitalizations and invasive procedures to address malignant ascites add complexity to the disease management, but seldom provide durable relief of pain and discomfort. Targeting the IL-8 axis could mitigate these complications by reducing neovascularization, decreasing fluid leakage, and alleviating symptoms associated with ascites and obstruction.
6. Preclinical Evidence Targeting the IL-8 Pathway
Due to its multifaceted role in PC pathogenesis, IL-8 has become an appealing target for therapeutic intervention in oncology (Figure 2). Inhibiting IL-8 or its receptors could disrupt inflammatory feedback loops sustaining the TME, reduce neutrophil and MDSC recruitment, and restore adaptive immune responses. IL-8 has been compared to other notable chemotactic agents, such as C5a, fMet-Leu-Phe, platelet-activating factor, and leukotriene B4 [30]. Unlike these agents, which are rapidly inactivated by oxidation or hydrolysis, IL-8 exhibits sustained activity [30,87]. IL-8 blockade could not only limit tumor proliferation and dissemination, but may alleviate systemic complications such as malignant ascites, fibrosis, and catabolic wasting. These changes would improve patients’ quality of life, alleviate debilitating symptoms, and increase eligibility for investigational therapies. The ultimate result would be better clinical outcomes for patients with PC.
A key potential drawback to IL-8-directed monotherapy is the redundancy of cytokines produced simultaneously in pathological conditions, suggesting that other cytokines with overlapping functions that will remain unaffected by IL-8 blockade. For instance, the elevations of both IL-8 and IL-6 found in PC have been the subject of current clinical investigation [16,17,73,88]. In the study by Alraouji et al., the authors investigated the effects of tocilizumab, an IL-6 receptor alpha agonistic antibody, on IL-8 production and the proangiogenic potential of triple-negative breast cancer cells [89]. The methods included treating triple-negative breast cancer cell lines with tocilizumab and measuring IL-8 through ELISA and RT-qPCR. Additionally, the study also assessed the impact on angiogenesis by conducting tube formation assays using human umbilical vein endothelial cells (HUVEC). The results showed that tocilizumab significantly reduced IL-8 production in triple-negative breast cancer cells, and treated HUVEC cells exhibited a marked decrease in angiogenesis, as evidenced by the reduced tube formation in vitro. By reducing IL-8 and angiogenesis, as well as reversing tumor EMT [22], tocilizumab contributes to a less aggressive tumor phenotype, highlighting its promise as an adjunctive treatment in managing malignancies.
IL-8 blockade has been studied in animal models [90,91]. In a dextran sodium sulfate-induced model of acute colitis in mice, those expressing the human IL-8 gene developed more tumors that were associated with higher quantities of myeloid cells that promote angiogenesis and tumor growth [29]. This finding reinforces the effects of IL-8, potentially impacting neutrophils and endothelial cells to promote tumorigenesis. In another study, Li et al. investigated an anti-IL-8 antibody in a humanized pancreatic cancer mouse model [44], finding enhanced myeloid cell activation and potentiation of the antitumor activity of an anti-PD-1 antibody. Such findings highlight the potential synergy between IL-8 blockade and immune checkpoint inhibitors, improving the immune response against tumors.
7. Clinical Trials of the Interleukin-8/CXCR1/2 Pathway and Immunotherapy Combinations
The IL-8/CXCR1/2 axis has emerged as a critical target in cancer therapy due to its multifaceted roles in tumor growth, angiogenesis, metastasis, and immune suppression (CXCR1/2 inhibitors, Table 2, Figure 2). BMS-986253 (HuMax-IL8) is a fully human monoclonal antibody designed to neutralize human IL-8. In preclinical studies, IL-8 blockade with BMS-986253 has been shown to reduce the recruitment of polymorphonuclear MDSC, revert mesenchymal transition, and enhance susceptibility of cancer cells to immune mediated lysis [92]. In a phase I clinical trial, Bilusic et al. evaluated the safety, tolerability, pharmacokinetics, and preliminary efficacy of BMS-986253 in patients with metastatic or unresectable solid tumors [27]. The study enrolled 15 patients who received escalating doses of BMS-986253 every two weeks to determine the maximum tolerated dose. The trial included detailed monitoring of adverse events and pharmacokinetic analysis, with secondary aim to assess tumor response. Results indicated that BMS-986253 was generally well-tolerated, with treatment-related adverse events occurring in 33% of patients, primarily grade 1, with no dose-limiting toxicities observed. Preliminary efficacy results showed stable disease in some patients, suggesting potential clinical activity. The investigation underscored the role of IL-8 targeting in solid tumors. The authors emphasized the need for further studies to confirm these findings and to explore combination therapies with other immunotherapeutic agents. Several ongoing studies are investigating the combination of BMS-986253 and other immunotherapies including nivolumab for hormone-sensitive prostate cancer (MAGIC-8, NCT03689699), a phase II trial of nivolumab in combination with BMS-986253 or cabiralizumab in advanced hepatocellular carcinoma (NCT04050462), and an investigational immunotherapy study of BMS-986253 with nivolumab in patients with advanced cancers (NCT03400332).

Table 2.
Review of IL-8 blockade agents and the mechanism of action of monoclonal antibodies and small-molecule inhibitors that impede the IL-8 axis. The completed and ongoing studies are included, as well as the reported adverse event rates for each trial.
SX-682 is a small-molecule inhibitor of CXCR1/2 and that has also been found to reduce MDSC recruitment. In preclinical CRC models, as KRAS (Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) mutations were linked to CXCR2 axis activation and promotion of an immunosuppressive TME, SX-682 was shown to abrogate MDSC trafficking as well as enhance the efficacy of anti-PD1 (nivolumab) therapy in these models [93]. The STOPTRAFFIC-1 trial (NCT04599140) is a phase I/II study evaluating SX-682 in combination with nivolumab for refractory RAS-mutated microsatellite-stable (MSS) metastatic CRC. Patients either received a dose-escalating regimen of SX-682 in combination with nivolumab, or SX-682 alone every four weeks. The primary objectives of the STOPTRAFFIC-1 trial include determining the safety profile of SX-682 alone and in combination with nivolumab, including the maximum tolerated dose (MTD) and recommended phase II dose. Secondary objectives include overall response rate, PFS, OS, and pharmacokinetic profiles. Efficacy results from the ongoing phase I/II trial are pending [93]. Another ongoing study (NCT04477343) is evaluating SX-682 in combination with nivolumab as maintenance therapy for unresectable pancreatic adenocarcinoma (PDAC). This phase I trial involves a dose-escalation design to determine the MTD of SX-682 when combined with nivolumab. Nine of the planned 20 patients have been enrolled, with initial dose-level completed without dose-limiting toxicities and increase dosing regimen ongoing [94].
As another small-molecule antagonist of CXCR2, AZD5069, reduces neutrophil recruitment to inflammatory sites [96]. Multiple human trials have been completed to evaluate AZD5069 as a potential therapeutic agent in inflammatory conditions including asthma, chronic obstructive pulmonary disease, and bronchiectasis [97,98,99]. Although these studies were not associated with improved clinical outcomes, they demonstrated that the therapy was well-tolerated, with infections and nasopharyngitis being the most common observed adverse events. A phase I/II study is evaluating AZD5069 in combination with durvalumab in patients with advanced hepatocellular carcinoma [100]. The study aims to determine the recommended phase II dose and assess the antitumor efficacy of the combination. The first dose cohort has been completed with no dose-limiting toxicities, and the second dose cohort is currently recruiting [100].
Finally, navarixin, a small-molecule antagonist of CXCR1/2, has shown potential benefits in multiple solid tumors by inhibiting CXCR1/2-mediated pathways [95]. Preclinical evidence suggests that CXCR2 inhibition can reduce tumor progression, lineage plasticity, and enhance the efficacy of immune checkpoint inhibitors [101,102]. A phase II study (NCT03473925) evaluated navarixin in combination with pembrolizumab in patients with advanced or metastatic castration-resistant prostate cancer (CRPC), microsatellite-stable colorectal cancer (MSS CRC) or non-small-cell lung cancer (NSCLC). Median PFS ranged from 1.8 to 2.4 months. The study was closed early due to lack of efficacy. Treatment-related adverse events occurred in 67% of patients, with dose-limiting toxicities including grade 4 neutropenia and grade 3 transaminase elevation, hepatitis, and pneumonitis. Given the high rates of treatment related adverse events, perhaps using a small molecule antagonist of the CXC motif chemokine receptor is too broad, allowing for problematic on target but off-tissue specificities. One additional navarixin study is ongoing and we await their results. This phase 2 basket study (NCT05453825) is investigating navarixin monotherapy or in combination with chemotherapy in patients with select advanced solid tumors, including colorectal cancer. The primary endpoints are objective response rate and PFS, with secondary endpoints including OS and disease control rate [103].
The preclinical and early clinical evidence supports IL-8 and CXCR1/2 inhibition as a rational and promising therapeutic strategy, particularly in tumors characterized by a proinflammatory, neutrophil- and MDSC-enriched microenvironment, such as PC. Given its ability to attenuate immunosuppressive myeloid infiltration, reverse epithelial–mesenchymal transition, and enhance susceptibility to immune-mediated killing, IL-8 blockade is especially well-suited as an adjunct to other modalities but may have efficacy as monotherapy, especially for symptomatic control of malignant ascites.
Timing and context of IL-8 pathway inhibition may be critical. Data from animal models and early-phase clinical trials suggest that its maximal benefit may occur when combined with immune checkpoint inhibitors (ICIs), particularly PD-1/PD-L1 blockade. In these settings, IL-8 inhibition appears to reprogram the TME toward immune activation and improve response rates to ICIs, as demonstrated in murine pancreatic and colorectal cancer models [22,90,91] and supported by ongoing trials such as STOPTRAFFIC-1 (NCT04599140) and the MAGIC-8 study (NCT03689699). These findings imply that IL-8 blockade may sensitize tumors that are otherwise refractory to ICIs by reducing myeloid-driven resistance mechanisms. Conversely, the integration of IL-8 pathway inhibition with chemotherapy is less well-defined. Cytotoxic chemotherapy itself can induce immunogenic cell death and transiently deplete immunosuppressive myeloid cells, potentially overlapping with the mechanism of IL-8 inhibition. Whether this results in synergy or redundancy remains an open question, but clinical trials combining navarixin or AZD5069 with chemotherapy are underway (NCT05453825), and their outcomes will inform this strategy.
The rationale for combining IL-8/CXCR1/2 inhibitors with ICIs stems from the ability of the IL-8/CXCR1/2 axis to create an immunosuppressive tumor microenvironment (TME). IL-8 promotes the recruitment of myeloid-derived suppressor cells (MDSCs), neutrophils, and tumor-associated macrophages (TAMs) into the TME. These cells, in turn, suppress T-cell activity, inhibit dendritic cell maturation, and promote angiogenesis, effectively shielding the tumor from immune attack.
ICIs, such as anti-PD-1/PD-L1 and anti-CTLA-4 antibodies, reinvigorate exhausted T cells and unleash their cytotoxic potential. However, the efficacy of ICIs is often limited by the presence of an immunosuppressive TME. By simultaneously targeting the IL-8/CXCR1/2 axis, we aim to disrupt the immunosuppressive barrier and enhance the ability of ICIs to elicit a robust anti-tumor immune response. In essence, IL-8/CXCR1/2 inhibitors can “prime” the TME, making it more susceptible to ICI therapy.
Future Directions and Challenges
While the combination of IL-8/CXCR1/2 inhibitors with ICIs holds significant promise, several challenges remain. These include identifying predictive biomarkers to select patients most likely to benefit from this combination therapy, optimizing the dosing and scheduling of IL-8/CXCR1/2 inhibitors in combination with ICIs, and managing potential toxicities associated with combination therapy. Future research should focus on addressing these challenges to maximize the therapeutic potential of targeting the IL-8/CXCR1/2 axis in combination with immunotherapy. Further investigation into the impact on the tumor vasculature by limiting angiogenesis may also prove beneficial.
8. Conclusions
This review highlights IL-8 as a central mediator of tumor progression, immune suppression, and inflammation in PC, positioning it as a compelling therapeutic target. IL-8 drives key pathological processes such as EMT, neutrophil recruitment, angiogenesis, and fibrosis—contributing to tumor growth and cause complications like malignant ascites formation and bowel obstructions. Preclinical and early clinical data support IL-8 blockade, particularly in combination with immune checkpoint inhibitors, as a promising approach to reprogram the TME and improve clinical outcomes. These insights highlight the need for continued translational and clinical research to validate IL-8-targeted strategies and integrate them into effective treatment paradigms for PC.
Author Contributions
Conceptualization, C.S., N.D., Z.L., Y.F., V.S.D., A.D.D., K.X., A.H.Z., D.L.B. and P.L.W.; resources, C.S., N.D. and P.L.W.; data curation, C.S., N.D. and P.L.W.; writing—original draft preparation, C.S., N.D. and P.L.W.; writing—review and editing, C.S., N.D., V.S.D., A.D.D., K.X., A.H.Z., D.L.B. and P.L.W.; visualization, C.S., N.D. and P.L.W.; supervision, N.D. and P.L.W.; project administration, N.D. and P.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Institute of Health (NIH) [R37CA232209], CA230972, BC132245_W81XWH-14-0258, W81XWH2210009_BC210533 and W81XWH2211069_ BC211396 and BC240179 from the Department of Defense, MetaVivor 713943, The Pennsylvania Breast Cancer Coalition, the Glimmer of Hope Foundation, and the David Downing Fund. The Hillman Cancer Center Luminex and Flow Cytometry Cores are supported by Cancer Center Support Grant P30CA047904.
Acknowledgments
The authors thank Sarah Carey, Jade Chang, and Jacalyn Newman, of Allegheny Health Network’s Health System Publication Support Office (HSPSO) for their assistance in editing and formatting the manuscript. The HSPSO is funded by Highmark Health (Pittsburgh, PA, USA), and all work was performed in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3, accessed 1 March 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PC | peritoneal carcinomatosis |
| EMT | epithelial-to-mesenchymal transition |
| MET | mesenchymal-to-epithelial transition |
| pEMT | partial epithelial-to-mesenchymal transition |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| FGF2 | fibroblast growth factor-2 |
| MCP-1 | monocyte chemoattractant protein-1 (also known as CCL2) |
| IFNγ | interferon gamma |
| IFNα | interferon alpha |
| IL-2 | interleukin-2 |
| IL-10 | interleukin-10 |
| IL-1 | interleukin-1 |
| IL-1R | interleukin-1 receptor |
| TNFR | tumor necrosis factor receptor |
| TRAF2 | tumor necrosis factor receptor-associated factor 2 |
| NF-κB | nuclear factor kappa B |
| NK | natural killer cells |
| PI3K | phosphoinositide 3-kinase |
| PLCβ | phospholipase C beta |
| cAMP | cyclic adenosine monophosphate |
| ROS | reactive oxygen species |
| NET | neutrophil extracellular trap |
| TME | tumor microenvironment |
| AKT | Ak strain transforming pathway |
| CRC | colorectal cancer |
| ERRα | estrogen-related receptor alpha |
| VEGF | vascular endothelial growth factor |
| MMP | matrix metalloproteinase |
| MMP-9 | matrix metalloproteinase-9 |
| ECM | extracellular matrix |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| OSM | oncostatin M |
| MDSC | myeloid-derived stem cells |
| TAMs | tissue-associated macrophages |
| ARG1 | arginase-1 |
| NLR | neutrophil to lymphocyte ratio |
| OS | overall survival |
| PFS | progression free survival |
| CEA | carcinoembryonic antigen |
| IL-17 | interleukin-17 |
| TGF-β | transforming growth factor-beta |
| NE | neutrophil elastase |
| HMGB1 | high-mobility group box 1 |
| ELISA | enzyme linked immunosorbent assay |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| CAFs | cancer-associated fibroblasts |
| PD-L1 | programmed death-ligand 1 |
| PD-1 | Programmed Cell Death Protein 1 |
| microRNA | micro-ribonucleic acid |
| HUVEC | human umbilical vein endothelial cells |
| mAB | monoclonal antibody |
| KRAS | Ki-ras2 Kirsten rat sarcoma viral oncogene homolog |
| MSS | microsatellite stable |
| MTD | maximum tolerated dose |
| CRPC | castration-resistant prostate cancer |
| NSCLC | non-small cell lung cancer |
| ICIs | immune checkpoint inhibitors |
| MSS CRC | microsatellite stable colorectal cancer |
| TNF-α | Tumor Necrosis Factor alpha |
| COPD | Chronic obstructive pulmonary disease |
| SMI | Small-molecule inhibitor |
| mCRC | mucinous colorectal cancer |
| DNA | deoxyribonucleic acid |
| PFN | profilin-1 |
| GzmB | granzyme B |
| TSC1/2 | Tuberous Sclerosis Complex ½ |
| Rheb | Ras homolog enriched in brain |
| mTORC | mammalian target of rapamycin complex 1 |
| TANs | tumor-associated neutrophils |
| PGE2 | prostaglandin E2 |
| Bv8 | prokineticin 2 |
| IL-1β | interleukin-1 beta |
| HIF-1α | hypoxia inducible factor-1 alpha |
| TLR4 | toll-like receptor-4 |
References
- Wagner, P.L.; Knotts, C.M.; Donneberg, V.S.; Dadgar, N.; Pico, C.X.C.; Xiao, K.; Zaidi, A.; Schiffman, S.C.; Allen, C.J.; Donnenberg, A.D.; et al. Characterizing the Immune Environment in Peritoneal Carcinomatosis: Insights for Novel Immunotherapy Strategies. Ann. Surg. Oncol. 2024, 31, 2069–2077. [Google Scholar] [CrossRef]
- Ng, D.; Ali, A.; Lee, K.; Eymael, D.; Abe, K.; Luu, S.; Kazazian, K.; Lu, Y.Q.; Brar, S.; Conner, J.; et al. Investigating the mechanisms of peritoneal metastasis in gastric adenocarcinoma using a novel ex vivo peritoneal explant model. Sci. Rep. 2022, 12, 11499. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Guiral, D.; Hubner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Primers 2021, 7, 91. [Google Scholar] [CrossRef]
- Foster, J.M.; Zhang, C.; Rehman, S.; Sharma, P.; Alexander, H.R. The contemporary management of peritoneal metastasis: A journey from the cold past of treatment futility to a warm present and a bright future. CA Cancer J. Clin. 2023, 73, 49–71. [Google Scholar] [CrossRef]
- Morano, W.F.; Aggarwal, A.; Love, P.; Richard, S.D.; Esquivel, J.; Bowne, W.B. Intraperitoneal immunotherapy: Historical perspectives and modern therapy. Cancer Gene Ther. 2016, 23, 373–381. [Google Scholar] [CrossRef]
- Thadi, A.; Khalili, M.; Morano, W.F.; Richard, S.D.; Katz, S.C.; Bowne, W.B. Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis. Vaccines 2018, 6, 54. [Google Scholar] [CrossRef]
- Zeltsman, M.; Mayor, M.; Jones, D.R.; Adusumilli, P.S. Surgical immune interventions for solid malignancies. Am. J. Surg. 2016, 212, 682–690.e5. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Point, G.R.; Cunetta, M.; Thorn, M.; Guha, P.; Espat, N.J.; Boutros, C.; Hanna, N.; Junghans, R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016, 23, 142–148. [Google Scholar] [CrossRef]
- Ornella, M.S.C.; Badrinath, N.; Kim, K.A.; Kim, J.H.; Cho, E.; Hwang, T.H.; Kim, J.J. Immunotherapy for Peritoneal Carcinomatosis: Challenges and Prospective Outcomes. Cancers 2023, 15, 2383. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kim, I.H. Molecular Mechanisms and Potential Rationale of Immunotherapy in Peritoneal Metastasis of Advanced Gastric Cancer. Biomedicines 2022, 10, 1376. [Google Scholar] [CrossRef]
- Lewis, C.R.; Dadgar, N.; Yellin, S.A.; Donnenberg, V.S.; Donnenberg, A.D.; Bartlett, D.L.; Allen, C.J.; Wagner, P.L. Regional Immunotherapy for Peritoneal Carcinomatosis in Gastroesophageal Cancer: Emerging Strategies to Re-Condition a Maladaptive Tumor Environment. Cancers 2023, 15, 5107. [Google Scholar] [CrossRef]
- Fanotto, V.; Salani, F.; Vivaldi, C.; Scartozzi, M.; Ribero, D.; Puzzoni, M.; Montagnani, F.; Leone, F.; Vasile, E.; Bencivenga, M.; et al. Primary Tumor Resection for Metastatic Colorectal, Gastric and Pancreatic Cancer Patients: In Search of Scientific Evidence to Inform Clinical Practice. Cancers 2023, 15, 5107. [Google Scholar] [CrossRef]
- Aggarwal, V.; Montoya, C.A.; Donnenberg, V.S.; Sant, S. Interplay between tumor microenvironment and partial EMT as the driver of tumor progression. iScience 2021, 24, 102113. [Google Scholar] [CrossRef]
- Vlaeminck-Guillem, V.; Bienvenu, J.; Isaac, S.; Grangier, B.; Golfier, F.; Passot, G.; Bakrin, N.; Rodriguez-Lafrasse, C.; Gilly, F.N.; Glehen, O. Intraperitoneal cytokine level in patients with peritoneal surface malignancies. A study of the RENAPE (French Network for Rare Peritoneal Malignancies). Ann. Surg. Oncol. 2013, 20, 2655–2662. [Google Scholar] [CrossRef]
- Salmon, H.; Remark, R.; Gnjatic, S.; Merad, M. Host tissue determinants of tumour immunity. Nat. Rev. Cancer 2019, 19, 215–227. [Google Scholar] [CrossRef]
- Park, H.; Lewis, C.; Dadgar, N.; Sherry, C.; Evans, S.; Ziobert, S.; Omstead, A.; Zaidi, A.; Xiao, K.; Ghosh, S.; et al. Wagner, Intra-pleural and intra-peritoneal tocilizumab therapy for managing malignant pleural effusions and ascites: The Regional Immuno-Oncology Trial (RIOT)−2 study protocol. Surg. Oncol. Insight 2024, 1, 100045. [Google Scholar] [CrossRef]
- Dadgar, N.; Sherry, C.; Zimmerman, J.; Park, H.; Lewis, C.; Donnenberg, A.; Zaidi, A.H.; Fan, Y.; Xiao, K.; Bartlett, D.; et al. Targeting interleukin-6 as a treatment approach for peritoneal carcinomatosis. J. Transl. Med. 2024, 22, 402. [Google Scholar] [CrossRef] [PubMed]
- Catherine Lewis, N.D.; Najafi, M.; Park, H.; Sherry, C.; Lucas, A.; Zaidi, A.; Xiao, K.; Omstead, A.; Donnenberg, A.; Bartlett, D.L.; et al. Intra-tumoral immunomodulatory therapy for advanced abdominal cancers using lipopolysaccharide: The Regional Immuno-Oncology Trial-1 (RIOT-1) protocol (NCT05751837). Surg. Oncol. Insight 2024, 1, 100042. [Google Scholar] [CrossRef]
- Morisaki, T.; Hikichi, T.; Onishi, H.; Morisaki, T.; Kubo, M.; Hirano, T.; Yoshimura, S.; Kiyotani, K.; Nakamura, Y. Intranodal Administration of Neoantigen Peptide-loaded Dendritic Cell Vaccine Elicits Epitope-specific T Cell Responses and Clinical Effects in a Patient with Chemorefractory Ovarian Cancer with Malignant Ascites. Immunol. Investig. 2021, 50, 562–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, W.S.; Kim, C.W.; Lee, S.J.; Yang, H.; Kong, S.J.; Ning, J.; Yang, K.M.; Kang, B.; Kim, W.R.; et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J. Immunother. Cancer Homepage 2020, 8, e000857. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, J.H.; Kwack, K.; Choi, S.W. Natural Killer Cell Therapy: A New Treatment Paradigm for Solid Tumors. Cancers 2019, 11, 1534. [Google Scholar] [CrossRef]
- Donnenberg, V.S.; Luketich, J.D.; Sultan, I.; Lister, J.; Bartlett, D.L.; Ghosh, S.; Donnenberg, A.D. A maladaptive pleural environment suppresses preexisting anti-tumor activity of pleural infiltrating T cells. Front. Immunol. 2023, 14, 1157697. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Clauser, K.R.; Tam, W.L.; Frose, J.; Ye, X.; Eaton, E.N.; Reinhardt, F.; Donnenberg, V.S.; Bhargava, R.; Carr, S.A.; et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014, 16, 1105–1117. [Google Scholar] [CrossRef]
- Kwong, T.T.; Xiong, Z.; Zhang, Y.; Wu, H.; Cao, J.; Wong, P.P.-C.; Liu, X.; Wang, J.; Wong, C.H.; Tse, G.M.-K.; et al. Overcoming immunotherapy resistance in hepatocellular carcinoma by targeting myeloid IL-8/CXCR2 signaling. Mol. Ther. 2025, 33, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marte, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Kuno, K.; Akiyama, M.; Kasahara, T.; Nakanishi, I.; Mukaida, N.; Matsushima, K. Expression of recombinant rabbit IL-8 in Escherichia coli and establishment of the essential involvement of IL-8 in recruiting neutrophils into lipopolysaccharide-induced inflammatory site of rabbit skin. Int. Immunol. 1993, 5, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Yang, M.H.; Tsai, M.L.; Lan, H.Y.; Su, S.H.; Chang, S.C.; Teng, H.W.; Yang, S.H.; Lan, Y.T.; Chiou, S.H.; et al. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology 2011, 141, 279–291.e5. [Google Scholar] [CrossRef] [PubMed]
- Eikawa, S.; Ohue, Y.; Kitaoka, K.; Aji, T.; Uenaka, A.; Oka, M.; Nakayama, E. Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6- and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6. J. Immunol. 2010, 185, 6734–6740. [Google Scholar] [CrossRef]
- Mukaida, N.; Matsushima, K. Regulation of IL-8 production and the characteristics of the receptors for IL-8. Cytokines 1992, 4, 41–53. [Google Scholar]
- Rodrigues, I.S.S.; Martins-Filho, A.; Micheli, D.C.; Lima, C.A.; Tavares-Murta, B.M.; Murta, E.F.C.; Nomelini, R.S. IL-6 and IL-8 as Prognostic Factors in Peritoneal Fluid of Ovarian Cancer. Immunol. Investig. 2020, 49, 510–521. [Google Scholar] [CrossRef]
- Arici, A. Local cytokines in endometrial tissue: The role of interleukin-8 in the pathogenesis of endometriosis. Ann. New York Acad. Sci. 2002, 955, 101–109; discussion 118, 396–406. [Google Scholar] [CrossRef]
- Arici, A.; Seli, E.; Zeyneloglu, H.B.; Senturk, L.M.; Oral, E.; Olive, D.L. Interleukin-8 induces proliferation of endometrial stromal cells: A potential autocrine growth factor. J. Clin. Endocrinol. Metab. 1998, 83, 1201–1205. [Google Scholar] [CrossRef]
- Ko, Y.C.; Mukaida, N.; Kasahara, T.; Muto, S.; Matsushima, K.; Kusano, E.; Asano, Y.; Itoh, Y.; Yamagishi, Y.; Kawai, T. Specific increase in interleukin-8 concentrations in dialysis fluid of patients with peritonitis receiving continuous ambulatory peritoneal dialysis. J. Clin. Pathol. 1995, 48, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, D.; Eiber, R.M.; Jochum, M.; Billing, A. Perioperative pattern of peritoneal interleukin 8, tumour necrosis factor-alpha, and granulocyte elastase release in human secondary peritonitis. Cytokine 1997, 9, 288–292. [Google Scholar] [CrossRef]
- Siff, T.; Parajuli, P.; Razzaque, M.S.; Atfi, A. Cancer-Mediated Muscle Cachexia: Etiology and Clinical Management. Trends Endocrinol. Metab. 2021, 32, 382–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Chandra, V.; Sanchez, E.R.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354. [Google Scholar] [CrossRef]
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Bar, J.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Kahani, H.; et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-alpha and the NF-kappaB pathway. Stem Cell Res. Ther. 2015, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, X.L.; Wei, Y.Q.; Wei, X.W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhai, J.; Shen, Y.; Zhang, T.; Wang, Y.; He, Y.; You, Q.; Shen, L. Tumor-derived IL-8 facilitates lymph node metastasis of gastric cancer via PD-1 up-regulation in CD8(+) T cells. Cancer Immunol. Immunother. 2022, 71, 3057–3070. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Vidal, F.; Hoarau-Vechot, J.; Bonneau, C.; Darai, E.; Touboul, C.; Rafii, A. Surgical peritoneal stress creates a pro-metastatic niche promoting resistance to apoptosis via IL-8. J. Transl. Med. 2018, 16, 271. [Google Scholar] [CrossRef]
- Geng, R.; Tan, X.; Wu, J.; Pan, Z.; Yi, M.; Shi, W.; Liu, R.; Yao, C.; Wang, G.; Lin, J.; et al. RNF183 promotes proliferation and metastasis of colorectal cancer cells via activation of NF-kappaB-IL-8 axis. Cell Death Dis. 2017, 8, e2994. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, L.; Lu, S.; Sun, X.; Liu, Y.; Li, H.; Wang, X.; Zhao, C.; Zhang, H.; Wang, Y. Autocrine IL-8 promotes F-actin polymerization and mediate mesenchymal transition via ELMO1-NF-kappaB-Snail signaling in glioma. Cancer Biol. Ther. 2015, 16, 898–911. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Lai, W.; Zeng, Y.; Xu, H.; Lan, Q.; Su, P.; Chu, Z. Interaction with tumor-associated macrophages promotes PRL-3-induced invasion of colorectal cancer cells via MAPK pathway-induced EMT and NF-kappaB signaling-induced angiogenesis. Oncol. Rep. 2019, 41, 2790–2802. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, X.; Yu, T.; Tang, Q.; Zhou, Z.; Wang, H.; Huang, W.; Huang, T.; Liang, F. Downregulation of VAP-1 in OSCC suppresses tumor growth and metastasis via NF-kappaB/IL-8 signaling and reduces neutrophil infiltration. J. Oral Pathol. Med. 2022, 51, 332–341. [Google Scholar] [CrossRef]
- Ding, S.; Tang, Z.; Jiang, Y.; Huang, H.; Luo, P.; Qing, B.; Zhang, S.; Tang, R. IL-8 Is Involved in Estrogen-Related Receptor alpha-Regulated Proliferation and Migration of Colorectal Cancer Cells. Dig. Dis. Sci. 2017, 62, 3438–3446. [Google Scholar] [CrossRef]
- Chen, M.B.; Hajal, C.; Benjamin, D.C.; Yu, C.; Azizgolshani, H.; Hynes, R.O.; Kamm, R.D. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl. Acad. Sci. USA 2018, 115, 7022–7027. [Google Scholar] [CrossRef]
- Yuen, K.C.; Liu, L.F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E., 3rd; Koeppen, H.; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef]
- Huang, X.; Nepovimova, E.; Adam, V.; Sivak, L.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K. Neutrophils in Cancer immunotherapy: Friends or foes? Mol. Cancer 2024, 23, 107. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.I.; Castillo, M.D.; Litzinger, M.; Hamilton, D.H.; Palena, C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011, 71, 5296–5306. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, Y.; Huang, M.; Shen, J. Elevated circulating IL-8 correlates with poor prognosis in urological cancers: A meta-analysis and bioinformatic validation. Ann. Med. 2025, 57, 2486592. [Google Scholar] [CrossRef]
- Milovanovic, J.; Todorovic-Rakovic, N.; Radulovic, M. Interleukin-6 and interleukin-8 serum levels in prognosis of hormone-dependent breast cancer. Cytokine 2019, 118, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycka, B.; Mackowiak-Matejczyk, B.; Terlikowska, K.M.; Kulesza-Bronczyk, B.; Kinalski, M.; Terlikowski, S.J. Serum levels of IL-6, IL-8 and CRP as prognostic factors in epithelial ovarian cancer. Eur. Cytokine Netw. 2013, 24, 106–113. [Google Scholar] [CrossRef]
- Feng, L.; Qi, Q.; Wang, P.; Chen, H.; Chen, Z.; Meng, Z.; Liu, L. Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J. Int. Med. Res. 2018, 46, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Carranza-Rua, O.; Alfaro, C.; Onate, C.; Martin-Algarra, S.; Perez, G.; Landazuri, S.F.; Gonzalez, A.; Gross, S.; Rodriguez, I.; et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin. Cancer Res. 2014, 20, 5697–5707. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Raghav, K.; Lieu, C.H.; Jiang, Z.Q.; Eng, C.; Vauthey, J.N.; Chang, G.J.; Qiao, W.; Morris, J.; Hong, D.; et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br. J. Cancer 2015, 112, 1088–1097. [Google Scholar] [CrossRef]
- Vano, Y.A.; Oudard, S.; By, M.A.; Tetu, P.; Thibault, C.; Aboudagga, H.; Scotte, F.; Elaidi, R. Optimal cut-off for neutrophil-to-lymphocyte ratio: Fact or Fantasy? A prospective cohort study in metastatic cancer patients. PLoS ONE 2018, 13, e0195042. [Google Scholar] [CrossRef]
- Ittiamornlert, P.; Ruengkhachorn, I. Neutrophil-lymphocyte ratio as a predictor of oncologic outcomes in stage IVB, persistent, or recurrent cervical cancer patients treated by chemotherapy. BMC Cancer 2019, 19, 51. [Google Scholar] [CrossRef]
- Murakami, Y.; Saito, H.; Shimizu, S.; Kono, Y.; Shishido, Y.; Miyatani, K.; Matsunaga, T.; Fukumoto, Y.; Fujiwara, Y. Neutrophil-to-Lymphocyte Ratio as a Prognostic Indicator in Patients With Unresectable Gastric Cancer. Anticancer Res. 2019, 39, 2583–2589. [Google Scholar] [CrossRef]
- Bowen, R.C.; Little, N.A.B.; Harmer, J.R.; Ma, J.; Mirabelli, L.G.; Roller, K.D.; Breivik, A.M.; Signor, E.; Miller, A.B.; Khong, H.T. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 32171–32189. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.G.; Demuth, C.; Sorensen, B.S.; Johansen, J.S.; Nielsen, D.; Pallisgaard, N.; Hoegdall, E.; Pfeiffer, P.; Jensen, B.V. Total cell-free DNA, carcinoembryonic antigen, and C-reactive protein for assessment of prognosis in patients with metastatic colorectal cancer. Tumor Biol. 2018, 40, 1010428318811207. [Google Scholar] [CrossRef]
- Benoy, I.H.; Salgado, R.; Van Dam, P.; Geboers, K.; Van Marck, E.; Scharpe, S.; Vermeulen, P.B.; Dirix, L.Y. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin. Cancer Res. 2004, 10, 7157–7162. [Google Scholar] [CrossRef]
- Tiainen, L.; Hamalainen, M.; Luukkaala, T.; Tanner, M.; Lahdenpera, O.; Vihinen, P.; Jukkola, A.; Karihtala, P.; Moilanen, E.; Kellokumpu-Lehtinen, P.L. Low Plasma IL-8 Levels During Chemotherapy Are Predictive of Excellent Long-Term Survival in Metastatic Breast Cancer. Clin. Breast Cancer 2019, 19, e522–e533. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Y.; Dai, Z.J.; Wu, C.J.; Ji, Y.H.; Xu, J. IL-6, IL-8 and TNF-alpha levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, J. Correlations of MRP1 gene with serum TGF-beta1 and IL-8 in breast cancer patients during chemotherapy. J. BUON 2018, 23, 1302–1308. [Google Scholar] [PubMed]
- Ocal, O.; Schutte, K.; Kupcinskas, J.; Morkunas, E.; Jurkeviciute, G.; de Toni, E.N.; Khaled, N.B.; Berg, T.; Malfertheiner, P.; Klumpen, H.J.; et al. Baseline Interleukin-6 and -8 predict response and survival in patients with advanced hepatocellular carcinoma treated with sorafenib monotherapy: An exploratory post hoc analysis of the SORAMIC trial. J. Cancer Res. Clin. Oncol. 2022, 148, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Onate, C.; Perez, G.; Alfaro, C.; Martin-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carleton, M.; Zhou, M.; Chen, T.; Feng, Y.; Huang, S.P.; Walsh, A.M.; Baxi, V.; Pandya, D.; Baradet, T.; et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020, 26, 688–692. [Google Scholar] [CrossRef]
- Donnenberg, V.S.; Luketich, J.D.; Popov, B.; Bartlett, D.L.; Donnenberg, A.D. A common secretomic signature across epithelial cancers metastatic to the pleura supports IL-6 axis therapeutic targeting. Front. Immunol. 2024, 15, 1404373. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, Z.; Huang, L.; Wang, L.; Miao, Y.; Wu, J. IL-8 promotes cell migration through regulating EMT by activating the Wnt/beta-catenin pathway in ovarian cancer. J. Cell Mol. Med. 2020, 24, 1588–1598. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Q.; Du, H.; Hao, H. CXCL8 and the peritoneal metastasis of ovarian and gastric cancer. Front. Immunol. 2023, 14, 1159061. [Google Scholar] [CrossRef]
- Sun, F.; Wang, J.; Sun, Q.; Li, F.; Gao, H.; Xu, L.; Zhang, J.; Sun, X.; Tian, Y.; Zhao, Q.; et al. Interleukin-8 promotes integrin beta3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 449. [Google Scholar] [CrossRef]
- De Larco, J.E.; Wuertz, B.R.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Villalba, M.; Olivera, I.; Cirella, A.; Eguren-Santamaria, I.; Berraondo, P.; Schalper, K.A.; de Andrea, C.E.; et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin. Cancer Res. 2021, 27, 2383–2393. [Google Scholar] [CrossRef]
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of Interleukin-8 and Neutrophil Extracellular Trap (NET) Formation in the Tumor Microenvironment: Is There a Pathogenic Role? J. Immunol. Res. 2019, 2019, 6252138. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Polese, A.; Magrini, F.; Fiorentini, C.; Olivari, M.T. Negative influences of ascites on the cardiac function of cirrhotic patients. Am. J. Med. 1975, 59, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Masse, E.M.; Herzberg, K.T.; Meyers, M.S.; Yeo, K.T.; Yeo, T.K.; Sioussat, T.M.; Dvorak, H.F. Pathogenesis of ascites tumor growth: Vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995, 55, 360–368. [Google Scholar] [PubMed]
- Wang, J.; Lin, S.; Brown, J.M.; van Wagoner, D.; Fiocchi, C.; Rieder, F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol. Rev. 2021, 302, 211–227. [Google Scholar] [CrossRef]
- Xin, S.; Liu, X.; He, C.; Gao, H.; Wang, B.; Hua, R.; Gao, L.; Shang, H.; Sun, F.; Xu, J. Inflammation accelerating intestinal fibrosis: From mechanism to clinic. Eur. J. Med. Res. 2024, 29, 335. [Google Scholar] [CrossRef]
- Laval, G.; Marcelin-Benazech, B.; Guirimand, F.; Chauvenet, L.; Copel, L.; Durand, A.; Francois, E.; Gabolde, M.; Mariani, P.; Rebischung, C.; et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J. Pain Symptom Manag. 2014, 48, 75–91. [Google Scholar] [CrossRef]
- Henry, J.C.; Pouly, S.; Sullivan, R.; Sharif, S.; Klemanski, D.; Abdel-Misih, S.; Arradaza, N.; Jarjoura, D.; Schmidt, C.; Bloomston, M. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery 2012, 152, 747–756; discussion 756–757. [Google Scholar] [CrossRef]
- Izzedine, H.; Mathian, A.; Amoura, Z.; Ng, J.H.; Jhaveri, K.D. Anticancer Drug-Induced Capillary Leak Syndrome. Kidney Int. Rep. 2022, 7, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Dewald, B.; Gerber, N.; Baggiolini, M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J. Clin. Investig. 1991, 87, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, V.S.; Wagner, P.L.; Luketich, J.D.; Bartlett, D.L.; Donnenberg, A.D. Localized Intra-Cavitary Therapy to Drive Systemic Anti-Tumor Immunity. Front. Immunol. 2022, 13, 846235. [Google Scholar] [CrossRef]
- Alraouji, N.N.; Aboussekhra, A. Tocilizumab inhibits IL-8 and the proangiogenic potential of triple negative breast cancer cells. Mol. Carcinog. 2021, 60, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mian, B.M.; Dinney, C.P.; Bermejo, C.E.; Sweeney, P.; Tellez, C.; Yang, X.D.; Gudas, J.M.; McConkey, D.J.; Bar-Eli, M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin. Cancer Res. 2003, 9, 3167–3175. [Google Scholar]
- Kim, J.H.; Frantz, A.M.; Anderson, K.L.; Graef, A.J.; Scott, M.C.; Robinson, S.; Sharkey, L.C.; TD, O.B.; Dickerson, E.B.; Modiano, J.F. Interleukin-8 promotes canine hemangiosarcoma growth by regulating the tumor microenvironment. Exp. Cell Res. 2014, 323, 155–164. [Google Scholar] [CrossRef]
- Dominguez, C.; McCampbell, K.K.; David, J.M.; Palena, C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2017, 2, e94296. [Google Scholar] [CrossRef]
- Johnson, B.; Kopetz, S.; Hwang, H.; Yuan, Y.; DePinho, R.A.; Zebala, J.; Overman, M.J. STOPTRAFFIC-1: A phase I/II trial of SX-682 in combination with nivolumab for refractory RAS-mutated microsatellite stable (MSS) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2022, 40, TPS3638. [Google Scholar] [CrossRef]
- Dunne, R.F.; Ullman, N.A.; Belt, B.A.; Ruffolo, L.I.; Burchard, P.; Hezel, A.F.; Zittel, J.; Wang, W.; Ramsdale, E.E.; Kaul, V.; et al. A phase I study to evaluate the safety and tolerability of SX-682 in combination with PD-1 inhibitor as maintenance therapy for unresectable pancreatic adenocarcinoma. J. Clin. Oncol. 2022, 40, TPS631-TPS631. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller, W.H., Jr.; Hansen, A.R.; Lee, J.S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: A phase 2 randomized trial. Investig. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef]
- Nicholls, D.J.; Wiley, K.; Dainty, I.; MacIntosh, F.; Phillips, C.; Gaw, A.; Mardh, C.K. Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J. Pharmacol. Exp. Ther. 2015, 353, 340–350. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef]
- Kirsten, A.M.; Forster, K.; Radeczky, E.; Linnhoff, A.; Balint, B.; Watz, H.; Wray, H.; Salkeld, L.; Cullberg, M.; Larsson, B. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm. Pharmacol. Ther. 2015, 31, 36–41. [Google Scholar] [CrossRef] [PubMed]
- De Soyza, A.; Pavord, I.; Elborn, J.S.; Smith, D.; Wray, H.; Puu, M.; Larsson, B.; Stockley, R. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur. Respir. J. 2015, 46, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.J.; Basu, B.; Hubner, R.; Ma, Y.T.; Meyer, T.; Palmer, D.H.; Pinato, D.J.J.; Plummer, E.R.; Ross, P.J.; Samson, A.; et al. A phase I/II study of the CXCR2 inhibitor, AZD5069, in combination with durvalumab, in patients (pts) with advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 2023, 41, TPS631-TPS631. [Google Scholar] [CrossRef]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Bullock, K.; Richmond, A. Suppressing MDSC Recruitment to the Tumor Microenvironment by Antagonizing CXCR2 to Enhance the Efficacy of Immunotherapy. Cancers 2021, 13, 6293. [Google Scholar] [CrossRef] [PubMed]
- Oberstein, P.E.; Pelster, M.; Culm, K.; Chamberlain Santos, V.; Koustenis, A.; Mockbee, C.M.; Youssoufian, H.; Krauss, J.C. ONCX-NAV-G201: A phase 2 basket study of navicixizumab monotherapy or in combination with chemotherapy in patients with select advanced solid tumors—Colorectal Cancer Cohort (trial in progress). J. Clin. Oncol. 2023, 41, TPS263-TPS263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).