Age-Stratified Clinicopathological Features and Efficacy of Adjuvant Chemotherapy in Resectable Gastric Cancer: An East-West Population-Based Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
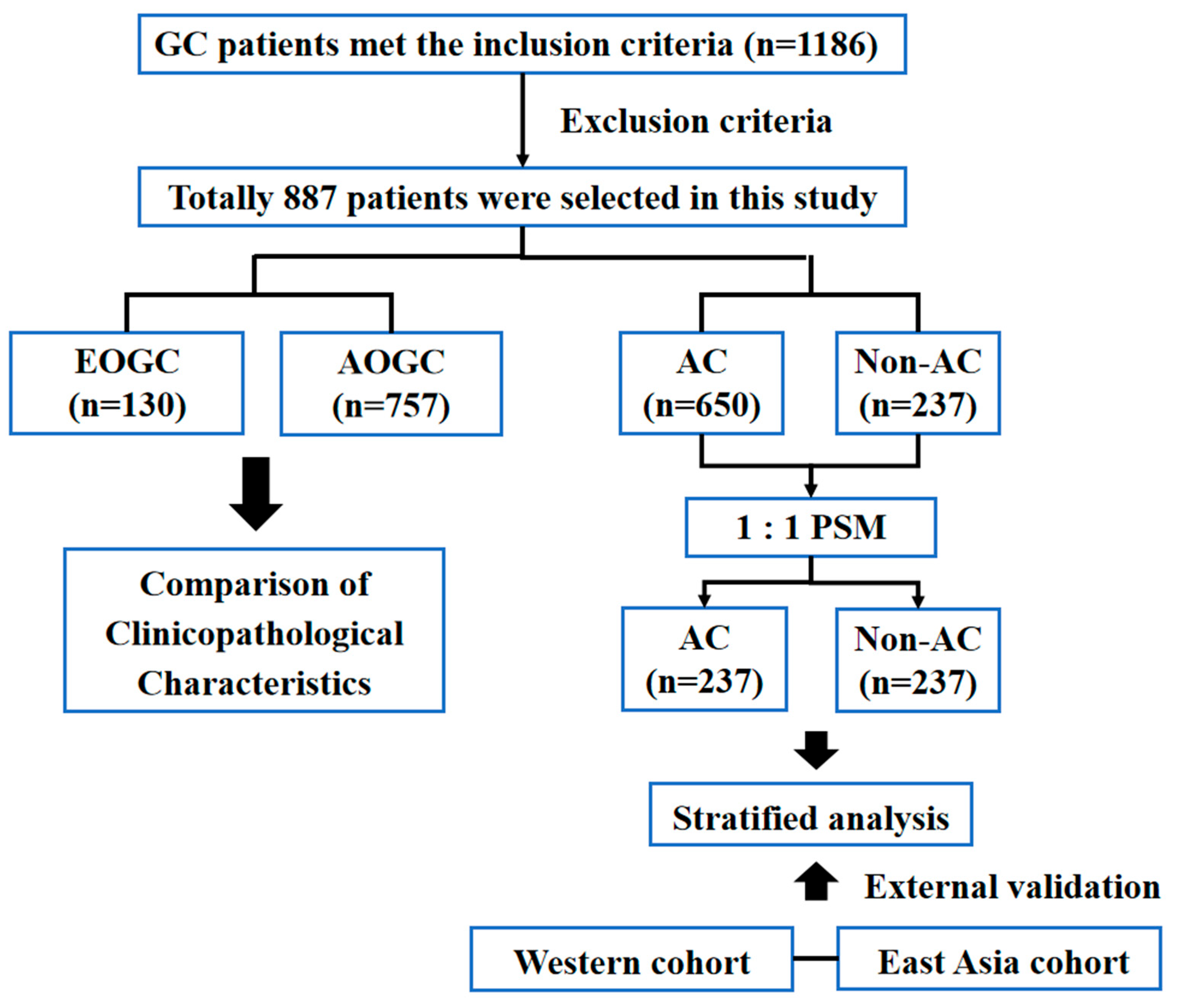
2.1. Patient Enrollment
2.2. Clinical Characteristics
2.3. Propensity Score Matching (PSM)
2.4. Statistical Analysis
3. Results
3.1. Patient Clinicopathological Characteristics
3.2. Prognostic Factors in Univariate and Multivariate Analyses
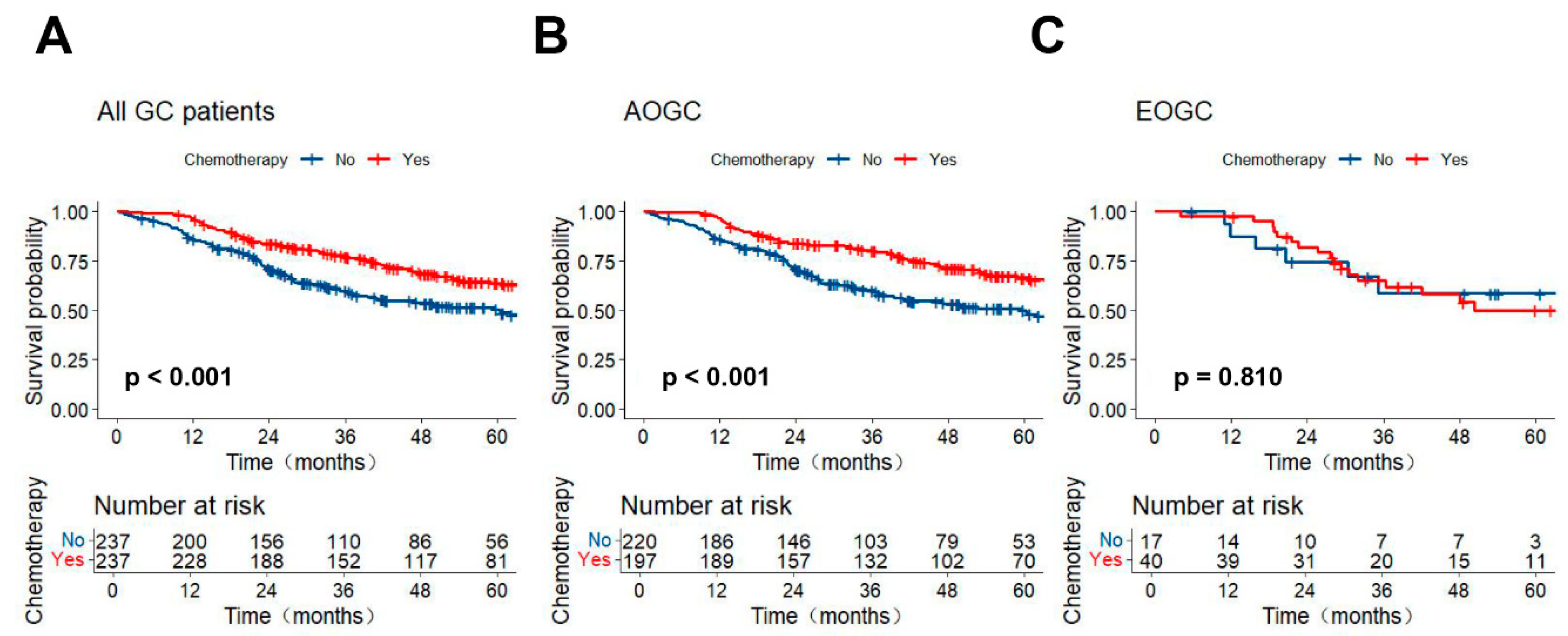
3.3. Stratified Survival Analysis of AC in the Different Age Groups
3.4. External Validation of the Efficacy of AC Stratified by Different Age Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOGC | Early-onset gastric cancer |
| AC | Adjuvant chemotherapy |
| AOGC | Average-onset gastric cancer |
| OS | Overall survival |
| PNI | perineural invasion |
| GC | Gastric cancer |
| Hp | Helicobacter pylori |
| SEER | Surveillance, Epidemiology, and End Results |
| ACRG | Asian Cancer Research Group |
| SMC | Samsung Medical Center |
| LVI | Lymphovascular invasion |
| CEA | Carcinoembryonic antigen |
| CA 19-9 | Carbohydrate antigen 19-9 |
| PSM | Propensity score matching |
| PH | proportional hazard |
| SMD | Standardized mean differences |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Setia, N.; Wang, C.X.; Lager, A.; Maron, S.; Shroff, S.; Arndt, N.; Peterson, B.; Kupfer, S.S.; Ma, C.; Misdraji, J.; et al. Morphologic and molecular analysis of early-onset gastric cancer. Cancer 2021, 127, 103–114. [Google Scholar] [CrossRef]
- Herrera-Pariente, C.; Capó-García, R.; Díaz-Gay, M.; Carballal, S.; Muñoz, J.; Llach, J.; Sánchez, A.; Bonjoch, L.; Arnau-Collell, C.; de Lima, Y.S.; et al. Identification of New Genes Involved in Germline Predisposition to Early-Onset Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 1310. [Google Scholar] [CrossRef]
- Ning, F.-L.; Zhang, N.-N.; Zhao, Z.-M.; Du, W.-Y.; Zeng, Y.-J.; Abe, M.; Pei, J.-P.; Zhang, C.-D. Global, Regional, and National Burdens with Temporal Trends of Early-, Intermediate-, and Later-Onset Gastric Cancer from 1990 to 2019 and Predictions up to 2035. Cancers 2022, 14, 5417. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Gan, L.; Chen, Z.; Wang, X.; Zhang, J.; Chen, J.; Tan, C.; Sheng, W.; Xu, M. Trends, clinicopathological features, surgical treatment patterns and prognoses of early-onset versus late-onset gastric cancer: A retrospective cohort study. J. Adv. Res. 2024; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhao, X.; Liu, Y.; Wang, N.; Zhang, L.; Zhu, X.; Dong, Q.; Liu, J.; Shi, Y. The clinicopathological characteristics of early-onset gastric cancer and its evolutionary trends: A retrospective study. Am. J. Cancer Res. 2022, 12, 2757–2769. [Google Scholar]
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’ADdario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef] [PubMed]
- Juo, Y.-Y.; Gibbons, M.A.M.; Dutson, E.; Lin, A.Y.; Yanagawa, J.; Hines, O.J.; Eibl, G.; Chen, Y. Obesity Is Associated with Early Onset of Gastrointestinal Cancers in California. J. Obes. 2018, 2018, 7014073. [Google Scholar] [CrossRef]
- Derks, S.; Bass, A.J. Mutational signatures in Helicobacter pylori-induced gastric cancer: Lessons from new sequencing technologies. Gastroenterology 2014, 147, 267–269. [Google Scholar] [CrossRef] [PubMed]
- A Lumish, M.; Walch, H.; Maron, S.B.; Chatila, W.; Kemel, Y.; Maio, A.; Ku, G.Y.; Ilson, D.H.; Won, E.; Li, J.; et al. Clinical and molecular characteristics of early-onset vs average-onset esophagogastric cancer. J. Natl. Cancer Inst. 2024, 116, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Yu, J.; Zhang, X.; Nie, Y. Emerging trends in early-onset gastric cancer. Chin. Med. J. 2024, 137, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Zhang, Q.; Li, Q.; Zhong, H.; Wang, Y.; Yang, H.; Li, H.; Wang, X.; Li, K.; et al. Multi-institutional development and validation of a nomogram to predict prognosis of early-onset gastric cancer patients. Front. Immunol. 2022, 13, 1007176. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Wang, B.; Xu, F.; Ma, F.; Qu, Y.; Jiang, D.; Li, K.; Feng, J.; Tian, S.; et al. Proteomic characterization of gastric cancer response to chemotherapy and targeted therapy reveals potential therapeutic strategies. Nat. Commun. 2022, 13, 5723. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, H.; Zhang, L.; You, J.; Jin, Z.; Zhang, B. Association between adjuvant chemotherapy and survival in stage I gastric cancer patients after curative resection. Gastroenterol. Rep. 2023, 11, goad070. [Google Scholar] [CrossRef]
- Mazurek, M.; Szewc, M.; Sitarz, M.Z.; Dudzińska, E.; Sitarz, R. Gastric Cancer: An Up-to-Date Review with New Insights into Early-Onse t Gastric Cancer. Cancers 2024, 16, 3163. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, R.; Zhu, H.; Ge, X.; Wang, Y.; Wang, X.; Miao, L. Comparison of treatment strategies and survival of early-onset gastric cancer: A population-based study. Sci. Rep. 2022, 12, 6288. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Oh, S.C.; Sohn, B.H.; Cheong, J.-H.; Kim, S.B.; Lee, J.E.; Park, K.C.; Lee, S.H.; Park, J.-L.; Park, Y.-Y.; Lee, H.-S.; et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat. Commun. 2018, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.-H.; Wang, S.C.; Park, S.; Porembka, M.R.; Christie, A.L.; Kim, H.; Kim, H.S.; Zhu, H.; Hyung, W.J.; Noh, S.H.; et al. Development and validation of a prognostic and predictive 32-gene signature for gastric cancer. Nat. Commun. 2022, 13, 774. [Google Scholar] [CrossRef] [PubMed]
- Mun, D.G.; Bhin, J.; Kim, S.; Kim, H.; Jung, J.H.; Jung, Y.; Jang, Y.E.; Park, J.M.; Kim, H.; Jung, Y.; et al. Proteogenomic Characterization of Human Early-Onset Gastric Cancer. Cancer Cell 2019, 35, 111–124.e10. [Google Scholar] [CrossRef]
- Milne, A.N.; Offerhaus, G.J. Early-onset gastric cancer: Learning lessons from the young. World J. Gastrointest. Oncol. 2010, 2, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Sundar, R.; Nakayama, I.; Markar, S.R.; Shitara, K.; van Laarhoven, H.W.M.; Janjigian, Y.Y.; Smyth, E.C. Gastric cancer. Lancet 2025, 405, 2087–2102. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, S.; Wu, W.; Kuo, Z.C.; Wei, Z.; Meng, S.; Chen, C.; Zhang, C.; He, Y. Gastric cancer in young patients: A separate entity with aggressive features and poor prognosis. J. Cancer Res. Clin. Oncol. 2020, 146, 2937–2947. [Google Scholar] [CrossRef]
- Das, G.; Bhutia, K.D.; Purkayastha, J.; Talukdar, A. Early-Onset Gastric Cancer from a Geographic Area of High Gastric Cancer Incidence in North-East India: A Retrospective Study. Indian. J. Surg. Oncol. 2023, 14, 308–311. [Google Scholar] [CrossRef]
- Takatsu, Y.; Hiki, N.; Nunobe, S.; Ohashi, M.; Honda, M.; Yamaguchi, T.; Nakajima, T.; Sano, T. Clinicopathological features of gastric cancer in young patients. Gastric Cancer 2016, 19, 472–478. [Google Scholar] [CrossRef]
- Chu, H.; Chen, X.; Liu, X.; Deng, C.; Bi, B.; He, Y.; Huo, M.; Zhang, C. Clinicopathological characteristics and prognosis of adolescents and young adults with gastric cancer after gastrectomy: A propensity score matching analysis. Front. Oncol. 2023, 13, 1204400. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, X.; Jiao, Y.; Lei, Y.; Li, X.; Bi, F.; Guo, F.; Wang, G.; Liu, M. A Distinct Clinicopathological Feature and Prognosis of Young Gastric Cancer Patients Aged ≤ 45 Years Old. Front. Oncol. 2021, 11, 674224. [Google Scholar] [CrossRef]
- Di Domenico, M.; Castoria, G.; Bilancio, A.; Migliaccio, A.; Auricchio, F. Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res. 1996, 56, 4516–4521. [Google Scholar]
- Wang, Z.; Butler, L.M.; Wu, A.H.; Koh, W.; Jin, A.; Wang, R.; Yuan, J. Reproductive factors, hormone use and gastric cancer risk: The Singapore Chinese Health Study. Int. J. Cancer 2016, 138, 2837–2845. [Google Scholar] [CrossRef]
- Hirahashi, M.; Yao, T.; Matsumoto, T.; Nishiyama, K.-I.; Oya, M.; Iida, M.; Tsuneyoshi, M. Intramucosal gastric adenocarcinoma of poorly differentiated type in the young is characterized by Helicobacter pylori infection and antral lymphoid hyperplasia. Mod. Pathol. 2007, 20, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Huang, H.; Zhao, L.; Wang, T.; Zhang, X.; Wang, W.; Zhang, Y.; Guo, C.; Zhao, D.; Chen, Y. Clinicopathological characteristics, survival outcomes, and genetic alterations of younger patients with gastric cancer: Results from the China National Cancer Center and cBioPortal datasets. Cancer Med. 2022, 11, 3057–3073. [Google Scholar] [CrossRef] [PubMed]
- Al-Refaie, W.B.; Hu, C.Y.; Pisters, P.W.; Chang, G.J. Gastric adenocarcinoma in young patients: A population-based appraisal. Ann. Surg. Oncol. 2011, 18, 2800–2807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gan, L.; Xu, M.-D.; Huang, M.; Zhang, X.; Gong, Y.; Wang, X.; Yu, G.; Guo, W. The prognostic value of age in non-metastatic gastric cancer after gastrectomy: A retrospective study in the U.S. and China. J. Cancer 2018, 9, 1188–1199. [Google Scholar] [CrossRef]
- Pisanu, A.; Podda, M.; Cois, A.; Uccheddu, A. Gastric cancer in the young: Is it a different clinical entity? A retrospective cohort study. Gastroenterol. Res. Pract. 2014, 2014, 125038. [Google Scholar] [CrossRef]
- Santoro, R.; Carboni, F.; Lepiane, P.; Ettorre, G.M.; Santoro, E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br. J. Surg. 2007, 94, 737–742. [Google Scholar] [CrossRef]
- Braga-Neto, M.B.; Carneiro, J.G.; Barbosa, A.M.d.C.; Silva, I.S.; Maia, D.C.; Maciel, F.S.; de Alcântara, R.J.A.; Vasconscelos, P.R.L.; Braga, L.L.B.C. Clinical characteristics of distal gastric cancer in young adults from Northeastern Brazil. BMC Cancer 2018, 18, 131. [Google Scholar] [CrossRef]
- Saito, H.; Takaya, S.; Fukumoto, Y.; Osaki, T.; Tatebe, S.; Ikeguchi, M. Clinicopathologic characteristics and prognosis of gastric cancer in young patients. Yonago Acta Med. 2012, 55, 57–61. [Google Scholar]

| Characteristic | EOGC n = 130 (%) | AOGC n = 757 (%) | p Value |
|---|---|---|---|
| Sex | <0.001 | ||
| Male | 68 (52.3) | 534 (70.5) | |
| Female | 62 (47.7) | 223 (29.5) | |
| Differentiation | <0.001 | ||
| Well-Moderate | 9 (6.9) | 242 (32.0) | |
| Poor | 121 (93.1) | 515 (68.0) | |
| Lauren Type | <0.001 | ||
| Intestinal | 6 (4.6) | 221 (29.2) | |
| Mixed | 17 (13.1) | 210 (27.7) | |
| Diffuse | 107 (82.3) | 326 (43.1) | |
| Location | <0.001 | ||
| Upper | 21 (16.1) | 241 (31.9) | |
| Middle | 52 (40.0) | 175 (23.1) | |
| Lower | 49 (37.7) | 325 (42.9) | |
| Overlap | 8 (6.2) | 16 (2.1) | |
| T stage | 0.382 | ||
| T1–2 | 13 (10.0) | 82 (10.8) | |
| T3 | 83 (63.9) | 518 (68.4) | |
| T4 | 34 (26.1) | 157 (20.7) | |
| N stage | 0.318 | ||
| N0 | 30 (23.1) | 210 (27.7) | |
| N+ | 100 (76.9) | 547 (72.3) | |
| PNI | 0.022 | ||
| No | 47 (36.2) | 359 (47.4) | |
| Yes | 83 (63.8) | 398 (52.6) | |
| LVI | 0.024 | ||
| No | 91 (70.00) | 447 (59.0) | |
| Yes | 39 (30.00) | 310 (41.0) | |
| CEA | 0.131 | ||
| ≤5 | 120 (92.3) | 660 (87.2) | |
| >5 | 10 (7.7) | 97 (12.8) | |
| CA-199 | 0.200 | ||
| ≤37 | 114 (87.7) | 626 (82.7) | |
| >37 | 16 (12.3) | 131 (17.3) | |
| Adjuvant chemotherapy | <0.001 | ||
| No | 17 (13.1) | 220 (29.1) | |
| Yes | 113 (86.9) | 537 (70.9) |
| Variables | OS | |||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age | 0.305 | |||
| EOGC | Reference | - | ||
| AOGC | 1.187 (0.86–1.65) | - | ||
| Sex | 0.936 | |||
| Male | Reference | - | ||
| Female | 1.010 (0.80–1.28) | - | ||
| Differentiation | ||||
| Well—Moderate | Reference | 0.532 | - | |
| Poor | 1.040 (0.92–1.18) | - | ||
| Lauren Type | 0.140 | |||
| Intestinal | Reference | - | ||
| Mixed | 1.307 (0.95–1.80) | - | ||
| Diffuse | 1.308 (0.99–1.73) | - | ||
| Location | 0.028 | 0.082 | ||
| Upper | Reference | Reference | ||
| Middle | 1.319 (0.98–1.77) | 1.186 (0.88–1.60) | ||
| Lower | 1.010 (0.77–1.33) | 0.922 (0.70–1.22) | ||
| Overlap | 1.907 (1.10–3.30) | 1.670 (0.96–2.89) | ||
| T stage | <0.001 | <0.001 | ||
| T1–2 | Reference | Reference | ||
| T3 | 2.677 (1.56–4.60) | 2.749 (1.57–4.81) | ||
| T4 | 5.197 (2.96–9.11) | 4.328 (2.42–7.75) | ||
| N stage | <0.001 | <0.001 | ||
| N0 | Reference | Reference | ||
| N+ | 2.173 (1.63–2.90) | 2.664 (1.97–3.61) | ||
| PNI | <0.001 | 0.001 | ||
| Negative | Reference | Reference | ||
| Positive | 1.899 (1.51–2.39) | 1.490 (1.18–1.91) | ||
| LVI | <0.001 | 0.242 | ||
| Negative | Reference | Reference | ||
| Positive | 1.511 (1.21–1.89) | 1.150 (0.91–1.45) | ||
| CEA | 0.282 | |||
| ≤5 | Reference | - | ||
| >5 | 1.200 (0.86–1.67) | - | ||
| CA19-9 | 0.015 | 0.411 | ||
| ≤37 | Reference | Reference | ||
| >37 | 1.408 (1.07–1.86) | 1.125 (0.85–1.49) | ||
| Adjuvant chemotherapy | 0.001 | <0.001 | ||
| No | Reference | Reference | ||
| Yes | 0.544 (0.43–0.68) | 0.456 (0.36–0.58) | ||
| Characteristic | Before PSM | p Value | SMD | After PSM | p Value | SMD | ||
|---|---|---|---|---|---|---|---|---|
| Non-AC n = 237 (%) | AC n = 650 (%) | Non-AC n = 237 (%) | AC n = 237 (%) | |||||
| Age | <0.001 | 0.269 | <0.001 | 0.259 | ||||
| ≤45 | 17 (7.2) | 113 (17.4) | 17 (7.2) | 40 (16.9) | ||||
| >45 | 220 (92.8) | 537 (82.6) | 220 (92.8) | 197 (83.1) | ||||
| Sex | 0.667 | 0.039 | 0.921 | 0.009 | ||||
| Male | 164 (69.2) | 438 (67.4) | 164 (69.2) | 165 (69.6) | ||||
| Female | 73 (30.8) | 212 (32.6) | 73 (30.8) | 72 (30.4) | ||||
| Differentiation | 0.048 | 0.152 | 0.373 | 0.083 | ||||
| Well-Moderate | 79 (33.3) | 172 (26.5) | 79 (33.3) | 70 (29.5) | ||||
| Poor | 158 (66.7) | 478 (73.5) | 158 (66.7) | 167 (70.5) | ||||
| Lauren Type | 0.321 | 0.116 | 0.325 | 0.142 | ||||
| Intestinal | 69 (29.1) | 158 (24.3) | 69 (29.1) | 55 (23.2) | ||||
| Mixed | 60 (25.3) | 167 (25.7) | 60 (25.3) | 62 (26.2) | ||||
| Diffuse | 108 (45.6) | 325 (50.0) | 108 (45.6) | 120 (50.6) | ||||
| Location | 0.267 | 0.156 | 0.294 | 0.171 | ||||
| Upper | 62 (26.2) | 200 (30.8) | 62 (26.2) | 81 (34.2) | ||||
| Middle | 56 (23.6) | 171 (26.3) | 56 (23.6) | 48 (20.3) | ||||
| Lower | 111 (46.8) | 263 (40.5) | 111 (46.8) | 100 (42.2) | ||||
| Overlap | 8 (3.4) | 16 (2.5) | 8 (3.4) | 8 (3.4) | ||||
| T stage | 0.027 | 0.193 | 1.000 | 0.001 | ||||
| T1–2 | 15 (6.3) | 80 (12.3) | 15 (6.3) | 15 (6.3) | ||||
| T3 | 164 (69.2) | 437 (67.2) | 164 (69.2) | 164 (69.2) | ||||
| T4 | 58 (24.5) | 133 (20.5) | 58 (24.5) | 58 (24.5) | ||||
| N stage | <0.001 | 0.427 | 1.000 | 0.001 | ||||
| N0 | 95 (40.1) | 145 (22.3) | 95 (40.1) | 95 (40.1) | ||||
| N+ | 142 (59.9) | 505 (77.7) | 142 (59.9) | 142 (59.9) | ||||
| PNI | 0.359 | 0.075 | 0.581 | 0.051 | ||||
| No | 115 (48.5) | 291 (44.8) | 115 (48.5) | 109 (46.0) | ||||
| Yes | 122 (51.5) | 359 (55.2) | 122 (51.5) | 128 (54.0) | ||||
| LVI | 0.294 | 0.085 | 0.774 | 0.026 | ||||
| No | 151 (63.7) | 387 (59.5) | 151 (63.7) | 154 (65.0) | ||||
| Yes | 86 (36.3) | 263 (40.5) | 86 (36.3) | 83 (35.0) | ||||
| CEA | 0.498 | 0.061 | 0.487 | 0.066 | ||||
| ≤5 | 205 (86.5) | 575 (88.5) | 205 (86.5) | 210 (88.6) | ||||
| >5 | 32 (13.5) | 75 (11.5) | 32 (13.5) | 27 (11.4) | ||||
| CA 19-9 | 0.022 | 0.190 | 0.160 | 0.136 | ||||
| ≤37 | 186 (78.5) | 554 (85.2) | 186 (78.5) | 198 (83.5) | ||||
| >37 | 51 (21.5) | 96 (14.8) | 51 (21.5) | 39 (16.5) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Guo, J.; Xiong, Z.; Zhong, B.; Huang, D.; Xu, H.; Chen, S.; Lian, L. Age-Stratified Clinicopathological Features and Efficacy of Adjuvant Chemotherapy in Resectable Gastric Cancer: An East-West Population-Based Study. Curr. Oncol. 2025, 32, 480. https://doi.org/10.3390/curroncol32090480
Deng Z, Guo J, Xiong Z, Zhong B, Huang D, Xu H, Chen S, Lian L. Age-Stratified Clinicopathological Features and Efficacy of Adjuvant Chemotherapy in Resectable Gastric Cancer: An East-West Population-Based Study. Current Oncology. 2025; 32(9):480. https://doi.org/10.3390/curroncol32090480
Chicago/Turabian StyleDeng, Zijian, Jianping Guo, Zhizhong Xiong, Bin Zhong, Dayin Huang, Haoyang Xu, Shi Chen, and Lei Lian. 2025. "Age-Stratified Clinicopathological Features and Efficacy of Adjuvant Chemotherapy in Resectable Gastric Cancer: An East-West Population-Based Study" Current Oncology 32, no. 9: 480. https://doi.org/10.3390/curroncol32090480
APA StyleDeng, Z., Guo, J., Xiong, Z., Zhong, B., Huang, D., Xu, H., Chen, S., & Lian, L. (2025). Age-Stratified Clinicopathological Features and Efficacy of Adjuvant Chemotherapy in Resectable Gastric Cancer: An East-West Population-Based Study. Current Oncology, 32(9), 480. https://doi.org/10.3390/curroncol32090480





