How Musculoskeletal Tumor Management Changed During the COVID-19 Pandemic: Data from a Nationwide Questionnaire Survey of Hospitals Specializing in Musculoskeletal Tumors in Japan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Presentation and Statistics
3. Results
3.1. Demographic Data
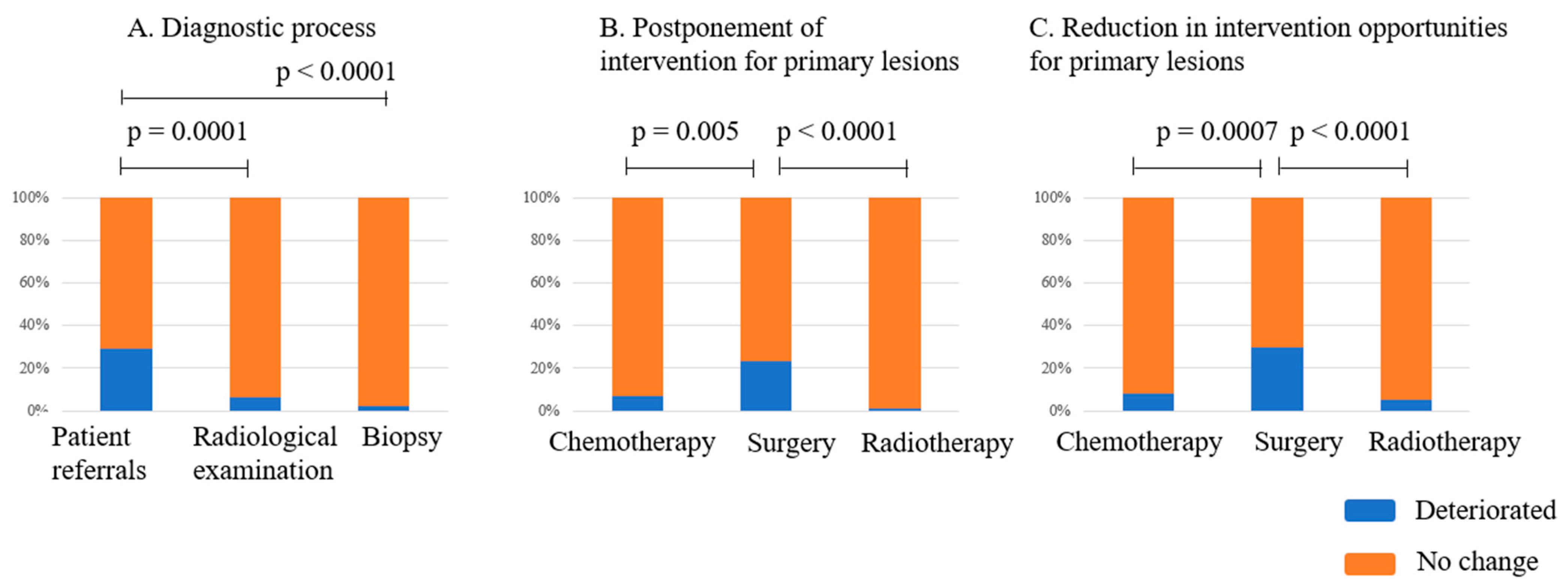
3.2. Impact on the Diagnostic Process
3.3. Impact on the Treatment Quality of Primary Malignant Musculoskeletal Tumors
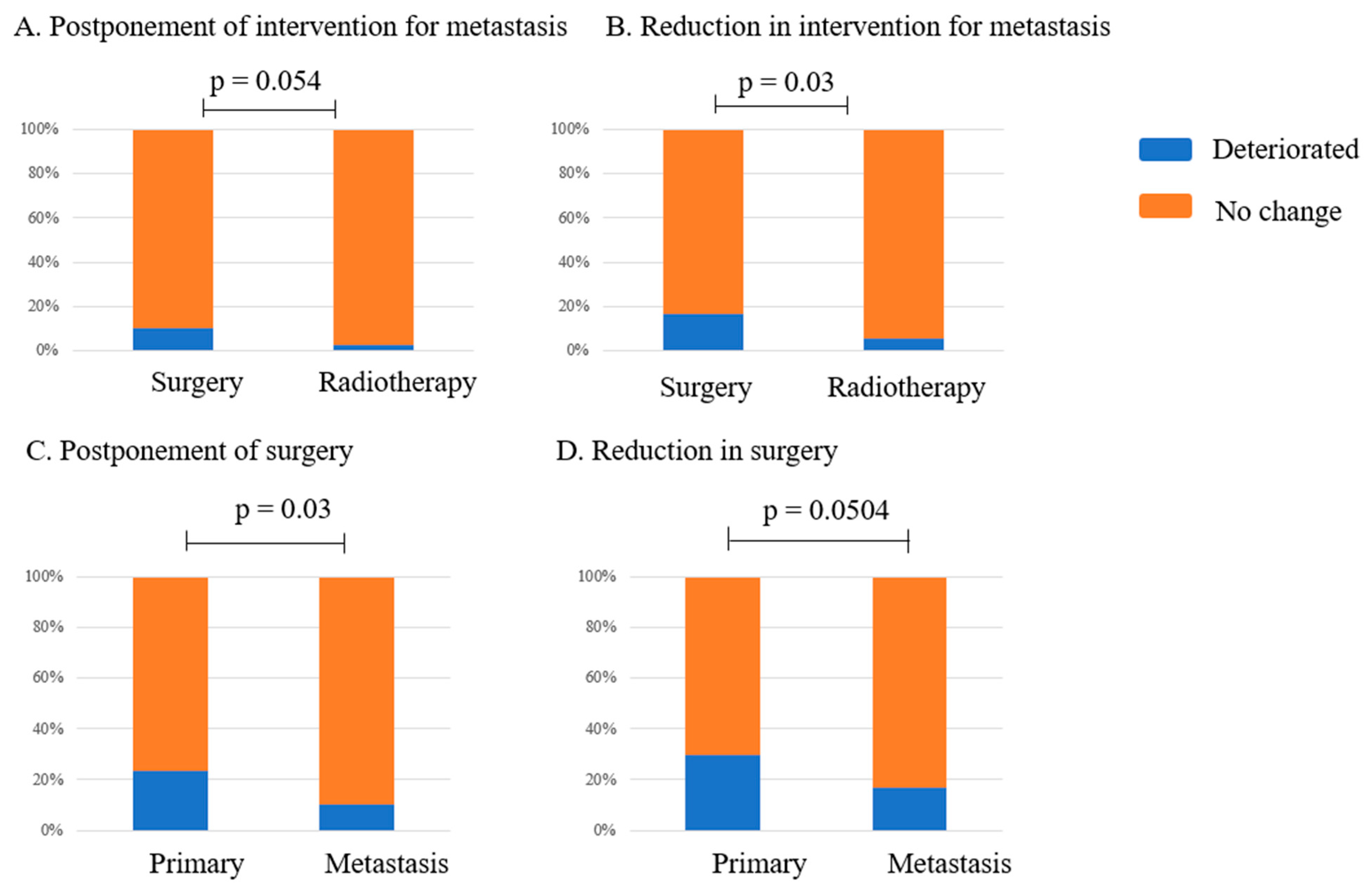
3.4. Changes in the Management Status of Metastatic Bone Tumors
3.5. Change in the Quality of Palliative Care
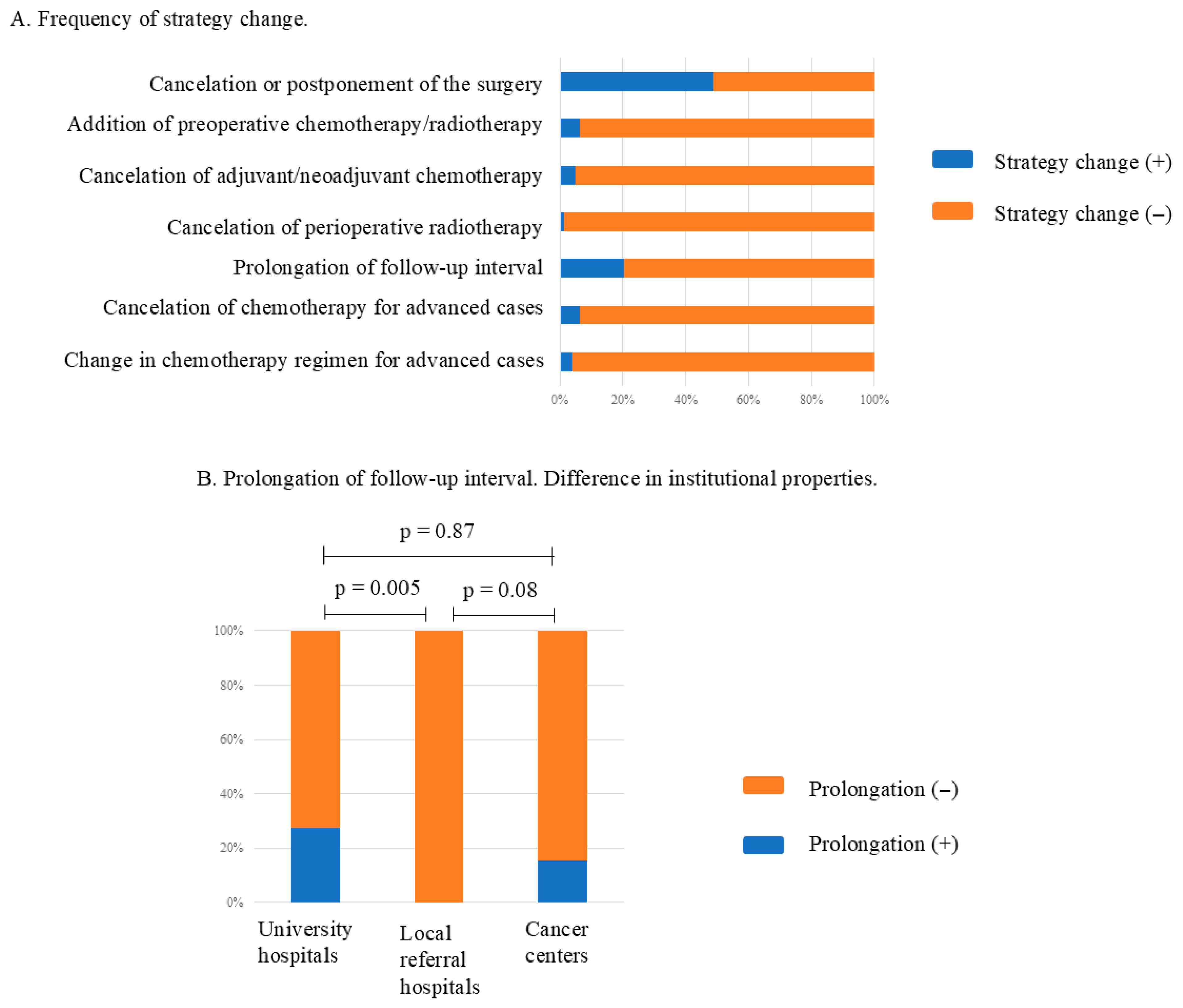
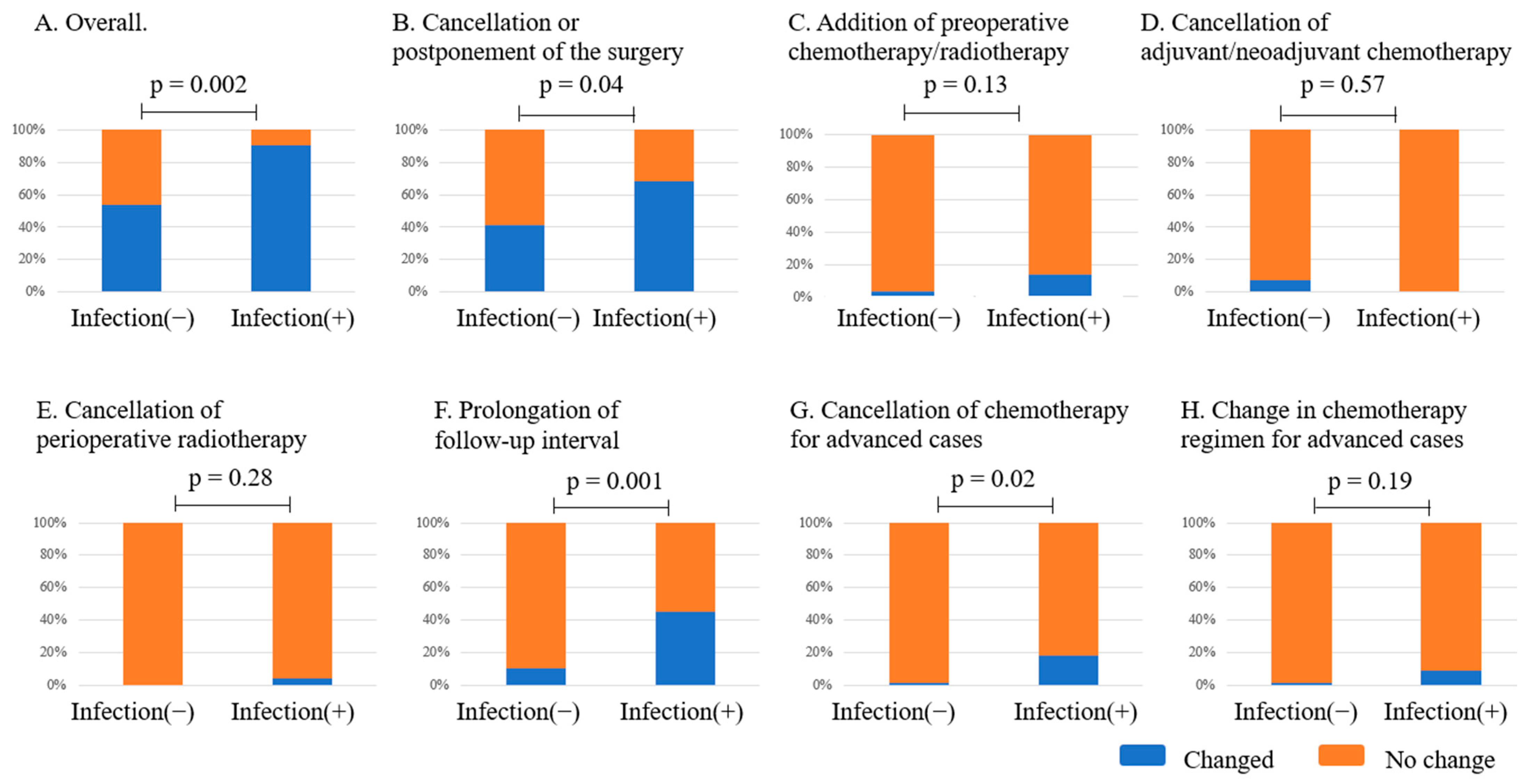
3.6. Impact on Treatment Strategies
4. Discussion
4.1. Institutional Function
4.1.1. Outpatients’ Clinic and Diagnosis
4.1.2. Surgical Intervention
4.1.3. Chemotherapy and Radiotherapy
4.1.4. Terminal Care
4.2. The Effect of COVID-19-Related Events
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: An international, prospective, cohort study. Lancet Oncol. 2021, 22, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.J.; Goodman, H.J.; Kenan, S.; Kenan, S. Did COVID-19 related delays in surgical management lead to patient morbidity in the orthopaedic oncological population? Bone Jt. Open 2021, 2, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Putro, Y.A.P.; Magetsari, R.; Mahyudin, F.; Basuki, M.H.; Saraswati, P.A.; Huwaidi, A.F. Impact of the COVID-19 on the surgical management of bone and soft tissue sarcoma: A systematic review. J. Orthop. 2023, 38, 1–6. [Google Scholar] [CrossRef]
- Carrillo-García, J.; Lacerenza, S.; Hindi, N.; García, I.C.; Marquina, G.; Cano Cano, J.M.; Trufero, J.M.; Tripero, A.R.S.; García, T.L.; Rioboo, M.J.C.; et al. Delays in diagnosis and surgery of sarcoma patients during the COVID-19 outbreak in Spain. Ther. Adv. Med. Oncol. 2024, 16, 17588359231220611. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nagata, K.; Adejuyigbe, B.; Shinozaki, T.; Yamada, K. Trends in inpatient orthopedic surgery during the COVID-19 pandemic in Japan: A nationwide data study. BMC Musculoskelet. Disord. 2024, 25, 503. [Google Scholar] [CrossRef]
- Nogami, Y.; Komatsu, H.; Makabe, T.; Hasegawa, Y.; Yokoyama, Y.; Kawana, K.; Okamoto, A.; Mikami, M.; Katabuchi, H.; The COVID-19 Task Force of the Japan Society of Gynecologic Oncology. Impact of COVID-19 on gynecologic cancer treatment in Japan: A nationwide survey by the Japan Society of Gynecologic Oncology (JSGO). J. Gynecol. Oncol. 2022, 33, e8. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Konishi, H.; Sato, Y.; Igarashi, M.; Yanagibashi, T.; Konno, R.; Saya, H.; Doki, Y.; Kakizoe, T. Impact of coronavirus disease 2019 on the number of newly diagnosed cancer patients and examinations and surgeries performed for cancer in Japan: A nationwide study. BMC Cancer 2022, 22, 1303. [Google Scholar] [CrossRef]
- Kurokawa, T.; Ozaki, A.; Bhandari, D.; Kotera, Y.; Sawano, T.; Kanemoto, Y.; Kanzaki, N.; Ejiri, T.; Saito, H.; Kaneda, Y.; et al. Association between COVID-19 incidence and postponement or cancellation of elective surgeries in Japan until September 2020: A cross-sectional, web-based survey. BMJ Open 2022, 12, e059886. [Google Scholar] [CrossRef]
- Callegaro, D.; Raut, C.P.; Keung, E.Z.; Kim, T.; Le Pechoux, C.; Martin Broto, J.; Gronchi, A.; Swallow, C.; Gladdy, R. Strategies for care of patients with gastrointestinal stromal tumor or soft tissue sarcoma during COVID-19 pandemic: A guide for surgical oncologists. J. Surg. Oncol. 2021, 123, 12–23. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Hindi, N.; Aguiar, S., Jr.; Badilla-González, R.; Castro-Oliden, V.; Chacón, M.; Correa-Generoso, R.; Álava, E.; Donati, D.M.; Eriksson, M.; et al. Sarcoma European and Latin American Network (SELNET) Recommendations on prioritization in sarcoma care during the COVID-19 pandemic. Oncologist 2020, 25, e1562–e1573. [Google Scholar] [CrossRef]
- Olshinka, N.; Mottard, S. Musculoskeletal oncology: Patient triage and management during the COVID-19 pandemic. Curr. Oncol. 2020, 27, e512–e515. [Google Scholar] [CrossRef]
- Tiwari, V.; Sharma, P.K.; Sampath Kumar, V.; Poudel, R.R.; Meena, S.; Banjara, R. Changes in the management of malignant bone tumors in the COVID-19 pandemic in developing countries. Cureus 2022, 14, e25245. [Google Scholar] [CrossRef]
- Bunzli, S.; O’Brien, P.; Aston, W.; Ayerza, M.A.; Chan, L.; Cherix, S.; de las Heras, J.; Donati, D.; Eyesan, U.; Fabbri, N.; et al. Life or limb: An international qualitative study on decision making in sarcoma surgery during the COVID-19 pandemic. BMJ Open 2021, 11, e047175. [Google Scholar] [CrossRef]
- Ryu, J.H.; Rahman, J.; Deo, S.; Flint, M. Effects of time to treatment initiation on outcomes for soft tissue sarcomas. J. Surg. Oncol. 2023, 127, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shinozaki, T.; Ito, J.; Nakajima, S.; Nakagawa, K.; Furuya, T.; Wada, K.; Kobayashi, N.; Shiba, N.; Kajino, Y.; et al. The influence of COVID-19 epidemic on the number of orthopaedic surgeries in Japan. J. Orthop. Sci. 2024, 29, 1319–1328. [Google Scholar] [CrossRef]
- Ban, Y.; Hoshi, M.; Oebisu, N.; Shimatani, A.; Takada, N.; Iwai, T.; Nakamura, H. Impact of the COVID-19 pandemic on bone and soft tissue tumor treatment: A single-institution study. PLoS ONE 2023, 18, e0283835. [Google Scholar] [CrossRef] [PubMed]
- Tsagkaris, C.; Trygonis, N.; Spyrou, V.; Koulouris, A. Telemedicine in care of sarcoma patients beyond the COVID-19 pandemic: Challenges and opportunities. Cancers 2023, 15, 3700. [Google Scholar] [CrossRef]
- Prajapati, A.; Gupta, S.; Nayak, P.; Gulia, A.; Puri, A. The effect of COVID-19: Adopted changes and their impact on management of musculoskeletal oncology care at a tertiary referral centre. J. Clin. Orthop. Trauma 2021, 23, 101651. [Google Scholar] [CrossRef]
- Gulia, A.; Tiwari, A.; Arora, R.S.; Gupta, S.; Raja, A. Sarcoma care practice in India during COVID pandemic: A nationwide survey. Indian J. Orthop. 2020, 54, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.L.; Harahap, W.A.; Aryandono, T. Perspectives on how to navigate cancer surgery in the breast, head and neck, skin, and soft tissue tumor in limited-resource countries during COVID-19 pandemic. Int. J. Surg. 2020, 79, 206–212. [Google Scholar] [CrossRef]
- Shibata, H.; Kato, S.; Sekine, I.; Abe, K.; Araki, N.; Iguchi, H.; Izumi, T.; Inaba, Y.; Osaka, I.; Kato, S.; et al. Diagnosis and treatment of bone metastasis: Comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open 2016, 1, e000037. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowicz, D.; Kotrych, D.; Starszak, K.; Prowans, P.; Zacha, S.; Kami’nski, A.; Brodecki, A.; Kotrych, K. Delay in Diagnosis and Treatment of Bone Sarcoma—Systematic Review. Cancers 2025, 17, 981. [Google Scholar] [CrossRef] [PubMed]
| Q1 | What is the Category of Your Hospital? |
| Q2 | Did your hospital experience COVID-19 infections in patients undergoing treatment for bone/soft tissue tumors? If so, what was the status of patients’ treatment processes? |
| Q3 | Did an outbreak cluster of COVID-19 occur in your hospital? |
| Q4 | Did your department experience COVID-19 infections in the musculoskeletal team staff? |
| Q5 | Did patient referrals change during the COVID-19 pandemic? |
| Q6 | Did the hospital restrict the performance of radiological examinations (CT, MRI) during the COVID-19 pandemic? |
| Q7 | Did the interval between the first visit and the performance of a biopsy change? |
| Q8 | Did the performance of the following treatment process for primary malignant/intermediate bone and soft tissue tumors change during the COVID-19 pandemic? |
| |
| |
| |
| |
| |
| |
| Q9 | Did the performance of the following treatment process for metastatic bone tumors change during the COVID-19 pandemic? |
| |
| |
| |
| |
| Q10 | Did the performance of palliative care change during the COVID-19 pandemic? |
| Q11 | Was there any occasion to change the following aspects of the treatment strategy during the COVID-19 pandemic? |
| |
| |
| |
| |
| |
| |
|
| n | % | ||
|---|---|---|---|
| Q5 | Patient referrals | ||
| No change | 50 | 64.1 | |
| Decrease | 23 | 29.5 | |
| Increase | 5 | 6.4 | |
| Q6 | Institutional restrictions on radiological examinations | ||
| No | 73 | 93.6 | |
| Yes | 5 | 6.4 | |
| Q7 | The interval between the first visit and the biopsy | ||
| No change | 76 | 97.4 | |
| Prolonged | 2 | 2.6 |
| Q8 | n | % | |
|---|---|---|---|
| A | The interval between the decision to apply and the day of chemotherapy | ||
| No change | 66 | 84.6 | |
| Prolonged | 5 | 6.4 | |
| Not performed in the institution | 7 | 8.9 | |
| B | The application frequency of chemotherapy | ||
| No change | 62 | 79.5 | |
| Decreased | 6 | 7.7 | |
| Increased | 3 | 3.8 | |
| Not performed at the hospital | 7 | 8.9 | |
| C | The interval between the decision to apply and the day of surgery | ||
| No change | 53 | 67.9 | |
| Decreased | 6 | 7.7 | |
| Prolonged | 18 | 23.1 | |
| Not performed at the hospital | 1 | 1.3 | |
| D | The application frequency of surgery | ||
| No change | 50 | 64.1 | |
| Decreased | 23 | 29.5 | |
| Increased | 4 | 5.1 | |
| Not performed at the hospital | 1 | 1.3 | |
| E | The interval between the decision to apply and the day of radiotherapy | ||
| No change | 69 | 88.5 | |
| Decreased | 3 | 3.8 | |
| Prolonged | 1 | 1.2 | |
| Not performed in the institution | 5 | 6.4 | |
| F | The application frequency of radiotherapy | ||
| No change | 67 | 85.9 | |
| Decreased | 4 | 5.1 | |
| Increased | 2 | 2.5 | |
| Not performed at the hospital | 5 | 6.4 |
| Q9 | n | % | |
|---|---|---|---|
| A | The interval between the decision to apply and the day of surgery | ||
| No change | 68 | 87.1 | |
| Decreased | 2 | 2.6 | |
| Prolonged | 8 | 10.3 | |
| B | The application frequency of surgery | ||
| No change | 60 | 77.0 | |
| Decreased | 13 | 16.7 | |
| Increased | 5 | 6.4 | |
| C | The interval between the decision to apply and the day of radiotherapy | ||
| No change | 68 | 87.1 | |
| Decreased | 3 | 3.8 | |
| Prolonged | 2 | 2.6 | |
| Not performed at the hospital | 5 | 6.4 | |
| D | The application frequency of radiotherapy | ||
| No change | 68 | 87.1 | |
| Decreased | 4 | 5.1 | |
| Increased | 1 | 1.3 | |
| Not performed at the hospital | 5 | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morii, T.; Iwata, S.; Yamaga, K.; Okamoto, M.; Ando, K.; Tanaka, T.; Nishida, J. How Musculoskeletal Tumor Management Changed During the COVID-19 Pandemic: Data from a Nationwide Questionnaire Survey of Hospitals Specializing in Musculoskeletal Tumors in Japan. Curr. Oncol. 2025, 32, 453. https://doi.org/10.3390/curroncol32080453
Morii T, Iwata S, Yamaga K, Okamoto M, Ando K, Tanaka T, Nishida J. How Musculoskeletal Tumor Management Changed During the COVID-19 Pandemic: Data from a Nationwide Questionnaire Survey of Hospitals Specializing in Musculoskeletal Tumors in Japan. Current Oncology. 2025; 32(8):453. https://doi.org/10.3390/curroncol32080453
Chicago/Turabian StyleMorii, Takeshi, Shintaro Iwata, Kensaku Yamaga, Masanori Okamoto, Kosei Ando, Takaaki Tanaka, and Jun Nishida. 2025. "How Musculoskeletal Tumor Management Changed During the COVID-19 Pandemic: Data from a Nationwide Questionnaire Survey of Hospitals Specializing in Musculoskeletal Tumors in Japan" Current Oncology 32, no. 8: 453. https://doi.org/10.3390/curroncol32080453
APA StyleMorii, T., Iwata, S., Yamaga, K., Okamoto, M., Ando, K., Tanaka, T., & Nishida, J. (2025). How Musculoskeletal Tumor Management Changed During the COVID-19 Pandemic: Data from a Nationwide Questionnaire Survey of Hospitals Specializing in Musculoskeletal Tumors in Japan. Current Oncology, 32(8), 453. https://doi.org/10.3390/curroncol32080453






