Focused Ultrasound for Sarcomas: A Narrative Review
Simple Summary
Abstract
1. Introduction
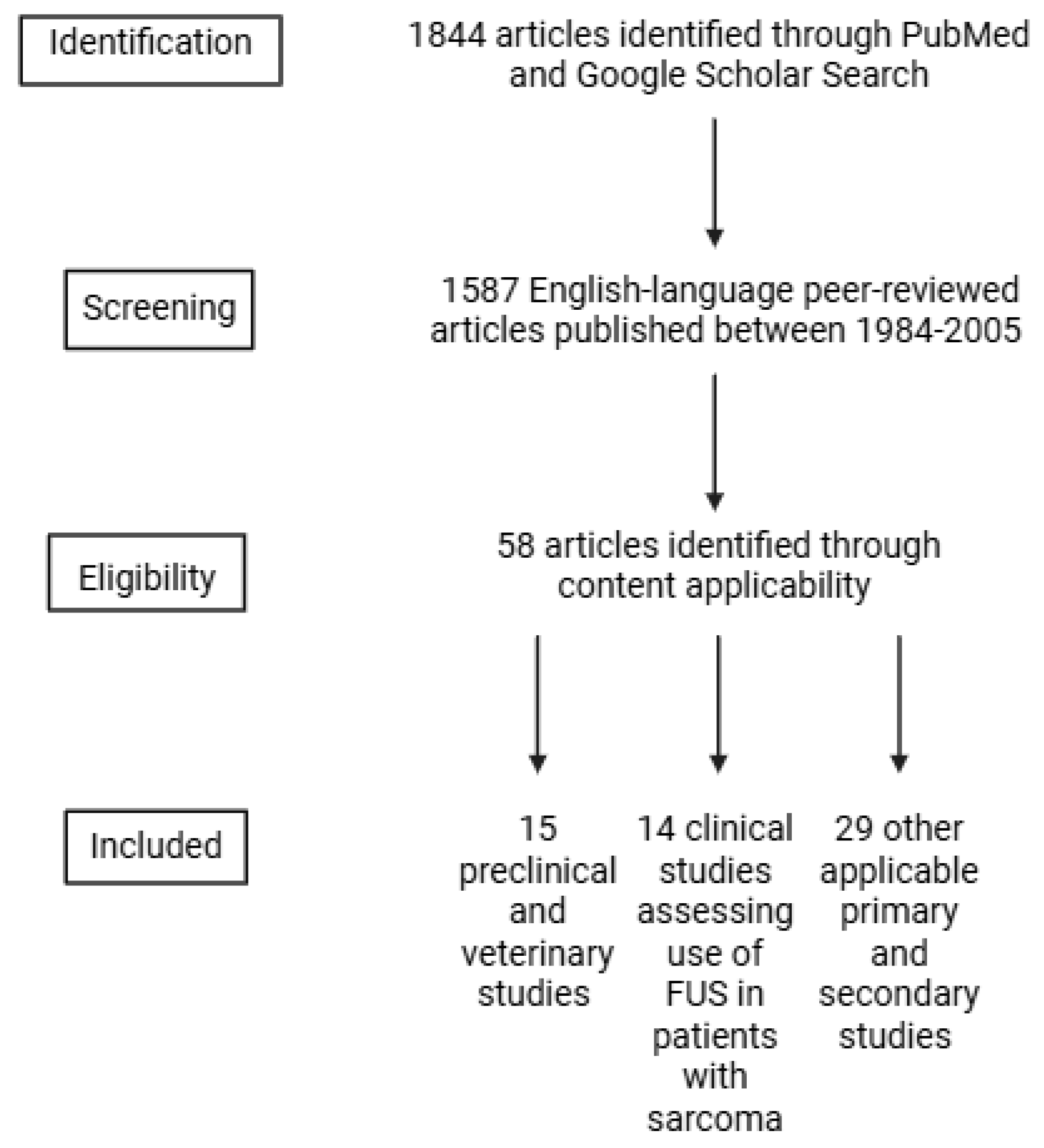
2. Methods
3. Mechanisms of Focused Ultrasound for Sarcoma Treatment
3.1. High Intensity Focused Ultrasound (HIFU) Thermal Ablation
3.2. Histotripsy
3.3. Sonodynamic Therapy (SDT)
3.4. Hyperthermia
4. Preclinical and Veterinary Studies
4.1. S180 Sarcoma Cell Line
4.2. Rhabdomyosarcoma and Leiomyosarcoma
4.3. Osteosarcoma and Soft Tissue Sarcomas
5. Clinical Studies
5.1. Osteosarcoma
5.2. Uterine Sarcoma and Leiomyoma
5.3. Myxofibrosarcoma
5.4. Spindle Cell Sarcoma
5.5. Pediatric Sarcomas
5.6. Other
6. Ongoing Clinical Trials
7. Focused Ultrasound as a Surgical Adjunct in Sarcoma Care
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIFU | High Intensity Focused Ultrasound |
| SDT | Sonodynamic Therapy |
| S180 | Sarcoma 180 cell line |
| NPVR | Non-Perfused Volume Ratio |
| SCS | Spindle Cell Sarcoma |
| MRI-HIFU or MRgFUS | Magnetic Resonance Imaging-guided High Intensity Focused Ultrasound or Magnetic Resonance-guided Focused Ultrasound |
| HT | Hyperthermia |
References
- Italiano, A.; Mathoulin-Pelissier, S.; Le Cesne, A.; Terrier, P.; Bonvalot, S.; Collin, F.; Michels, J.; Blay, J.; Coindre, J.; Bui, B. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer 2010, 117, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Ruff, S.M.; Grignol, V.P.; Contreras, C.M.; Pollock, R.E.; Beane, J.D. Morbidity and Mortality after Surgery for Retroperitoneal Sarcoma. Curr. Oncol. 2022, 30, 492–505. [Google Scholar] [CrossRef]
- Crombé, A.; Roulleau-Dugage, M.; Italiano, A. The diagnosis, classification, and treatment of sarcoma in this era of artificial intelligence and immunotherapy. Cancer Commun. 2022, 42, 1288–1313. [Google Scholar] [CrossRef]
- Zhou, Y.-F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Zhu, X.; Wang, J.; Xu, F.; Lin, Z.; Gong, C.; He, M.; Zhang, L. MRI features and clinical outcomes of unexpected uterine sarcomas in patients who underwent high-intensity focused ultrasound ablation for presumed uterine fibroids. Int. J. Hyperth. 2021, 38, 39–45. [Google Scholar] [CrossRef]
- Krishna, V.; Sammartino, F.; Rezai, A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, P.-H. Magnetic resonance image-guided versus ultrasound-guided high-intensity focused ultrasound in the treatment of breast cancer. Chin. J. Cancer 2013, 32, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.V.; Yarmolenko, P.S.; Celik, H.; Eranki, A.; Partanen, A.; Smitthimedhin, A.; Kim, A.; Oetgen, M.; Santos, D.; Patel, J.; et al. Comparison of Noninvasive High-Intensity Focused Ultrasound with Radiofrequency Ablation of Osteoid Osteoma. J. Pediatr. 2017, 190, 222–228.e1. [Google Scholar] [CrossRef]
- Jones, T.A.; Chin, J.; Mcleod, D.; Barkin, J.; Pantuck, A.; Marks, L.S. High Intensity Focused Ultrasound for Radiorecurrent Prostate Cancer: A North American Clinical Trial. J. Urol. 2018, 199, 133–139. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Cosgrove, G.R.; Lipsman, N.; Lozano, A.M.; Chang, J.W.; Halpern, C.; Ghanouni, P.; Eisenberg, H.; Fishman, P.; Taira, T.; Schwartz, M.L.; et al. Magnetic resonance imaging–guided focused ultrasound thalamotomy for essential tremor: 5-year follow-up results. J. Neurosurg. 2023, 138, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, J.-Y.; Lee, D.-H.; Kim, W.-H.; Hwang, J.H. Current and Future Clinical Applications of High-Intensity Focused Ultrasound (HIFU) for Pancreatic Cancer. Gut Liver 2010, 4, S57–S61. [Google Scholar] [CrossRef]
- Yu, W.; Tang, L.; Lin, F.; Yao, Y.; Shen, Z.; Zhou, X. High-intensity focused ultrasound: Noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg. Oncol. 2015, 24, 9–15. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Zhao, G.-C.; Zheng, C.-X.; Yuan, L.; Yuan, G.-B. Managing spindle cell sarcoma with surgery and high-intensity focused ultrasound: A case report. World J. Clin. Cases 2023, 11, 6551–6557. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, H.; Zhang, L.; Li, K.; Su, H.; Jin, C.; Zhou, K.; Bai, J.; Wu, F.; Wang, Z. Primary Bone Malignancy: Effective Treatment with High-Intensity Focused Ultrasound Ablation. Radiology 2010, 255, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tang, L.; Lin, F.; Jiang, L.; Shen, Z. Significance of HIFU in local unresectable recurrence of soft tissue sarcoma, a single-center, respective, case series in China. Surg. Oncol. 2019, 30, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Mendiratta-Lala, M.; Wiggermann, P.; Pech, M.; Serres-Créixams, X.; White, S.B.; Davis, C.; Ahmed, O.; Parikh, N.D.; Planert, M.; Thormann, M.; et al. The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors. Radiology 2024, 312, e233051. [Google Scholar] [CrossRef]
- McHale, A.P.; Callan, J.F.; Nomikou, N.; Fowley, C.; Callan, J.F. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. In Therapeutic Ultrasound; Springer Science and Business Media LLC: Cham, Switzerland, 2016; Volume 880, pp. 429–450. [Google Scholar]
- Yamaguchi, T.; Kitahara, S.; Kusuda, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Current Landscape of Sonodynamic Therapy for Treating Cancer. Cancers 2021, 13, 6184. [Google Scholar] [CrossRef]
- Su, X.; Wang, P.; Wang, X.; Cao, B.; Li, L.; Liu, Q. Apoptosis of U937 Cells Induced by Hematoporphyrin Monomethyl Ether-Mediated Sonodynamic Action. Cancer Biother. Radiopharm. 2013, 28, 207–217. [Google Scholar] [CrossRef]
- Tinkov, S.; Coester, C.; Serba, S.; Geis, N.A.; Katus, H.A.; Winter, G.; Bekeredjian, R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: In-vivo characterization. J. Control. Release 2010, 148, 368–372. [Google Scholar] [CrossRef]
- Todorova, M.; Agache, V.; Mortazavi, O.; Chen, B.; Karshafian, R.; Hynynen, K.; Man, S.; Kerbel, R.S.; Goertz, D.E. Antitumor effects of combining metronomic chemotherapy with the antivascular action of ultrasound stimulated microbubbles. Int. J. Cancer 2012, 132, 2956–2966. [Google Scholar] [CrossRef]
- Goertz, D.E.; Todorova, M.; Mortazavi, O.; Agache, V.; Chen, B.; Karshafian, R.; Hynynen, K.; Woloschak, G.E. Antitumor Effects of Combining Docetaxel (Taxotere) with the Antivascular Action of Ultrasound Stimulated Microbubbles. PLoS ONE 2012, 7, e52307. [Google Scholar] [CrossRef]
- Gao, Z.; Zheng, J.; Yang, B.; Wang, Z.; Fan, H.; Lv, Y.; Li, H.; Jia, L.; Cao, W. Sonodynamic therapy inhibits angiogenesis and tumor growth in a xenograft mouse model. Cancer Lett. 2013, 335, 93–99. [Google Scholar] [CrossRef]
- Collins, V.G.; Hutton, D.; Hossain-Ibrahim, K.; Joseph, J.; Banerjee, S. The abscopal effects of sonodynamic therapy in cancer. Br. J. Cancer 2024, 132, 409–420. [Google Scholar] [CrossRef]
- Sebeke, L.C.; Gómez, J.D.C.; Heijman, E.; Rademann, P.; Simon, A.C.; Ekdawi, S.; Vlachakis, S.; Toker, D.; Mink, B.L.; Schubert-Quecke, C.; et al. Hyperthermia-induced doxorubicin delivery from thermosensitive liposomes via MR-HIFU in a pig model. J. Control. Release 2022, 343, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Goertz, D.E.; Hynynen, K. Focused Ultrasound Hyperthermia Mediated Drug Delivery Using Thermosensitive Liposomes and Visualized With In Vivo Two-Photon Microscopy. Theranostics 2017, 7, 2718–2731. [Google Scholar] [CrossRef]
- Wang, X.B.; Liu, Q.H.; Wang, P.; Tang, W.; Hao, Q. Study of cell killing effect on S180 by ultrasound activating protoporphyrin IX. Ultrasonics 2008, 48, 135–140. [Google Scholar] [CrossRef]
- Chida, S.; Okada, K.; Suzuki, N.; Komori, C.; Shimada, Y. Infiltration by macrophages and lymphocytes in transplantable mouse sarcoma after irradiation with high-intensity focused ultrasound. Anticancer. Res. 2009, 29, 3877–3882. [Google Scholar] [PubMed]
- Tang, W.; Liu, Q.; Wang, X.; Wang, P.; Zhang, J.; Cao, B. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics 2009, 49, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, P.; Hu, J.; Jia, Y.; Wu, L.; Chen, X.; Liu, Q.; Wang, X. A new sensitizer DVDMS combined with multiple focused ultrasound treatments: An effective antitumor strategy. Sci. Rep. 2015, 5, 17485. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Vaezy, S.; Noble, M.L.; Chi, E.Y.; Walker, C.; Martin, R.W.; Fujimoto, V.Y. Treatment of Uterine Leiomyosarcoma in a Xenograft Nude Mouse Model Using High-Intensity Focused Ultrasound: A Potential Treatment Modality for Recurrent Pelvic Disease. Gynecol. Oncol. 2002, 86, 344–350. [Google Scholar] [CrossRef]
- Wunker, C.; Piorkowska, K.; Keunen, B.; Babichev, Y.; Wong, S.M.; Regenold, M.; Dunne, M.; Nomikos, J.; Siddiqui, M.; Pichardo, S.; et al. Magnetic Resonance-Guided High Intensity Focused Ultrasound Generated Hyperthermia: A Feasible Treatment Method in a Murine Rhabdomyosarcoma Model. J. Vis. Exp. 2023, 13, e64544. [Google Scholar] [CrossRef]
- de Smet, M.M.; Hijnen, N.M.M.; Langereis, S.; Elevelt, A.M.; Heijman, E.; Dubois, L.; Lambin, P.; Grüll, H. Magnetic Resonance Guided High-Intensity Focused Ultrasound Mediated Hyperthermia Improves the Intratumoral Distribution of Temperature-Sensitive Liposomal Doxorubicin. Investig. Radiol. 2013, 48, 395–405. [Google Scholar] [CrossRef]
- Hijnen, N.; Kneepkens, E.; de Smet, M.; Langereis, S.; Heijman, E.; Grüll, H. Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proc. Natl. Acad. Sci. USA 2017, 114, E4802–E4811. [Google Scholar] [CrossRef]
- Seward, M.C.; Daniel, G.B.; Ruth, J.D.; Dervisis, N.; Partanen, A.; Yarmolenko, P.S. Feasibility of targeting canine soft tissue sarcoma with MR-guided high-intensity focused ultrasound. Int. J. Hyperth. 2018, 35, 205–215. [Google Scholar] [CrossRef]
- Carroll, J.; Coutermarsh-Ott, S.; Klahn, S.L.; Tuohy, J.; Barry, S.L.; Allen, I.C.; Hay, A.N.; Ruth, J.; Dervisis, N. High intensity focused ultrasound for the treatment of solid tumors: A pilot study in canine cancer patients. Int. J. Hyperth. 2022, 39, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Evripidou, N.; Panayiotou, S.; Spanoudes, K.; Damianou, C. Treatment of canine and feline sarcoma using MR-guided focused ultrasound system. J. Ultrasound 2022, 25, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Ruger, L.; Yang, E.; Gannon, J.; Sheppard, H.; Coutermarsh-Ott, S.; Ziemlewicz, T.J.; Dervisis, N.; Allen, I.C.; Daniel, G.B.; Tuohy, J.; et al. Mechanical High-Intensity Focused Ultrasound (Histotripsy) in Dogs With Spontaneously Occurring Soft Tissue Sarcomas. IEEE Trans. Biomed. Eng. 2022, 70, 768–779. [Google Scholar] [CrossRef]
- Ruger, L.; Yang, E.; Coutermarsh-Ott, S.; Vickers, E.; Gannon, J.; Nightengale, M.; Hsueh, A.; Ciepluch, B.; Dervisis, N.; Vlaisavljevich, E.; et al. Histotripsy ablation for the treatment of feline injection site sarcomas: A first-in-cat in vivo feasibility study. Int. J. Hyperth. 2023, 40, 2210272. [Google Scholar] [CrossRef]
- Ruger, L.N.; Hay, A.N.; Gannon, J.M.; Sheppard, H.O.; Coutermarsh-Ott, S.L.; Daniel, G.B.; Kierski, K.R.; Ciepluch, B.J.; Vlaisavljevich, E.; Tuohy, J.L. Histotripsy Ablation of Spontaneously Occurring Canine Bone Tumors. IEEE Trans. Biomed. Eng. 2022, 70, 331–342. [Google Scholar] [CrossRef]
- Ruger, L.N.; Hay, A.N.; Vickers, E.R.; Coutermarsh-Ott, S.L.; Gannon, J.M.; Covell, H.S.; Daniel, G.B.; Laeseke, P.F.; Ziemlewicz, T.J.; Kierski, K.R.; et al. Characterizing the Ablative Effects of Histotripsy for Osteosarcoma: In Vivo Study in Dogs. Cancers 2023, 15, 741. [Google Scholar] [CrossRef]
- Alfaro, G.; Lomeli, C.; Ocadiz, R.; Ortega, V.; Barrera, R.; Ramirez, M.; Nava, G. Immunologic and genetic characterization of S180, a cell line of murine origin capable of growing in different inbred strains of mice. Vet. Immunol. Immunopathol. 1992, 30, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T.; Rincic, M. Cytogenomic Characterization of Murine Cell Line Sarcoma 180 = S-180. Int. J. Mol. Sci. 2025, 26, 1127. [Google Scholar] [CrossRef]
- Fukunishi, H.; Funaki, K.; Ikuma, K.; Kaji, Y.; Sugimura, K.; Kitazawa, R.; Kitazawa, S. Unsuspected uterine leiomyosarcoma: Magnetic resonance imaging findings before and after focused ultrasound surgery; official journal of the International Gynecological Cancer Society: International journal of gynecological cancer, 2007; Volume 17, pp. 724–728. [Google Scholar] [CrossRef]
- Samuel, A.; Fennessy, F.M.; Tempany, C.M.; Stewart, E.A. Avoiding treatment of leiomyosarcomas: The role of magnetic resonance in focused ultrasound surgery. Fertility and sterility 2008, 90(3), 850.e9–850.e12. [Google Scholar] [CrossRef]
- Li, C.; Wu, P.; Liang, Z.; Fan, W.; Huang, J.; Zhang, F. Osteosarcoma: Limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound. Cancer Biol. Ther. 2009, 8, 1102–1108. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Fan, W.; Huang, J.; Zhang, F.; Wu, P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 2010, 116, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Marina, N.; Bernstein, M. High-intensity focused ultrasound (HIFU) is not indicated for treatment of primary bone sarcomas. Cancer 2011, 117(12), 2822–2823. [Google Scholar] [CrossRef]
- Hu, X.; Cai, H.; Zhou, M.; He, H.; Tian, W.; Hu, Y.; Chen, L.; Deng, Y. New clinical application of high-intensity focused ultrasound: Local control of synovial sarcoma. World J. Surg. Oncol. 2013, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Staruch, R.M.; Koral, K.; Xie, X.; Chopra, R.; Laetsch, T.W. Pediatric Sarcomas Are Targetable by MR-Guided High Intensity Focused Ultrasound (MR-HIFU): Anatomical Distribution and Radiological Characteristics. Pediatr. Blood Cancer 2016, 63, 1753–1760. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, H.H.; Kang, S.Y.; Park, E.J.; Park, D.H.; Son, K.; Han, J.K. Portable ultrasound-guided high-intensity focused ultrasound with functions for safe and rapid ablation: Prospective clinical trial for uterine fibroids-short-term and long-term results. European radiology 2020, 30(3), 1554–1563. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Zhong, X.; Shen, H.; Yuan, Y. High-intensity focused ultrasound treatment as an alternative regimen for myxofibrosarcoma. Dermatol. Ther. 2021, 34, e14816. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized Trial of Hyperthermia and Radiation for Superficial Tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.; Foca, F.; Oboldi, D.; Diano, D.; Bazzocchi, A.; Fabbri, L.; Mercatali, L.; Vanni, S.; Maltoni, M.; Bianchini, D.; et al. 3-T magnetic resonance-guided high-intensity focused ultrasound (3 T-MR-HIFU) for the treatment of pain from bone metastases of solid tumors. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Simões Corrêa Galendi, J.; Yeo, S.Y.; Grüll, H.; Bratke, G.; Akuamoa-Boateng, D.; Baues, C.; Bos, C.; Verkooijen, H.M.; Shukri, A.; Stock, S.; et al. Early economic modeling of magnetic resonance image-guided high intensity focused ultrasound compared to radiotherapy for pain palliation of bone metastases. Front. Oncol. 2022, 12, 987546. [Google Scholar] [CrossRef] [PubMed]
- Slotman, D.J.; Bartels, M.M.; Ferrer, C.J.; Bos, C.; Bartels, L.W.; Boomsma, M.F.; Phernambucq, E.C.; Nijholt, I.M.; Morganti, A.G.; Siepe, G.; et al. Focused Ultrasound and RadioTHERapy for non-invasive palliative pain treatment in patients with bone metastasis: A study protocol for the three armed randomized controlled FURTHER trial. Trials 2022, 23, 1061. [Google Scholar] [CrossRef] [PubMed]
| Reference | Sarcoma Type | Animal Model (n) | Focused Ultrasound Mechanism | FUS Parameters | Findings |
|---|---|---|---|---|---|
| [29] | S180 | N/A | SDT | 30 s, 2.2 MHz, 3 W/cm2 | Protoporphyrin IX and FUS increased free fatty acid concentration and decreased antioxidant enzyme activity. |
| [30] | S180 | Mouse (n = 25) | Thermal ablation | 10 s, 3 MHz, 10 W | Using HIFU for partial ablation had an immunological antitumor effect but was insufficient to completely eliminate tumor. |
| [31] | S180 | N/A | SDT | 3 min,1.75 MHz,1.4 ± 0.07 W/cm2 | Apoptosis mechanisms of FUS and of hematoporphyrin differ, which could explain the synergistic effect as treatment. |
| [32] | S180 | N/A | SDT | 30/60/90 s, 1.1 MHz, 2 W | Sinoporphyrin sodium and FUS resulted in tumor tissue destruction, cancer cell apoptosis, inhibited angiogenesis, and suppressed cancer cell proliferation. |
| [33] | Uterine leiomyosarcoma | Mouse (n = 65) | Thermal ablation | 2.0 MHz, 2000 W/cm2 | HIFU treatment completely reduced tumor volume in all mice. No metastasis was observed after 3 months. |
| [34] | Rhabdomyosarcoma | Mouse (n = 65) | Hyperthermia (HT) | 10/20 min, 2.5 MHz | HT increased the intratumoral concentration of doxorubicin. Heating the tumor for 20 min has the most consistent delivery. |
| [35] | Rhabdomyosarcoma | Rat (n = 12) | Hyperthermia (HT) | 20 s, 1.44 MHz, 5–10 W | HT allowed for more homogenous and widespread delivery of radiolabeled liposomes across tumor |
| [36] | Rhabdomyosarcoma | Rat (n = 113) | Hyperthermia (HT) and ablation | Hyperthermia: 15 min, 1.44 MHz, 10–15 W Ablation: >240 cumulative mins, 1.44 MHz, 35 W | Hyperthermia ensured homogenous drug delivery across tumor when compared to sham and ablation-only groups. The combination of hyperthermia and ablation led to the highest concentration of homogenous delivery. |
| [37] | STS | Canine (n = 53) | Thermal ablation | N/A | Targetability of most STS in dogs confirmed. Truncal and axillary tumors had the highest targetability, while head and spine had lower targetability. |
| [38] | STS, mast cell tumor, osteosarcoma, thyroid carcinoma | Canine (n = 20) | Thermal ablation | 40–50 s, 5–10 MHz | Thermal ablation increases immune activity in tumor after treatment, particularly in T-cell activation. Thermal damage noted after treatment. |
| [39] | STS | Canine (n = 6) Feline (n = 4) | Thermal ablation | 10/60 s, 2.6 MHz, 50/75 W | Thermal ablation caused coagulative necrosis, but some cancer cells remained intact. FUS should be used with radiotherapy or chemotherapy to eliminate remaining cells. |
| [40] | STS | Canine (n = 10) | Histotripsy | 500 kHz, PNP: 22.60 ± 7.21 MPa | Histotripsy was well tolerated and feasible in canine STS. Pro-inflammatory changes were noted in the TME. |
| [41] | STS | Feline (n = 3) | Histotripsy | 1 MHz, PNP: 29.59 ± 6.08 MPa | Histotripsy was well tolerated and is feasible in feline STS. Pro-inflammatory changes were noted in the TME. |
| [42] | Osteosarcoma | Canine (n = 5) | Histotripsy | 500 kHz, PNP: 29.59 ± 8.17 MPa | Histotripsy is safe and effective for canine osteosarcoma. |
| [43] | Osteosacoma | Canine (n = 10) | Histotripsy | 500 kHz, PNP: 26.57 ± 3.82 MPa | Histotripsy ablation is safe and feasible in lytic or prolieferative canine OS and chondrosarcoma (n = 1), with or without soft tissue extension. More extensive tissue destruction was observed after histotripsy of 1000 PPP compared to 500 PPP. Radiographic changes within the tumor ablation zone were noticeable on post-histotripsy CT scan. |
| Reference | Title | Sarcoma Type | Focused Ultrasound Mechanism | N | Findings |
|---|---|---|---|---|---|
| [46] | Unsuspected uterine leiomyosarcoma: magnetic resonance imaging findings before and after focused ultrasound surgery | Uterine leiomyosarcoma | Thermal ablation | 1 | Six months after FUS treatment, tumor decreased in size. However, a circular area of the tumor showed increased intensity on T2-weight imaging. |
| [47] | Avoiding treatment of leiomyosarcomas: the role of magnetic resonance in focused ultrasound surgery | Uterine leiomyosarcoma | N/A | 1 | 47-year-old female received expedited diagnosis because of MRI screening that was performed for MRgFUS. |
| [48] | Osteosarcoma: limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound | Osteosarcoma | Thermal ablation | 7 | Complete response in 3 patients, partial response in 3 patients. One patient had pulmonary metastasis 5 months post- HIFU. Median survival: 68 months Five-year survival rate: 71.4% |
| [49] | Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound | Osteosarcoma | Thermal ablation | 25 | 100% of patients had significant pain relief 87.5% had complete pain relief Primary bone tumors group: Six (46.2%) complete response Five (38.4%) partial response One moderate response One progressive disease 84.6% total response rate Metastatic bone tumors group: Five (41.7%) complete response Four (33.3%) partial response One moderate response One stable disease One progressive disease 75.0% total response rate |
| [15] | Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation | Typical osteosarcoma, periosteal osteosarcoma, periosteal sarcoma, chondrosarcoma, Ewing sarcoma | Thermal ablation | Typical Osteosarcoma: 6 Periosteal Osteosarcoma: 1 Periosteal Sarcoma: 1 Chondrosarcoma: 10 Ewing Sarcoma: 3 | Sixty-nine patients had complete ablation of their tumors. Eleven patients had >50% tumor ablation. Overall survival rates at 1, 2, 3, 4, and 5 yrs: 89.8%, 72.3%, 60.5%, 50.5%, and 50.5% Patients with stage IIb disease survival rates: 93.3%, 82.4%, 75.0%, 63.7%, and 63.7% Patients with stage III disease: 79.2%, 42.2%, 21.1%, 15.8%, and 15.8% Only five (7%) of the 69 patients who underwent complete ablation had local cancer recurrence. Forty adverse events were recorded. |
| [50] | High-intensity focused ultrasound (HIFU) is not indicated for treatment of primary bone sarcomas. | Osteosarcoma | Thermal ablation | N/A | Commentary on [47] paper: Authors argue that surgical remission is the most important prognostic factor for osteosarcomas, so surgery should not have been withheld. HIFU should not be advertised as a safe alternative to surgery unless it has the same rates of local control as surgery. |
| [51] | New clinical application of high-intensity focused ultrasound: local control of synovial sarcoma. | Spindle cell sarcoma | Thermal ablation | 1 | 51-year-old male patient with recurrent synovial sarcoma (treated with lumpectomy and multiple cycles of chemotherapy) of left chest wall underwent 5 cycles of HIFU treatment, which completely ablated tumor. No adverse events reported. |
| [13] | High-intensity focused ultrasound: noninvasive treatment for local unresectable recurrence of osteosarcoma | Osteosarcoma | Thermal ablation | 27 | Two (7.4%) complete response Twelve (44.4%) partial response Nine (33.3%) stable disease Four (14.8%) progression Response rate: 51.8% Local disease control rate: 85.2%. Patients without pulmonary metastasis had better local disease control rate, longer local disease progression-free time, progression-free time, and overall survival time than patients with pulmonary metastasis. |
| [52] | Pediatric Sarcomas Are Targetable by MR-Guided High Intensity Focused Ultrasound (MR-HIFU): Anatomical Distribution and Radiological Characteristics | Pediatric sarcomas | Thermal ablation | 121 | Primary lesions: 64% targetable by MR-HIFU Majority of targetable tumors were osteosarcomas (31%) and Ewing sarcoma (21%) Metastatic tumors: 14% targetable Relapsed disease: 35% at least one targetable tumor Most metastases at diagnosis (79%) and lesions in recurrent disease (66%) were in the chest and not targetable 2/2 difficulties in ultrasound transmission and respiratory motion. |
| [16] | Significance of HIFU in local unresectable recurrence of soft tissue sarcoma, a single-center, respective, case series in China | Recurrent local, unresectable STS | Thermal ablation | Lipoblastoma: 8 Undifferentiated pleomorphic sarcoma: 7 Fibrosarcoma: 6 Chondrosarcoma: 4 Synovial sarcoma: 3 Leiomyosarcoma: 3 Aggressive fibromatosis: 2 Alveolar rhabdo- myosarcoma: 1 Clear cell sarcoma: 1 Primitive neuroectodermal tumor: 1 | Zero complete response 47.3% partial response 33.3% had stable disease 19.4% had disease progression Twelve months after HIFU treatment: 38.9% partial response, 16.7% stable disease, 44.4% progression. Median LPFS, PFS, and OS were 13 months, 10 months, and 20 months, respectively. Twenty-seven patients had disease progression after 12-months, 16 of these patients had metastasis prior to secondary relapse of local recurrence, 9 had metastases after local secondary relapse, and 2 had simultaneous metastases and local secondary relapse. 33 patients reported pain prior to receiving HIFU; 9 achieved complete remission of pain, 16 achieved partial remission, and 8 reported no improvement in pain. |
| [53] | Portable ultrasound-guided high-intensity focused ultrasound with functions for safe and rapid ablation: prospective clinical trial for uterine fibroids-short-term and long-term results | Uterine leiomyoma | Thermal ablation | 59 | At 1-, 3-, and 5-months, fibroid volume shrinkage was 17.3%, 33.3%, and 45.1% At 3 month follow-up, 30.8% of patients reported >50% improvement in menorrhagia symptoms. Significant QOL improvement reported without changes in physical condition. Twenty-six patients satisfied with HIFU treatment and patient satisfaction was negatively correlated with residual tumor volume. Five patients underwent surgical myomectomy or hysterectomy, and one patient underwent hormonal intrauterine de-vice insertion due to recurrence of symptoms. |
| [5] | MRI features and clinical outcomes of unexpected uterine sarcomas in patients who underwent high-intensity focused ultrasound ablation for presumed uterine fibroids | Uterine sarcoma | Thermal ablation | 17 | Eleven patients with presumed uterine fibroids were diagnosed with uterine sarcoma prior to HIFU treatment. Six patients with presumed uterine fibroids were diagnosed with uterine sarcoma after treatment. There were no significant differences between histological type, margin of lesions, or enhancement of lesions on MRI that could explain the cause of misdiagnosis. |
| [54] | High-intensity focused ultrasound treatment as an alternative regimen for myxofibrosarcoma | Recurrent myxofibrosarcoma | Thermal ablation | 1 | After 5 cycles of low-power HIFU: Complete ablation of the tumor occurred. No tumor relapse was noted on serial MRIs during a 30 month follow-up period. No complications from HIFU treatment, possibly due to multiple, lower power treatment. |
| [14] | Managing spindle cell sarcoma with surgery and high-intensity focused ultrasound: A case report | Spindle cell sarcoma | Thermal ablation | 1 | After 5 cycles of HIFU ablation: Complete ablation of the tumor occurred. No complications from HIFU treatment reported. |
| Clinical Trial Name | clinicaltrials.gov ID | Status | Study Type | Study Location | Patient Population | Estimated Completion Date | Estimated Enrollment | Tumor Type(s) | Treatment Type | Study Overview |
|---|---|---|---|---|---|---|---|---|---|---|
| Focused Ultrasound to Promote Immune Responses for Undifferentiated Pleomorphic Sarcoma | NCT04123535 | Recruiting | Interventional | San Francisco, USA | Adults | July 2025 | 20 | Newly diagnosed or metastatic undifferentiated pleomorphic sarcomas | MRgFUS | Primary outcome: Evaluate rate and severity of adverse events from MRgFUS of undifferentiated pleomorphic sarcoma. Secondary outcomes: Measure immune responses to MRgFUS via serological analysis and multiplex immunohistochemistry assays of tumor specimens. Additionally, the immune responses to MRgFUS will be compared to the immune responses prior to receiving ultrasound in the same patients or to a comparison group of archived samples that did not undergo ultrasound. |
| HIFU Ablation of Soft Tissue Sarcoma | NCT05111964 | Recruiting | Interventional | Oxford, UK | Adults | October 2025 | 12–16 with a minimum of 10 | Soft tissue sarcomas and unresectable small symptomatic intra-abdominal desmoid tumors | High Intensity Focused Ultrasound (HIFU) ablation | Primary outcome: Measure safety and feasibility of HIFU ablation of soft tissue sarcomas and small symptomatic desmoid tumors. Secondary outcome: Radiological response using MRI and 18F-FDG PET will also be used to measure efficacy. Post-resection histology will also be completed to allow for histopathological correlation. Tertiary outcome: Exploration of immune response during HIFU ablation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchimanchi, N.; Sul, N.; Gajula, S.; Mercante, M.; Tocco, E.; Mayhew, M.M.; Dengel, L.T.; Cavalcante, L.; Hadley, L.; Witt, R.G. Focused Ultrasound for Sarcomas: A Narrative Review. Curr. Oncol. 2025, 32, 452. https://doi.org/10.3390/curroncol32080452
Kuchimanchi N, Sul N, Gajula S, Mercante M, Tocco E, Mayhew MM, Dengel LT, Cavalcante L, Hadley L, Witt RG. Focused Ultrasound for Sarcomas: A Narrative Review. Current Oncology. 2025; 32(8):452. https://doi.org/10.3390/curroncol32080452
Chicago/Turabian StyleKuchimanchi, Nidhi, Nicolle Sul, Sai Gajula, Margaret Mercante, Emily Tocco, Mackenzie M. Mayhew, Lynn T. Dengel, Ludimila Cavalcante, Lauren Hadley, and Russell Gardner Witt. 2025. "Focused Ultrasound for Sarcomas: A Narrative Review" Current Oncology 32, no. 8: 452. https://doi.org/10.3390/curroncol32080452
APA StyleKuchimanchi, N., Sul, N., Gajula, S., Mercante, M., Tocco, E., Mayhew, M. M., Dengel, L. T., Cavalcante, L., Hadley, L., & Witt, R. G. (2025). Focused Ultrasound for Sarcomas: A Narrative Review. Current Oncology, 32(8), 452. https://doi.org/10.3390/curroncol32080452







