Multi-Level Oncological Management of a Rare, Combined Mediastinal Tumor: A Case Report
Simple Summary
Abstract
1. Introduction
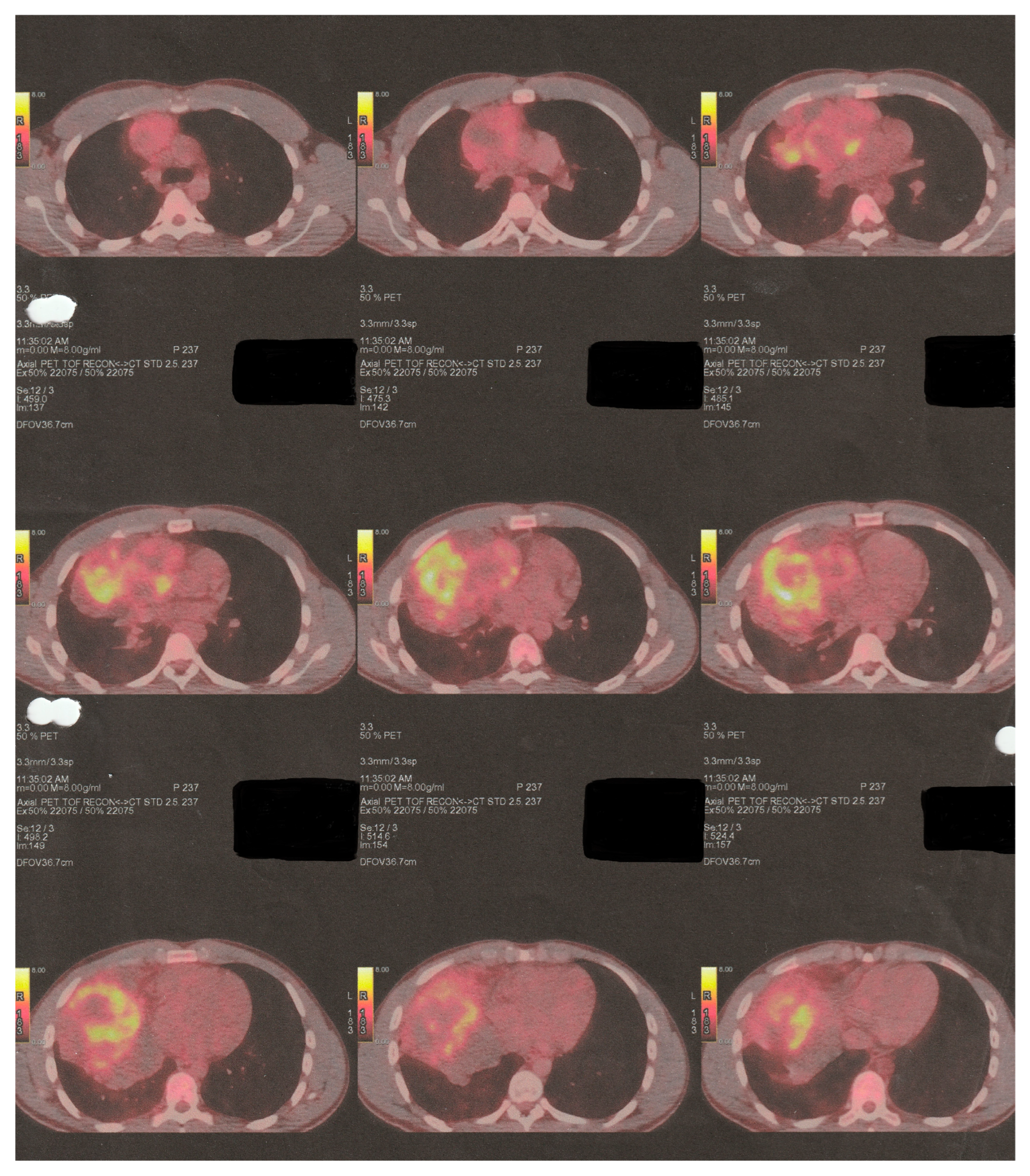
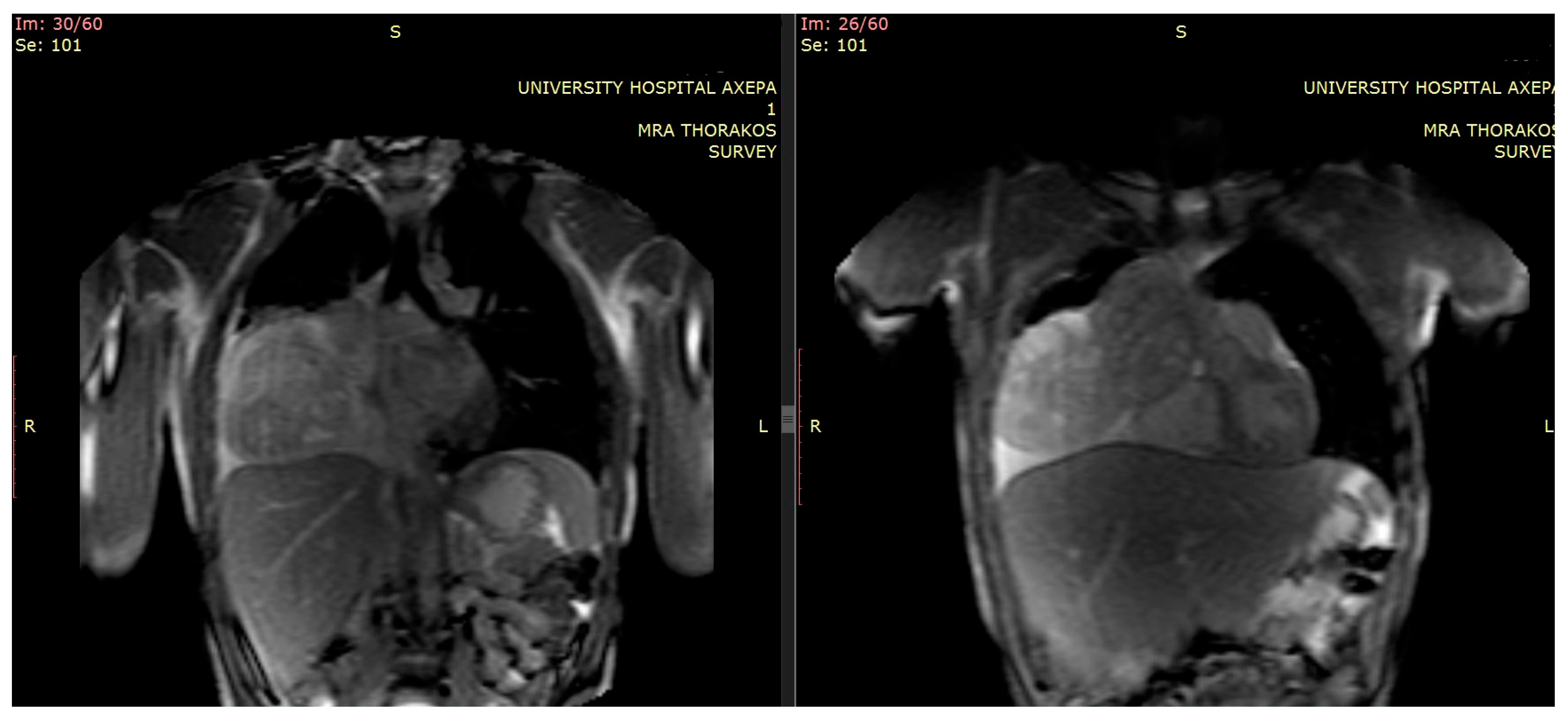
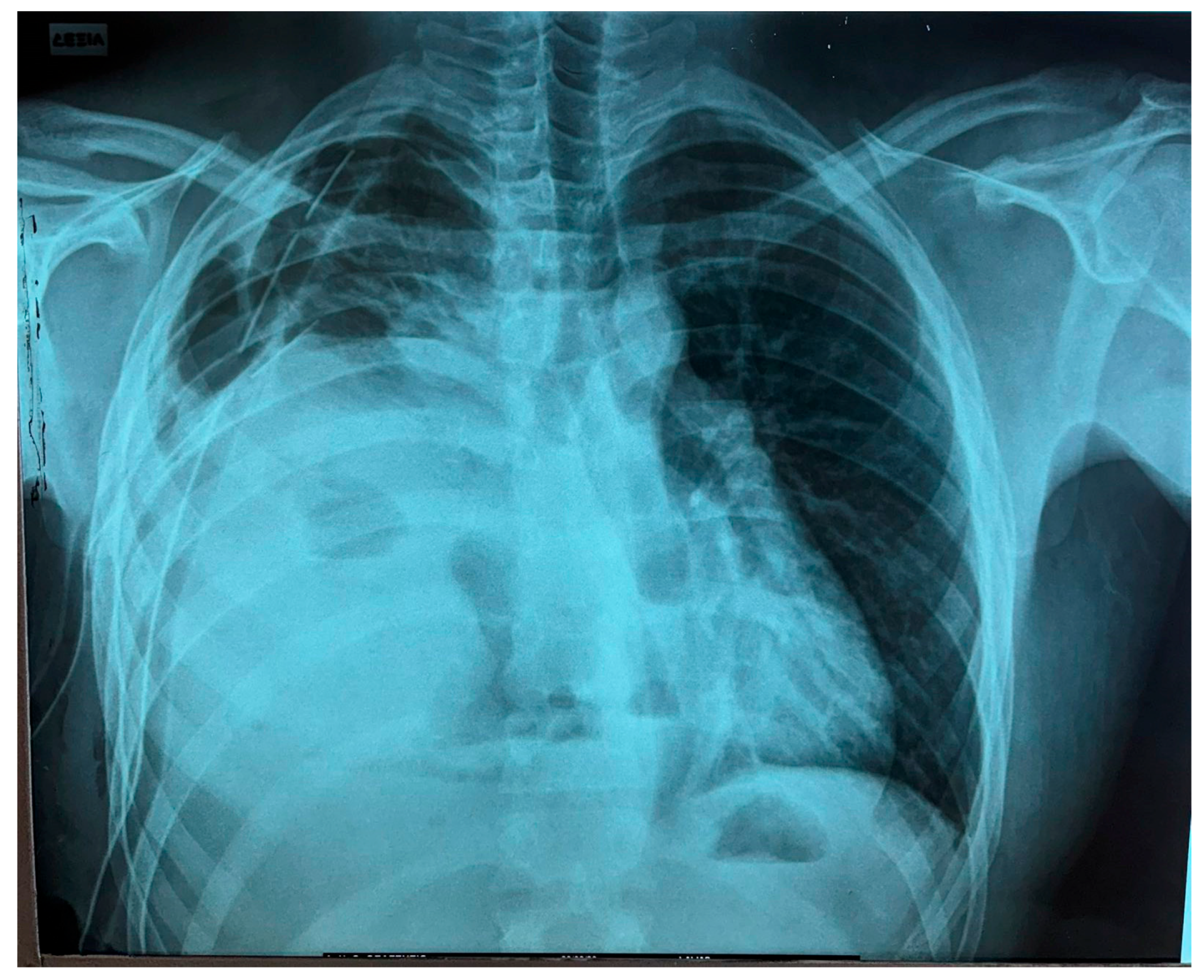
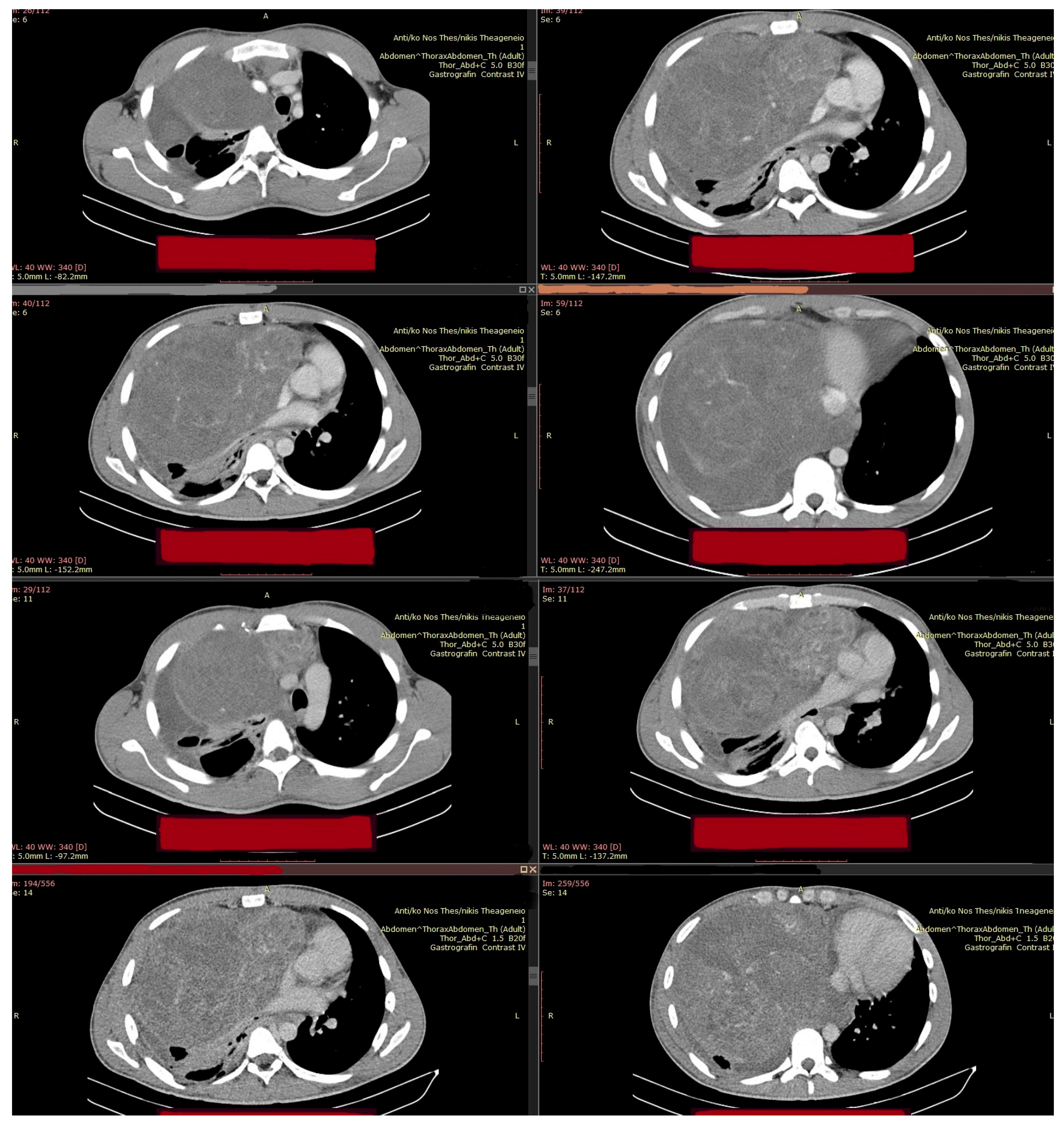
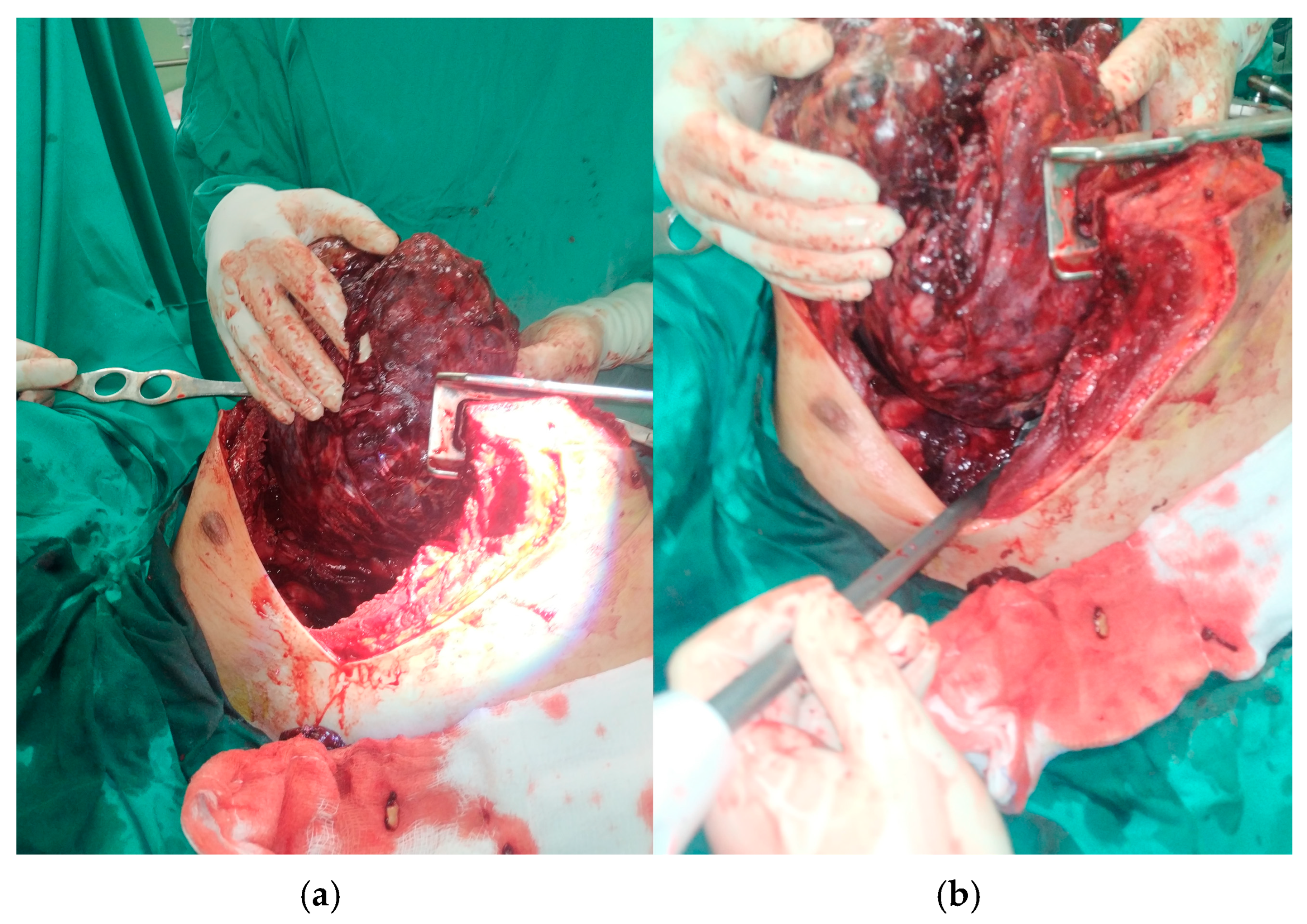
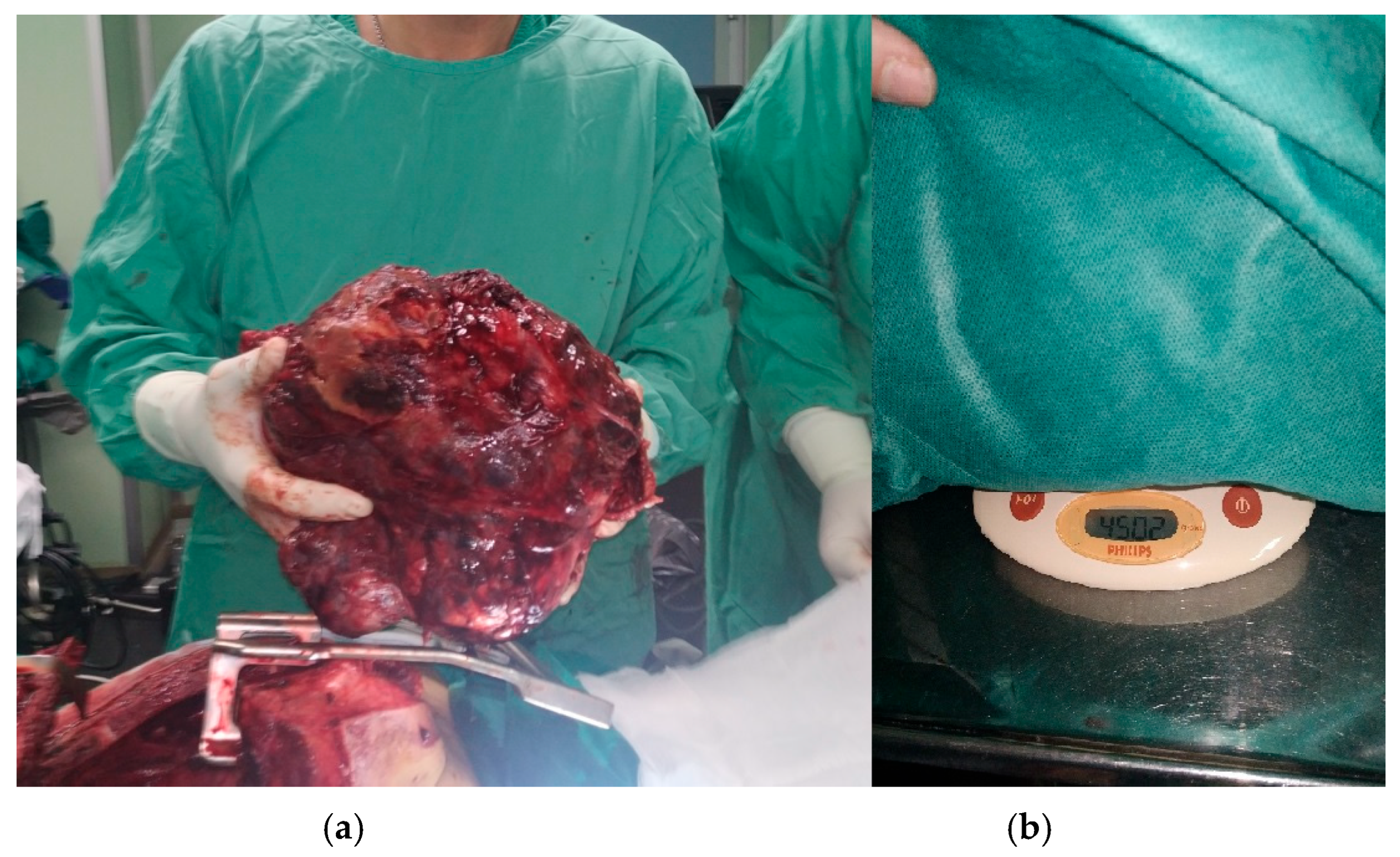
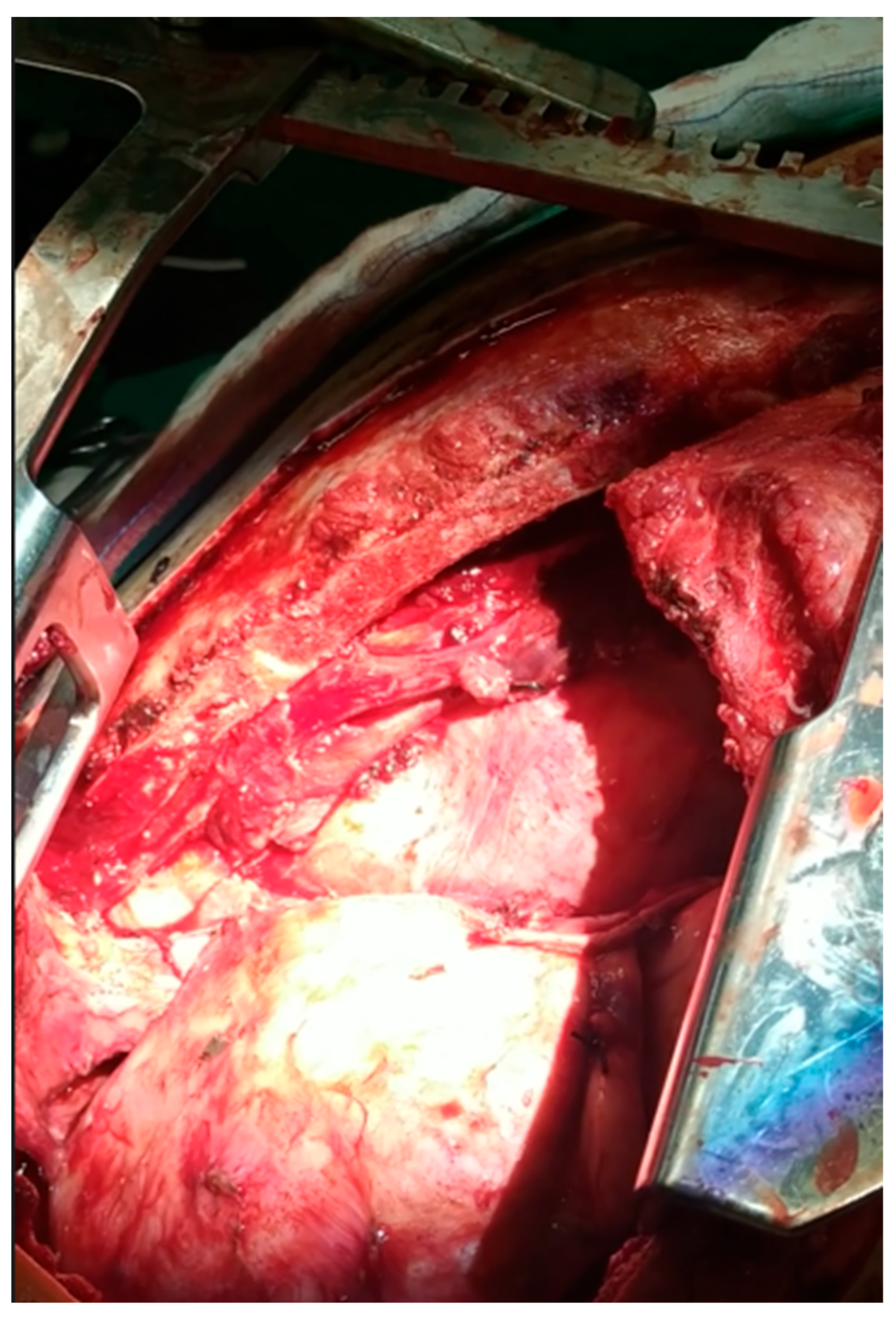
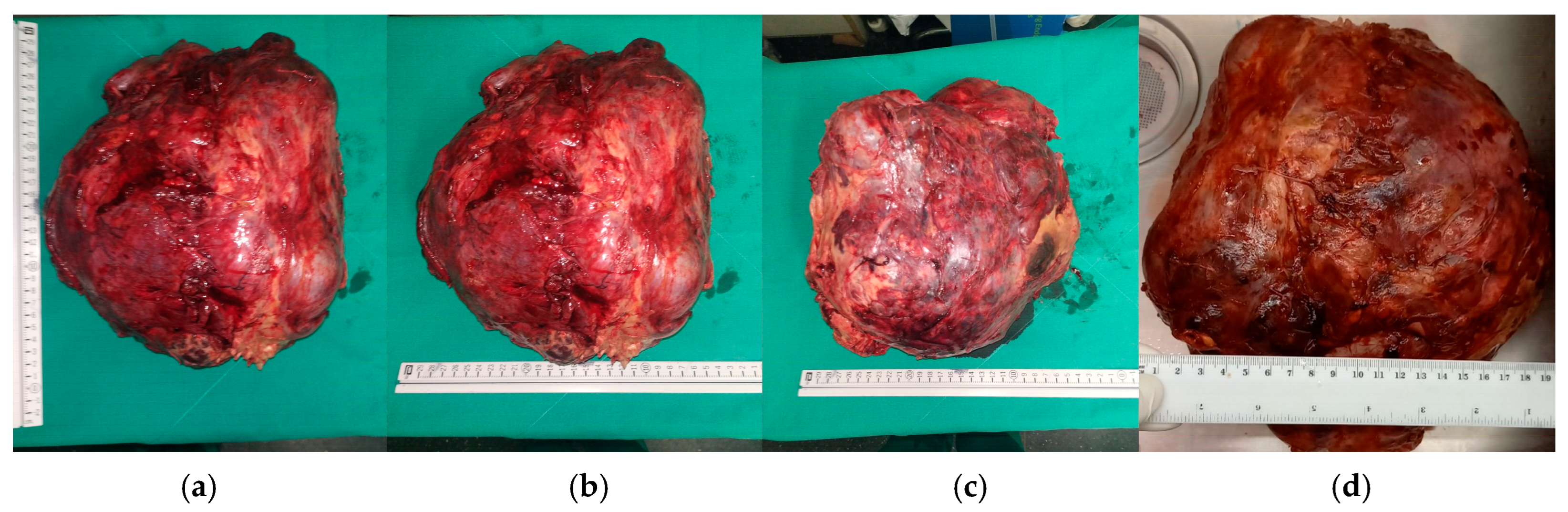
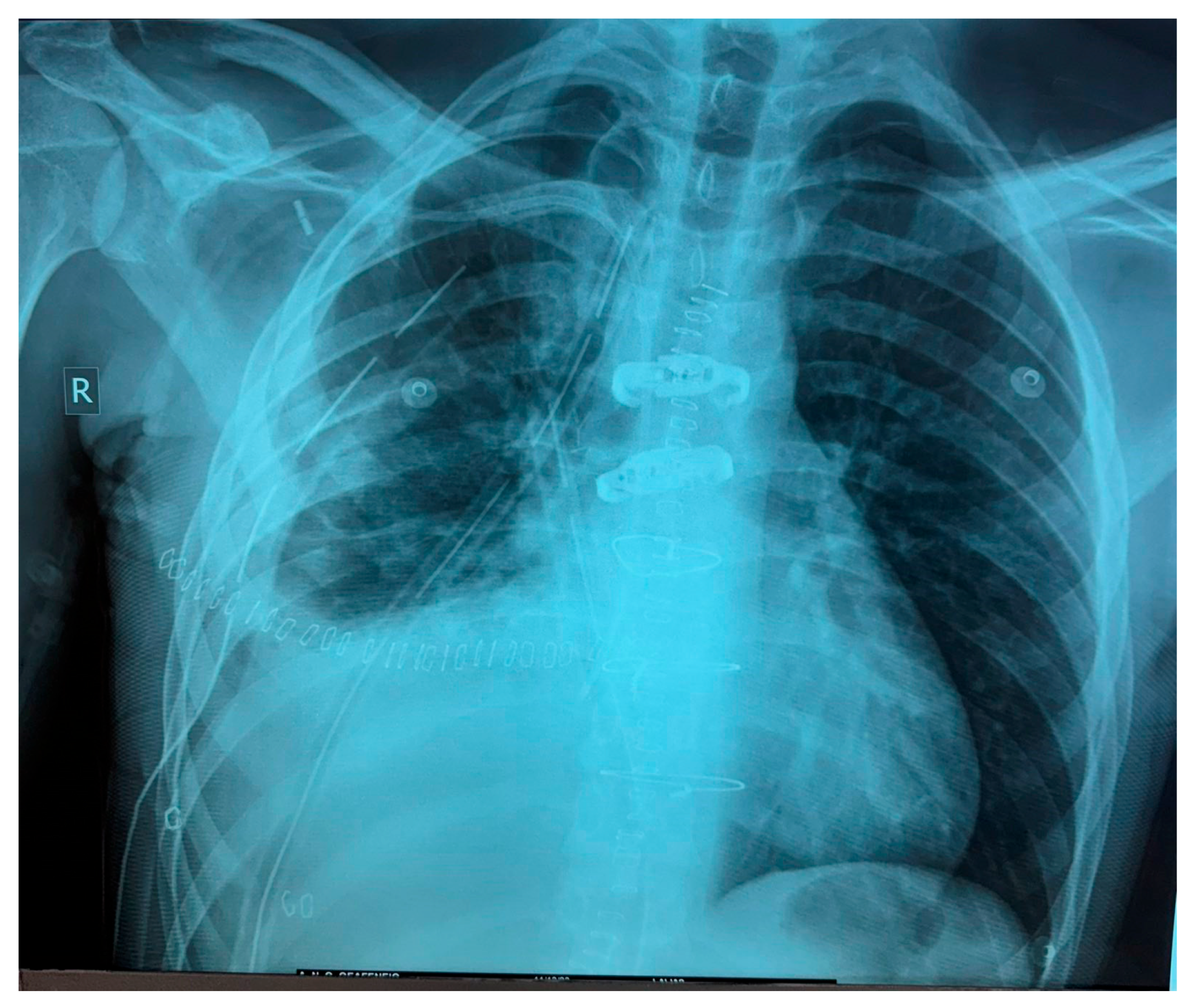
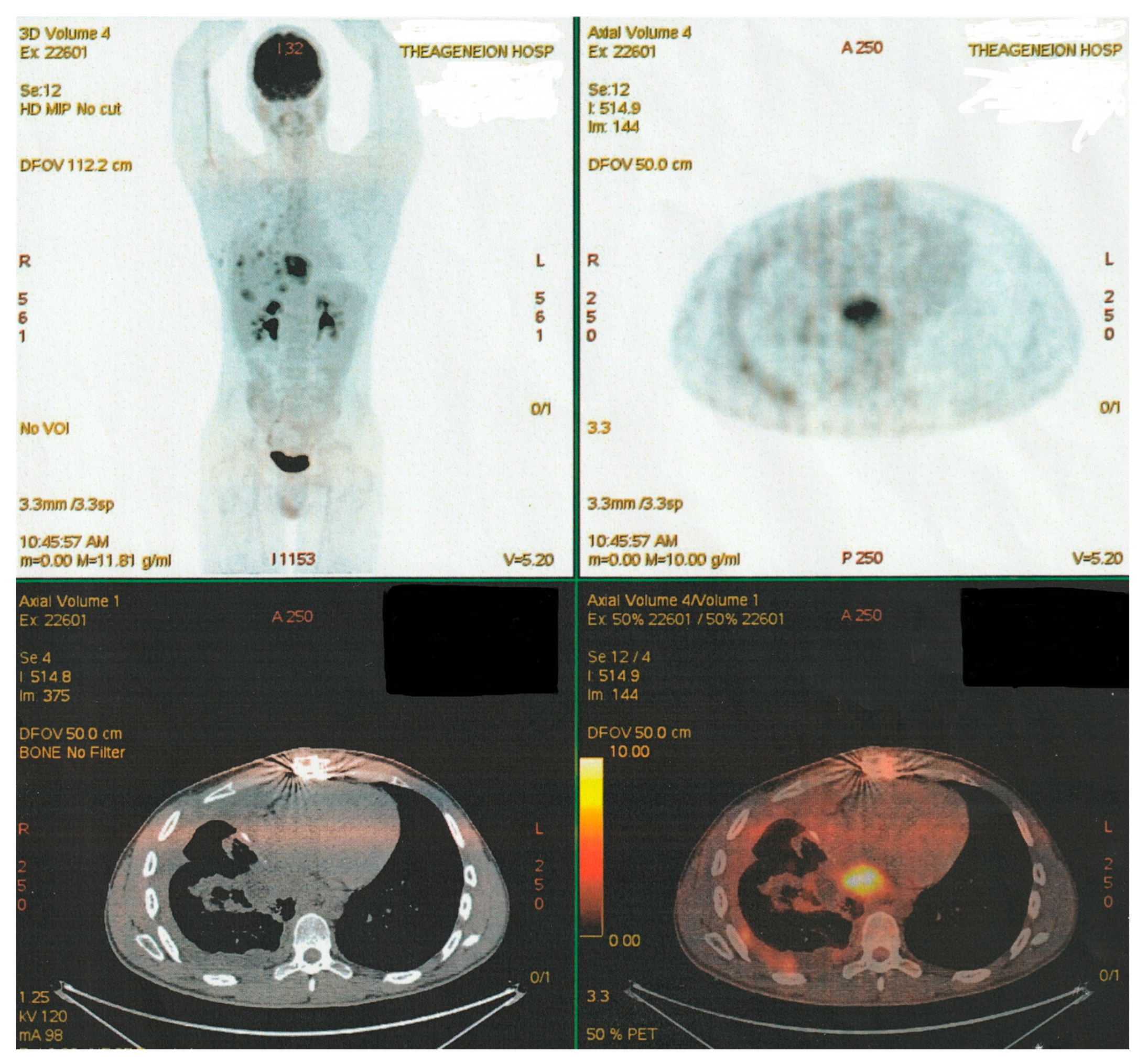
2. Clinical Presentation
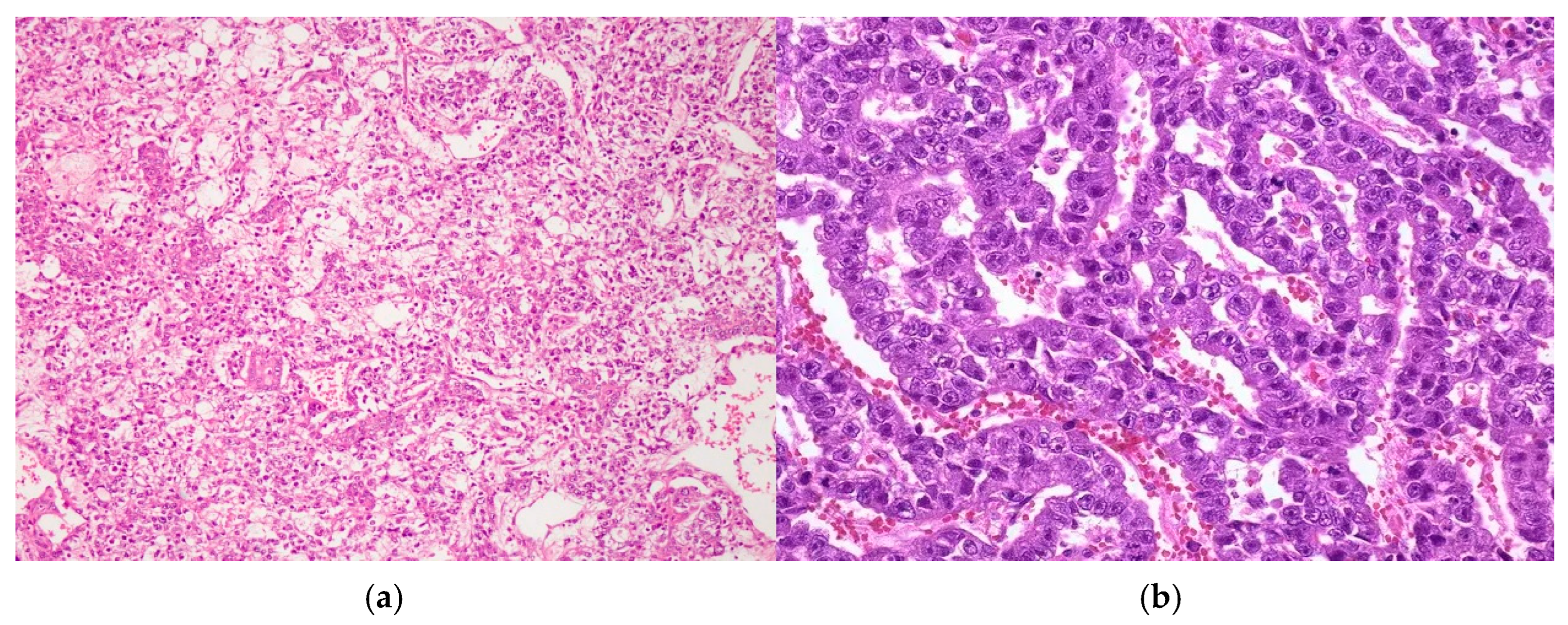
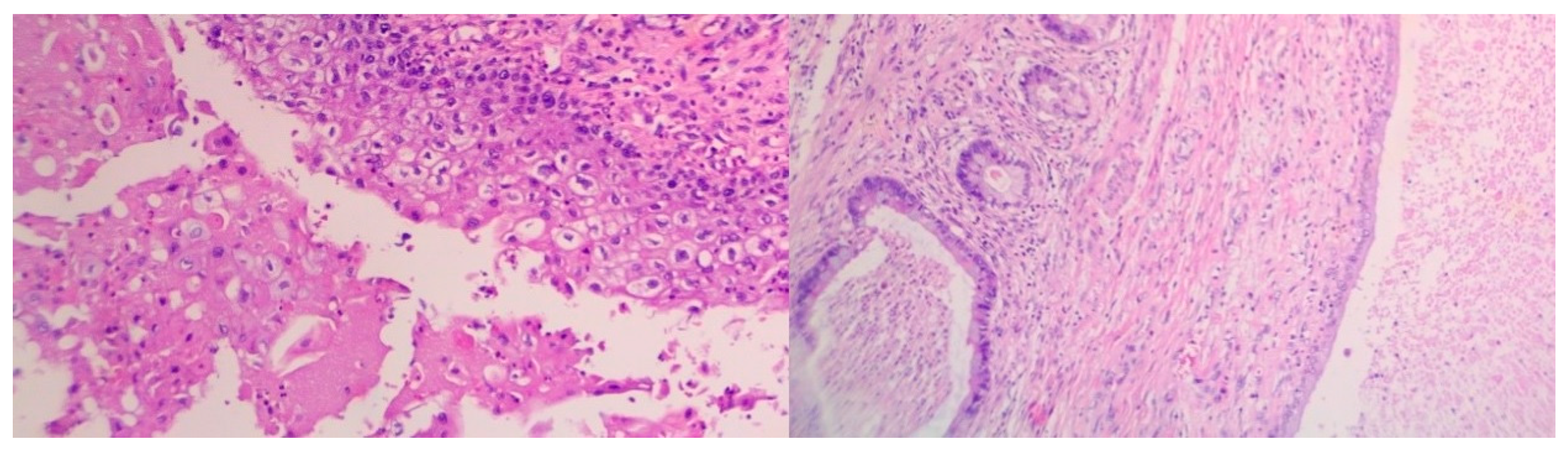
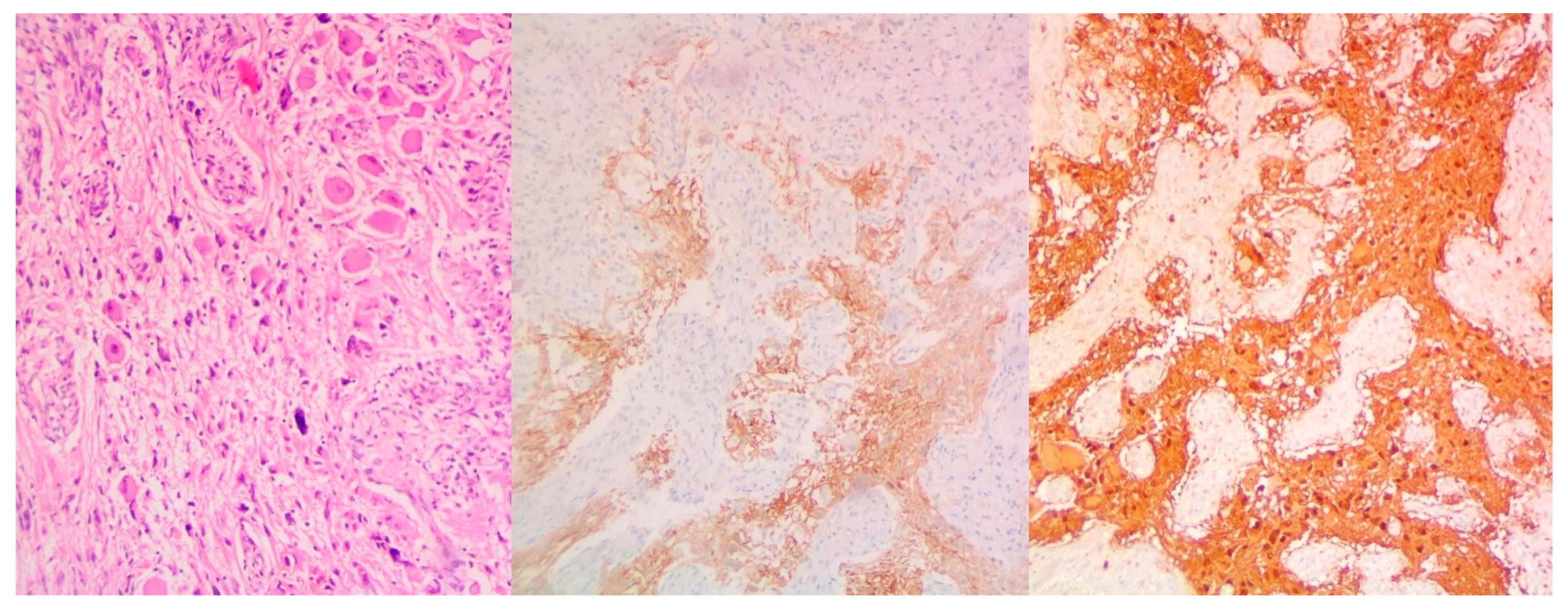
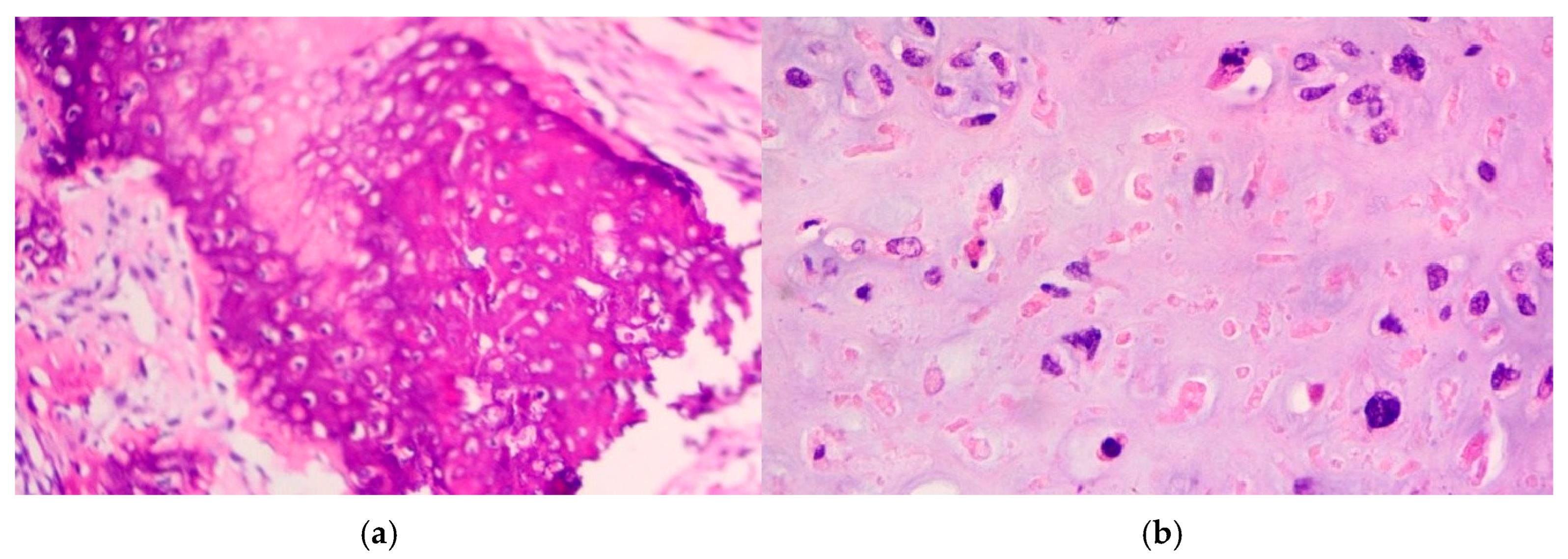
- Extensive necrosis and non-viable embryonal carcinoma, suggesting a partial chemotherapeutic response.
- Residual teratoma of postpubertal type, consistent with earlier findings.
- Scattered foci of high-grade somatic-type malignancies, including:
- ○
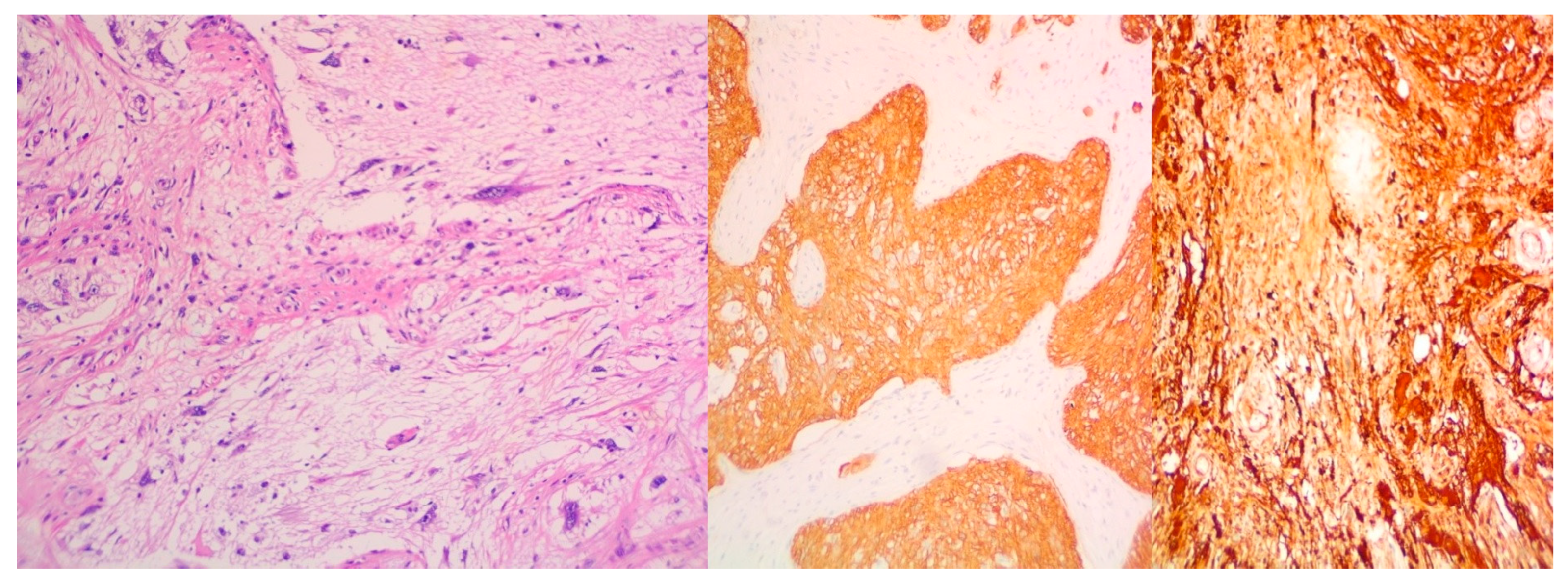
- Rhabdomyosarcoma (Desmin+, Sarcomeric Actin+);
- ○
- Chondrosarcoma (S100+);
- ○
- Osteosarcoma; and
- ○
- High-grade glial malignant elements, morphologically and immunohistochemically consistent with glioblastoma (GFAP+, NSE+, S100+).
- ❖
- Rhabdomyosarcoma tends to be aggressive and may respond to vincristine-, actinomycin D-, and cyclophosphamide-based regimens, though its chemosensitivity is less predictable.
- ❖
- Osteo- and chondrosarcoma components are typically chemoresistant, particularly when arising within a GCT.
- ❖
- Glioblastoma-like elements are extremely rare in this setting and are universally aggressive, with little data supporting responsiveness to standard glioma protocols in extraneural locations.
3. Discussion
- Somatic transformation may emerge from various germ cell lineages (teratomatous, embryonal);
- Transformation may occur along multiple differentiation pathways;
- Routine imaging or serologic monitoring alone is insufficient to capture progression in such biologically diverse tumors.
- ✓
- Detecting early signs of somatic transformation;
- ✓
- Differentiating among components with distinct therapeutic vulnerabilities;
- ✓
- Guiding the selection of non-standard chemotherapy protocols or other targeted agents (e.g., sarcoma- or glioma-directed therapy).
4. Conclusions
- ➢
- Preoperative biopsy may underestimate tumor heterogeneity, necessitating a high index of suspicion and readiness for definitive surgery post-chemotherapy, while post-chemotherapy resection is essential in the management of mediastinal tumors, even in apparent responders. Physicians must not over-rely on limited biopsy results when imaging or clinical behavior suggest a more aggressive or atypical course.
- ➢
- Tumor markers (aFP, β-HCG) may not reflect recurrence.
- ➢
- Somatic transformation should be suspected in cases with atypical clinical behavior or incomplete radiologic regression. Such developments challenge existing treatment paradigms. Incomplete responses should raise suspicion and prompt the consideration of aggressive surgery.
- ➢
- Histological surprise remains a real risk in mediastinal tumors, especially in young patients. Surgeons must be prepared for unexpected intraoperative findings and the possibility that complete resection is both diagnostic and therapeutic. Surgical resection should not be delayed if imaging suggests residual viable tumor post-chemotherapy, even when biopsy suggests a partial response. Upfront surgery or early post-chemotherapy resection may offer both therapeutic and definitive diagnostic benefits.
- ➢
- Multimodal imaging follow-up (CT and MRI) every 3–6 months in the first two years is crucial to detect relapse early, succeeded with annual follow-up, up to 5 years, with prompt evaluation of new symptoms.
- ➢
- Multidisciplinary collaboration is non-negotiable. Pathologists, thoracic surgeons, oncologists, and radiologists must be involved early and throughout treatment to recognize rare variants and guide dynamic management.
- ➢
- New treatment strategies are urgently needed, potentially involving molecular characterization and targeted therapy trials for STM components.
- ➢
- A centralized registry for rare mediastinal tumors would help consolidate clinical experience, improve diagnostic accuracy, and support future research into therapeutic innovations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aFP | Alpha-Fetoprotein |
| ASCT | autologous stem cell transplantation |
| CT | Computer Tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| EGGCT | Extragonadal Germ-Cell tumor |
| FNA | Fine-Needle Aspiration |
| G-CSF | human Granulocyte Colony-Stimulating factor |
| GCT | Germ-Cell tumor |
| HDCT | high-dose chemotherapy |
| IGCCCG | International Germ Cell Cancer Collaborative Group |
| IVC | Inferior Vena Cava |
| LDH | Lactate Dehydrogenase |
| MDT | multidisciplinary tumor board |
| MGCT | Mixed Germ-Cell tumor |
| MRI | Magnetic Resonance Imaging |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| pmNSGCT | primary mediastinal Non-Seminomatous Germ-Cell tumor |
| RBC | red blood cells |
| RLL | Right Lower Lobe |
| SUV | standardized Uptake Value |
| SVC | Superior Vena Cava |
| VATS | Video-Assisted Thoracic Surgery |
| β-HCG | beta-human Chorionic Gonadotropin |
References
- Marx, A.; Chan, J.K.; Chalabreysse, L.; Dacic, S.; Detterbeck, F.; French, C.A.; Hornick, J.L.; Inagaki, H.; Jain, D.; Lazar, A.J.; et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J. Thorac. Oncol. 2022, 17, 200–213. [Google Scholar] [CrossRef]
- Palade, E.; Delaca, G.B.; Titu, I.-M.; Ciulic, S.A.; Cismaru, G.; Stef, A.; Manole, S. Defining “Giant” Mediastinal Tumors: Proposal of a New Clinical–Radiological Classification and Case Report. Diagnostics 2025, 15, 159. [Google Scholar] [CrossRef]
- Damek, A.; Kurch, L.; Franke, F.C.; Attarbaschi, A.; Beishuizen, A.; Cepelova, M.; Ceppi, F.; Daw, S.; Dieckmann, K.; Fernández-Teijeiro, A.; et al. Hodgkin lymphoma: Hypodense lesions in mediastinal masses. Sci. Rep. 2024, 14, 14591. [Google Scholar] [CrossRef]
- Sen, I.; Kalra, M.; Gloviczki, P. Interventions for superior vena cava syndrome. J. Cardiovasc. Surg. 2022, 63, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Peng, Q.; Tang, W.; Wu, N.; Yang, L.; Qi, L.; Li, J.; Huang, Y. Magnetic Resonance Elastography of Anterior Mediastinal Tumors. J. Magn. Reson. Imaging 2025, 61, 841–848. [Google Scholar] [CrossRef]
- Collaud, S.; Stork, T.; Kaman, H.; Bauer, S.; Pöttgen, C.; Schildhaus, H.-U.; Schmack, B.; Aigner, C. Giant middle mediastinal lesions: When tumor size correlates with mesenchymal origin-a retrospective single-center analysis. Mediastinum 2023, 7, 24. [Google Scholar] [CrossRef]
- Lott-Limbach, A.A.; Wakely, P.E., Jr. Mediastinal sarcomas: Experience using fine needle aspiration cytopathology. Mediastinum 2020, 4, 14. [Google Scholar] [CrossRef]
- Kang, K.; Wang, S.; Li, B.; Wang, M.; Xiong, F. Single-port thoracoscopic resection of a posterior mediastinal Mullerian cyst in a woman. J. Int. Med. Res. 2022, 50, 3000605221096275. [Google Scholar] [CrossRef] [PubMed]
- Renaud, L.; Donzel, M.; Decroocq, J.; Decazes, P.; Galtier, J.; Burroni, B.; Veresezan, E.-L.; Sesboüé, C.; Dartigues, P.; Chassagne-Clément, C.; et al. Primary mediastinal B-cell lymphoma (PMBCL): The LYSA pragmatic guidelines. Eur. J. Cancer 2025, 220, 115369. [Google Scholar] [CrossRef]
- Özdemir, Ö. Anterior mediastinal masses and thymic cysts. World J. Clin. Cases 2025, 13, 95035. [Google Scholar] [CrossRef] [PubMed]
- Brcic, L.; Roden, A.C. Histopathological features of giant mediastinal tumors-a literature review. Mediastinum 2023, 7, 37. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, K.; Zhang, X. Primary mediastinal yolk sac tumor: A case report and literature review. Clin. Case Rep. 2023, 11, e7781. [Google Scholar] [CrossRef]
- Camus, V.; Molina, T.J.; Desmots, F.; Blanc-Durand, P.; Kanoun, S.; Moslemi, A.; Ruminy, P.; Le Gouill, S.; Ghesquieres, H.; Obéric, L.; et al. Interim PET after 4 cycles predicts outcome in histomolecularly confirmed primary mediastinal B-cell lymphoma. Blood Adv. 2025, 9, 2232–2246. [Google Scholar] [CrossRef] [PubMed]
- Sakura, M.; Tsukamoto, T.; Yonese, J.; Nakaishi, M.; Ohkubo, Y.; Maezawa, T.; Takimoto, K.; Fukui, I.; Nakagawa, K.; Okumura, S.; et al. The result of VIP chemotherapy as an induction therapy in 6 patients with non-seminomatous extragonadal germ cell tumor. Nihon Hinyokika Gakkai Zasshi 2004, 95, 634–637. [Google Scholar] [CrossRef][Green Version]
- Arteta, A.A.; Santiago-Pacheco, V.; Villada, J.C. Primary mediastinal tumors in children and adults: A clinicopathological spectrum. Mediastinum 2025, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, C.; Nichols, C.R.; Droz, J.-P.; Schmoll, H.-J.; Horwich, A.; Gerl, A.; Fossa, S.D.; Beyer, J.; Pont, J.; Kanz, L.; et al. Extragonadal Germ Cell Tumors of the Mediastinum and Retroperitoneum: Results from an International Analysis. J. Clin. Oncol. 2002, 20, 1864–1873. [Google Scholar] [CrossRef]
- Kessler, R.C.; Merikangas, K.R. The National Comorbidity Survey Replication (NCS-R): Background and aims. Int. J. Methods Psychiatr. Res. 2004, 13, 60–68. [Google Scholar] [CrossRef]
- Al Kindi, A.H.; A Al Kindi, F.; Al Riyami, M.; Khalil, E. Giant Mediastinal Myxoid Pleomorphic Liposarcoma. Sultan Qaboos Univ. Med. J. 2023, 23, 271–273. [Google Scholar] [CrossRef]
- Kim, H.K. Selective Mediastinal Lymph Node Dissection: Precision Surgery in the Era of Precision Medicine. J. Thorac. Oncol. 2023, 18, 834–837. [Google Scholar] [CrossRef]
- Bordas-Martinez, J.; Vercher-Conejero, J.; Rodriguez-González, G.; Notta, P.; Cabeza, C.M.; Cubero, N.; Lopez-Lisbona, R.; Diez-Ferrer, M.; Tebé, C.; Santos, S.; et al. Mediastinal staging lymph node probability map in non-small cell lung cancer. Respir. Res. 2025, 26, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-F.; Bai, C.-M.; Yang, D.; Chen, S.-C. Clinical analysis of primary mediastinal germ cell tumors. Zhonghua Zhong Liu Za Zhi 2007, 29, 531–534. [Google Scholar] [PubMed]
- Dunne, E.G.; Fick, C.N.; Jones, D.R. Mediastinal Staging in Non-Small-Cell Lung Cancer: Saying Goodbye to Mediastinoscopy. J. Clin. Oncol. 2023, 41, 3785–3790. [Google Scholar] [CrossRef]
- Baskin, A.S.; Burapachaisri, K.; Guha, S.; Velotta, J.B. The Landmark Series: Advances in Preoperative Mediastinal Lymph Node Staging for Non-small Cell Lung Cancer (NSCLC). Ann. Surg. Oncol. 2025, 32, 3175–3186. [Google Scholar] [CrossRef]
- McKenney, J.K.; Heerema-McKenney, A.; Rouse, R.V. Extragonadal germ cell tumors: A review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv. Anat. Pathol. 2007, 14, 69–92. [Google Scholar] [CrossRef]
- Ozgun, G.; Nappi, L. Primary Mediastinal Germ Cell Tumors: A Thorough Literature Review. Biomedicines 2023, 11, 487. [Google Scholar] [CrossRef]
- Wang, X.; Huang, P.; Wang, Z.; Liu, Y.; Fan, B.; Dong, W. Radiomics in thymic epithelial tumors: A scoping review of current status and advances. BMC Cancer 2025, 25, 493. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Kanda, S.; Nihonmatu, R.; Gomi, D.; Sekiguchi, N.; Noguchi, T.; Fukushima, T.; Kobayashi, T.; Yamamoto, H.; Takeda, T. Primary mediastinal germ cell tumors—A retrospective analysis of >30 years of experience in a single institution. Thorac. Cancer 2021, 12, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Carter, Y.M. Mediastinal Nonseminoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Azuma, Y.; Tochigi, N.; Sano, A.; Sakai, T.; Iyoda, A. Thoracoscopic resection of bilateral multiple superior mediastinal neurofibromas. J. Cardiothorac. Surg. 2021, 16, 310. [Google Scholar] [CrossRef]
- Azuma, Y.; Tochigi, N.; Sano, A.; Sakai, T.; Iyoda, A. Transformation of a low-grade glioma into a glioblastoma along with the development of lung and mediastinal lymph node metastases after repeated craniotomy: A case report. Ibrain 2023, 10, 385–389. [Google Scholar] [CrossRef]
- Dean, L.W.; Wong, N.C.; Dason, S.; Isharwal, S.; Donoghue, M.; Jia, L.; Tap, W.D.; Joseph, G.; Funt, S.A.; McHugh, D.J.; et al. Clinical features and outcomes of secondary somatic malignancy (SSM) associated with primary mediastinal nonseminomatous germ cell tumors (PM-NSGCT). J. Clin. Oncol. 2019, 37, 531. [Google Scholar] [CrossRef]
- Aoshima, H.; Omata, M.; Shidei, H.; Ogihara, A.; Mitsuboshi, S.; Isaka, T.; Matsumoto, T.; Kanzaki, M. Robotic resection of mediastinal tumors: Surgical approach and procedure. BMC Surg. 2025, 25, 115. [Google Scholar] [CrossRef] [PubMed]
| Days 1 and 15 | Days 1–5 | |
|---|---|---|
| Bleomycin® | 30,000 IU i.v. | |
| Cisplatin® | 20 mg/m2 i.v. | |
| Etoposide® | 100 mg/m2 i.v. |
| Days 1–5 | Day 1 | Days 1–5 | |
|---|---|---|---|
| Cisplatin® | 20 mg/m2 i.v. | ||
| Etoposide® | 75 mg/m2 i.v. | ||
| Ifosfamide® | 1200 mg/m2 i.v. | ||
| Mesna® | 120 mg/m2 i.v. | 1200 mg/m2 i.v. |
| Days 2–5 | Day 1 | Hour 0/4/8 | |
|---|---|---|---|
| Cisplatin® | 25 mg/m2 i.v. | ||
| Ifosfamide® | 1500 mg/m2 i.v. | ||
| Paclitaxel® and Denosumab® | 250 mg/m2 i.v. | ||
| Mesna® | 500 mg/m2 i.v. |
| Day 1 | Days 1–2 | Days 1–5 | |||
|---|---|---|---|---|---|
| Vincristine® | 2 mg max i.v. | ||||
| Doxorubicin® | 37.5 mg/m2 i.v. | ||||
| Cyclophosphamide® | 1200 mg/m2 i.v. | ||||
| Ifosfamide® | 100 mg/m2 i.v. | ||||
| Before Cyclophosphamide® | 2 and 6 h post-infusion (VDC) | Days 1–5 | Continuous infusion with Ifosfamide® | After (IE) infusion | |
| Mesna® | 240 mg/m2 i.v. bolus | 480 mg/m2 i.v. | 360 mg/m2 i.v. bolus | 1800 mg/m2 i.v. | 400 mg/m2 i.v. bolus |
| 30 min before Doxorubicin® infusion | |||||
| Dexrazoxane® | 750 mg/m2 i.v. | ||||
| Biomarker | Exon/Region | Genomic Alteration | Classification | Method |
|---|---|---|---|---|
| HRR Status (Non-Gene Biomarker) | - | - | Not Detected | Next-Generation Sequencing (NGS) |
| TMB (Tumor Mutational Burden) (Non-Gene Biomarker) | - | - | 7.68 muts/MB | Next-Generation Sequencing (NGS) |
| PTEN (Gene) | Exon 5 | c.394G>A (p.G1325) | Likely Pathogenic | Next-Generation Sequencing (NGS) |
| TP53 (Gene) | Exon 5 | c.454_466del (p.P152Afs*14) | Likely Pathogenic | Next-Generation Sequencing (NGS) |
| PIK3R1 (Gene) | Exon 10 | c.1126G>A (p.G376R) | Likely Pathogenic | Next-Generation Sequencing (NGS) |
| HIST1H2BO (Gene) | - | c.109_110delAG | Variant of Uncertain Significance (VUS) | Next-Generation Sequencing (NGS) |
| SMARCB1 (Gene) | - | c.790A>G | Variant of Uncertain Significance (VUS) | Next-Generation Sequencing (NGS) |
| ROS1 (Gene) | - | c.5270G>A | Variant of Uncertain Significance (VUS) | Next-Generation Sequencing (NGS) |
| KDM5A (Gene) | - | c.2150G>T | Variant of Uncertain Significance (VUS) | Next-Generation Sequencing (NGS) |
| MSI (Non-Gene Biomarker) | - | Stable | MSI-Stable | Immunohistochemistry |
| HER2 (Non-Gene Biomarker) | - | Negative | HER2-Negative | Immunohistochemistry |
| PD-L1 (Non-Gene Biomarker) | - | CPS < 1% | Low Expression | Immunohistochemistry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theocharidis, V.; Rallis, T.; Gogakos, A.; Paliouras, D.; Lazopoulos, A.; Koutourini, M.; Tzinevi, M.; Vildiridi, A.; Dimopoulos, P.; Kasarakis, D.; et al. Multi-Level Oncological Management of a Rare, Combined Mediastinal Tumor: A Case Report. Curr. Oncol. 2025, 32, 423. https://doi.org/10.3390/curroncol32080423
Theocharidis V, Rallis T, Gogakos A, Paliouras D, Lazopoulos A, Koutourini M, Tzinevi M, Vildiridi A, Dimopoulos P, Kasarakis D, et al. Multi-Level Oncological Management of a Rare, Combined Mediastinal Tumor: A Case Report. Current Oncology. 2025; 32(8):423. https://doi.org/10.3390/curroncol32080423
Chicago/Turabian StyleTheocharidis, Vasileios, Thomas Rallis, Apostolos Gogakos, Dimitrios Paliouras, Achilleas Lazopoulos, Meropi Koutourini, Myrto Tzinevi, Aikaterini Vildiridi, Prokopios Dimopoulos, Dimitrios Kasarakis, and et al. 2025. "Multi-Level Oncological Management of a Rare, Combined Mediastinal Tumor: A Case Report" Current Oncology 32, no. 8: 423. https://doi.org/10.3390/curroncol32080423
APA StyleTheocharidis, V., Rallis, T., Gogakos, A., Paliouras, D., Lazopoulos, A., Koutourini, M., Tzinevi, M., Vildiridi, A., Dimopoulos, P., Kasarakis, D., Kousidis, P., Nikolaidou, A., Vrochidis, P., Mironidou-Tzouveleki, M., & Barbetakis, N. (2025). Multi-Level Oncological Management of a Rare, Combined Mediastinal Tumor: A Case Report. Current Oncology, 32(8), 423. https://doi.org/10.3390/curroncol32080423






