Different Master Regulators Define Proximal and Distal Gastric Cancer: Insights into Prognosis and Opportunities for Targeted Therapy
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Acquisition
- The Cancer Genome Atlas (TCGA): We accessed RNA sequencing (RNA-seq) data and corresponding clinical annotations for GC patients from the Genomic Data Commons (GDC) Data Portal (https://portal.gdc.cancer.gov/ accessed on 15 February 2025). Specifically, the TCGA Stomach Adenocarcinoma (TCGA-STAD) project was utilized. The GDC provides harmonized genomic data, facilitating integrative analyses across various studies. Tumor localization in TCGA was assigned based on clinical metadata fields, including “primary site” and “site of resection or biopsy.” Tumors located at the gastroesophageal junction or cardia were classified as proximal gastric cancer, while those involving the antrum or pylorus were categorized as distal gastric cancer.
- Gene Expression Omnibus (GEO): We retrieved microarray-based gene expression datasets GSE62254 and GSE15459 (https://www.ncbi.nlm.nih.gov/geo/ accessed on 1 March 2025), which include comprehensive clinical annotations for GC patients.
2.2. Patient Cohort Selection
- Inclusion criteria for this study were as follows:
- Histologically confirmed diagnosis of gastric adenocarcinoma.
- Availability of tumor localization data, categorizing tumors as proximal (esophagogastric junction and cardia) or distal (antrum and pylorus).
- Comprehensive clinical and gene expression data.
2.3. Data Preprocessing and Normalization
2.4. Differential Expression Analysis
2.5. Master Regulator Inference
2.6. Functional Enrichment Analysis
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
| Characteristic | Proximal GC (n = 207) | Distal GC (n = 649) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| Diffuse histology | 135 (65%) | 227 (35%) | <0.001 | 3.59 (2.64–4.89) |
| Poorly differentiated | 149 (72%) | 311 (48%) | <0.001 | 2.86 (2.06–3.98) |
| T3/T4 stage | 141 (68%) | 318 (49%) | <0.001 | 2.26 (1.64–3.12) |
| N+ lymph node involvement | 124 (60%) | 273 (42%) | 0.014 | 2.06 (1.50–2.83) |
| Metastasis (M1) | 37 (18%) | 65 (10%) | 0.021 | 1.98 (1.27–3.08) |
| Intestinal subtype | 50 (24%) | 493 (76%) | <0.001 | 0.14 (0.10–0.20) |
3.2. Differential Gene Expression Analysis
3.3. Identification of Location-Specific Master Regulators
3.4. Functional Enrichment of MR Target Genes
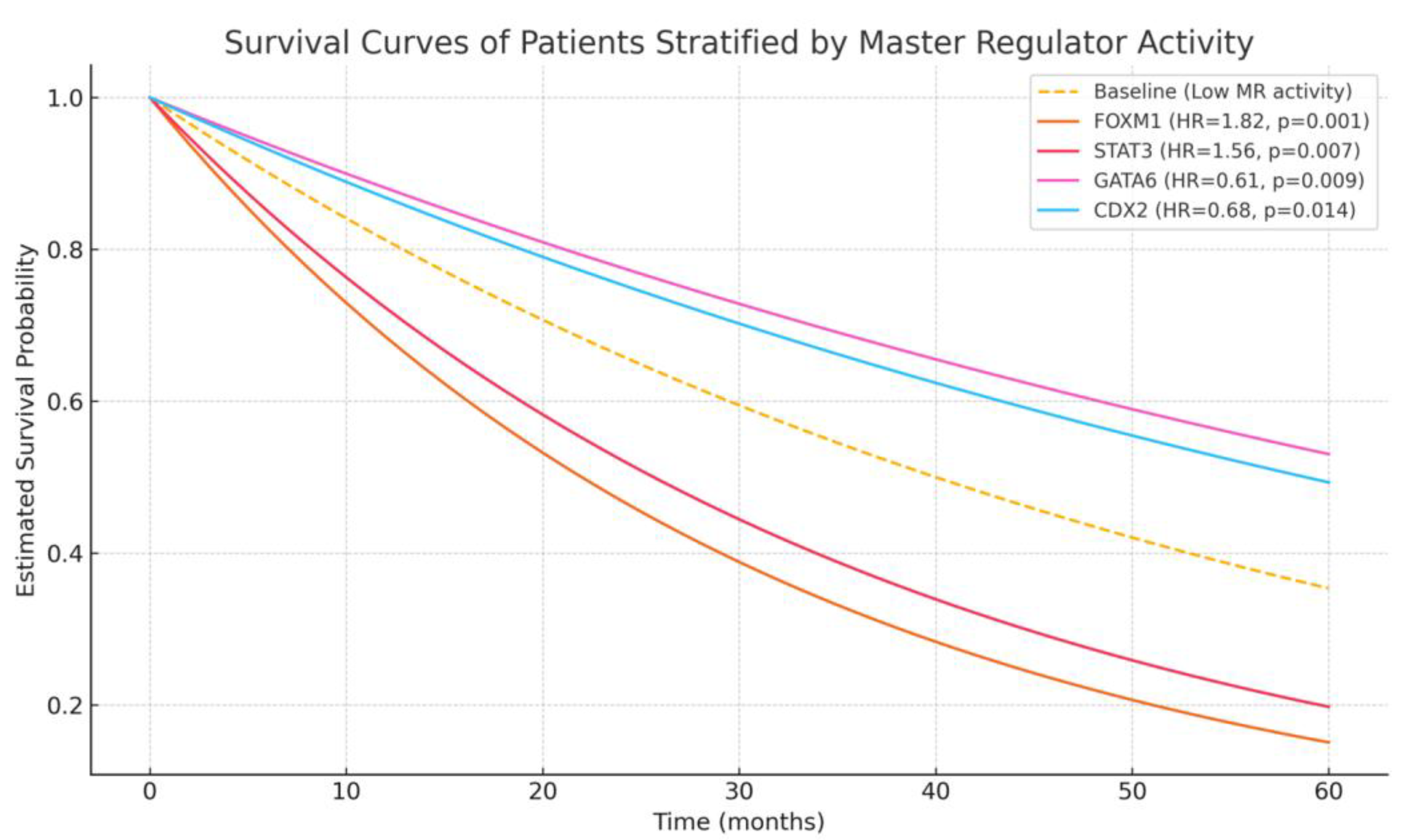
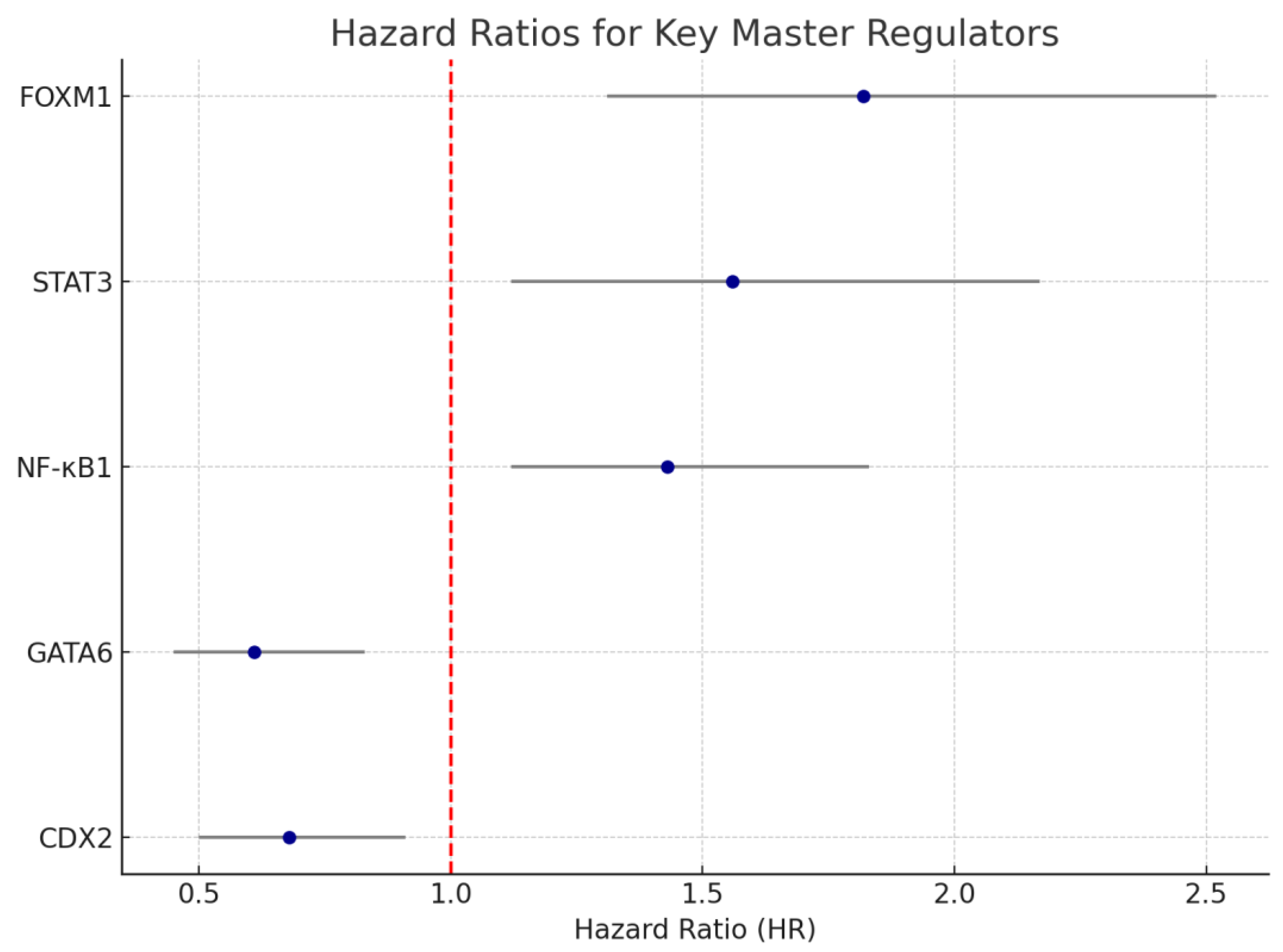
3.5. Correlation of MR Activity with Clinical and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, N.; Fan, Y.; Yang, T.; Yang, Z.; Fan, D. The Burden of Gastric Cancer and Possible Risk Factors from 1990 to 2021, and Projections until 2035: Findings from the Global Burden of Disease Study 2021. Biomark. Res. 2025, 13, 5. [Google Scholar] [CrossRef]
- Hsu, J.-T.; Lin, Y.-N.; Chen, Y.-F.; Kou, H.-W.; Wang, S.-Y.; Chou, W.-C.; Wu, T.-R.; Yeh, T.-S. A Comprehensive Overview of Gastric Cancer Management from a Surgical Point of View. Biomed. J. 2024, 100817. [Google Scholar] [CrossRef]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2019, 76, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, D.; Hu, X.; Zhang, G.; Li, Z.; Zhao, Y.; Liu, Z.; Wang, Y. Analysis of Immune Status in Gastric Adenocarcinoma with Different Infiltrating Patterns and Origin Sites. Front. Immunol. 2022, 13, 978715. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yang, H.; Huang, S.; Zhou, T.; Zhang, X.; Zu, G. Comparison of the Overall Survival of Proximal and Distal Gastric Cancer after Gastrectomy: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2021, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Li, Y.; Tang, S.; Zhang, Y.; Chen, Y.; Khan, S.A. Comparison on Clinicopathological Features, Treatments and Prognosis between Proximal Gastric Cancer and Distal Gastric Cancer: A National Cancer Data Base Analysis. J. Cancer 2019, 10, 3145–3153. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kang, S.J.; Choi, W.; Seo, A.N.; Eom, B.W.; Kang, B.; Kim, B.J.; Min, B.-H.; Tae, C.H.; Choi, C.I.; et al. Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-Based, Multidisciplinary Approach (Update of 2022 Guideline). J. Gastric Cancer 2025, 25, 5–114. [Google Scholar] [CrossRef]
- Yoo, S.; Chen, Q.; Wang, L.; Wang, W.; Chakravarthy, A.; Busuttil, R.; Boussioutas, A.; Liu, D.; She, J.; Fenton, T.R.; et al. Molecular and Cellular Heterogeneity of Gastric Cancer Explained by Methylation-Driven Key Regulators. bioRxiv 2020. [Google Scholar] [CrossRef]
- Begolli, R.; Patouna, A.; Vardakas, P.; Xagara, A.; Apostolou, K.; Kouretas, D.; Giakountis, A. Deciphering the Landscape of GATA-Mediated Transcriptional Regulation in Gastric Cancer. Antioxidants 2024, 13, 1267. [Google Scholar] [CrossRef]
- Yu, B.; Dai, W.; Pang, L.; Sang, Q.; Li, F.; Yu, J.; Feng, H.; Li, J.; Hou, J.; Yan, C.; et al. The Dynamic Alteration of Transcriptional Regulation by Crucial TFs during Tumorigenesis of Gastric Cancer. Mol. Med. 2022, 28, 41. [Google Scholar] [CrossRef] [PubMed]
- Ooki, A.; Osumi, H.; Yoshino, K.; Yamaguchi, K. Potent Therapeutic Strategy in Gastric Cancer with Microsatellite Instability-High and/or Deficient Mismatch Repair. Gastric Cancer 2024, 27, 907. [Google Scholar] [CrossRef] [PubMed]
- Accordino, G.; Lettieri, S.; Bortolotto, C.; Benvenuti, S.; Gallotti, A.; Gattoni, E.; Agustoni, F.; Pozzi, E.; Rinaldi, P.; Primiceri, C.; et al. Cancers Review From Interconnection between Genes and Microenvironment to Novel Immunotherapeutic Approaches in Upper Gastro-Intestinal Cancers-A Multidisciplinary Perspective. Cancers 2020, 12, 2105. [Google Scholar] [CrossRef]
- Díaz del Arco, C.; Fernández Aceñero, M.J.; Ortega Medina, L. Molecular Classifications in Gastric Cancer: A Call for Interdisciplinary Collaboration. Int. J. Mol. Sci. 2024, 25, 2649. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The Sva Package for Removing Batch Effects and Other Unwanted Variation in High-Throughput Experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Mercatelli, D.; Lopez-Garcia, G.; Giorgi, F.M. Corto: A Lightweight R Package for Gene Network Inference and Master Regulator Analysis. Bioinformatics 2020, 36, 3916–3917. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R Package for Comparing Biological Themes among Gene Clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New Perspectives on Genomes, Pathways, Diseases and Drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.; Aljabban, J.; Rudeski-Rohr, T.; Lessans, S.; Nakkina, S.P.; Hadley, D.; Zhu, X.; Altomare, D.A. Meta-Analysis Reveals the Prognostic Relevance of Nuclear and Membrane-Associated Bile Acid Receptors in Gastric Cancer. Clin. Transl. Gastroenterol. 2021, 12, e00295. [Google Scholar] [CrossRef]
- Jafari, N.; Abediankenari, S.; Hosseini-Khah, Z.; Valizadeh, S.M.; Torabizadeh, Z.; Zaboli, E.; Ghasemi, M.; Fakheri, H.; Hosseini, V.; Shekarriz, R.; et al. Expression Patterns of Seven Key Genes, Including β-Catenin, Notch1, GATA6, CDX2, MiR-34a, MiR-181a and MiR-93 in Gastric Cancer. Sci. Rep. 2020, 10, 12342. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Sun, S.; Luo, Z.; Zhang, P.; Xu, Y.; Qiu, Z.; Huang, C. Single-Cell Transcriptome Profiling Identifies Tumor Heterogeneity in Epithelial, Stromal and Immune Cells Between Distal and Proximal Gastric Cancer. iScience 2023. [Google Scholar] [CrossRef]
- Gulmann, C.; Hegarty, H.; Grace, A.; Leader, M.; Patchett, S.; Kay, E. Differences in Proximal (Cardia) versus Distal (Antral) Gastric Carcinogenesis via Retinoblastoma Pathway. World J. Gastroenterol. 2004, 10, 17. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Barni, S.; Steccanella, F.; Sgroi, G.; Passalacqua, R.; Tomasello, G. Prognostic Role of Primary Tumor Location in Non-Metastatic Gastric Cancer: A Systematic Review and Meta-Analysis of 50 Studies. Ann. Surg. Oncol. 2017, 24, 2655–2668. [Google Scholar] [CrossRef]
- Yaprak, G.; Tataroglu, D.; Dogan, B.; Pekyurek, M. Prognostic Factors for Survival in Patients with Gastric Cancer: Single-Centre Experience. N. Clin. Istanb. 2019, 7, 146. [Google Scholar] [CrossRef]
- Kulig, P.; Pach, R.; Majewska, O.; Kulig, J. Clinicopathological Prognostic Factors Determining Outcomes of Treatment in Gastric Cancer Surgery. In Vivo 2022, 36, 2927. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, N.; Jia, Z.; Le, X.; Dai, B.; Wei, D.; Huang, S.; Tan, D.; Xie, K. Critical Role and Regulation of Transcription Factor Foxm1 in Human Gastric Cancer Angiogenesis and Progression. Cancer Res. 2009, 69, 3501–3509. [Google Scholar] [CrossRef]
- Li, X.; Qiu, W.; Liu, B.; Yao, R.; Liu, S.; Yao, Y.; Liang, J. Forkhead Box Transcription Factor 1 Expression in Gastric Cancer: FOXM1 Is a Poor Prognostic Factor and Mediates Resistance to Docetaxel. J. Transl. Med. 2013, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, D.; Yu, Q.; Li, L.; Wu, P. Prognostic Value of FOXM1 in Solid Tumors: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 32298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Van Boxel-Dezaire, A.H.H.; Cheon, H.; Yang, J.; Stark, G.R. STAT3 Activation in Response to IL-6 Is Prolonged by the Binding of IL-6 Receptor to EGF Receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16975–16980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.M.; Putoczki, T.L.; Ernst, M. STAT3-Activating Cytokines: A Therapeutic Opportunity for Inflammatory Bowel Disease? J. Interferon Cytokine Res. 2015, 35, 340. [Google Scholar] [CrossRef]
- Hodge, D.R.; Hurt, E.M.; Farrar, W.L. The Role of IL-6 and STAT3 in Inflammation and Cancer. Eur. J. Cancer 2005, 41, 2502–2512. [Google Scholar] [CrossRef]
- Matsuda, T. The Physiological and Pathophysiological Role of IL-6/STAT3-Mediated Signal Transduction and STAT3 Binding Partners in Therapeutic Applications. Biol. Pharm. Bull. 2023, 46, 364–378. [Google Scholar] [CrossRef]
- Pan, Y.M.; Wang, C.G.; Zhu, M.; Xing, R.; Cui, J.T.; Li, W.M.; Yu, D.D.; Wang, S.B.; Zhu, W.; Ye, Y.J.; et al. STAT3 Signaling Drives EZH2 Transcriptional Activation and Mediates Poor Prognosis in Gastric Cancer. Mol. Cancer 2016, 15, 79. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618. [Google Scholar] [CrossRef]
- Ni, Y.; Low, J.T.; Silke, J.; O’Reilly, L.A. Digesting the Role of JAK-STAT and Cytokine Signaling in Oral and Gastric Cancers. Front. Immunol. 2022, 13, 835997. [Google Scholar] [CrossRef]
- Chen, M.; Wang, T.; Tian, D.; Hai, C.; Qiu, Z. Induction, Growth, Drug Resistance, and Metastasis: A Comprehensive Summary of the Relationship between STAT3 and Gastric Cancer. Heliyon 2024, 10, e37263. [Google Scholar] [CrossRef]
- Rah, B.; Rather, R.A.; Bhat, G.R.; Baba, A.B.; Mushtaq, I.; Farooq, M.; Yousuf, T.; Dar, S.B.; Parveen, S.; Hassan, R.; et al. JAK/STAT Signaling: Molecular Targets, Therapeutic Opportunities, and Limitations of Targeted Inhibitions in Solid Malignancies. Front. Pharmacol. 2022, 13, 821344. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S. Preclinical Development and Clinical Studies of Targeted JAK/STAT Combined Anti-PD-1/PD-L1 Therapy. Int. Immunopharmacol. 2024, 130, 111717. [Google Scholar] [CrossRef]
- Wang, K.N.; Zhou, K.; Zhong, N.N.; Cao, L.M.; Li, Z.Z.; Xiao, Y.; Wang, G.R.; Huo, F.Y.; Zhou, J.J.; Liu, B.; et al. Enhancing Cancer Therapy: The Role of Drug Delivery Systems in STAT3 Inhibitor Efficacy and Safety. Life Sci. 2024, 346, 122635. [Google Scholar] [CrossRef]
- Aquino-Acevedo, A.N.; Orengo-Orengo, J.A.; Cruz-Robles, M.E.; Saavedra, H.I. Mitotic Kinases Are Emerging Therapeutic Targets against Metastatic Breast Cancer. Cell Div. 2024, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular Mechanisms of Chemo- and Radiotherapy Resistance and the Potential Implications for Cancer Treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Seo, E.H.; Bae, D.H.; Haam, K.; Jang, H.R.; Park, J.L.; Kim, J.H.; Kim, M.; Kim, S.Y.; Jeong, H.Y.; et al. Methylation of the CDX2 Promoter in Helicobacter Pylori-Infected Gastric Mucosa Increases with Age and Its Rapid Demethylation in Gastric Tumors Is Associated with Upregulated Gene Expression. Carcinogenesis 2020, 41, 1341–1352. [Google Scholar] [CrossRef]
- Ansari, S.; Gantuya, B.; Tuan, V.P.; Yamaoka, Y. Diffuse Gastric Cancer: A Summary of Analogous Contributing Factors for Its Molecular Pathogenicity. Int. J. Mol. Sci. 2018, 19, 2424. [Google Scholar] [CrossRef] [PubMed]
- Oz Puyan, F.; Can, N.; Ozyilmaz, F.; Usta, U.; Sut, N.; Tastekin, E.; Altaner, S. The Relationship among PDX1, CDX2, and Mucin Profiles in Gastric Carcinomas; Correlations with Clinicopathologic Parameters. J. Cancer Res. Clin. Oncol. 2011, 137, 1749–1762. [Google Scholar] [CrossRef]
- Wang, X.T.; Wei, W.Y.; Kong, F.B.; Lian, C.; Luo, W.; Xiao, Q.; Xie, Y.B. Prognostic Significance of Cdx2 Immunohistochemical Expression in Gastric Cancer: A Meta-Analysis of Published Literatures. J. Exp. Clin. Cancer Res. 2012, 31, 98. [Google Scholar] [CrossRef]
- Deng, X.; Jiang, P.; Chen, J.; Li, J.; Li, D.; He, Y.; Jiang, Y.; Zhang, Y.; Xu, S.; Li, X.; et al. GATA6 Promotes Epithelial-Mesenchymal Transition and Metastasis through MUC1/β-Catenin Pathway in Cholangiocarcinoma. Cell Death Dis. 2020, 11, 860. [Google Scholar] [CrossRef]
- Chia, N.Y.; Deng, N.; Das, K.; Huang, D.; Hu, L.; Zhu, Y.; Lim, K.H.; Lee, M.H.; Wu, J.; Sam, X.X.; et al. Regulatory Crosstalk between Lineage-Survival Oncogenes KLF5, GATA4 and GATA6 Cooperatively Promotes Gastric Cancer Development. Gut 2015, 64, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Jeon, M.S.; Nam, J.W.; Kang, J.K.; Lee, Y.J.; Kang, J.Y.; Kim, H.P.; Han, S.W.; Kang, G.H.; Kim, T.Y. Aberrant GATA2 Epigenetic Dysregulation Induces a GATA2/GATA6 Switch in Human Gastric Cancer. Oncogene 2018, 37, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Wei, K.L.; Chou, J.L.; Lu, C.K.; Hsieh, C.C.; Lin, J.M.J.; Deng, Y.F.; Hsu, W.T.; Wang, H.M.D.; Leung, C.H.; et al. Aberrant JAK/STAT Signaling Suppresses TFF1 and TFF2 through Epigenetic Silencing of GATA6 in Gastric Cancer. Int. J. Mol. Sci. 2016, 17, 1467. [Google Scholar] [CrossRef]
- Kiri, S.; Ryba, T. Cancer, Metastasis, and the Epigenome. Mol. Cancer 2024, 23, 154. [Google Scholar] [CrossRef]
- Blanchet, A.; Bourgmayer, A.; Kurtz, J.E.; Mellitzer, G.; Gaiddon, C. Isoforms of the P53 Family and Gastric Cancer: A Ménage à Trois for an Unfinished Affair. Cancers 2021, 13, 916. [Google Scholar] [CrossRef]
- Naeem, H.; Momal, U.; Imran, M.; Shahbaz, M.; Hussain, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Umar, M.; Mujtaba, A.; El-Ghorab, A.H.; et al. Anticancer Perspectives of Genistein: A Comprehensive Review. Int. J. Food Prop. 2023, 26, 3305–3341. [Google Scholar] [CrossRef]
- Penzo, M.; Massa, P.E.; Olivotto, E.; Bianchi, F.; Borzi, R.M.; Hanidu, A.; Xiang, L.I.; Jun, L.I.; Marcu, K.B. Sustained NF-ΚB Activation Produces a Short-Term Cell Proliferation Block in Conjunction with Repressing Effectors of Cell Cycle Progression Controlled by E2F or FoxM1. J. Cell. Physiol. 2009, 218, 215–227. [Google Scholar] [CrossRef]
- Koo, C.Y.; Muir, K.W.; Lam, E.W.F. FOXM1: From Cancer Initiation to Progression and Treatment. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2012, 1819, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Merjaneh, N.; Hajjar, M.; Lan, Y.W.; Kalinichenko, V.V.; Kalin, T.V. The Promise of Combination Therapies with FOXM1 Inhibitors for Cancer Treatment. Cancers 2024, 16, 756. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Chen, L.T.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; Yeh, K.H.; et al. A Phase 3 Study of Nivolumab in Previously Treated Advanced Gastric or Gastroesophageal Junction Cancer (ATTRACTION-2): 2-Year Update Data. Gastric Cancer 2020, 23, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Long, X.; Liu, P.S.; Xie, X. The Interplay of Oncogenic Signaling, Oxidative Stress and Ferroptosis in Cancer. Int. J. Cancer 2023, 153, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Zabeti Touchaei, A.; Vahidi, S.; Samadani, A.A. Decoding the Regulatory Landscape of LncRNAs as Potential Diagnostic and Prognostic Biomarkers for Gastric and Colorectal Cancers. Clin. Exp. Med. 2024, 24, 29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marano, L.; Sorrenti, S.; Malerba, S.; Skokowski, J.; Polom, K.; Girnyi, S.; Cwalinski, T.; Prete, F.P.; González-Ojeda, A.; Fuentes-Orozco, C.; et al. Different Master Regulators Define Proximal and Distal Gastric Cancer: Insights into Prognosis and Opportunities for Targeted Therapy. Curr. Oncol. 2025, 32, 424. https://doi.org/10.3390/curroncol32080424
Marano L, Sorrenti S, Malerba S, Skokowski J, Polom K, Girnyi S, Cwalinski T, Prete FP, González-Ojeda A, Fuentes-Orozco C, et al. Different Master Regulators Define Proximal and Distal Gastric Cancer: Insights into Prognosis and Opportunities for Targeted Therapy. Current Oncology. 2025; 32(8):424. https://doi.org/10.3390/curroncol32080424
Chicago/Turabian StyleMarano, Luigi, Salvatore Sorrenti, Silvia Malerba, Jaroslaw Skokowski, Karol Polom, Sergii Girnyi, Tomasz Cwalinski, Francesco Paolo Prete, Alejandro González-Ojeda, Clotilde Fuentes-Orozco, and et al. 2025. "Different Master Regulators Define Proximal and Distal Gastric Cancer: Insights into Prognosis and Opportunities for Targeted Therapy" Current Oncology 32, no. 8: 424. https://doi.org/10.3390/curroncol32080424
APA StyleMarano, L., Sorrenti, S., Malerba, S., Skokowski, J., Polom, K., Girnyi, S., Cwalinski, T., Prete, F. P., González-Ojeda, A., Fuentes-Orozco, C., Goyal, A., Vaithianathan, R., Vladimirov, M., Lori, E., Pironi, D., Abou-Mrad, A., Testini, M., Oviedo, R. J., & Vashist, Y. (2025). Different Master Regulators Define Proximal and Distal Gastric Cancer: Insights into Prognosis and Opportunities for Targeted Therapy. Current Oncology, 32(8), 424. https://doi.org/10.3390/curroncol32080424







