Immunologic Alteration After Total En-Bloc Spondylectomy with Anterior Spinal Column Reconstruction with Frozen Tumor-Containing Bone Autologous Grafts: A Case Report in a Prospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
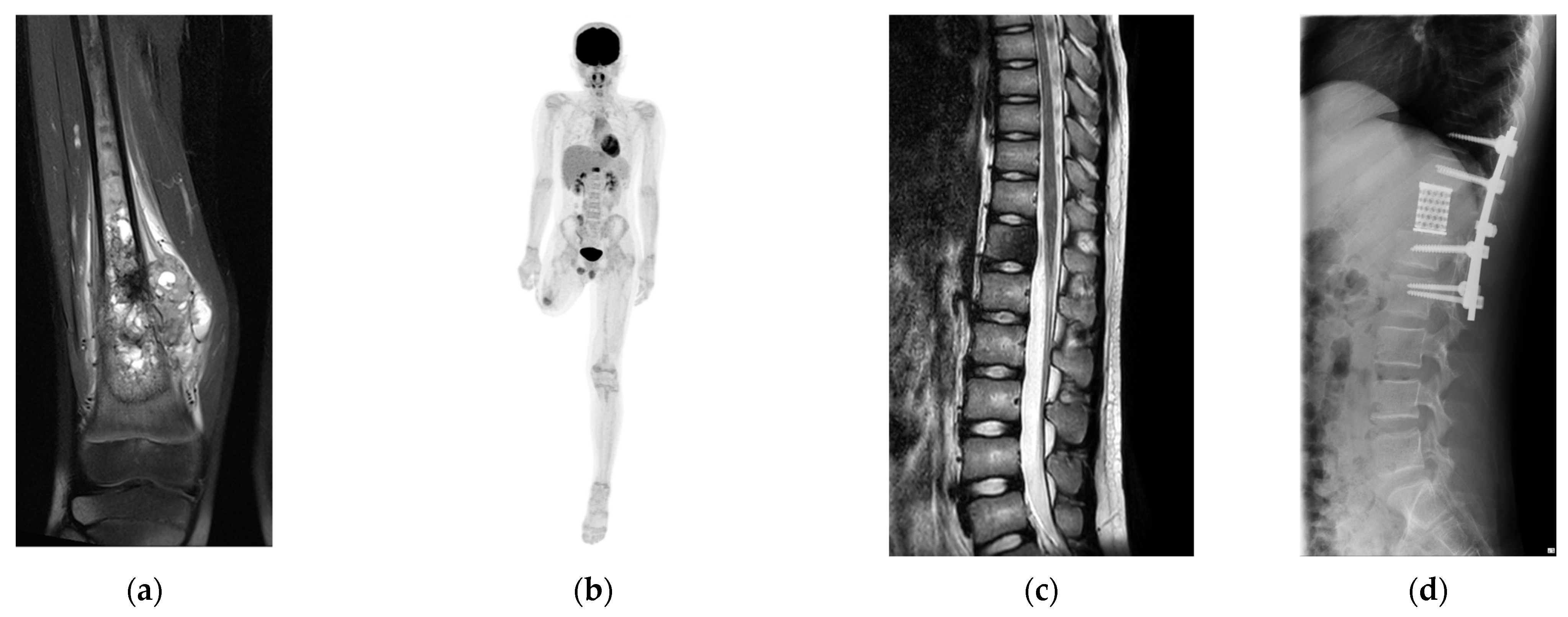
2.1. Patient
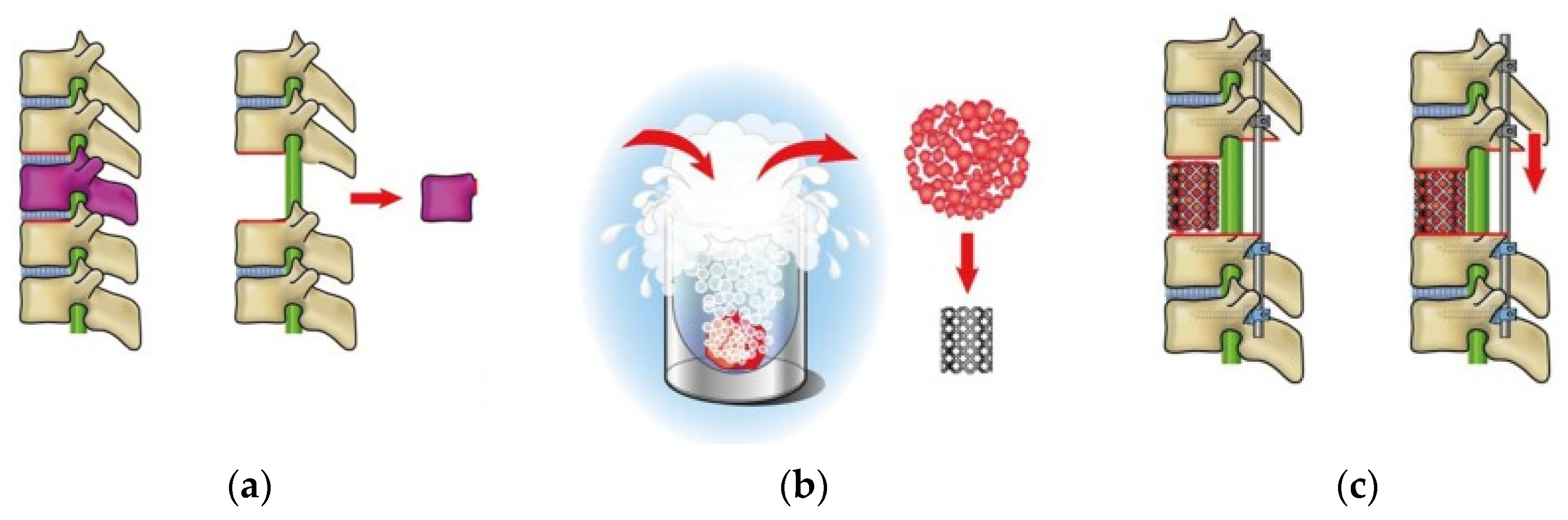
2.2. Tumor Resection and Reconstruction
2.3. RNA Extraction and the Assessment of Integrity for the Sequence
2.4. Immunome Sequencing Method
2.5. Data Analysis
3. Results
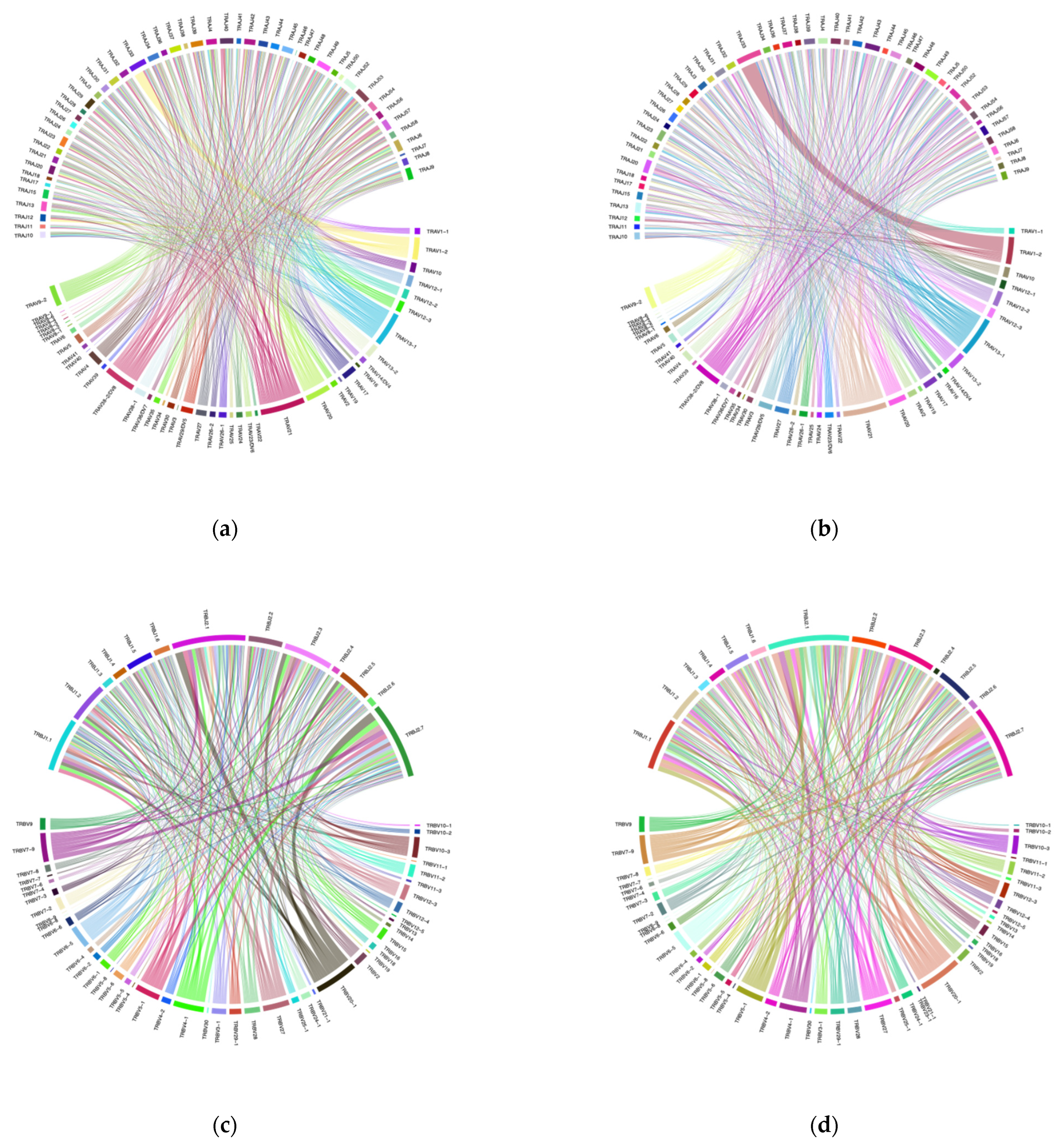
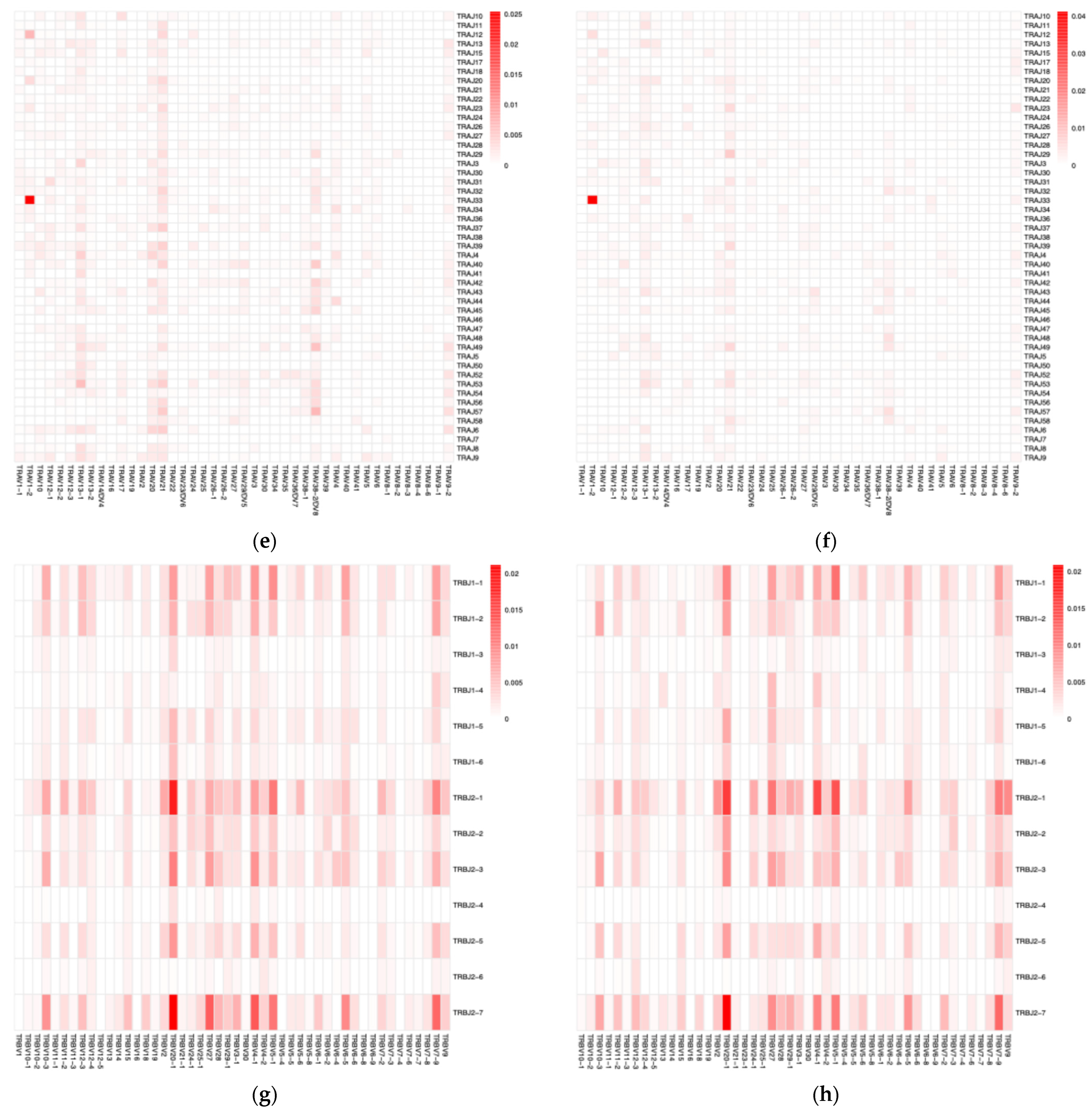
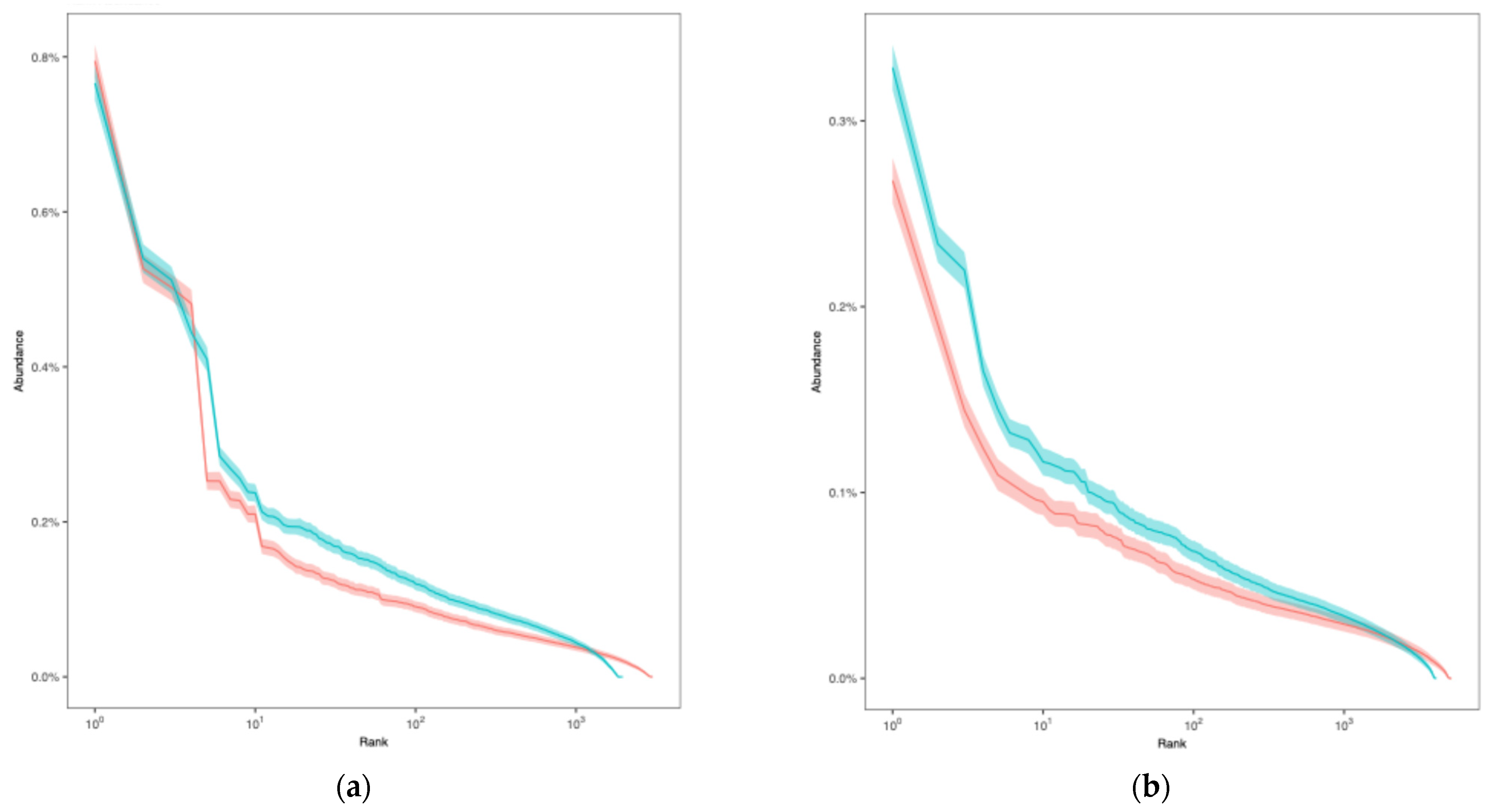
3.1. VJ Gene Recombinations
3.2. Diversity Analysis
3.3. Patient’s Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Abundance-based Coverage Estimator |
| CDR | Complementarity-Determining Region |
| DAMPs | Damage-Associated Molecular Patterns |
| HMGB | High Mobility Group Box Protein |
| ICI | Immune Checkpoint Inhibitor |
| IFN | Interferon |
| IL | Interleukin |
| IMGT | International ImMunoGeneTics |
| MMR-d | Mismatch Repair-deficient |
| MSI | Microsatellite Instability |
| TCR | T-cell Receptor |
| TES | Total En-Bloc Spondylectomy |
| TMB | Tumor Mutation Burden |
| TME | Tumor Microenvironment |
| TRA | T-cell Receptor α Chain |
| TRB | T-cell Receptor β Chain |
Appendix A
| Sample | Total Reads | Total Bases | Average Length | Q20 | Q30 | GC% | N% |
|---|---|---|---|---|---|---|---|
| TCR α chain (preoperative) | 3157530 | 938871105 | 297.34 | 96.84 | 88.23 | 48.11 | 0.08 |
| TCR β chain (preoperative) | 3261872 | 967060609 | 296.47 | 96.92 | 88.66 | 53.89 | 0.08 |
| TCR α chain (postoperative) | 3120218 | 928251874 | 297.5 | 96.84 | 88.17 | 48.08 | 0.08 |
| TCR β chain (postoperative) | 4182078 | 1235268425 | 295.37 | 97.06 | 89.22 | 54.42 | 0.08 |
References
- Shulman, S.; Yantorno, C.; Bronson, P. Cryo-Immunology: A Method of Immunization to Autologous Tissue. Proc. Soc. Exp. Biol. Med. 1967, 124, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Soanes, W.A.; Ablin, R.J.; Gonder, M.J. Remission of Metastatic Lesions Following Cryosurgery in Prostatic Cancer: Immunologic Considerations. J. Urol. 1970, 104, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Madan, R.A.; Pachynski, R.; Mulders, P.; Sheikh, N.A.; Trager, J.; Drake, C.G. Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. J. Natl. Cancer Inst. 2017, 109, djw261. [Google Scholar] [CrossRef]
- Mole, R.H. Whole Body Irradiation; Radiobiology or Medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal Effect of Radiotherapy Combined with Immune Checkpoint Inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef]
- Chandra, R.A.; Wilhite, T.J.; Balboni, T.A.; Alexander, B.M.; Spektor, A.; Ott, P.A.; Ng, A.K.; Hodi, F.S.; Schoenfeld, J.D. A Systematic Evaluation of Abscopal Responses Following Radiotherapy in Patients with Metastatic Melanoma Treated with Ipilimumab. Oncoimmunology 2015, 4, e1046028. [Google Scholar] [CrossRef]
- Kodet, O.; Němejcova, K.; Strnadová, K.; Havlínová, A.; Dundr, P.; Krajsová, I.; Štork, J.; Smetana, K., Jr.; Lacina, L. The Abscopal Effect in the Era of Checkpoint Inhibitors. Int. J. Mol. Sci. 2021, 22, 7204. [Google Scholar] [CrossRef]
- Mole, R.H. The Effect of X-Irradiation on the Basal Oxygen Consumption of the Rat. Q. J. Exp. Physiol. Cogn. Med. Sci. 1953, 38, 69–74. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Wan, S.L.; Sakayama, K.; Yamamoto, N.; Nishida, H.; Tomita, K. Reconstruction Using an Autograft Containing Tumour Treated by Liquid Nitrogen. J. Bone Jt. Surg. Br. 2005, 87, 218–225. [Google Scholar] [CrossRef]
- Takeuchi, A.; Tsuchiya, H.; Setsu, N.; Gokita, T.; Tome, Y.; Asano, N.; Minami, Y.; Kawashima, H.; Fukushima, S.; Takenaka, S.; et al. What Are the Complications, Function, and Survival of Tumor-Devitalized Autografts Used in Patients with Limb-sparing Surgery for Bone and Soft Tissue Tumors? A Japanese Musculoskeletal Oncology Group Multi-Institutional Study. Clin. Orthop Relat. Res. 2023, 481, 2110–2124. [Google Scholar] [CrossRef]
- Murakami, H.; Demura, S.; Kato, S.; Nishida, H.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Ota, T.; Shinmura, K.; Yokogawa, N.; et al. Increase of Il-12 Following Reconstruction for Total En Bloc Spondylectomy Using Frozen Autografts Treated with Liquid Nitrogen. PLoS ONE 2013, 8, e64818. [Google Scholar] [CrossRef]
- Ishii, T.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Fujii, M.; Igarashi, T.; Tsuchiya, H. Invasiveness Reduction of Recent Total En Bloc Spondylectomy: Assessment of the Learning Curve. Asian Spine J. 2016, 10, 522–527. [Google Scholar] [CrossRef]
- Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Ota, T.; Shinmura, K.; Yokogawa, N.; Fang, X.; et al. Systemic Antitumor Immune Response Following Reconstruction Using Frozen Autografts for Total En Bloc Spondylectomy. Spine J. 2014, 14, 1567–1571. [Google Scholar] [CrossRef]
- Roth, D.B. V(D)J Recombination: Mechanism, Errors, and Fidelity. Microbiol. Spectr. 2014, 2, 313–324. [Google Scholar] [CrossRef]
- Hozumi, N.; Tonegawa, S. Evidence for Somatic Rearrangement of Immunoglobulin Genes Coding for Variable and Constant Regions. Proc. Natl. Acad. Sci. USA 1976, 73, 3628–3632. [Google Scholar] [CrossRef]
- Mazzotti, L.; Gaimari, A.; Bravaccini, S.; Maltoni, R.; Cerchione, C.; Juan, M.; Navarro, E.A.-G.; Pasetto, A.; Nascimento Silva, D.; Ancarani, V.; et al. T-Cell Receptor Repertoire Sequencing and Its Applications: Focus on Infectious Diseases and Cancer. Int. J. Mol. Sci. 2022, 23, 8590. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and Using Diversity Indices: Insights for Ecological Applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Klein-Jöbstl, D.; Schornsteiner, E.; Mann, E.; Wagner, M.; Drillich, M.; Schmitz-Esser, S. Pyrosequencing Reveals Diverse Fecal Microbiota in Simmental Calves During Early Development. Front. Microbiol. 2014, 5, 622. [Google Scholar] [CrossRef] [PubMed]
- Spellerberg, I.F.; Fedor, P.J. A Tribute to Claude Shannon (1916–2001) and a Plea for More Rigorous Use of Species Richness, Species Diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Aran, A.; Garrigós, L.; Curigliano, G.; Cortés, J.; Martí, M. Evaluation of the TCR Repertoire as a Predictive and Prognostic Biomarker in Cancer: Diversity or Clonality? Cancers 2022, 14, 1771. [Google Scholar] [CrossRef]
- Arstila, T.P.; Casrouge, A.; Baron, V.; Even, J.; Kanellopoulos, J.; Kourilsky, P. A Direct Estimate of the Human Alphabeta T Cell Receptor Diversity. Science 1999, 286, 958–961. [Google Scholar] [CrossRef]
- Attaf, M.; Huseby, E.; Sewell, A.K. Aβ T Cell Receptors as Predictors of Health and Disease. Cell. Mol. Immunol. 2015, 12, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshimatsu, G.; Faustman, D.L. The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy. Cells 2022, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Ehab, J.; Hauptmann, E.; Gunturu, N.S.; Karalis, J.D.; Kent, D.O.; Heid, C.A.; Reznik, S.I.; Sarkaria, I.S.; Huang, H.; et al. The Current State of Tumor Microenvironment-Specific Therapies for Non-Small Cell Lung Cancer. Cancers 2025, 17, 1732. [Google Scholar] [CrossRef] [PubMed]
- Song, I.H.; Lee, S.B.; Jeong, B.K.; Park, J.; Kim, H.; Lee, G.; Cha, S.M.; Lee, H.; Gong, G.; Kwon, N.J.; et al. T Cell Receptor Clonotype in Tumor Microenvironment Contributes to Intratumoral Signaling Network in Patients with Colorectal Cancer. Immunol. Res. 2024, 72, 921–937. [Google Scholar] [CrossRef]
- Takahashi, H.; Hanaoka, K.; Wada, H.; Kojima, D.; Watanabe, M. The Current Status of T Cell Receptor (TCR) Repertoire Analysis in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 2698. [Google Scholar] [CrossRef]
- Porciello, N.; Franzese, O.; D’Ambrosio, L.; Palermo, B.; Nisticò, P. T-Cell Repertoire Diversity: Friend or Foe for Protective Antitumor Response? J. Exp. Clin. Cancer Res. 2022, 41, 356. [Google Scholar] [CrossRef]
- Campana, L.G.; Mansoor, W.; Hill, J.; Macutkiewicz, C.; Curran, F.; Donnelly, D.; Hornung, B.; Charleston, P.; Bristow, R.; Lord, G.M.; et al. T-Cell Infiltration and Clonality May Identify Distinct Survival Groups in Colorectal Cancer: Development and Validation of a Prognostic Model Based on The Cancer Genome Atlas (TCGA) and Clinical Proteomic Tumor Analysis Consortium (CPTAC). Cancers 2022, 14, 5883. [Google Scholar] [CrossRef]
- Manuel, M.; Olivier, T.; Thomas, B.; Gilles, C.; Anais, C.; Gilles, P.; Tioka, R.; Audrey, G.; Solène, P.; Jean-François, M.; et al. Lymphopenia Combined with Low Tcr Diversity (Divpenia) Predicts Poor Overall Survival in Metastatic Breast Cancer Patients. OncoImmunology 2012, 1, 432–440. [Google Scholar] [CrossRef]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune Checkpoint Inhibitors for the Treatment of Msi-H/Mmr-D Colorectal Cancer and a Perspective on Resistance Mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Kondou, R.; Iizuka, A.; Miyata, H.; Maeda, C.; Kanematsu, A.; Ashizawa, T.; Nagashima, T.; Urakami, K.; Shimoda, Y.; et al. Characterization of the Immunological Status of Hypermutated Solid Tumors in the Cancer Genome Analysis Project HOPE. Anticancer Res. 2022, 42, 3537–3549. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.R.; Shen, Y.; Qian, D.C.; Buchwald, Z.S. The Abscopal Effect: A Review of Pre-Clinical and Clinical Advances. Int. J. Mol. Sci. 2021, 22, 11061. [Google Scholar] [CrossRef]
- Shabalkina, A.V.; Izosimova, A.V.; Ryzhichenko, E.O.; Shurganova, E.V.; Myalik, D.S.; Maryanchik, S.V.; Ruppel, V.K.; Knyazev, D.I.; Khilal, N.R.; Barsova, E.V.; et al. Nodal Expansion, Tumor Infiltration and Exhaustion of Neoepitope-Specific Th Cells After Prophylactic Peptide Vaccination and Anti-CTLA4 Therapy in Mouse Melanoma B16. Int. J. Mol. Sci. 2025, 26, 6453. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Inoue, H.; Park, J.H.; Kiyotani, K.; Zewde, M.; Miyashita, A.; Jinnin, M.; Kiniwa, Y.; Okuyama, R.; Tanaka, R.; Fujisawa, Y.; et al. Intratumoral Expression Levels of Pd-L1, Gzma, and Hla-a Along with Oligoclonal T Cell Expansion Associate with Response to Nivolumab in Metastatic Melanoma. Oncoimmunology 2016, 5, e1204507. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in Mmr-Proficient and Mmr-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Yonezawa, N.; Murakami, H.; Demura, S.; Kato, S.; Miwa, S.; Yoshioka, K.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Oku, N.; et al. Abscopal Effect of Frozen Autograft Reconstruction Combined with an Immune Checkpoint Inhibitor Analyzed Using a Metastatic Bone Tumor Model. Int. J. Mol. Sci. 2021, 22, 1973. [Google Scholar] [CrossRef] [PubMed]
| Analyzed Points | Richness | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| CDR3 α chain (preoperative) | 2967 | 3017 | 3006 | 11.09 | 0.9993 |
| CDR3 α chain (postoperative) | 1865 | 1921 | 1940 | 10.46 | 0.9990 |
| CDR3 β chain (preoperative) | 5070 | 5154 | 5174 | 11.97 | 0.9997 |
| CDR3 β chain (postoperative) | 4050 | 4094 | 4101 | 11.63 | 0.9996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiba, H.; Kimura, H.; Terauchi, R.; Suzuki, N.; Kato, K.; Yagi, K.; Yamaguchi, M.; Murakami, K.; Suenaga, S.; Shirai, T.; et al. Immunologic Alteration After Total En-Bloc Spondylectomy with Anterior Spinal Column Reconstruction with Frozen Tumor-Containing Bone Autologous Grafts: A Case Report in a Prospective Study. Curr. Oncol. 2025, 32, 432. https://doi.org/10.3390/curroncol32080432
Aiba H, Kimura H, Terauchi R, Suzuki N, Kato K, Yagi K, Yamaguchi M, Murakami K, Suenaga S, Shirai T, et al. Immunologic Alteration After Total En-Bloc Spondylectomy with Anterior Spinal Column Reconstruction with Frozen Tumor-Containing Bone Autologous Grafts: A Case Report in a Prospective Study. Current Oncology. 2025; 32(8):432. https://doi.org/10.3390/curroncol32080432
Chicago/Turabian StyleAiba, Hisaki, Hiroaki Kimura, Ryu Terauchi, Nobuyuki Suzuki, Kenji Kato, Kiyoshi Yagi, Makoto Yamaguchi, Kiyoka Murakami, Shogo Suenaga, Toshiharu Shirai, and et al. 2025. "Immunologic Alteration After Total En-Bloc Spondylectomy with Anterior Spinal Column Reconstruction with Frozen Tumor-Containing Bone Autologous Grafts: A Case Report in a Prospective Study" Current Oncology 32, no. 8: 432. https://doi.org/10.3390/curroncol32080432
APA StyleAiba, H., Kimura, H., Terauchi, R., Suzuki, N., Kato, K., Yagi, K., Yamaguchi, M., Murakami, K., Suenaga, S., Shirai, T., Aso, A., Errani, C., & Murakami, H. (2025). Immunologic Alteration After Total En-Bloc Spondylectomy with Anterior Spinal Column Reconstruction with Frozen Tumor-Containing Bone Autologous Grafts: A Case Report in a Prospective Study. Current Oncology, 32(8), 432. https://doi.org/10.3390/curroncol32080432






