A Multimodal MRI-Based Model for Colorectal Liver Metastasis Prediction: Integrating Radiomics, Deep Learning, and Clinical Features with SHAP Interpretation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Image Acquisition and Preprocessing
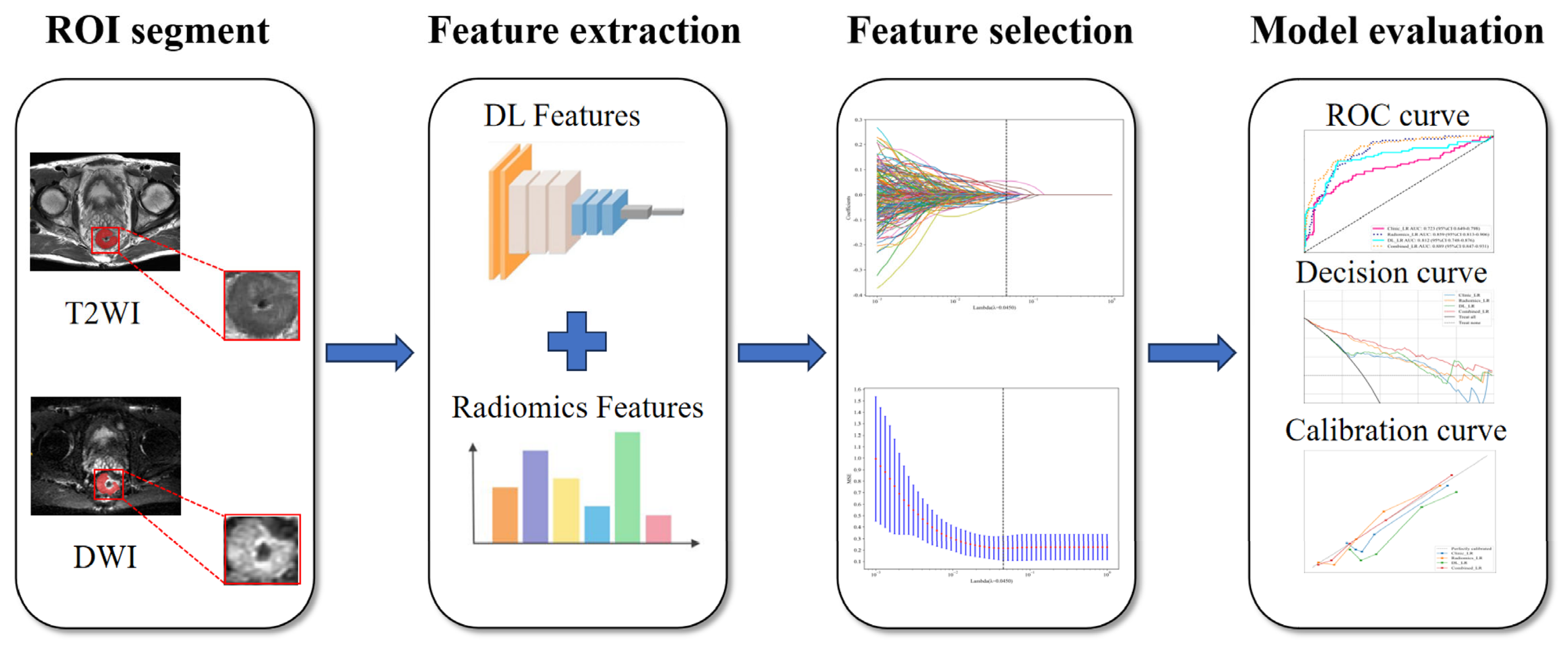
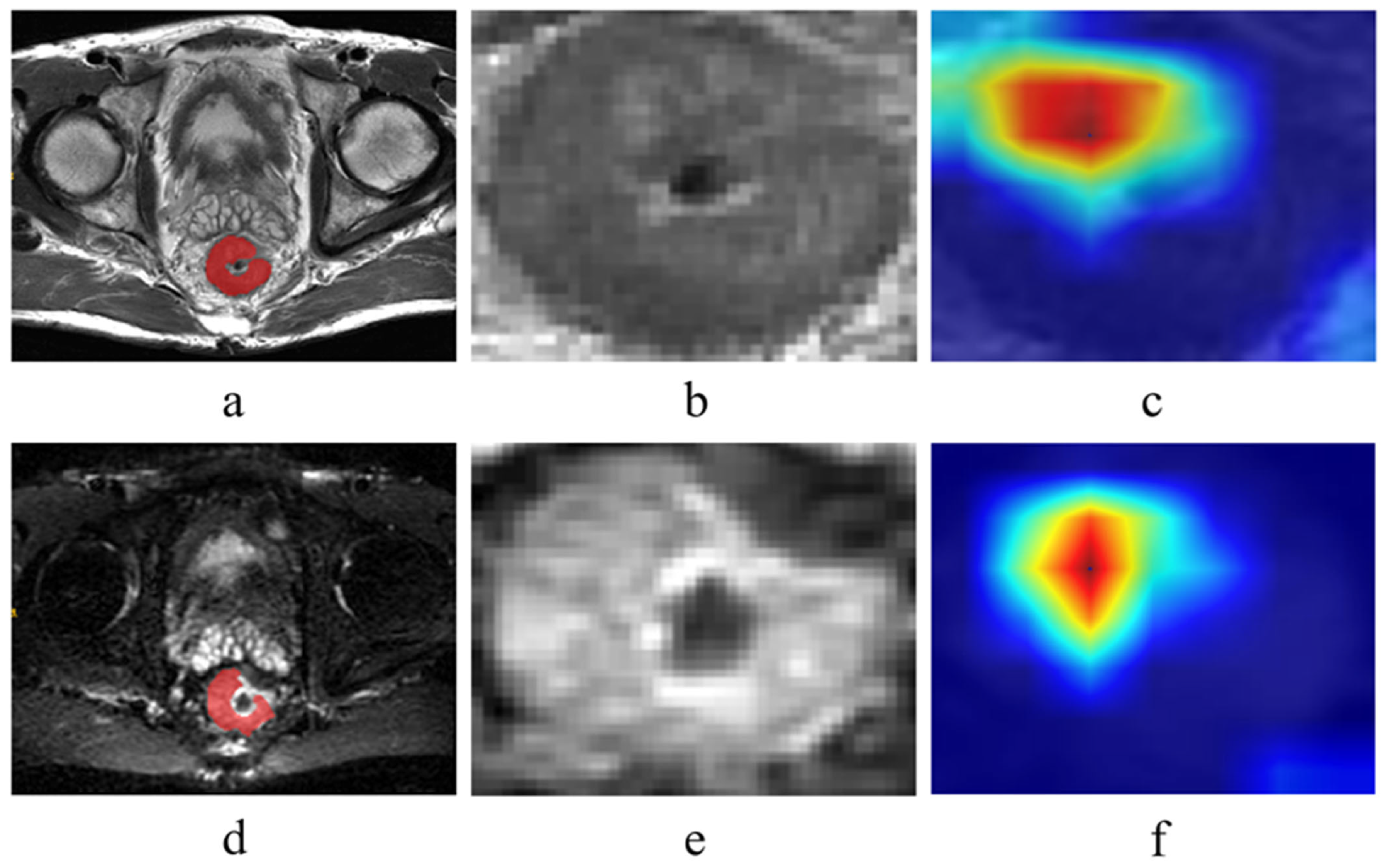
2.3. Tumor Segmentation and Feature Extraction
2.4. Feature Selection and Model Construction
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison Between Different Models
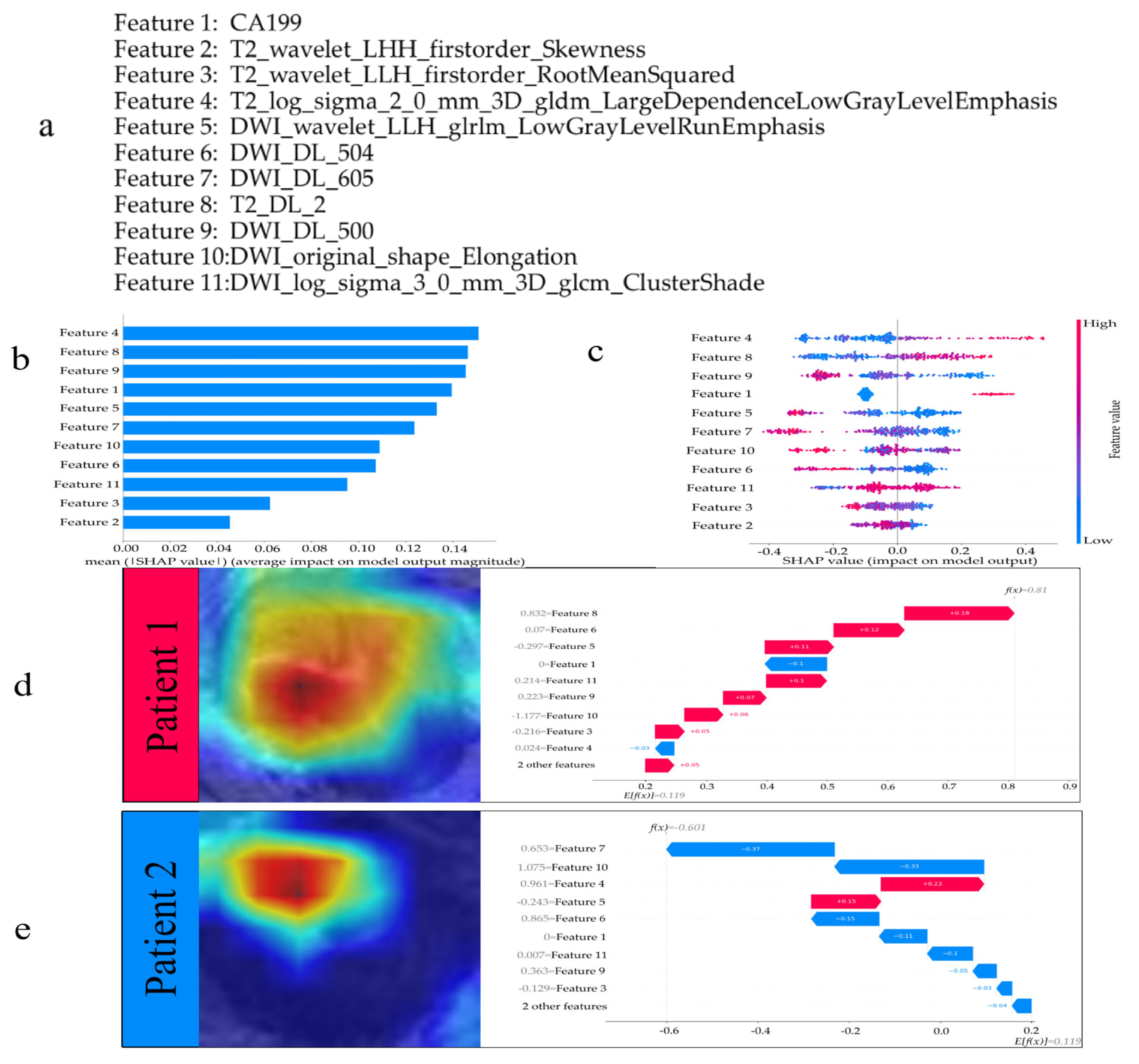
3.3. Interpreting the Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRLM | Colorectal Cancer Liver Metastasis |
| SHAP | SHapley Additive exPlanations |
| T2WI | T2-weighted Imaging |
| DWI | Diffusion-weighted Imaging |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| DCA | Decision Curve Analysis |
| CRC | Colorectal Cancer |
| CNNs | Convolutional Neural Networks |
| CEA | Carcinoembryonic Antigen |
| CA19-9 | Carbohydrate Antigen 19-9 |
| ROI | Region Of Interest |
| Grad-CAM | Gradient-weighted Class Activation Mapping |
| ICC | Intraclass Correlation Coefficients |
| LR | Logistic Regression |
| DL | Deep Learning |
| AUC | Area Under the Curve |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.-A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibañes, E.; Pawlik, T.M. Liver metastases. Nat. Rev. Dis. Primers 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.; Wang, L.; Jin, K.; Zhang, Y.; Wang, Y.; Sun, Y.; Tang, J. Genomic and Clinical Predictors of Conversion in Initially Unresectable Colorectal Cancer Liver Metastases. Ann. Surg. Oncol. 2025. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yue, L.; Fu, C.; Grimm, R.; Li, W.; Guo, W.; Tong, T. Whole tumor based texture analysis of magnetic resonance diffusion imaging for colorectal liver metastases: A prospective study for diffusion model comparison and early response biomarker. Eur. J. Radiol. 2024, 170, 111203. [Google Scholar] [CrossRef]
- Bera, K.; Braman, N.; Gupta, A.; Velcheti, V.; Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 2021, 19, 132–146. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Wen, Y.; Liang, H.; Zhong, J.-G.; Pang, P.-P. Magnetic resonance imaging-radiomics evaluation of response to chemo therapy for synchronous liver metastasis of colorectal cancer. World J. Gastroenterol. 2021, 27, 6465–6475. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Webster DR. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Chilamkurthy, S.; Ghosh, R.; Tanamala, S.; Biviji, M.; Campeau, N.G.; Venugopal, V.K.; Mahajan, V.; Rao, P.; Warier, P. Deep learning algorithms for detection of critical findings in head CT scans: A retrospective study. Lancet 2018, 392, 388–2396. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Y.; Liao, X.; Wang, E.; Cai, R.; Pan, Y.; Wang, D.; Lin, Y. Comparison of clinical, radiomics, deep learning, and fusion models for predicting early recurrence in locally advanced rectal cancer based on multiparametric MRI: A multicenter study. Eur. J. Radiol. 2025, 189, 112173. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of Compound Activity Predictions from Complex Machine Learning Models Using Local Approximations and Shapley Values. J. Med. Chem. 2019, 63, 8761–8777. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Jiang, Y.; Cheng, X.; He, W.; Yu, H.; Li, X.; Yang, J.; Yao, G.; Lu, Z.; et al. Multiparametric magnetic resonance imaging (MRI)-based radiomics model explained by the Shapley Additive exPlanations (SHAP) method for predicting complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A multicenter retrospective study. Quant. Imaging Med. Surg. 2024, 14, 4617–4634. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Baessler, B.; Bakas, S.; Cuocolo, R.; Fedorov, A.; Maier-Hein, L.; Mercaldo, N.; Müller, H.; Orlhac, F.; Pinto dos Santos, D.; et al. CheckList for EvaluAtion of Radiomics research (CLEAR): A step-by-step reporting guideline for authors and reviewers endorsed by ESR and EuSoMII. Insights Imaging 2023, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Su, J.; Liu, K.; Hu, C. Deep learning radiomics model based on PET/CT predicts PD-L1 expression in non-small cell lung cancer. Eur. J. Radiol. Open 2024, 12, 100549. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Hu, J.; Chen, S.; Zhao, R.; Peng, W.; Liu, Y.; Hu, S.; Zou, S.; Wang, S.; Zhao, X.; et al. Prediction of lymph node metastasis in stage T1–2 rectal cancers with MRI-based deep learning. Eur. Radiol. 2023, 33, 3638–3646. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- Long, B.; Li, R.; Wang, R.; Yin, A.; Zhuang, Z.; Jing, Y.; E, L. A computed tomography-based deep learning radiomics model for predicting the gender-age-physiology stage of patients with connective tissue disease-associated interstitial lung disease. Comput. Biol. Med. 2025, 191, 110128. [Google Scholar] [CrossRef]
- Yuan, J.; Li, B.; Zhang, C.; Wang, J.; Huang, B.; Ma, L. Machine Learning-Based CT Radiomics Model to Predict the Risk of Hip Fragility Fracture. Acad. Radiol. 2025, 32, 2854–2862. [Google Scholar] [CrossRef]
- Tao, Y.; Wei, Y.; Yu, Y.; Qin, X.; Huang, Y.; Liao, J. Development and Validation of a Nomogram Based on Multiparametric MRI for Predicting Lymph Node Metastasis in Endometrial Cancer: A Retrospective Cohort Study. Acad. Radiol. 2025, 32, 2751–2762. [Google Scholar] [CrossRef]
- Stocker, D.; Hectors, S.; Marinelli, B.; Carbonell, G.; Bane, O.; Hulkower, M.; Kennedy, P.; Ma, W.; Lewis, S.; Kim, E.; et al. Prediction of hepatocellular carcinoma response to radiation segmentectomy using an MRI-based machine learning approach. Abdom. Radiol. 2024, 50, 2000–2011. [Google Scholar] [CrossRef]
- Sherry, A.D.; Msaouel, P.; McCaw, Z.R.; Abi Jaoude, J.; Hsu, E.J.; Kouzy, R.; Patel, R.; Yang, Y.; Lin, T.A.; Taniguchi, C.M.; et al. Prevalence and implications of significance testing for baseline covariate imbalance in randomised cancer clinical trials: The Table 1 Fallacy. Eur. J. Cancer 2023, 194, 113357. [Google Scholar] [CrossRef]
- Cheng, M.; Guo, Y.; Zhao, H.; Zhang, H.; Liang, P.; Gao, J. CT-based deep learning radiomics analysis for preoperative Lauren classification in gastric cancer and explore the tumor microenvironment. Eur. J. Radiol. Open 2025, 15, 100667. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.-Y.; Yin, X.-P.; Gao, B.-L. Radiomics and Radiogenomics in Evaluation of Colorectal Cancer Live Metastasis. Front. Oncol. 2022, 11, 689509. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, H.; Saldanha, O.L.; Nebelung, S.; Kuhl, C.; Amygdalos, I.; Lang, S.A.; Wu, X.; Meng, X.; Truhn, D.; et al. An MRI Deep Learning Model Predicts Outcome in Rectal Cancer. Radiology 2023, 307, e222223. [Google Scholar] [CrossRef]
- Nazir, M.I.; Akter, A.; Hussen Wadud, M.A.; Uddin, M.A. Utilizing customized CNN for brain tumor prediction with explainable AI. Heliyon 2024, 10, e38997. [Google Scholar] [CrossRef]
- McCaw, Z.R.; Tian, L.; Wei, J.; Claggett, B.L.; Bretz, F.; Fitzmaurice, G.; Wei, L.J. Choosing clinically interpretable summary measures and robust analytic procedures for quantifying the treatment difference in comparative clinical studies. Stat. Med. 2021, 40, 6235–6242. [Google Scholar] [CrossRef]

| Characteristics | Training Set | Internal Test Set | External Validation Set | |||
|---|---|---|---|---|---|---|
| Metastatic Group n = 79 | Non-Metastatic Group n = 177 | Metastatic Group n = 43 | Non-Metastatic Group n = 68 | Metastatic Group n = 35 | Non-Metastatic Group n = 61 | |
| Age (mean ± SD) | 60.85 ± 9.92 | 63.80 ± 11.22 | 61.70 ± 12.06 | 63.12 ± 11.31 | 62.55 ± 10.52 | 61.63 ± 10.87 |
| Gender | ||||||
| Female | 19 (24.05%) | 52 (29.38%) | 17 (39.53%) | 27 (39.71%) | 15 (42.86%) | 30 (49.18%) |
| Male | 60 (75.95%) | 125 (70.62%) | 26 (60.47%) | 41 (60.29%) | 20 (57.14%) | 31 (50.82%) |
| CEA, ng/mL | ||||||
| ≤5 | 34 (43.04%) | 98 (55.37%) | 17 (39.53%) | 33 (48.53%) | 18 (51.43%) | 25 (40.98%) |
| >5 | 45 (56.96%) | 79 (44.63%) | 26 (60.47%) | 35 (51.47%) | 17 (48.57%) | 36 (59.02%) |
| CA19-9, U/mL | ||||||
| ≤37 | 49 (62.03%) | 159 (89.83%) | 30 (69.77%) | 56 (82.35%) | 12 (34.29%) | 18 (29.51%) |
| >37 | 30 (37.97%) | 18 (10.17%) | 13 (30.23%) | 12 (17.65%) | 23 (65.71%) | 43 (70.49%) |
| T-stage | ||||||
| T2 | 9 (11.39%) | 49 (27.68%) | 8 (18.60%) | 13 (19.12%) | 4 (11.43%) | 16 (26.23%) |
| T3 | 52 (65.82%) | 103 (58.19%) | 25 (58.14%) | 46 (67.65%) | 20 (57.14%) | 37 (60.66%) |
| T4 | 18 (16.80%) | 25 (14.12%) | 10 (23.26%) | 9 (13.24%) | 11 (31.43%) | 8 (13.11%) |
| N-stage | ||||||
| N0 | 9 (11.39%) | 21 (11.86%) | 4 (9.30%) | 7 (10.29%) | 6 (17.14%) | 4 (6.56%) |
| N1 | 27 (34.18%) | 56 (31.64%) | 12 (27.91%) | 19 (27.94%) | 16 (45.71%) | 39 (63.93%) |
| N2 | 43 (54.43%) | 100 (56.50%) | 27 (62.79%) | 42 (61.76%) | 13 (37.14%) | 18 (29.51%) |
| Characteristics | Univariate Analyses | Multivariate Analyses | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.72 (0.86~0.99) | 0.032 | 0.88 (0.77~1.00) | 0.094 |
| Gender (male) | 1.25 (0.71~1.82) | 0.491 | ||
| CEA (>5) | 1.86 (1.03~2.48) | 0.047 | 1.46 (0.80~1.59) | 0.539 |
| CA19-9 (>37) | 3.58 (2.29~6.65) | <0.001 | 3.28 (1.64~6.01) | <0.001 |
| T-stage | 1.72 (1.20~2.48) | 0.012 | 1.61 (0.92~2.59) | 0.183 |
| N-stage | 1.00 (0.73~1.38) | 0.964 | ||
| Models | AUC (95% CI) |
|---|---|
| Training Set | |
| Combined | 0.889 (95% CI: 0.847–0.931) |
| DL | 0.797 (95% CI: 0.738–0.856) |
| Radiomics | 0.859 (95% CI: 0.813–0.906) |
| Clinic | 0.723 (95% CI: 0.649–0.798) |
| Internal Test Set | |
| Combined | 0.838 (95% CI: 0.751–0.924) |
| DL | 0.729 (95% CI: 0.630–0.828) |
| Radiomics | 0.806 (95% CI: 0.721–0.891) |
| Clinic | 0.667 (95% CI: 0.557–0.778) |
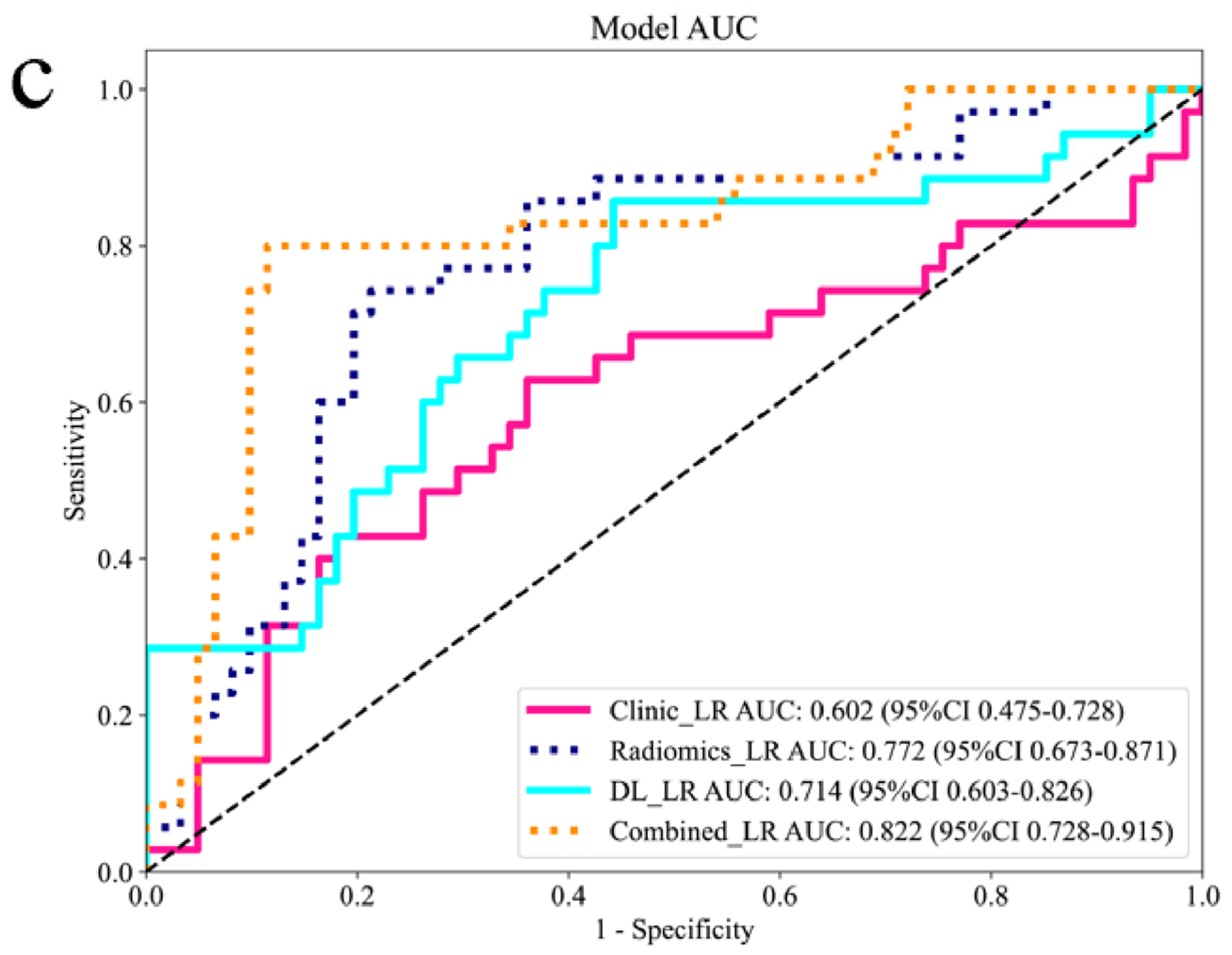
| External validation set | |
| Combined | 0.822 (95% CI: 0.728–0.915) |
| DL | 0.714 (95% CI: 0.603–0.826) |
| Radiomics | 0.772 (95% CI: 0.673–0.871) |
| Clinic | 0.602 (95% CI: 0.475–0.728) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Duan, F.; Chen, L.; Wang, R.; Li, K.; Sun, Q.; Fu, K. A Multimodal MRI-Based Model for Colorectal Liver Metastasis Prediction: Integrating Radiomics, Deep Learning, and Clinical Features with SHAP Interpretation. Curr. Oncol. 2025, 32, 431. https://doi.org/10.3390/curroncol32080431
Yan X, Duan F, Chen L, Wang R, Li K, Sun Q, Fu K. A Multimodal MRI-Based Model for Colorectal Liver Metastasis Prediction: Integrating Radiomics, Deep Learning, and Clinical Features with SHAP Interpretation. Current Oncology. 2025; 32(8):431. https://doi.org/10.3390/curroncol32080431
Chicago/Turabian StyleYan, Xin, Furui Duan, Lu Chen, Runhong Wang, Kexin Li, Qiao Sun, and Kuang Fu. 2025. "A Multimodal MRI-Based Model for Colorectal Liver Metastasis Prediction: Integrating Radiomics, Deep Learning, and Clinical Features with SHAP Interpretation" Current Oncology 32, no. 8: 431. https://doi.org/10.3390/curroncol32080431
APA StyleYan, X., Duan, F., Chen, L., Wang, R., Li, K., Sun, Q., & Fu, K. (2025). A Multimodal MRI-Based Model for Colorectal Liver Metastasis Prediction: Integrating Radiomics, Deep Learning, and Clinical Features with SHAP Interpretation. Current Oncology, 32(8), 431. https://doi.org/10.3390/curroncol32080431





