Novel Therapeutic Development for Nasopharyngeal Carcinoma
Simple Summary
Abstract
1. Introduction
1.1. Cancer Biology
1.2. Staging and Prognosis
1.3. Current Standard Therapy
2. Novel Therapeutics in NPC
2.1. Immune Cell-Activating Agents
2.1.1. Immune Checkpoint Inhibitors
PD-1/PD-L1 Inhibitors
Beyond PD-1/PD-L1
Bispecific Immune Checkpoint Inhibitors
2.1.2. T Cell Co-Stimulatory Pathways
2.1.3. Other Immune Modulating Agents
2.2. Therapeutic Vaccine
2.3. Adoptive Cell Therapy
2.4. Antibody–Drug Conjugates
2.5. Targeted Therapies
2.5.1. Receptor Tyrosine Kinase
2.5.2. Other Targets
3. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Rumgay, H.; Li, M.; Cao, S.; Chen, W. Nasopharyngeal Cancer Incidence and Mortality in 185 Countries in 2020 and the Projected Burden in 2040: Population-Based Global Epidemiological Profiling. JMIR Public Health Surveill. 2023, 9, e49968. [Google Scholar] [CrossRef]
- Guo, L.-F.; Dai, Y.-Q.; Yu, Y.-F.; Wu, S.-G. Gender-Specific Survival of Nasopharyngeal Carcinoma in Endemic and Non-Endemic Areas Based on the US SEER Database and a Chinese Single-Institutional Registry. Clin. Epidemiol. 2024, 16, 769–782. [Google Scholar] [CrossRef]
- Cantù, G. Nasopharyngeal carcinoma. A “different” head and neck tumour. Part A: From histology to staging. Acta Otorhinolaryngol. Ital. 2023, 43, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.J.; Gao, X.; Chen, C.-J.; Yang, X.R.; Diehl, S.R.; Goldstein, A.; Hsu, W.-L.; Liang, X.S.; Marti, D.; Liu, M.-Y.; et al. Association of human leukocyte antigens with nasopharyngeal carcinoma in high-risk multiplex families in Taiwan. Hum. Immunol. 2009, 70, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Siak, P.Y.; Leong, C.-O.; Cheah, S.-C. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front. Microbiol. 2023, 14, 1116143. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-S.; Kieff, E. Epstein-Barr virus latent genes. Exp. Mol. Med. 2015, 47, e131. [Google Scholar] [CrossRef]
- Ahmed, N.; Abusalah, M.; Farzand, A.; Absar, M.; Yusof, N.Y.; Rabaan, A.A.; AlSaihati, H.; Alshengeti, A.; Alwarthan, S.; Alsuwailem, H.S.; et al. Updates on Epstein-Barr Virus (EBV)-Associated Nasopharyngeal Carcinoma: Emphasis on the Latent Gene Products of EBV. Medicina 2022, 59, 2. [Google Scholar] [CrossRef]
- Chen, J. Roles of the PI3K/Akt pathway in Epstein-Barr virus-induced cancers and therapeutic implications. World J. Virol. 2012, 1, 154–161. [Google Scholar] [CrossRef]
- Raghupathy, R.; Hui, E.P.; Chan, A.T.C. Epstein-Barr virus as a paradigm in nasopharyngeal cancer: From lab to clinic. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, 149–153. [Google Scholar] [CrossRef]
- Gruhne, B.; Sompallae, R.; Marescotti, D.; Kamranvar, S.A.; Gastaldello, S.; Masucci, M.G. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2009, 106, 2313–2318. [Google Scholar] [CrossRef]
- Hau, P.M.; Lung, H.L.; Wu, M.; Tsang, C.M.; Wong, K.L.; Mak, N.K.; Lo, K.W. Targeting Epstein-Barr Virus in Nasopharyngeal Carcinoma. Front. Oncol. 2020, 10, 600. [Google Scholar] [CrossRef]
- Choy, E.Y.-W.; Siu, K.-L.; Kok, K.-H.; Lung, R.W.-M.; Tsang, C.M.; To, K.-F.; Kwong, D.L.-W.; Tsao, S.W.; Jin, D.-Y. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J. Exp. Med. 2008, 205, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, F.; Wu, W.; Wang, Y.; Ding, H.; Qian, L. Epstein-Barr virus-encoded microRNAs as regulators in host immune responses. Int. J. Biol. Sci. 2018, 14, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Tse, K.P.; Su, W.H.; Chang, K.P.; Tsang, N.M.; Yu, C.J.; Tang, P.; See, L.C.; Hsueh, C.; Yang, M.L.; Hao, S.P.; et al. Genome-wide association study reveals multiple nasopharyngeal carcinoma-associated loci within the HLA region at chromosome 6p21.3. Am. J. Hum. Genet. 2009, 85, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Zhou, T.; He, Y.Q.; Xue, W.Q.; Zhang, J.B.; Zheng, X.H.; Li, X.Z.; Zhang, S.D.; Zeng, Y.X.; Jia, W.H. Fine-mapping of HLA class I and class II genes identified two independent novel variants associated with nasopharyngeal carcinoma susceptibility. Cancer Med. 2018, 7, 6308–6316. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Chen, Y.; Liu, W.; Yu, J.; Wu, G. Methylation associated inactivation of RASSF1A and its synergistic effect with activated K-Ras in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 160. [Google Scholar] [CrossRef]
- Lao, T.D.; Thieu, H.H.; Nguyen, D.H.; Le, T.A.H. Hypermethylation of the RASSF1A gene promoter as the tumor DNA marker for nasopharyngeal carcinoma. Int. J. Biol. Markers 2022, 37, 31–39. [Google Scholar] [CrossRef]
- Leong, M.M.L.; Lung, M.L. The Impact of Epstein-Barr Virus Infection on Epigenetic Regulation of Host Cell Gene Expression in Epithelial and Lymphocytic Malignancies. Front. Oncol. 2021, 11, 629780. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Luo, W.R. Rethinking cancer. Zhonghua Zhong Liu Za Zhi 2025, 47, 463–467. [Google Scholar] [CrossRef]
- Pan, J.-J.; Mai, H.-Q.; Ng, W.T.; Hu, C.-S.; Li, J.-G.; Chen, X.-Z.; Chow, J.C.H.; Wong, E.; Lee, V.; Ma, L.-Y.; et al. Ninth Version of the AJCC and UICC Nasopharyngeal Cancer TNM Staging Classification. JAMA Oncol. 2024, 10, 1627–1635. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ouyang, Z.; He, S.; Qin, X.; Liang, X.; Huang, W.; Wang, R.; Hu, K. MRI-based deep learning and radiomics for predicting the efficacy of PD-1 inhibitor combined with induction chemotherapy in advanced nasopharyngeal carcinoma: A prospective cohort study. Transl. Oncol. 2025, 52, 102245. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Meng, M.; Bi, L.; Kim, J.; Feng, D.D.; Song, S. Prediction of 5-year progression-free survival in advanced nasopharyngeal carcinoma with pretreatment PET/CT using multi-modality deep learning-based radiomics. Front. Oncol. 2022, 12, 899351. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-F.; Han, F.; Zhao, C.; Huang, Y.; Chen, C.-Y.; Xiao, W.-W.; Li, J.-X.; Lu, T.-X. Treatment outcomes for different subgroups of nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Chin. J. Cancer 2011, 30, 565–573. [Google Scholar] [CrossRef] [PubMed]
- van Velsen, J.S.; van der Vegt, B.; Plaat, B.E.C.; Langendijk, J.A.; Epskamp-Kuijpers, C.C.H.J.; van Dijk, B.A.C.; Oosting, S.F. Nasopharyngeal carcinoma: Nationwide trends in subtype-specific incidence and survival over 3 decades in a non-endemic area. J. Cancer Res. Clin. Oncol. 2024, 150, 49. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Z.; Chen, L.; Li, Y.; Zhao, T.; Wei, Q. Characteristics of Early Death in Patients with Localized Nasopharyngeal Cancer: A Population-Based SEER Analysis. Front. Oncol. 2021, 11, 580220. [Google Scholar] [CrossRef]
- Alami, I.E.; Gihbid, A.; Charoute, H.; Khaali, W.; Brahim, S.M.; Tawfiq, N.; Cadi, R.; Belghmi, K.; El Mzibri, M.; Khyatti, M. Prognostic value of Epstein-Barr virus DNA load in nasopharyngeal carcinoma: A meta-analysis. Pan Afr. Med. J. 2022, 41, 6. [Google Scholar] [CrossRef]
- Colevas, A.D.; Cmelak, A.J.; Pfister, D.G.; Spencer, S.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Caudell, J.J.; Durm, G.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 2.2025. J. Natl. Compr. Canc Netw. 2025, 23, 2–11. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wen, Y.H.; Tang, J.; Wei, Y.; You, R.; Zhu, X.L.; Li, J.; Chen, L.; Ling, L.; Zhang, N.; et al. Endoscopic surgery compared with intensity-modulated radiotherapy in resectable locally recurrent nasopharyngeal carcinoma: A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 381–390. [Google Scholar] [CrossRef]
- You, R.; Liu, Y.P.; Xie, Y.L.; Lin, C.; Duan, C.Y.; Chen, D.P.; Pan, Y.; Qi, B.; Zou, X.; Guo, L.; et al. Hyperfractionation compared with standard fractionation in intensity-modulated radiotherapy for patients with locally advanced recurrent nasopharyngeal carcinoma: A multicentre, randomised, open-label, phase 3 trial. Lancet 2023, 401, 917–927. [Google Scholar] [CrossRef]
- Network, N.C.C. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 16 April 2025).
- Gong, L.; Kwong, D.L.; Dai, W.; Wu, P.; Wang, Y.; Lee, A.W.; Guan, X.Y. The Stromal and Immune Landscape of Nasopharyngeal Carcinoma and Its Implications for Precision Medicine Targeting the Tumor Microenvironment. Front. Oncol. 2021, 11, 744889. [Google Scholar] [CrossRef]
- Wang, X.; Han, F.; Huang, Y.; Xu, C.; Mao, Y.; Lin, L.; Chen, Y.; Chen, C. 920P Envafolimab plus chemoradiotherapy for locally advanced nasopharyngeal carcinoma (NPC), a prospective, single-armed phase II trial. Ann. Oncol. 2023, 34, S581. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, W.F.; Ai, X.; Lei, F.; Ma, D.; Ni, H.; Hou, X.; Wang, Q.; Yu, H.; Li, J. LBA3 First-line HLX07 vs. placebo combined with serplulimab and chemotherapy for nasopharyngeal cancer: A randomised, double-blind, multicentre phase II study. Ann. Oncol. 2024, 35, S1554. [Google Scholar] [CrossRef]
- Luo, J.; Xiao, W.; Hua, F.; Cao, Y.; Wang, D.; Wang, X. Efficacy and safety of PD-1 inhibitors in recurrent or metastatic nasopharyngeal carcinoma patients after failure of platinum-containing regimens: A systematic review and meta-analysis. BMC Cancer 2023, 23, 1172. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 2019, 20, 1425–1434. [Google Scholar] [CrossRef]
- Xu, R.-H.; Wang, F.; Chen, G.; Qiu, M.; Ma, J.; Liu, H.; Mo, X.; Li, Y.; Wan, X.; Luo, J.; et al. Neoadjuvant treatment of IBI310 (anti-CTLA-4 antibody) plus sintilimab (anti-PD-1 antibody) in patients with microsatellite instability-high/mismatch repair-deficient colorectal cancer: Results from a randomized, open-labeled, phase Ib study. JCO 2024, 42, 3505. [Google Scholar] [CrossRef]
- Chen, G.; Sun, D.C.; Ba, Y.; Zhang, Y.X.; Zhou, T.; Zhao, Y.Y.; Zhao, H.Y.; Fang, W.F.; Huang, Y.; Wang, Z.; et al. Anti-LAG-3 antibody LBL-007 plus anti-PD-1 antibody toripalimab in advanced nasopharyngeal carcinoma and other solid tumors: An open-label, multicenter, phase Ib/II trial. J. Hematol. Oncol. 2025, 18, 15. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Qu, S.; Liu, L.; Chen, L.; Yang, K.; Huang, X.; Li, J.; Wang, R.; Zhu, H.; et al. Anti-LAG-3 antibody LBL-007 in combination with anti-PD-1 antibody tislelizumab with or without chemotherapy in patients with advanced nasopharyngeal cancer and other malignant tumors: A phase Ib/II dose escalation/expansion study. JCO 2024, 42, 6033. [Google Scholar] [CrossRef]
- Andrzejczak, A.; Karabon, L. BTLA biology in cancer: From bench discoveries to clinical potentials. Biomark. Res. 2024, 12, 8. [Google Scholar] [CrossRef]
- Lan, Y.; Moustafa, M.; Knoll, M.; Xu, C.; Furkel, J.; Lazorchak, A.; Yeung, T.-L.; Hasheminasab, S.-M.; Jenkins, M.H.; Meister, S.; et al. Simultaneous targeting of TGF-β/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell 2021, 39, 1388–1403.e1310. [Google Scholar] [CrossRef]
- Chiang, C.L.; Lam, T.C.; Li, J.C.B.; Chan, K.S.K.; El Helali, A.; Lee, Y.Y.P.; Law, L.H.T.; Zheng, D.; Lo, A.W.I.; Kam, N.W.; et al. Efficacy, safety, and correlative biomarkers of bintrafusp alfa in recurrent or metastatic nasopharyngeal cancer patients: A phase II clinical trial. Lancet Reg. Health—West. Pac. 2023, 40, 100898. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Huang, J.; Hong, X.; Wu, B.; Ding, Q.; Peng, G.; Yang, K. 416P A study of AK104 (an anti-PD1 and anti-CTLA4 bispecific antibody) combined with chemotherapy for the treatment of anti-PD-1 resistant, recurrent, or metastatic nasopharyngeal carcinoma (R/M NPC). Ann. Oncol. 2024, 35, S1559. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, S.; Qu, S.; Guan, Y.; Zhao, Y.; Wang, J.; Wu, T.; Yu, X.; Xue, S.; Kang, X.; et al. Safety and efficacy of iparomlimab and tuvonralimab in combination with gemcitabine and cisplatin as first-line treatment for patients with recurrent or metastatic nasopharyngeal carcinoma: A multicenter, single-arm, phase 2 trial (DUBHE-N-302). JCO 2024, 42, 6026. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Y.; Fang, W.; Ma, Y.; Zhang, Y.; Wei, X.-L.; Yang, Y.; Yang, W.; Liu, F.; Lin, Z.; et al. Efficacy and safety of QL1706, a novel dual immune checkpoint blockade containing a mixture of anti-PD1 IgG4 and anti-CTLA4 IgG1 antibodies, for advanced nasopharyngeal carcinoma (NPC): Pooled cohort data from phase 1a/1b trials. JCO 2022, 40, 6034. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Zang, A.; Cheng, Y.; Zhang, Y.; Wang, X.; Chen, Z.; Qu, S.; He, J.; Chen, C.; et al. First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors. J. Hematol. Oncol. 2023, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Mascarelli, D.E.; Rosa, R.S.M.; Toscaro, J.M.; Semionatto, I.F.; Ruas, L.P.; Fogagnolo, C.T.; Lima, G.C.; Bajgelman, M.C. Boosting Antitumor Response by Costimulatory Strategies Driven to 4-1BB and OX40 T-cell Receptors. Front. Cell Dev. Biol. 2021, 9, 692982. [Google Scholar] [CrossRef]
- Hamid, O.; Chiappori, A.A.; Thompson, J.A.; Doi, T.; Hu-Lieskovan, S.; Eskens, F.A.L.M.; Ros, W.; Diab, A.; Spano, J.-P.; Rizvi, N.A.; et al. First-in-human study of an OX40 (ivuxolimab) and 4-1BB (utomilumab) agonistic antibody combination in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e005471. [Google Scholar] [CrossRef]
- Arch, R.H.; Thompson, C.B. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol. Cell Biol. 1998, 18, 558–565. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, Y.; Li, Y.; Tang, L.; Guo, Y.; Yuan, G.; Fu, Z.; Yu, J.-C.; Zhang, L.; Zhao, H. Anti-OX40 antibody BAT6026 in Patients with Advanced Solid Tumors: A Multi-Center Phase I Study. iScience 2025, 28, 112270. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, F.; Zhang, Y.; Liu, Q.; Xue, J.; Huang, Y.; Zhao, Y.; Yang, Y.; Fang, W.; Zhou, T.; et al. Preclinical characterization and phase 1 results of ADG106 in patients with advanced solid tumors and non-Hodgkin’s lymphoma. Cell Rep. Med. 2024, 5, 101414. [Google Scholar] [CrossRef]
- Wang, H.; Hu, J.; Zhou, W.; Qian, A. Metabolic reprogramming in the pathogenesis and progression of nasopharyngeal carcinoma: Molecular mechanisms and therapeutic implications. Am. J. Cancer Res. 2024, 14, 4049–4064. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Siak, P.Y.; Leong, C.O.; Cheah, S.C. Nasopharyngeal Carcinoma and Its Microenvironment: Past, Current, and Future Perspectives. Front. Oncol. 2022, 12, 840467. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Yin, S.; To, K.K.W.; Fu, L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol. Cancer 2023, 22, 44. [Google Scholar] [CrossRef]
- Mediavilla-Varela, M.; Castro, J.; Chiappori, A.; Noyes, D.; Hernandez, D.C.; Allard, B.; Stagg, J.; Antonia, S.J. A Novel Antagonist of the Immune Checkpoint Protein Adenosine A2a Receptor Restores Tumor-Infiltrating Lymphocyte Activity in the Context of the Tumor Microenvironment. Neoplasia 2017, 19, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Song, S.; Yang, L.; Ma, J.; Chung, J.; Dearie, A.; Chien, S.; Spalding, Q.; Choi, S.; Blanco, G.; et al. Abstract 6380: PLX-4545, a selective IKZF2 degrader, reprograms suppressive Tregs leading to tumor growth inhibition and combination benefit with immune checkpoint therapy. Cancer Res. 2025, 85, 6380. [Google Scholar] [CrossRef]
- DiMartino, J.F.; Thompson, P.; Huang, Y.; Freeman-Cook, K.; Farrell, C.; Yang, P. Abstract CT150: A first in humans trial of PLX-4545, a molecular glue degrader of IKZF2, in healthy volunteers, shows pharmacologic modulation of Tregs at well-tolerated doses. Cancer Res. 2025, 85, CT150. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lo, W.-F.; Lee, T.-H.; Ren, Y.; Hwang, S.-L.; Cheng, Y.-F.; Chen, C.-L.; Chang, Y.-S.; Lee, S.P.; Rickinson, A.B.; et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002, 62, 6952–6958. [Google Scholar]
- Nickles, E.; Dharmadhikari, B.; Yating, L.; Walsh, R.J.; Koh, L.P.; Poon, M.; Tan, L.K.; Wang, L.-Z.; Ang, Y.; Asokumaran, Y.; et al. Dendritic cell therapy with CD137L-DC-EBV-VAX in locally recurrent or metastatic nasopharyngeal carcinoma is safe and confers clinical benefit. Cancer Immunol. Immunother. 2022, 71, 1531–1543. [Google Scholar] [CrossRef]
- Hui, E.P.; Taylor, G.S.; Jia, H.; Ma, B.B.; Chan, S.L.; Ho, R.; Wong, W.L.; Wilson, S.; Johnson, B.F.; Edwards, C.; et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013, 73, 1676–1688. [Google Scholar] [CrossRef]
- Taylor, G.S.; Jia, H.; Harrington, K.; Lee, L.W.; Turner, J.; Ladell, K.; Price, D.A.; Tanday, M.; Matthews, J.; Roberts, C.; et al. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: A phase I trial in UK patients with EBV-positive cancer. Clin. Cancer Res. 2014, 20, 5009–5022. [Google Scholar] [CrossRef]
- Lin, M.C.; Lin, Y.C.; Chen, S.T.; Young, T.H.; Lou, P.J. Therapeutic vaccine targeting Epstein-Barr virus latent protein, LMP1, suppresses LMP1-expressing tumor growth and metastasis in vivo. BMC Cancer 2017, 17, 18. [Google Scholar] [CrossRef]
- Lei, L.; Li, J.; Liu, M.; Hu, X.; Zhou, Y.; Yang, S. CD40L-adjuvanted DNA vaccine carrying EBV-LMP2 antigen enhances anti-tumor effect in NPC transplantation tumor animal. Cent. Eur. J. Immunol. 2018, 43, 117–122. [Google Scholar] [CrossRef]
- Peng, X.; He, X.; Zhang, W.; Huang, H.; Li, X.; Chen, J.; Wei, Y.; Song, X. Safety, tolerability, and immunogenicity of WGc-043 in subjects with EBV-positive cancers: Results from an investigator-initiated trial. JCO 2024, 42, 139. [Google Scholar] [CrossRef]
- Huang, J.; Harris, E.; Lorch, J. Vaccination as a therapeutic strategy for Nasopharyngeal carcinoma. Oral Oncol. 2022, 135, 106083. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhan, Y.; Luo, J.; Wang, W.; Fan, S. Unveiling immune resistance mechanisms in nasopharyngeal carcinoma and emerging targets for antitumor immune response: Tertiary lymphoid structures. J. Transl. Med. 2025, 23, 38. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Hassouneh, F.; Alvarez-Heredia, P.; Pera, A.; Solana, R. Immune Checkpoint Inhibitors for Vaccine Improvements: Current Status and New Approaches. Pharmaceutics 2022, 14, 1721. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, V.; San Román, M.; Pozas, J.; Chamorro, J.; Rosero, D.I.; Guerrero, P.; Calvo, J.C.; González, C.; García de Quevedo, C.; Pérez de Aguado, P.; et al. Adoptive T cell therapy for solid tumors: Current landscape and future challenges. Front. Immunol. 2024, 15, 1352805. [Google Scholar] [CrossRef]
- Comoli, P.; De Palma, R.; Siena, S.; Nocera, A.; Basso, S.; Del Galdo, F.; Schiavo, R.; Carminati, O.; Tagliamacco, A.; Abbate, G.F.; et al. Adoptive transfer of allogeneic Epstein–Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boostsLMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann. Oncol. 2004, 15, 113–117. [Google Scholar] [CrossRef]
- Raab-Traub, N. Epstein–Barr virus in the pathogenesis of NPC. Semin. Cancer Biol. 2002, 12, 431–441. [Google Scholar] [CrossRef]
- Huang, J.; Fogg, M.; Wirth, L.J.; Daley, H.; Ritz, J.; Posner, M.R.; Wang, F.C.; Lorch, J.H. Epstein-Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer 2017, 123, 2642–2650. [Google Scholar] [CrossRef]
- Toh, H.C.; Yang, M.H.; Wang, H.M.; Hsieh, C.Y.; Chitapanarux, I.; Ho, K.F.; Hong, R.L.; Ang, M.K.; Colevas, A.D.; Sirachainan, E.; et al. Gemcitabine, carboplatin, and Epstein-Barr virus-specific autologous cytotoxic T lymphocytes for recurrent or metastatic nasopharyngeal carcinoma: VANCE, an international randomized phase III trial. Ann. Oncol. 2024, 35, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Lee, V.; Schuessler, A.; Beagley, L.; Rehan, S.; Tsang, J.; Li, V.; Tiu, R.; Smith, D.; Neller, M.A.; et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: Phenotype and effector function of T cells impact on clinical response. OncoImmunology 2017, 6, e1273311. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Cohen, R.B.; Park, J.C.; Pfister, D.G.; Massarelli, E.; Li, Z.; Xing, B.; Dinavahi, R.; Mehta, A. Safety of tabelecleucel with pembrolizumab in recurrent/metastatic Epstein–Barr virus-associated nasopharyngeal carcinoma. J. Clin. Oncol. 2024, 42, 6096. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.-Y.; He, J.; Li, Z.-L.; Tang, X.-F.; Chen, S.-P.; Xie, C.-M.; Li, Y.-Q.; Huang, L.-X.; Ye, S.-b.; et al. Phase I trial of adoptively transferred tumor-infiltrating lymphocyte immunotherapy following concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. OncoImmunology 2015, 4, e976507. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.J.; Rafiq, S.; Brentjens, R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016, 13, 370–383. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Yang, K.; Yang, Y.; Zhou, W.; Huang, Y.; Liang, X.; Su, J.; Jiang, L.; Li, J.; et al. EpCAM-targeting CAR-T cell immunotherapy is safe and efficacious for epithelial tumors. Sci. Adv. 2023, 9, eadg9721. [Google Scholar] [CrossRef]
- Dudaniec, K.; Westendorf, K.; Nössner, E.; Uckert, W. Generation of Epstein-Barr Virus Antigen-Specific T Cell Receptors Recognizing Immunodominant Epitopes of LMP1, LMP2A, and EBNA3C for Immunotherapy. Human Gene Ther. 2021, 32, 919–935. [Google Scholar] [CrossRef]
- Teppert, K.; Wang, X.; Anders, K.; Evaristo, C.; Lock, D.; Künkele, A. Joining Forces for Cancer Treatment: From “TCR versus CAR” to “TCR and CAR”. Int. J. Mol. Sci. 2022, 23, 14563. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody–drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, Y.; Tao, Y.; Liu, S. Nasopharyngeal Carcinoma: The Role of the EGFR in Epstein-Barr Virus Infection. Pathogens 2021, 10, 1113. [Google Scholar] [CrossRef]
- Wang, L.; Zhuang, H.; Xu, X.; Zhou, J.; Jiao, Y. Efficacy and survival analysis of nimotuzumab combined with concurrent chemoradiotherapy in the treatment of locally advanced nasopharyngeal carcinoma. Front. Oncol. 2023, 13, 1129649. [Google Scholar] [CrossRef]
- Han, F.; Wang, X.; Xiang, Y.; Tang, L.-Q.; Qu, S.; Shu, X.; Zhang, P.; Qiu, S.; Zhou, Y.; Guo, Y.; et al. Becotatug vedotin vs. chemotherapy in pre-heavily treated advanced nasopharyngeal carcinoma: A randomized, controlled, multicenter, open-label study. J. Clin. Oncol. 2025, 43, LBA6005. [Google Scholar] [CrossRef]
- Flieswasser, T.; Van den Eynde, A.; Van Audenaerde, J.; De Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 axis in oncology: The new kids on the block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef] [PubMed]
- Garmezy, B.; Kambhampati, S.; Curti, B.; Reimers, M.; Caimi, P.; Diefenbach, C.; Heath, E.; Jonasch, E.; Park, S.; Kornblum, N.; et al. 718 Phase 1/2 study of PRO1160, a CD70-directed antibody-drug conjugate, in patients with advanced solid tumors and hematologic malignancies. In Proceedings of the SITC 38th Annual Meeting (SITC 2023) Abstracts, San Diego, CA, USA, 1–5 November 2023; p. A813. [Google Scholar]
- Ma, Y.; Yang, Y.; Huang, Y.; Fang, W.; Xue, J.; Meng, X.; Fan, Y.; Fu, S.; Wu, L.; Zheng, Y.; et al. A B7H3-targeting antibody–drug conjugate in advanced solid tumors: A phase 1/1b trial. Nat. Med. 2025, 31, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, G.; Zhang, X.; Ma, W. Targeting HER3 to overcome EGFR TKI resistance in NSCLC. Front. Immunol. 2024, 14, 1332057. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, Y.; Zhao, Y.; Zhao, S.; Xue, J.; Yang, Y.; Fang, W.; Guo, Y.; Han, Y.; Yang, K.; et al. BL-B01D1, a first-in-class EGFR–HER3 bispecific antibody–drug conjugate, in patients with locally advanced or metastatic solid tumours: A first-in-human, open-label, multicentre, phase 1 study. Lancet Oncol. 2024, 25, 901–911. [Google Scholar] [CrossRef]
- Asiedu, M.K.; Beauchamp-Perez, F.D.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 2014, 33, 1316–1324. [Google Scholar] [CrossRef]
- Liang, R.; Yang, L.; Zhu, X. Nimotuzumab, an Anti-EGFR Monoclonal Antibody, in the Treatment of Nasopharyngeal Carcinoma. Cancer Control 2021, 28, 1073274821989301. [Google Scholar] [CrossRef]
- Wang, R.-J.; Ke, R.-Q.; Yu, Y.-F.; Lu, G.-Z.; Wu, S.-G. Addition of nimotuzumab to concurrent chemoradiotherapy after induction chemotherapy improves outcomes of patients with locally advanced nasopharyngeal carcinoma. Front. Pharmacol. 2024, 15, 1366853. [Google Scholar] [CrossRef]
- Bourhis, M.; Palle, J.; Galy-Fauroux, I.; Terme, M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front. Immunol. 2021, 12, 616837. [Google Scholar] [CrossRef]
- Lu, N.; Jiang, Y.F.; Xia, W.X.; Huang, Y.; Xie, C.M.; Xu, C.; Ye, Y.F.; Liu, G.Y.; Bei, W.X.; Ke, L.R.; et al. Efficacy and safety of sintilimab plus bevacizumab in metastatic nasopharyngeal carcinoma after failure of platinum-based chemotherapy: An open-label phase 2 study. EClinicalMedicine 2023, 62, 102136. [Google Scholar] [CrossRef]
- Yuan, L.; Jia, G.D.; Lv, X.F.; Xie, S.Y.; Guo, S.S.; Lin, D.F.; Liu, L.T.; Luo, D.H.; Li, Y.F.; Deng, S.W.; et al. Camrelizumab combined with apatinib in patients with first-line platinum-resistant or PD-1 inhibitor resistant recurrent/metastatic nasopharyngeal carcinoma: A single-arm, phase 2 trial. Nat. Commun. 2023, 14, 4893. [Google Scholar] [CrossRef]
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Feng, Z.; You-Ping, L.; You, R.; Zou, X.; Peng, L.; Hua, Y.; Chen, M. 892P Camrelizumab plus dalpiciclib in anti-PD-1 refractory recurrent or metastatic nasopharyngeal carcinoma. Ann. Oncol. 2024, 35, S633. [Google Scholar] [CrossRef]
- Gurizzan, C.; Farinatti, S.; Alberti, A.; Bonomo, P.; Resteghini, C.; Alfieri, S.; Bergamini, C.; Perri, F.; Moretti, G.; Galizia, D.; et al. 891P Pembrolizumab and olaparib in recurrent/metastatic, platinum resistant nasopharyngeal cancer: The POINT study. Ann. Oncol. 2024, 35, S633. [Google Scholar] [CrossRef]
- Colevas, A.D.; Siu, L.; Lim, D.W.T.; Gao, B.; Voon, P.J.; Khan, S.; Eng, L.; Ahn, M.J.; Lee, V.; Wang, H.M.; et al. 354O A phase Ib/II study of nanatinostat (Nstat) plus valganciclovir (VGCV) in EBV+ solid tumors and with pembrolizumab (PEM) in recurrent/metastatic nasopharyngeal carcinoma (R/M NPC). Ann. Oncol. 2023, 34, S1607. [Google Scholar] [CrossRef]
- Haverkos, B.; Alpdogan, O.; Baiocchi, R.; Brammer, J.E.; Feldman, T.A.; Capra, M.; Brem, E.A.; Nair, S.; Scheinberg, P.; Pereira, J.; et al. Targeted therapy with nanatinostat and valganciclovir in recurrent EBV-positive lymphoid malignancies: A phase 1b/2 study. Blood Adv. 2023, 7, 6339–6350. [Google Scholar] [CrossRef]
- Kansal, V.; Kinney, B.L.C.; Uppada, S.; Saba, N.F.; Stokes, W.A.; Buchwald, Z.S.; Schmitt, N.C. The expanding role of IAP antagonists for the treatment of head and neck cancer. Cancer Med. 2023, 12, 13958–13965. [Google Scholar] [CrossRef]
- Colevas, A.D.D.; Rudek, M.A.; Even, C.; Lee, V.H.-F.; Gillison, M.L.; Khan, S.A.; Lu, R.; Winters, E.; Biedermann, S.; Lai, S.; et al. Phase I/IIa clinical trial of a small molecule EBNA1 inhibitor, VK-2019, in patients with Epstein Barr virus–positive nasopharyngeal cancer with pharmacokinetic and pharmacodynamic correlative studies. JCO 2023, 41, 6035. [Google Scholar] [CrossRef]
- Sugiokto, F.G.; Li, R. Targeting EBV Episome for Anti-Cancer Therapy: Emerging Strategies and Challenges. Viruses 2025, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Suryani, L.; Lee, H.P.Y.; Teo, W.K.; Chin, Z.K.; Loh, K.S.; Tay, J.K. Precision Medicine for Nasopharyngeal Cancer-A Review of Current Prognostic Strategies. Cancers 2024, 16, 918. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zhou, X.; Zhu, S.; Kaya, N.A.; Chan, Y.S.; Ma, L.; Xu, M.; Zhai, W. An Integrative Analysis of Nasopharyngeal Carcinoma Genomes Unraveled Unique Processes Driving a Viral-Positive Cancer. Cancers 2023, 15, 1243. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-C.; Meng, X.; Hazawa, M.; Nagata, Y.; Varela, A.M.; Xu, L.; Sato, Y.; Liu, L.-Z.; Ding, L.-W.; Sharma, A.; et al. The genomic landscape of nasopharyngeal carcinoma. Nat. Genet. 2014, 46, 866–871. [Google Scholar] [CrossRef]
- Peng, H.; Chen, L.; Chen, Y.-P.; Li, W.-F.; Tang, L.-L.; Lin, A.-H.; Sun, Y.; Ma, J. The current status of clinical trials focusing on nasopharyngeal carcinoma: A comprehensive analysis of ClinicalTrials.gov database. PLoS ONE 2018, 13, e0196730. [Google Scholar] [CrossRef]
- Yu, Z.; Hong, S.; Yu, H.; Zhang, X.; Li, Z.; Chen, P.; Zhou, Y. Efficacy and safety of immune checkpoint inhibitors in the treatment of recurrent or metastatic nasopharyngeal carcinoma: A systematic review and meta-analysis. Chin. Med. J. 2024, 138, 531–539. [Google Scholar] [CrossRef]
- Yu, H.; Yin, X.; Mao, Y.; Chen, M.; Tang, Q.; Yan, S. The global burden of nasopharyngeal carcinoma from 2009 to 2019: An observational study based on the Global Burden of Disease Study 2019. Eur. Arch. Otorhinolaryngol. 2022, 279, 1519–1533. [Google Scholar] [CrossRef]
- Bossi, P.; Trama, A.; Bernasconi, A.; Grisanti, S.; Mohamad, I.; Galiana, I.L.; Ozyar, E.; Franco, P.; Vecchio, S.; Bonomo, P.; et al. Nasopharyngeal cancer in non-endemic areas: Impact of treatment intensity within a large retrospective multicentre cohort. Eur. J. Cancer 2021, 159, 194–204. [Google Scholar] [CrossRef]
- Xu, R.; Wong, C.H.L.; Chan, K.S.K.; Chiang, C.L. PD-L1 expression as a potential predictor of immune checkpoint inhibitor efficacy and survival in patients with recurrent or metastatic nasopharyngeal cancer: A systematic review and meta-analysis of prospective trials. Front. Oncol. 2024, 14, 1386381. [Google Scholar] [CrossRef]
- Economopoulou, P.; Pantazopoulos, A.; Spathis, A.; Kotsantis, I.; Kyriazoglou, A.; Kavourakis, G.; Zakopoulou, R.; Chatzidakis, I.; Anastasiou, M.; Prevezanou, M.; et al. Immunotherapy in Nonendemic Nasopharyngeal Carcinoma: Real-World Data from Two Nonendemic Regions. Cells 2021, 11, 32. [Google Scholar] [CrossRef]
- Strobl, M.A.R.; Gallaher, J.; Robertson-Tessi, M.; West, J.; Anderson, A.R.A. Treatment of evolving cancers will require dynamic decision support. Ann. Oncol. 2023, 34, 867–884. [Google Scholar] [CrossRef] [PubMed]
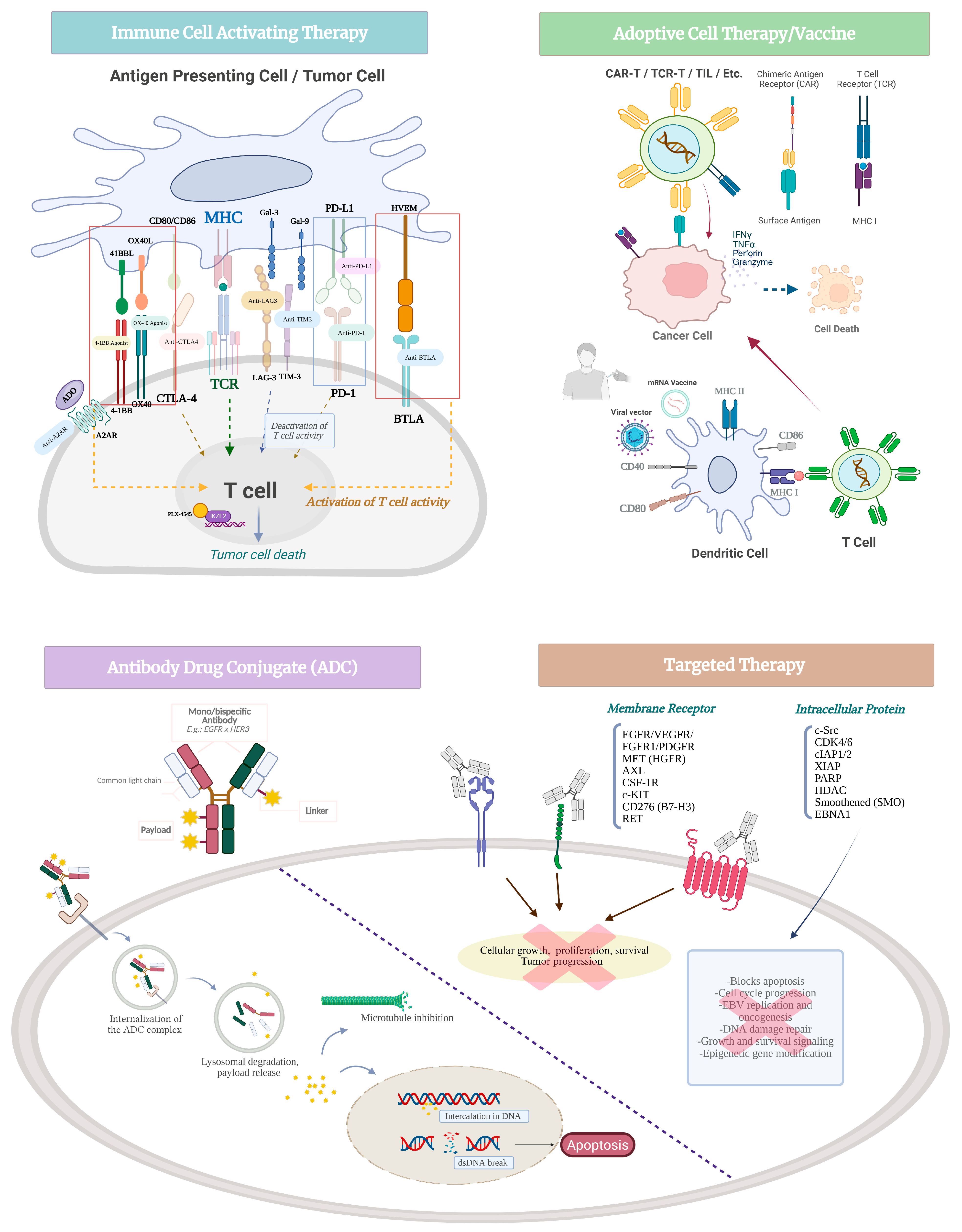
| Immune Cell Activating Therapy | |||||||||
| Drug Name | Mechanism | Phase | NCT ID | Regimen | N | Population | Key Outcome | G ≥ 3 TRAE (%) | |
| Sintilimab | Anti-PD-1 mAb | III | NCT03700476 | CCRT ± Sintilimab | 425 | LA-NPC | EFS HR 0.59; 36-mo EFS 86% vs. 76% | 74 | |
| Envafolimab | Anti-PD-L1 mAb | II | NCT05397769 | CCRT + Envafolimab | 36 | LA-NPC | ORR 94.4% | 0 | |
| Serplulimab | Anti-PD-1 mAb | II | NCT05513573 | Gem/Cis + Serplulimab | 25 | R/M NPC | ORR 72%; 12-mo PFS 52.8% | 32 | |
| INCB099280 | PD-L1 SMI | I | NCT04242199 | Monotherapy | 179 | Adv. solid tumors | ORR 16.0%; mDOR 16.8 mo | 13.4 | |
| Tagitanlimab | Anti-PD-L1 mAb | III | NCT05294172 | Gem/Cis ± Tagitanlimab | 358 | R/M NPC | PFS HR 0.47; ORR 81.7%; mDOR 11.7 mo | 3.9 | |
| IBI-310 | Anti-CTLA-4 mAb | Ib/II | NCT04945421 | IBI-310 + Sintilimab | 30 | R/M NPC | NR | NR | |
| LBL-007 | Anti-LAG-3 mAb | Ib/II | NCT05102006 | LBL-007 + Toripalimab | 30 | R/M NPC | ORR 33.3%; mPFS 10.8 mo; mDOR 15 mo | 11.3 | |
| Relatlimab | Anti-LAG-3 mAb | II | NCT06029270 | Nivolumab ± Relatlimab | 156 | R/M NPC | NR | NR | |
| TQB2618 | Anti-TIM-3 mAb | II | NCT05563480 | Penpulimab ± TQB2618 | 17 | R/M NPC | ORR 0%; mPFS 1.6 mo; 6-mo PFS 18.2% | 0 | |
| Tifcemalimab | Anti-BTLA mAb | I/II | NCT04929080 | JS004 ± Toripalimab | 149 | R/M HNSCC and NPC | NR | NR | |
| Bintrafusp alfa | PD-L1 × TGF-β fusion | II | NCT04396886 | Monotherapy | 38 | R/M NPC | ORR 23.7%; mOS 17.0 mo; mPFS 2.3 mo; | 42.4 | |
| Retlirafusp alfa | PD-L1 × TGF-β fusion | Ib | NCT04282070 | Arm 1 (post-chemo) Arm 2 (post-PD-1) | 54 | R/M NPC | Arm 1: ORR 33.3%; mPFS 5.3 mo Arm 2: ORR 4.2%; mPFS 1.4 mo | 18.5 | |
| TQB2858 | PD-L1 × TGF-β fusion | Ib/II | NCT05198531 | TQB2858 + Anlotinib | 90 | R/M NPC | NR | NR | |
| Cadonilimab | PD-1 × CTLA-4 BsAb | II | NCT05790200 | Cadonilimab + Chemo | 25 | PD-1-R R/M NPC | ORR 68%; mPFS 10.6 mo; mDOR 9.1 mo; 1-yr OS rate 75.6% | 48 | |
| SI-B003 | PD-1 × CTLA-4 BsAb | I | NCT04606472 | Monotherapy | 60 | Adv. solid tumors | ORR 16.1%; mPFS 3.7 mo | 3 | |
| QL1706 | PD-1 × CTLA-4 BsAb | I | NCT04296994, NCT05171790 | Monotherapy | 110 (NPC) | Adv. solid tumors | ORR 24.5%; mDOR 11.7 mo (NPC cohort) | 16 | |
| Vudalimab | PD-1 × CTLA-4 BsAb | I | NCT03517488 | Monotherapy | 77 | Adv. solid tumors | ORR 13.0% | 16.4 | |
| BGB-A445 | Anti-OX40 agonist mAb | I | NCT04215978 | Mono ± Tislelizumab | 59/32 | Adv. solid tumors | ORR 4%/23% | 41/53 | |
| BAT6026 | Anti-OX40 agonist mAb | I | NCT05105971 | Monotherapy | 30 | Adv. solid tumors | ORR 0%; mPFS 1.5 mo | 33.3 | |
| ADG106 | Anti-4-1BB mAb | Ib/II | NCT04775680 | ADG106 + Toripalimab | 25 | Adv. solid tumors | ORR 4.1% | 16 | |
| ILB2109 | A2AR SMI | Ib/IIa | NCT05955105 | ILB-2109 + Toripalimab | 200 | Adv. solid tumors | NR | NR | |
| DKY709 | IKZF2 degrader | Ib | NCT03891953 | DKY709 ± Spartalizumab | 98 | Adv. solid tumors | NR | NR | |
| PLX-4545 | IKZF2 degrader | I | ACTRN12623001265662 | Monotherapy | NR | Adv. solid tumors | NR | NR | |
| Vaccines | |||||||||
| Drug Name | Mechanism | Phase | NCT ID | Regimen | N | Population | Key Outcome | G ≥ 3 TRAE (%) | |
| CD137L-DC-EBV-VAX | DC vaccine | I | NCT03282617 | Monotherapy | 12 | R/M NPC | ORR 8.3%; mPFS 3.8 mo; mOS 20.8 mo | 0 | |
| KSD-101 | DC vaccine | I | NCT06370026, NCT06097793 | Monotherapy | 12 | EBV+ NPC | NR | NR | |
| DC-CIK | DC + CIK adoptive-cell vaccine | II | NCT01821495 | CCRT ± DC-CIK | 100 | LA-NPC | NR | NR | |
| Auto-DC ± Allo-DS | Autologous DC ± allogeneic dendritic-secretome | I/II | NCT05261750 | RT/CCRT + Auto-DC ± Allo-DC | 15 | R/M NPC | NR | NR | |
| MVA-EBNA1/LMP2 | Viral-vector (MVA) vaccine | Ib | NCT01800071 | Monotherapy | 18 | EBV+ NPC | 83% responded to vaccine-coded antigens | 0 | |
| VAC003 | Viral-vector (MVA) vaccine | II | NCT01094405 | Monotherapy | 25 | EBV+ R/M NPC | NR | NR | |
| WGc-043 | EBV-antigen mRNA vaccine | I | NCT05714748 | Monotherapy | 12 | EBV+ R/M NPC | ORR 16.7% | 0 | |
| Adoptive Cell Therapy | |||||||||
| Therapy Type | Target Antigen(S) | Phase | NCT ID | N | Population | Key Outcome | G ≥ 3 TRAE (%) | ||
| CTL (autologous) | LMP2, EBNA1 | III | NCT02578641 | 330 | R/M NPC | No benefit (chemo ± EBV CTL) | 0.6 | ||
| LMP2, EBNA1 | II | NCT00834093, NCT00431210 | 21 | R/M NPC | ORR 4.8%; mPFS 2.2 mo; mOS 16.7 mo | 0 | |||
| LMP1/2, BARF1, EBNA1 | I | NCT02065362 | 14 | EBV+ NPC | NR | NR | |||
| Multi EBV Ag | I | NCT00608257 | 8 | EBV+ R/M NPC | ORR 12.5%; EBV CTL + CD45 | 0 | |||
| CTL (allogeneic) | EBV Ag (Tabelecleucel) | Ib/II | NCT03769467 | 12 | EBV+ R/M NPC | SD 50% | 0 | ||
| CAR-T (autologous) | NR (U87) | I | NCT06614686 | 20 | R/M HNSCC | NR | NR | ||
| EBV gp350 (BRG01) | I | NCT05864924 | 11 | EBV+ R/M NPC | Tumor shrinkage in 75%; PFS > 6 mo 100% | 0 | |||
| NR | I | NCT05654077 | 24 | R/M NPC | NR | NR | |||
| LMP1 | I/II | NCT02980315 | 20 | EBV-associated tumors | NR | NR | |||
| EpCAM | I | NCT02915445 | 12 | EpCAM+ solid tumors | ORR 16.7% | 8.3 | |||
| Dual EBV Ag (BGT007) | I | NCT05616468 | 23 | R/M NPC | NR | NR | |||
| CAR-T (allogenic) | CD70 (CHT101) | I | NCT06383507 | 18 | Adv. solid tumors | NR | NR | ||
| MUC1-C (P-MUC1C-ALLO1) | I | NCT05239143 | 6 | Adv. solid tumors | ORR 16.7% | 0 | |||
| CAR-T (allogenic, γδ) | NKG2DL (CTM-N2D) | I | NCT04107142 | 10 | Adv. solid tumors | NR | NR | ||
| TCR-T | EBV Ag | I/II | NCT04509726 | 20 | EBV+ R/M NPC | NR | NR | ||
| LMP1/2, EBNA1 (YT-E001) | II | NCT03648697 | 20 | EBV+ R/M NPC | NR | NR | |||
| LMP2 | I | NCT03925896 | 27 | EBV+ R/M NPC | NR | NR | |||
| Mixed CAR-T/TCR-T | EBV Ag | I | NCT05587543 | 24 | EBV+ R/M NPC | NR | NR | ||
| TIL (autologous) | NR | I | NCT01462903 | 20 | LA-NPC | ORR 95% | 5 | ||
| Allo-CTL | EBV Ag + Pembrolizumab + Tabelecleucel | I/II | NCT03769467 | 12 | EBV+ R/M NPC | ORR 0% | 0 | ||
| Antibody Drug Conjugate | |||||||||
| Drug | Target | Payload | Phase | NCT ID | Regimen | N | Population | Key Outcome | G ≥ 3 TRAE (%) |
| Becotatug vedotin | EGFR | HY-15162 | IIa | NCT05126719 | Monotherapy | 61 | R/M NPC (post Pt/PD-1) | ORR: 39.3% (DL1), 55.2% (DL2); | 11.5 |
| I/II | NCT05688605 | MRG003 + HX008 | 30 | Adv. solid tumors | ORR 66.7%, 6-mo PFS rate 76.2% (NPC sub-cohort, n = 9) | 23.3 | |||
| GEN1160 | CD70 | DX-8951 | I/II | NCT05721222 | Monotherapy | 134 | R/M NPC, RCC, NHL | NR | NR |
| YL201 | B7-H3 | YL0010014 | I/II | NCT05434234 /NCT06057922 | Monotherapy | 312 | Adv. solid tumors | ORR 48.6%; mPFS: 7.8 mo (NPC sub-cohort, n = 70)) | 54.5 |
| TAK-500 | CCR2 | TAK-676 | I/II | NCT05070247 | TAK-500 ± Pembrolizumab | 61 | Adv. solid tumors | NR | NR |
| BL-B01D1 | EGFR x HER3 | Ed-04 | I | NCT05194982 | Monotherapy | 195 | Adv. solid tumors | ORR 38%, mPFS 6.8 mo (NPC sub-cohort, n = 42) | 71 |
| GEN1286 | EGFR x MET | DX-8951 | I/II | NCT06685068 | Monotherapy | 260 | Adv. solid tumors | NR | NR |
| Target Therapy | |||||||||
| Drug | Molecular Target | Class | Phase | NCT ID | Regimen | N | Population | Key Outcome | G ≥ 3 TRAE (%) |
| Nimotuzumab | EGFR | mAb | III | NCT06561763 | Toripalimab + Nimotuzumab | 416 | LA-NPC | NR | NR |
| Pimurutamab | EGFR | mAb | II | NCT05513573 | Pimurutamab + HLX10 + Chemo | 75 | R/M NPC | ORR 72%; 12-mo PFS 63.0% | 28.0 |
| Anlotinib | VEGFR/FGFR/PDGFR/KIT/RET | TKI | II | NCT03906058 | Monotherapy | 39 | R/M NPC | ORR 20.5%; mPFS 5.7 mo | 23.7 |
| Cabozantinib | VEGFR/MET/RET/KIT/Tie2/AXL/FLT3 | TKI | II | NCT05904080 | Nivolumab/Ipilimumab ± Cabozantinib | 50 | R/M NPC | NR | NR |
| Apatinib | VEGFR2 | TKI | II | NCT04586088 | Apatinib + Camrelizumab | 58 | R/M NPC | ORR 65.5%; mPFS 10.4 mo | 58.6 |
| Surufatinib | VEGFR/FGFR1/CSF-1R | TKI | II | NCT04955886 | Surufatinib + Toripalimab | 14 | R/M NPC | NR | NR |
| Axitinib | VEGFR/PDGFRβ/KIT | TKI | II | NCT01249547 | Monotherapy | 40 | R/M NPC | mOS 10.4 mo, 1-yr OS rate 45.4% | 8 |
| TKI | II | NCT04562441 | Axitinib + Avelumab (PD-L1) | 13 | ICI-naïve R/M NPC | ORR 7.7%; mPFS 5.4 mo, mOS 15.0 mo | NR | ||
| Dalpiciclib | CDK4/6 | SMI | II | NCT05724355 | Dalpiciclib + Camrelizumab | 34 | R/M NPC (PD-1 resistant) | ORR 32.4%; mDOR 10.4 mo, mPFS 6.7 mo | 76.5 |
| Niraparib | PARP | SMI | II | NCT05162872 | Niraparib + Sintilimab | 99 | R/M NPC | NR | NR |
| Fuzuloparib | PARP | SMI | II | NCT04978012 | Fuzuloparib + Camrelizumab | 48 | R/M NPC | NR | NR |
| Olaparib | PARP | SMI | III | NCT04825990 | Olaparib + Pembrolizumab | 34 | R/M NPC (Pt resistant) | ORR 13%; mPFS 4 mo | 22 |
| Nanatinostat | HDAC | HA | I | NCT05166577 | Nanatinostat + Valganciclovir | 15 | EBV+ R/M NPC | ORR 6.7% | 0 |
| Tolinapant | cIAP1/2, XIAP | NPM | I | NCT05245682 | Tolinapant + RT | 10 | LA-HNSCC | NR | 0 |
| Taladegib | SMO (Hh) | SMI | II | NCT05199584 | Monotherapy | 44 | PTCH1-mutated solid tumors | NR | NR |
| VK 2019 | EBNA1 | SMI | II | NCT04925544 | Monotherapy | 22 | EBV+ R/M NPC | ORR 4.5% | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, Y.; Kim, S.; Park, J.C. Novel Therapeutic Development for Nasopharyngeal Carcinoma. Curr. Oncol. 2025, 32, 479. https://doi.org/10.3390/curroncol32090479
Kim J, Lee Y, Kim S, Park JC. Novel Therapeutic Development for Nasopharyngeal Carcinoma. Current Oncology. 2025; 32(9):479. https://doi.org/10.3390/curroncol32090479
Chicago/Turabian StyleKim, Jongwoo, Yunjoo Lee, Seoin Kim, and Jong Chul Park. 2025. "Novel Therapeutic Development for Nasopharyngeal Carcinoma" Current Oncology 32, no. 9: 479. https://doi.org/10.3390/curroncol32090479
APA StyleKim, J., Lee, Y., Kim, S., & Park, J. C. (2025). Novel Therapeutic Development for Nasopharyngeal Carcinoma. Current Oncology, 32(9), 479. https://doi.org/10.3390/curroncol32090479






