Clinical Impact of CTLA-4 Single-Nucleotide Polymorphism in DLBCL Patients Treated with CAR-T Cell Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Endpoints
2.3. Statistical Analysis
2.4. CTLA4 Gene Analysis
3. Results
3.1. Prevalence of the CTLA4 A17 Allele in European DLBCL Patients
3.2. Baseline Clinical Characteristics of the DLBCL Patient Cohort
3.3. Clinical Characteristics and CAR-T Cell Therapy
3.4. Treatment Outcomes—Univariate Analysis
3.5. Treatment Outcomes—Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in Refractory Diffuse Large B-Cell Lymphoma: Results from the International SCHOLAR-1 Study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Goy, A.; Dunleavy, K. CAR T-Cell Therapy in Highly Aggressive B-Cell Lymphoma: Emerging Biological and Clinical Insights. Blood 2022, 140, 1461–1469. [Google Scholar] [CrossRef]
- Johnson, P.C.; Abramson, J.S. Engineered T Cells: CAR T Cell Therapy and Beyond. Curr. Oncol. Rep. 2022, 24, 23–31. [Google Scholar] [CrossRef]
- Westin, J.; Sehn, L.H. CAR T Cells as a Second-Line Therapy for Large B-Cell Lymphoma: A Paradigm Shift? Blood 2022, 139, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Dave, H.; Jerkins, L.; Hanley, P.J.; Bollard, C.M.; Jacobsohn, D. Driving the CAR to the Bone Marrow Transplant Program. Curr. Hematol. Malig. Rep. 2019, 14, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Cappell, K.M.; Kochenderfer, J.N. Long-Term Outcomes Following CAR T Cell Therapy: What We Know so Far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef]
- Negishi, S.; Girsch, J.H.; Siegler, E.L.; Bezerra, E.D.; Miyao, K.; Sakemura, R.L. Treatment Strategies for Relapse after CAR T-Cell Therapy in B Cell Lymphoma. Front. Pediatr. 2023, 11, 1305657. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Westin, J.R.; Tam, C.S.; Borchmann, P.; Jaeger, U.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Andreadis, C.; et al. Correlative Analyses of Patient and Clinical Characteristics Associated with Efficacy in Tisagenlecleucel-Treated Relapsed/Refractory Diffuse Large B-Cell Lymphoma Patients in the Juliet Trial. Blood 2019, 134, 4103. [Google Scholar] [CrossRef]
- Fioretti, S.; Matson, C.A.; Rosenberg, K.M.; Singh, N.J. Host B Cells Escape CAR-T Immunotherapy by Reversible Downregulation of CD19. Cancer Immunol. Immunother. 2023, 72, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Locke, F.L.; Rossi, J.M.; Neelapu, S.S.; Jacobson, C.A.; Miklos, D.B.; Ghobadi, A.; Oluwole, O.O.; Reagan, P.M.; Lekakis, L.J.; Lin, Y.; et al. Tumor Burden, Inflammation, and Product Attributes Determine Outcomes of Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv. 2020, 4, 4898–4911. [Google Scholar] [CrossRef]
- Shah, N.N.; Fry, T.J. Mechanisms of Resistance to CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef]
- Seipel, K.; Abbühl, M.; Bacher, U.; Nilius, H.; Daskalakis, M.; Pabst, T. Clinical Impact of Single Nucleotide Polymorphism in CD-19 on Treatment Outcome in FMC63-CAR-T Cell Therapy. Cancers 2023, 15, 3058. [Google Scholar] [CrossRef]
- Nirschl, C.J.; Drake, C.G. Molecular Pathways: Co-Expression of Immune Checkpoint Molecules: Signaling Pathways and Implications for Cancer Immunotherapy. Clin. Cancer Res. 2013, 19, 4917–4924. [Google Scholar] [CrossRef]
- Hossen, M.M.; Ma, Y.; Yin, Z.; Xia, Y.; Du, J.; Huang, J.Y.; Huang, J.J.; Zou, L.; Ye, Z.; Huang, Z. Current Understanding of CTLA-4: From Mechanism to Autoimmune Diseases. Front. Immunol. 2023, 14, 1198365. [Google Scholar] [CrossRef]
- Monne, M.; Piras, G.; Palmas, A.; Arru, L.; Murineddu, M.; Latte, G.; Noli, A.; Gabbas, A. Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) Gene Polymorphism and Susceptibility to Non-Hodgkin’s Lymphoma. Am. J. Hematol. 2004, 76, 14–18. [Google Scholar] [CrossRef]
- Cheng, T.-Y.; Lin, J.-T.; Chen, L.-T.; Shun, C.-T.; Wang, H.-P.; Lin, M.-T.; Wang, T.-E.; Cheng, A.-L.; Wu, M.-S. Association of T-Cell Regulatory Gene Polymorphisms with Susceptibility to Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. J. Clin. Oncol. 2006, 24, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Shi, Z.; Gao, L.; Zhang, L.; Wei, S.; Chen, Y.; Lu, C.; Wang, J.; Zuo, L.; Zhang, L. Impact of the Cytotoxic T-Lymphocyte Associated Antigen-4 Rs231775 A/G Polymorphism on Cancer Risk. Heliyon 2023, 9, e23164. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhou, H.; Feng, Y.; Chen, Y.; Zhu, L.; Mi, Y. Comprehensive Analysis of 29,464 Cancer Cases and 35,858 Controls to Investigate the Effect of the Cytotoxic T-Lymphocyte Antigen 4 Gene Rs231775 A/G Polymorphism on Cancer Risk. Front. Oncol. 2022, 12, 878507. [Google Scholar] [CrossRef] [PubMed]
- Anjos, S.; Nguyen, A.; Ounissi-Benkalha, H.; Tessier, M.-C.; Polychronakos, C. A Common Autoimmunity Predisposing Signal Peptide Variant of the Cytotoxic T-Lymphocyte Antigen 4 Results in Inefficient Glycosylation of the Susceptibility Allele. J. Biol. Chem. 2002, 277, 46478–46486. [Google Scholar] [CrossRef]
- Mäurer, M.; Loserth, S.; Kolb-Mäurer, A.; Ponath, A.; Wiese, S.; Kruse, N.; Rieckmann, P. A Polymorphism in the Human Cytotoxic T-Lymphocyte Antigen 4 (CTLA4) Gene (Exon 1 +49) Alters T-Cell Activation. Immunogenetics 2002, 54, 1–8. [Google Scholar] [CrossRef]
- Agarwal, S.; Aznar, M.A.; Rech, A.J.; Good, C.R.; Kuramitsu, S.; Da, T.; Gohil, M.; Chen, L.; Hong, S.-J.A.; Ravikumar, P.; et al. Deletion of the Inhibitory Co-Receptor CTLA-4 Enhances and Invigorates Chimeric Antigen Receptor T Cells. Immunity 2023, 56, 2388–2407.e9. [Google Scholar] [CrossRef]
- Nydegger, A.; Novak, U.; Kronig, M.-N.; Legros, M.; Zeerleder, S.; Banz, Y.; Bacher, U.; Pabst, T. Transformed Lymphoma Is Associated with a Favorable Response to CAR-T-Cell Treatment in DLBCL Patients. Cancers 2021, 13, 6073. [Google Scholar] [CrossRef]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A Real-World Comparison of Tisagenlecleucel and Axicabtagene Ciloleucel CAR T Cells in Relapsed or Refractory Diffuse Large B Cell Lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef]
- Kennedy, A.; Robinson, M.A.; Hinze, C.; Waters, E.; Williams, C.; Halliday, N.; Dovedi, S.; Sansom, D.M. The CTLA-4 Immune Checkpoint Protein Regulates PD-L1:PD-1 Interaction via Transendocytosis of Its Ligand CD80. EMBO J. 2023, 42, e111556. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-Cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Butler, S.C.; Cui, J.; Cillo, A.R.; Cardello, C.; Liu, C.; Brunazzi, E.A.; Baessler, A.; Xie, B.; Kunning, S.R.; et al. LAG-3 and PD-1 Synergize on CD8+ T Cells to Drive T Cell Exhaustion and Hinder Autocrine IFN-γ-Dependent Anti-Tumor Immunity. Cell 2024, 187, 4355–4372.e22. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Jasek, M.; Karabon, L. Immune Checkpoint Molecules-Inherited Variations as Markers for Cancer Risk. Front. Immunol. 2020, 11, 606721. [Google Scholar] [CrossRef]
- Ansell, S.M.; Hurvitz, S.A.; Koenig, P.A.; LaPlant, B.R.; Kabat, B.F.; Fernando, D.; Habermann, T.M.; Inwards, D.J.; Verma, M.; Yamada, R.; et al. Phase I Study of Ipilimumab, an Anti-CTLA-4 Monoclonal Antibody, in Patients with Relapsed and Refractory B-Cell Non-Hodgkin Lymphoma. Clin. Cancer Res. 2009, 15, 6446–6453. [Google Scholar] [CrossRef]
- Tuscano, J.M.; Maverakis, E.; Groshen, S.; Tsao-Wei, D.; Luxardi, G.; Merleev, A.A.; Beaven, A.; DiPersio, J.F.; Popplewell, L.; Chen, R.; et al. A Phase I Study of the Combination of Rituximab and Ipilimumab in Patients with Relapsed/Refractory B-Cell Lymphoma. Clin. Cancer Res. 2019, 25, 7004–7013. [Google Scholar] [CrossRef] [PubMed]
| All | CTLA4 A17hom | CTLA4 T17Ahet | CTLA4 T17hom | p * | |
|---|---|---|---|---|---|
| Patients, n (%) | 111 (100) | 15 (14) | 46 (41) | 50 (45) | |
| Age at ID, years, median (range) | 62 (24–79) | 60 (43–75) | 64 (34–78) | 60 (24–79) | 0.08 1 |
| M:F (ratio) | 59:51 (1.2) | 6:9 (0.67) | 27:19 (1.7) | 26:24 (1.4) | 0.44 2 |
| Initial diagnosis | 111 (100) | 0.17 2 | |||
| DLBCL, n (%) | 102 (92) | 13 (92) | 44 (96) | 45 (90) | |
| de novo, n (% | 66 (65) | 4 (31) | 31 (70) | 31 (69) | |
| transformed, n (%) | 36 (35) | 9 (69) | 13 (30) | 14 (31) | |
| PMBCL, n (%) | 2 (2) | 0 | 0 | 2 (4) | |
| CLL/SLL, n (%) | 1 (1) | 1 (4) | 0 | 0 | |
| FL, n (%) | 6 (5) | 1 (4) | 2 (4) | 3 (6) | |
| Initial stage | 91 (100) | 0.36 2 | |||
| I, n (%) | 5 (4) | 0 | 2 (5) | 2 (5) | |
| II, n (%) | 16 (18) | 1 (10) | 7 (18) | 8 (19) | |
| III, n (%) | 17 (19) | 5 (45) | 3 (8) | 9 (21) | |
| IV, n (%) | 54 (59) | 5 (45) | 26 (69) | 23 (55) | |
| Risk (IPI) | 110 (100) | 0.67 2 | |||
| Low risk (0–1), n (%) | 3 (3) | 1 (7) | 1 (2) | 1 (2) | |
| Low–intermediate risk (2), n (%) | 8 (7) | 1 (7) | 3 (7) | 4 (6) | |
| High–intermediate risk (3), n(%) | 35 (32) | 5 (33) | 10 (22) | 20 (40) | |
| High risk (4–5), n (%) | 33 (30) | 4 (27) | 17 (40) | 12 (24) | |
| Nd, n (%) | 31 (28) | 3 (20) | 15 (33) | 13 (26) | |
| Chemo-TX lines | 111 (100) | 0.26 2 | |||
| 1, n (%) | 5 (5) | 1 (7) | 3 (7) | 1 (2) | |
| 2, n (%) | 39 (35) | 3 (20) | 19 (41) | 17 (34) | |
| 3, n (%) | 58 (53) | 8 (53) | 20 (43) | 30 (60) | |
| >3, n (%) | 9 (7) | 3 (20) | 4 (9) | 2 (4) | |
| Radiotherapy, n (%) | 47 (42) | 7 (47) | 19 (41) | 21 (42) | 0.93 2 |
| ASCT, n (%) | 42 (38) | 9 (60) | 13 (28) | 20 (40) | 0.08 2 |
| Parameter | All | CTLA4 A17hom | CTLA4 T17Ahet | CTLA4 T17hom | p * |
|---|---|---|---|---|---|
| Patients n (%) | 111 (100) | 15 (14) | 46 (41) | 50 (45) | |
| Age at CAR-T, years, median (range) | 66 (25–82) | 67 (44–77) | 71 (35–80) | 64 (25–82) | 0.19 1 |
| Time ID to CAR-T, years, median (range) | 4 (1–26) | 7 (1–13) | 7 (1–19) | 4 (1–26) | 0.37 1 |
| Remission Status pre CAR-T | 0.10 2 | ||||
| CR | 8 (7) | 1 (7) | 2 (4) | 5 (11) | |
| PR | 37 (35) | 8 (57) | 13 (29) | 16 (33) | |
| SD | 5 (5) | 2 (14) | 1 (2) | 2 (4) | |
| PD | 57 (53) | 3 (22) | 29 (65) | 25 (52) | |
| Bridging chemo-tx | 45 (41) | 4 (27) | 16 (35) | 25 (50) | 0.16 2 |
| Bridging radio-tx | 18 (16) | 1 (7) | 7 (15) | 10 (20) | 0.56 2 |
| LDH pre CAR-T (U/L) median | 331 | 326 | 346 | 304 | 0.35 1 |
| (range) | (135–3949) | (177–609) | (170–2312) | (135–3949) | |
| Elevated LDH (>250 U/L), n (%) | 67 (60) | 8 (53) | 33 (72) | 26 (52) | 0.12 2 |
| CAR-T cell product | 0.001 2 | ||||
| Tisa-cel | 63 (57) | 10 (67) | 27 (59) | 26 (52) | |
| Axi-cel | 42 (38) | 2 (13) | 16 (35) | 24 (48) | |
| Liso-cel | 6 (5) | 3 (20) | 3 (6) | 0 |
| Parameter | All | CTLA4 A17hom | CTLA4 T17Ahet | CTLA4 T17hom | p * |
|---|---|---|---|---|---|
| Patients, n (%) | 111 (100) | 15 (14) | 46 (41) | 50 (45) | |
| CRS, n (%) | 92 (83) | 7 (47) | 42 (91) | 43 (86) | 0.006 2 |
| Grade 1 | 61 (55) | 5 (33) | 29 (63) | 27 (54) | |
| Grade 2 | 27 (24) | 2 (13) | 12 (26) | 13 (26) | |
| Grade 3 | 4 (4) | 0 | 1 (2) | 3 (6) | |
| Time to CRS, days, median (range) | 2 (0–17) | 2 (0–4) | 2 (0–12) | 2 (0–17) | 0.31 1 |
| ICANS, n (%) | 39 (36) | 6 (40) | 19 (41) | 14 (29) | 0.29 2 |
| Grade 1 | 12 (11) | 4 (26) | 6 (13) | 2 (4) | |
| Grade 2 | 7 (6) | 0 | 5 (11) | 2 (4) | |
| Grade 3 | 14 (13) | 1 (7) | 6 (13) | 7 (15) | |
| Grade 4 | 6 (6) | 1 (7) | 2 (4) | 3 (6) | |
| Time to ICANS, days, median (range) | 6 (0–15) | 7 (6–11) | 5 (0–15) | 6 (2–12) | 0.58 1 |
| CRP peak, mg/L median (range) | 43 (2–328) | 25 (4–98) | 48 (3–288) | 43 (2–328) | 0.06 1 |
| Time to CRP peak, days, median (range) | 3 (0–28) | 3 (0–10) | 3 (0–28) | 3 (0–22) | 0.62 1 |
| IL-6 peak, pg/mL, median | 606 | 31 | 1189 | 719 | 0.005 1 |
| (range) | (4–157,117) | (12–700) | (4–142,180) | (7–157,117) | |
| Time to IL-6 peak, days, median (range) | 4 (0–39) | 4 (0–7) | 5 (1–39) | 4 (0–21) | 0.311 |
| Ferritin peak, ug/L, median | 1210 | 572 | 1199 | 1367 | 0.022 1 |
| (range) | (99–13,393) | (161–4073) | (99–9791) | (190–13,393) | |
| Time to ferritin peak days, median (range) | 10 (0–44) | 9 (2–31) | 10 (0–21) | 10 (0–44) | 0.62 1 |
| CAR T plasma peak, copies/µg cfDNA, | 4751 | 4976 | 5662 | 4516 | 0.39 1 |
| median (range) | (30–218,000) | (1000–140,000) | (30–218,000) | (37–91,000) | |
| Time to CAR T peak, days, median (range) | 9 (2–83) | 10 (7–83) | 9 (2–20) | 9 (2–27) | 0.72 1 |
| CAR T persistence 6 months, median | 96 | 171 | 82 | 67 | 0.09 1 |
| (range) | (0–4071) | (65–4071) | (0–2069) | (0–2317) | |
| Best response after CAR T cell therapy | 0.45 2 | ||||
| CR, n (%) | 61 (57) | 11 (85) | 25 (56) | 26 (52) | |
| PR, n (%) | 26 (24) | 2 (15) | 10 (22) | 14 (28) | |
| SD, n (%) | 6 (6) | 0 | 3 (7) | 3 (6) | |
| PD, n (%) | 14 (13) | 0 | 7 (15) | 7 (14) | |
| Relapse, n (%) | 48 (43) | 3 (20) | 17 (37) | 28 (56) | 0.025 2 |
| Death, n (%) | 53 (48) | 3 (20) | 24 (54) | 26 (52) | 0.069 2 |
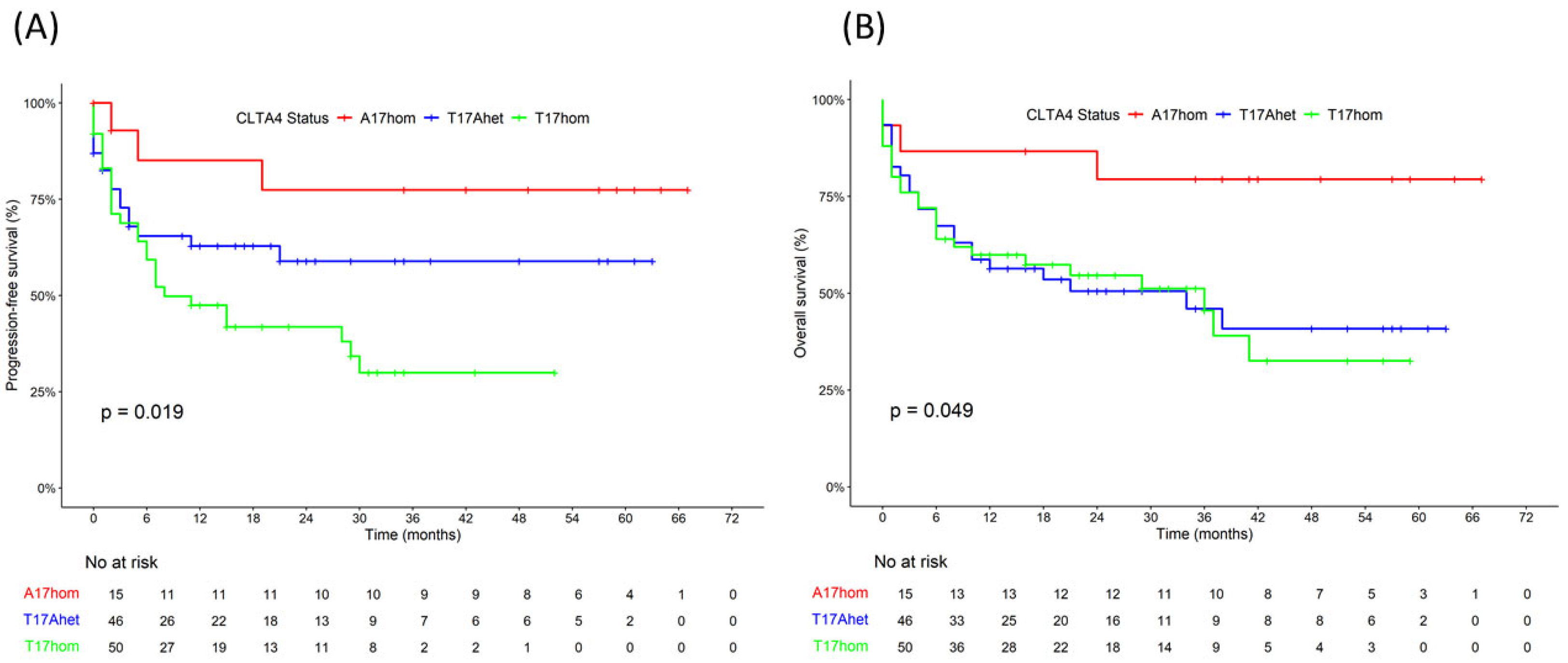
| Four-year PFS rate (%) | 48 | 77 | 59 | 30 | 0.019 3 |
| Four-year OS rate (%) | 45 | 79 | 41 | 33 | 0.049 3 |
| Parameter | All Tisa-cel n (%) | CTLA4 A17hom | CTLA4 T17Ahet | CTLA4 T17hom | p * |
|---|---|---|---|---|---|
| Patients, n (%) | 63 (100) | 10 (16) | 27 (44) | 26 (41) | |
| CRS, n (%) | 54 (86) | 5 (50) | 26 (96) | 23 (88) | 0.049 2 |
| Grade 1 | 35 (55) | 3 (33) | 18 (67) | 14 (54) | |
| Grade 2 | 16 (25) | 2 (20) | 7 (26) | 7 (27) | |
| Grade 3 | 3 (5) | 0 | 1 (4) | 2 (8) | |
| ICANS, n (%) | 22 (35) | 3 (33) | 11 (41) | 8 (31) | 0.59 2 |
| Grade 1 | 7 (11) | 2 (20) | 3 (11) | 2 (8) | |
| Grade 2 | 5 (8) | 0 | 3 (11) | 2 (8) | |
| Grade 3 | 6 (10) | 0 | 4 (15) | 2 (8) | |
| Grade 4 | 5 (8) | 1 (10) | 2 (7) | 2 (8) | |
| CRP peak, mg/L median | 43 | 19 | 48 | 44 | 0.16 1 |
| (range) | (2–328) | (4–98) | (3–288) | (2–328) | |
| IL-6 peak, pg/mL, median | 480 | 26 | 677 | 696 | 0.004 1 |
| (range) | (4–157,117) | (12–700) | (4–142,180) | (7–157,117) | |
| Ferritin peak, µg/L, median | 1113 | 494 | 948 | 1393 | 0.15 1 |
| (range) | (99–13,393) | (161–4073) | (99–9791) | (190–13,393) | |
| CAR T plasma peak, copies/µg cfDNA, | 4212 | 4520 | 3157 | 4860 | 0.76 1 |
| median (range) | (30–218,000) | (1000–140,000) | (30–218,000) | (37–91,000) | |
| CAR T persistence 6 months, median | 228 | 171 | 245 | 227 | 0.95 1 |
| (range) | (0–4071) | (65–4071) | (0–2069) | (0–2317) | |
| Best response after CAR T cell therapy | 0.25 2 | ||||
| CR, n (%) | 34 (54) | 8 (80) | 14 (52) | 12 (46) | |
| PR, n (%) | 19 (30) | 2 (20) | 7 (26) | 8 (31) | |
| SD, n (%) | 4 (6) | 0 | 3 (11) | 1 (4) | |
| PD, n (%) | 6 (10) | 0 | 3 (11) | 3 (12) | |
| Relapse, n (%) | 28 (44) | 2 (20) | 11 (41) | 15 (58) | 0.156 2 |
| Death, n (%) | 34 (48) | 3 (33) | 16 (59) | 15(58) | 0.29 2 |
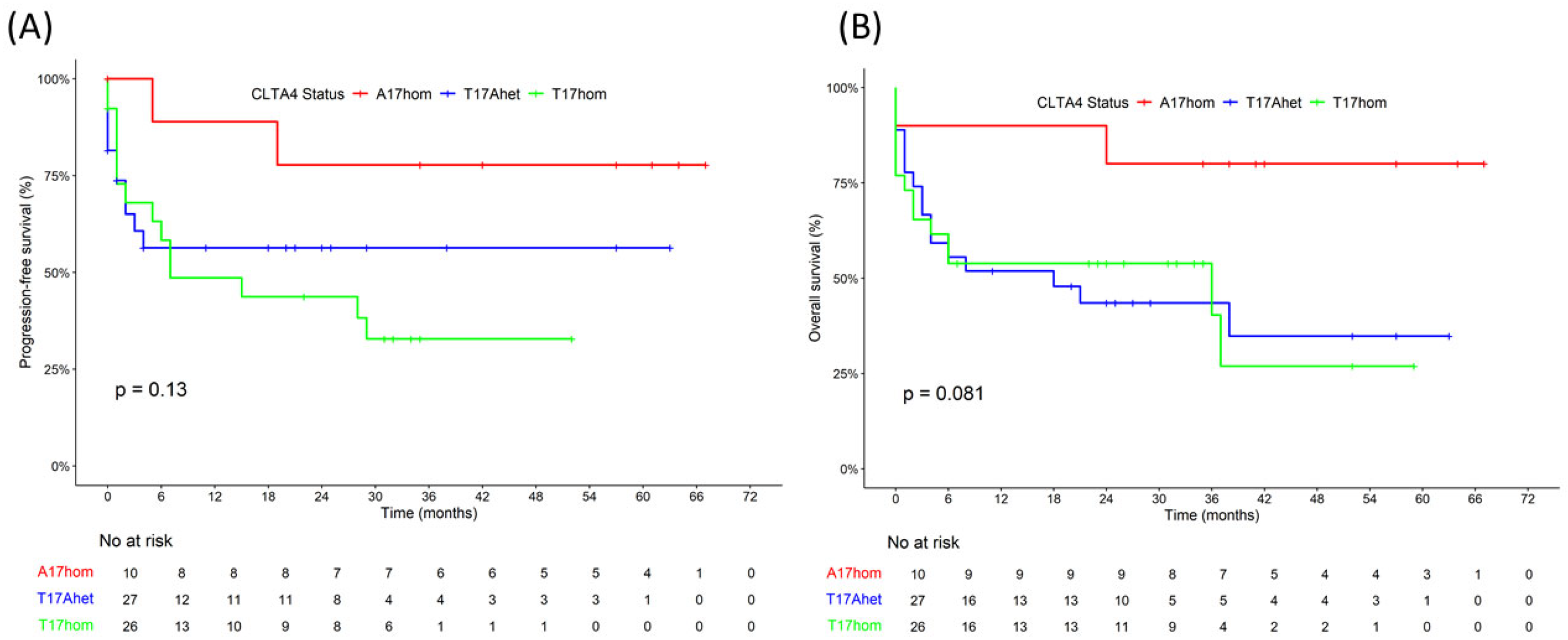
| Four-year PFS rate (%) | 52 | 78 | 54 | 30 | 0.13 3 |
| Four-year OS rate (%) | 45 | 80 | 30 | 25 | 0.08 3 |
| Predictors | PFS | OS | ||
|---|---|---|---|---|
| HR | p-Value | HR | p-Value | |
| CTLA4 A17hom vs. T17hom | 0.31 | 0.06 | 0.27 | 0.04 |
| CTLA4 A17hom vs. T17Ahet | 0.51 | 0.30 | 0.34 | 0.09 |
| Age > 65 vs. age < 65 | 0.82 | 0.56 | 1.83 | 0.04 |
| Female vs. male | 0.80 | 0.47 | 0.98 | 0.94 |
| Axi-cel vs. Tisa-cel | 0.89 | 0.70 | 0.70 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seipel, K.; Shaforostova, I.; Nilius, H.; Bacher, U.; Pabst, T. Clinical Impact of CTLA-4 Single-Nucleotide Polymorphism in DLBCL Patients Treated with CAR-T Cell Therapy. Curr. Oncol. 2025, 32, 425. https://doi.org/10.3390/curroncol32080425
Seipel K, Shaforostova I, Nilius H, Bacher U, Pabst T. Clinical Impact of CTLA-4 Single-Nucleotide Polymorphism in DLBCL Patients Treated with CAR-T Cell Therapy. Current Oncology. 2025; 32(8):425. https://doi.org/10.3390/curroncol32080425
Chicago/Turabian StyleSeipel, Katja, Inna Shaforostova, Henning Nilius, Ulrike Bacher, and Thomas Pabst. 2025. "Clinical Impact of CTLA-4 Single-Nucleotide Polymorphism in DLBCL Patients Treated with CAR-T Cell Therapy" Current Oncology 32, no. 8: 425. https://doi.org/10.3390/curroncol32080425
APA StyleSeipel, K., Shaforostova, I., Nilius, H., Bacher, U., & Pabst, T. (2025). Clinical Impact of CTLA-4 Single-Nucleotide Polymorphism in DLBCL Patients Treated with CAR-T Cell Therapy. Current Oncology, 32(8), 425. https://doi.org/10.3390/curroncol32080425








