Sleep Quality Moderates the Impact of Place-Based Social Adversity on Physical Health in Women with Breast Cancer Transitioning from Active Treatment to Survivorship
Simple Summary
Abstract
1. Introduction
1.1. Social Adversity and Health Consequences of Breast Cancer
1.2. Need to Both Address Health Equity and Identify Individual-Level Moderators
1.3. Modifiable Psychosocial and Behavioral Factors as Buffers
1.4. Study Aim
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.3. Statistical Analysis Plan
3. Results
3.1. Sample Characteristics and Bivariate Correlations
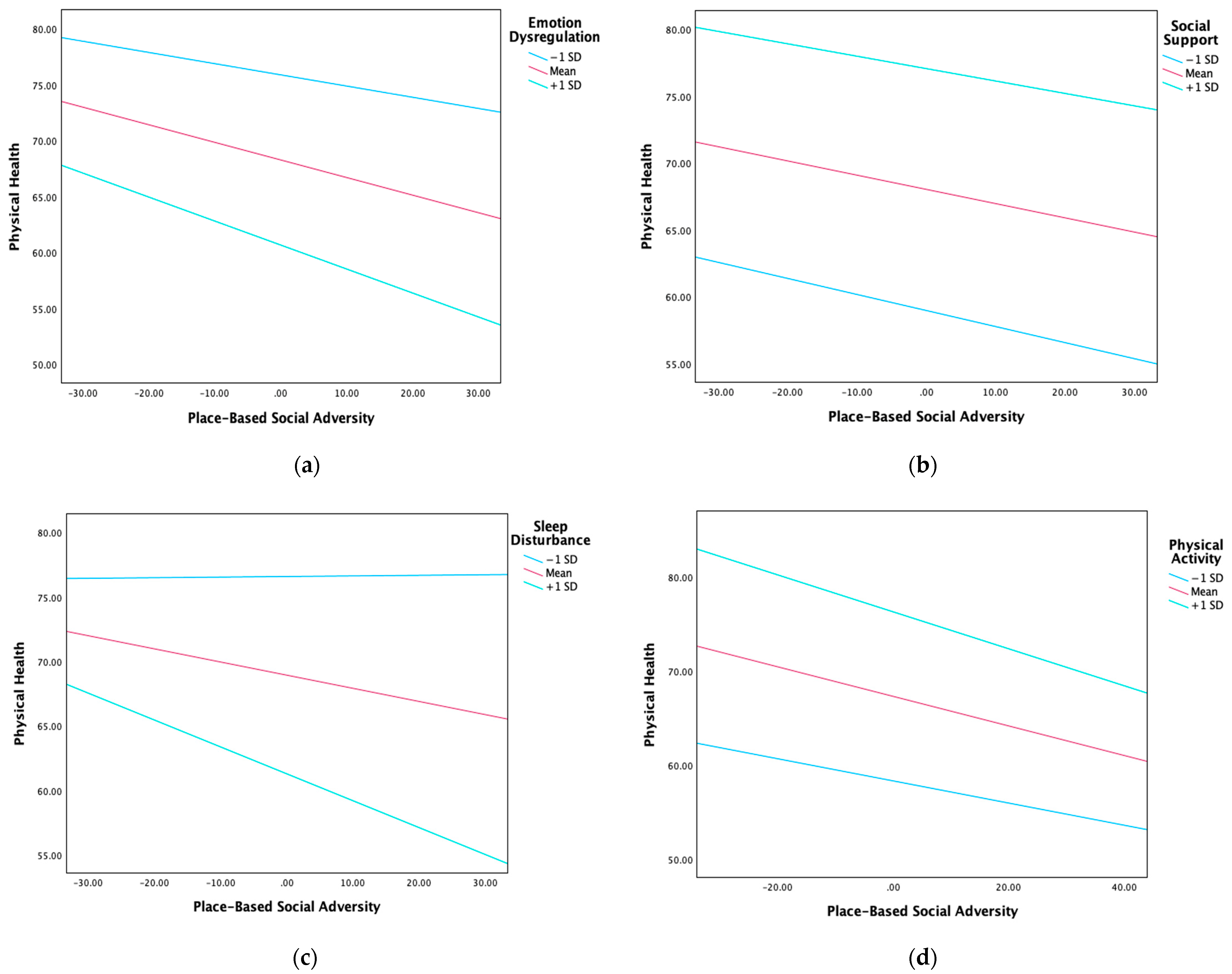
3.2. Moderation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2025. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2025-cancer-facts-figures.html (accessed on 10 June 2025).
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Soldato, D.; Arecco, L.; Agostinetto, E.; Franzoi, M.A.; Mariamidze, E.; Begijanashvili, S.; Brunetti, N.; Spinaci, S.; Solinas, C.; Vaz-Luis, I.; et al. The Future of Breast Cancer Research in the Survivorship Field. Oncol. Ther. 2023, 11, 199–229. [Google Scholar] [CrossRef]
- Thompson, B.; Hohl, S.D.; Molina, Y.; Paskett, E.D.; Fisher, J.L.; Baltic, R.D.; Washington, C.M. Breast cancer disparities among women in underserved communities in the USA. Curr. Breast Cancer Rep. 2018, 10, 131–141. [Google Scholar] [CrossRef]
- Parker, P.A.; Youssef, A.; Walker, S.; Basen-Engquist, K.; Cohen, L.; Gritz, E.R.; Wei, Q.X.; Robb, G.L. Short-term and long-term psychosocial adjustment and quality of life in women undergoing different surgical procedures for breast cancer. Ann. Surg. Oncol. 2007, 14, 3078–3089. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef]
- Frazelle, M.L.; Friend, P.J. Optimizing the teachable moment for health promotion for cancer survivors and their families. J. Adv. Pract. Oncol. 2016, 7, 422–433. [Google Scholar]
- Braveman, P.; Egerter, S.; Williams, D.R. The social determinants of health: Coming of age. Annu. Rev. Public Health 2011, 32, 381–398. [Google Scholar] [CrossRef]
- Garg, A.; Jack, B.; Zuckerman, B. Addressing the social determinants of health within the patient-centered medical home: Lessons from pediatrics. JAMA 2013, 309, 2001–2002. [Google Scholar] [CrossRef]
- Veličković, K.; Borrebaeck, C.A.K.; Bendahl, P.-O.; Hegardt, C.; Johnsson, P.; Richter, C.; Rydén, L.; Hallberg, I.R. One-year recovery from breast cancer: Importance of tumor and treatment-related factors, resilience, and sociodemographic factors for health-related quality of life. Front. Oncol. 2022, 12, 891850. [Google Scholar] [CrossRef]
- Williams, A.D.; Moo, T.-A. The impact of socioeconomic status and social determinants of health on disparities in breast cancer incidence, treatment, and outcomes. Curr. Breast Cancer Rep. 2023, 15, 30–36. [Google Scholar] [CrossRef]
- McClendon, J.; Chang, K.; JBoudreaux, M.; Oltmanns, T.F.; Bogdan, R. Black-White racial health disparities in inflammation and physical health: Cumulative stress, social isolation, and health behaviors. Psychoneuroendocrinology 2021, 131, 105251. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Finney Rutten, L.J.; Wilson, P.M.; Fan, C.; Boyd, C.M.; Jacobson, D.J.; Rocca, W.A.; Sauver, J.L.S. Neighborhood socioeconomic disadvantage is associated with multimorbidity in a geographically-defined community. BMC Public Health 2020, 20, 13. [Google Scholar] [CrossRef]
- Kind, A.J.H.; Buckingham, W.R. Making neighborhood-disadvantage metrics accessible—The neighborhood atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- Babatunde, O.A.; Zahnd, W.E.; Eberth, J.M.; Lawson, A.B.; Adams, S.A.; Boakye, E.A.; Jefferson, M.S.; Allen, C.G.; Pearce, J.L.; Li, H.; et al. Association between neighborhood social deprivation and stage at diagnosis among breast cancer patients in South Carolina. Int. J. Environ. Res. Public Health 2021, 18, 11824. [Google Scholar] [CrossRef]
- Wang, C.; Frost, J.; Tang, M.; Shah, R.; Kim, E.; Shamamian, P.E.; Montalmant, K.E.; Oleru, O.; Seyidova, N.; Henderson, P.W. Neighborhood deprivation is associated with increased postoperative complications after implant-based breast reconstruction. Clin. Breast Cancer 2024, 24, 604–610. [Google Scholar] [CrossRef]
- Barber, L.E.; Maliniak, M.L.; Nash, R.; Moubadder, L.; Haynes, D.; Ward, K.C.; McCullough, L.E. A comparison of three area-level indices of neighborhood deprivation and socioeconomic status and their applicability to breast cancer mortality. J. Urban Health 2024, 101, 75–79. [Google Scholar] [CrossRef]
- Roy, A.M.; George, A.; Attwood, K.; Alaklabi, S.; Patel, A.; Omilian, A.R.; Yao, S.; Gandhi, S. Effect of Neighborhood Deprivation Index on breast cancer survival in the United States. Breast Cancer Res. Treat. 2023, 202, 139–153. [Google Scholar] [CrossRef]
- You, K.-L.; Sereika, S.M.; Bender, C.M.; Hamilton, J.B.; Mazanec, S.R.; Brufsky, A.; Rosenzweig, M.Q. Health-related quality of life over chemotherapy course among individuals with early-stage breast cancer: The association of social determinants of health and neighborhood socioeconomic disadvantage. Support. Care Cancer 2024, 32, 224. [Google Scholar] [CrossRef]
- Hassan, A.M.; Nguyen, H.T.; Corkum, J.P.; Liu, J.; Kapur, S.K.; Chu, C.K.; Tamirisa, N.; Offodile, A.C. Area deprivation index is associated with variation in quality of life and psychosocial well-being following breast cancer surgery. Ann. Surg. Oncol. 2023, 30, 80–87. [Google Scholar] [CrossRef]
- Wu, C.; Ashing, K.T.; Jones, V.C.; Barcelo, L. The association of neighborhood context with health outcomes among ethnic minority breast cancer survivors. J. Behav. Med. 2018, 41, 52–61. [Google Scholar] [CrossRef]
- Culbertson, M.G.; Bennett, K.; Kelly, C.M.; Sharp, L.; Cahir, C. The psychosocial determinants of quality of life in breast cancer survivors: A scoping review. BMC Cancer 2020, 20, 948. [Google Scholar] [CrossRef]
- Pezzolato, M.; Spada, G.E.; Fragale, E.; Cutica, I.; Masiero, M.; Marzorati, C.; Pravettoni, G. Predictive models of psychological distress, quality of life, and adherence to medication in breast cancer patients: Aa scoping review. Patient Prefer. Adherence 2023, 17, 3461–3473. [Google Scholar] [CrossRef]
- Wells, K.J.; Drizin, J.H.; Ustjanauskas, A.E.; Vázquez-Otero, C.; Pan-Weisz, T.M.; Ung, D.; Carrizosa, C.; Laronga, C.; Roetzheim, R.G.; Johnson, K.; et al. The psychosocial needs of underserved breast cancer survivors and perspectives of their clinicians and support providers. Support. Care Cancer 2022, 30, 105–116. [Google Scholar] [CrossRef]
- Gallo, L.C.; Matthews, K.A. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychol. Bull. 2003, 129, 10–51. [Google Scholar] [CrossRef]
- Syrowatka, A.; Motulsky, A.; Kurteva, S.; Hanley, J.A.; Dixon, W.G.; Meguerditchian, A.N.; Tamblyn, R. Predictors of distress in female breast cancer survivors: A systematic review. Breast Cancer Res. Treat. 2017, 165, 229–245. [Google Scholar] [CrossRef]
- Umberson, D.; Montez, J.K. Social relationships and health: A flashpoint for health policy. J. Health Soc. Behav. 2010, 51, S54–S66. [Google Scholar] [CrossRef]
- Saban, K.L.; Mathews, H.L.; Bryant, F.B.; Tell, D.; Joyce, C.; DeVon, H.A.; Janusek, L.W. Perceived discrimination is associated with the inflammatory response to acute laboratory stress in women at risk for cardiovascular disease. Brain Behav. Immun. 2018, 73, 625–632. [Google Scholar] [CrossRef]
- Sturgeon, J.A.; Arewasikporn, A.; Okun, M.A.; Davis, M.C.; Ong, A.D.; Zautra, A.J. The psychosocial context of financial stress: Implications for inflammation and psychological health. Psychosom. Med. 2016, 78, 134–143. [Google Scholar] [CrossRef]
- Zheng, Z.; Han, X.; Zhao, J.; Banegas, M.P.; Tucker-Seeley, R.; Rai, A.; Fedewa, S.A.; Song, W.; Jemal, A.; Yabroff, K.R. Financial hardship, healthcare utilization, and health among U.S. cancer survivors. Am. J. Prev. Med. 2020, 59, 68–78. [Google Scholar] [CrossRef]
- Mahindru, A.; Patil, P.; Agrawal, V. Role of physical activity on mental health and well-being: A review. Cureus 2023, 15, e33475. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Gostian-Ropotin, L.A.; Beltrán-Velasco, A.I.; Belando-Pedreño, N.; Simón, J.A.; López-Mora, C.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Sporting mind: The interplay of physical activity and psychological health. Sports 2024, 12, 37. [Google Scholar] [CrossRef]
- Thompson, R.A. Emotion regulation: A theme in search of definition. Monogr. Soc. Res. Child. Dev. 1994, 59, 25. [Google Scholar] [CrossRef]
- Brandão, T.; Tavares, R.; Schulz, M.S.; Matos, P.M. Measuring emotion regulation and emotional expression in breast cancer patients: A systematic review. Clin. Psychol. Rev. 2016, 43, 114–127. [Google Scholar] [CrossRef]
- Baziliansky, S.; Cohen, M. Emotion regulation and psychological distress in cancer survivors: A systematic review and meta-analysis. Stress Health 2021, 3, 3–18. [Google Scholar] [CrossRef]
- Conley, C.C.; Bishop, B.T.; Andersen, B.L. Emotions and emotion regulation in breast cancer survivorship. Healthcare 2016, 4, 56. [Google Scholar] [CrossRef]
- Finck, C.; Barradas, S.; Zenger, M.; Hinz, A. Quality of life in breast cancer patients: Associations with optimism and social support. Int. J. Clin. Health Psychol. 2018, 18, 27–34. [Google Scholar] [CrossRef]
- Cheng, H.; Sit, J.W.H.; Chan, C.W.H.; So, W.K.W.; Choi, K.C.; Cheng, K.K.F. Social support and quality of life among Chinese breast cancer survivors: Findings from a mixed methods study. Eur. J. Oncol. Nurs. 2013, 17, 788–796. [Google Scholar] [CrossRef]
- Chou, A.F.; Stewart, S.L.; Wild, R.C.; Bloom, J.R. Social support and survival in young women with breast carcinoma. Psychooncology 2012, 21, 125–133. [Google Scholar] [CrossRef]
- Hughes, S.; Jaremka, L.M.; Alfano, C.M.; Glaser, R.; Povoski, S.P.; Lipari, A.M.; Farrar, W.B.; Yee, L.D.; Carson, W.E.; Malarkey, W.B.; et al. Social support predicts inflammation, pain, and depressive symptoms: Longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology 2014, 42, 38–44. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Cao, P.; Ren, G. Resilience and quality of life: Exploring the mediator role of social support in patients with breast cancer. Med. Sci. Monit. 2017, 23, 5969–5979. [Google Scholar] [CrossRef]
- Thompson, T.; Pérez, M.; Kreuter, M.; Margenthaler, J.; Colditz, G.; Jeffe, D.B. Perceived social support in African American breast cancer patients: Predictors and effects. Soc. Sci. Med. 2017, 192, 134–142. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Fritzson, E.; Ligus, K.; Park, C.L. Social support buffers the effect of social deprivation on comorbidity burden in adults with cancer. Ann. Behav. Med. 2024, 58, 701–706. [Google Scholar] [CrossRef]
- de Boer, M.C.; Wörner, E.A.; Verlaan, D.; van Leeuwen, P.A.M. The mechanisms and effects of physical activity on breast cancer. Clin. Breast Cancer 2017, 17, 272–278. [Google Scholar] [CrossRef]
- Koevoets, E.W.; Schagen, S.B.; de Ruiter, M.B.; Geerlings, M.I.; Witlox, L.; van der Wall, E.; Stuiver, M.M.; Sonke, G.S.; Velthuis, M.J.; Jobsen, J.J.; et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: A randomized controlled trial (PAM study). Breast Cancer Res. 2022, 24, 36. [Google Scholar] [CrossRef]
- Bucciarelli, V.; Bianco, F.; Di Blasio, A.; Morano, T.; Tuosto, D.; Mucedola, F.; Di Santo, S.; Cimini, A.; Napolitano, G.; Bucci, I.; et al. Cardiometabolic profile, physical activity, and quality of life in breast cancer survivors after different physical exercise protocols: A 34-month follow-up study. J. Clin. Med. 2023, 12, 4795. [Google Scholar] [CrossRef]
- Hooshmand Moghadam, B.; Golestani, F.; Bagheri, R.; Cheraghloo, N.; Eskandari, M.; Wong, A.; Nordvall, M.; Suzuki, K.; Pournemati, P. The effects of high-intensity interval training vs. Moderate-intensity continuous training on inflammatory markers, body composition, and physical fitness in overweight/obese survivors of breast cancer: A randomized controlled clinical trial. Cancers 2021, 13, 4386. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Anton, P.M.; Verhulst, S.; Vicari, S.K.; Robbs, R.S.; McAuley, E. Effects of a multicomponent physical activity behavior change intervention on fatigue, anxiety, and depressive symptomatology in breast cancer survivors: Randomized trial. Psychooncology 2017, 26, 1901–1906. [Google Scholar] [CrossRef]
- Spei, M.-E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Ratcliff, C.G.; Lam, C.Y.; Arun, B.; Valero, V.; Cohen, L. Ecological momentary assessment of sleep, symptoms, and mood during chemotherapy for breast cancer. Psychooncology 2014, 23, 1220–1228. [Google Scholar] [CrossRef]
- Liu, L.; Mills, P.J.; Rissling, M.; Fiorentino, L.; Natarajan, L.; Dimsdale, J.E.; Sadler, G.R.; Parker, B.A.; Ancoli-Israel, S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav. Immun. 2012, 26, 706–713. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Carter, P.; Stuifbergan, A.; Parmelee, B.; Kesler, S. Relationships between self-reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psychooncology 2018, 27, 1937–1943. [Google Scholar] [CrossRef]
- Lourenço, A.; Dantas, A.A.G.; de Souza, J.C.; Araujo, C.M.; Araujo, D.N.; Lima, I.N.D.F.; Dantas, D.d.S. Sleep quality is associated with Disability and Quality of life in breast cancer survivors: A cross-sectional pilot study. Eur. J. Cancer Care 2020, 30, e13339. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Zhou, E.S.; Poole, E.M.; Zhang, X.; Michels, K.B.; Eliassen, A.H.; Chen, W.Y.; Holmes, M.D.; Tworoger, S.S.; Schernhammer, E.S. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br. J. Cancer 2017, 116, 1239–1246. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Park, C.L.; Lee, J.W.; Harel, O.; Sanft, T.; Fritzson, E.; Salafia, C.; Ligus, K.; Gnall, K.; Magin, Z.E. Physical health and function trajectories in adults with cancer: Psychosocial predictors of class membership. J. Cancer Surviv. 2025, 19, 1173–1183. [Google Scholar] [CrossRef]
- Robert Graham Center. Social Deprivation Index (SDI). Robert Graham Center. 2018. Available online: https://www.graham-center.org/maps-data-tools/social-deprivation-index.html (accessed on 6 June 2025).
- Hagell, P.; Westergren, A.; Årestedt, K. Beware of the origin of numbers: Standard scoring of the SF-12 and SF-36 summary measures distorts measurement and score interpretations. Res. Nurs. Health 2017, 40, 378–386. [Google Scholar] [CrossRef]
- Kaufman, E.A.; Xia, M.; Fosco, G.; Yaptangco, M.; Skidmore, C.R.; Crowell, S.E. The difficulties in emotion regulation scale short form (DERS-SF): Validation and replication in adolescent and adult samples. J. Psychopathol. Behav. Assess. 2016, 38, 443–455. [Google Scholar] [CrossRef]
- Sherbourne, C.D.; Stewart, A.L. The MOS social support survey. Soc. Sci. Med. 1991, 32, 705–714. [Google Scholar] [CrossRef]
- Godin, G. The Godin-Shephard leisure-time physical activity questionnaire. Health Fitness J. Can. 2011, 1, 18–22. [Google Scholar] [CrossRef]
- Yu, L.; Buysse, D.J.; Germain, A.; Moul, D.E.; Stover, A.; Dodds, N.E.; Johnston, K.L.; Pilkonis, P.A. Development of short forms from the PROMISTM sleep disturbance and Sleep-Related Impairment item banks. Behav. Sleep Med. 2011, 10, 6–24. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Press: New York, NY, USA, 2017. [Google Scholar]
- Patel, N.P.; Grandner, M.A.; Xie, D.; Branas, C.C.; Gooneratne, N. “Sleep disparity” in the population: Poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health 2010, 10, 475. [Google Scholar] [CrossRef]
- Sosso, F.A.E.; Holmes, S.D.; Weinstein, A.A. Influence of socioeconomic status on objective sleep measurement: A systematic review and meta-analysis of actigraphy studies. Sleep Health 2021, 7, 417–428. [Google Scholar] [CrossRef]
- Seabrook, J.A.; Avison, W.R. Socioeconomic status and cumulative disadvantage processes across the life course: Implications for health outcomes. Can. Rev. Sociol. 2012, 49, 50–68. [Google Scholar] [CrossRef]
- Johnson, J.A.; Rash, J.A.; Campbell, T.S.; Savard, J.; Gehrman, P.R.; Perlis, M.; Carlson, L.E.; Garland, S.N. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med. Rev. 2016, 27, 20–28. [Google Scholar] [CrossRef]
- Cheng, P.; Luik, A.I.; Fellman-Couture, C.; Peterson, E.; Joseph, C.L.M.; Tallent, G.; Tran, K.M.; Ahmedani, B.K.; Roehrs, T.; Roth, T.; et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: A randomized trial. Psychol. Med. 2019, 49, 491–500. [Google Scholar] [CrossRef]
- Zhou, E.S.; Ritterband, L.M.; Bethea, T.N.; Robles, Y.P.; Heeren, T.C.; Rosenberg, L. Effect of culturally tailored, internet-delivered cognitive behavioral therapy for insomnia in Black women: A randomized clinical trial. JAMA Psychiatry 2022, 79, 538–549. [Google Scholar] [CrossRef]
| Variable | M (SD) or N (%) | Range |
|---|---|---|
| Age | 56.09 (12.30) | 24–80 |
| Race a | ||
| White | 225 (88.2%) | - |
| Black or African American | 15 (5.9%) | - |
| American Indian or Alaska Native | 7 (2.7%) | - |
| Asian | 5 (2.0%) | - |
| Not reported or unknown | 4 (1.6%) | - |
| Ethnicity | ||
| Hispanic/Latinx | 19 (7.5%) | - |
| Not Hispanic/Latinx | 210 (82.4%) | - |
| Not reported or unknown | 26 (10.2%) | - |
| Highest level of education | ||
| No formal education | 1 (0.4%) | - |
| Grade school | 2 (0.8%) | - |
| High school graduate | 21 (8.2%) | - |
| Some college or associate degree | 66 (25.9%) | - |
| Bachelor’s degree | 68 (26.7%) | - |
| Graduate or professional degree | 5 (2.0%) | - |
| Master’s degree | 72 (28.2%) | - |
| Doctoral degree or professional degree | 15 (5.9%) | - |
| Not reported | 5 (2.0%) | - |
| Breast cancer stage | ||
| I | 141 (55.3%) | - |
| II | 90 (35.3%) | - |
| III | 19 (7.5%) | - |
| Missing | 5 (2.0%) | - |
| SDI | 29.59 (29.60) | 1.00–98.00 |
| Physical health | 68.54 (28.03) | 0.00–100.00 |
| Emotion dysregulation | 1.87 (0.59) | 1.00–4.50 |
| Social support | 78.57 (21.52) | 11.84–100.00 |
| Sleep disturbance | 23.45 (8.19) | 8.00–40.00 |
| Physical activity b | 29.06 (26.20) | 0.00–123.00 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. Age | - | |||||
| 2. Social adversity | .01 | - | ||||
| 3. Emotion dysregulation | −.25 ** | −.01 | - | |||
| 4. Social support | .06 | −.10 | −.31** | - | ||
| 5. Physical activity | −.19 ** | −.07 | .07 | .13 | - | |
| 6. Sleep disturbance | −.22 ** | .14 * | .34 ** | −.24 ** | .03 | - |
| 7. Physical health | .07 | −.21 ** | −.28 ** | .33 ** | .30 ** | −.30 ** |
| Emotion Dysregulation Model | Social Support Model | Sleep Disturbance Model | Physical Activity Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | Bootstrapped 95% CI | B | SE | Bootstrapped 95% CI | B | SE | Bootstrapped 95% CI | B | SE | Bootstrapped 95% CI | |
| Age | −0.15 | 0.16 | −0.46, 0.17 | 0.01 | 0.16 | −0.30, 0.32 | −0.10 | 0.15 | −0.40, 0.20 | 0.05 | 0.16 | −0.27, 0.37 |
| Chemotherapy | −7.51 | 3.49 | −14.30, −0.55 | −6.62 | 3.43 | −13.45, 0.22 | −5.71 | 3.43 | −12.44, 0.97 | −6.34 | 3.70 | −13.33, 1.12 |
| Radiation | 11.13 | 4.07 | 3.10, 19.32 | 14.63 | 4.31 | 6.19, 23.01 | 10.62 | 4.14 | 2.22, 18.68 | 12.31 | 4.28 | 3.83, 20.68 |
| Hormone therapy | 0.82 | 4.20 | −7.53, 9.15 | −1.64 | 4.16 | −9.50, 6.41 | 3.69 | 4.18 | −4.45, 11.89 | 2.29 | 4.18 | −5.69, 10.36 |
| Social adversity | −0.16 | 0.06 | −0.27, −0.05 | −0.11 | 0.06 | −0.23, 0.01 | −0.12 | 0.06 | −0.24, −0.01 | −0.16 | 0.06 | −0.27, −0.04 |
| Psychosocial factor | −13.25 | 3.04 | −19.32, −7.59 | 0.42 | 0.08 | 0.27, 0.58 | −0.94 | 0.22 | −1.36, −0.51 | 0.34 | 0.07 | 0.20, 0.49 |
| Social adversity × psychosocial factor | −0.10 | 0.09 | −0.26, 0.08 | 0.001 | 0.003 | −0.004, 0.01 | −0.01 | 0.01 | −0.03, −0.0002 | −0.002 | 0.002 | −0.001, 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.L.; Gnall, K.E.; Salafia, C.; Bellizzi, K.M. Sleep Quality Moderates the Impact of Place-Based Social Adversity on Physical Health in Women with Breast Cancer Transitioning from Active Treatment to Survivorship. Curr. Oncol. 2025, 32, 420. https://doi.org/10.3390/curroncol32080420
Park CL, Gnall KE, Salafia C, Bellizzi KM. Sleep Quality Moderates the Impact of Place-Based Social Adversity on Physical Health in Women with Breast Cancer Transitioning from Active Treatment to Survivorship. Current Oncology. 2025; 32(8):420. https://doi.org/10.3390/curroncol32080420
Chicago/Turabian StylePark, Crystal L., Katherine E. Gnall, Caroline Salafia, and Keith M. Bellizzi. 2025. "Sleep Quality Moderates the Impact of Place-Based Social Adversity on Physical Health in Women with Breast Cancer Transitioning from Active Treatment to Survivorship" Current Oncology 32, no. 8: 420. https://doi.org/10.3390/curroncol32080420
APA StylePark, C. L., Gnall, K. E., Salafia, C., & Bellizzi, K. M. (2025). Sleep Quality Moderates the Impact of Place-Based Social Adversity on Physical Health in Women with Breast Cancer Transitioning from Active Treatment to Survivorship. Current Oncology, 32(8), 420. https://doi.org/10.3390/curroncol32080420





