Bone Health and Endocrine Therapy with Ovarian Function Suppression in Premenopausal Early Breast Cancer: A Real-Life Monocenter Experience with Denosumab
Simple Summary
Abstract
1. Introduction
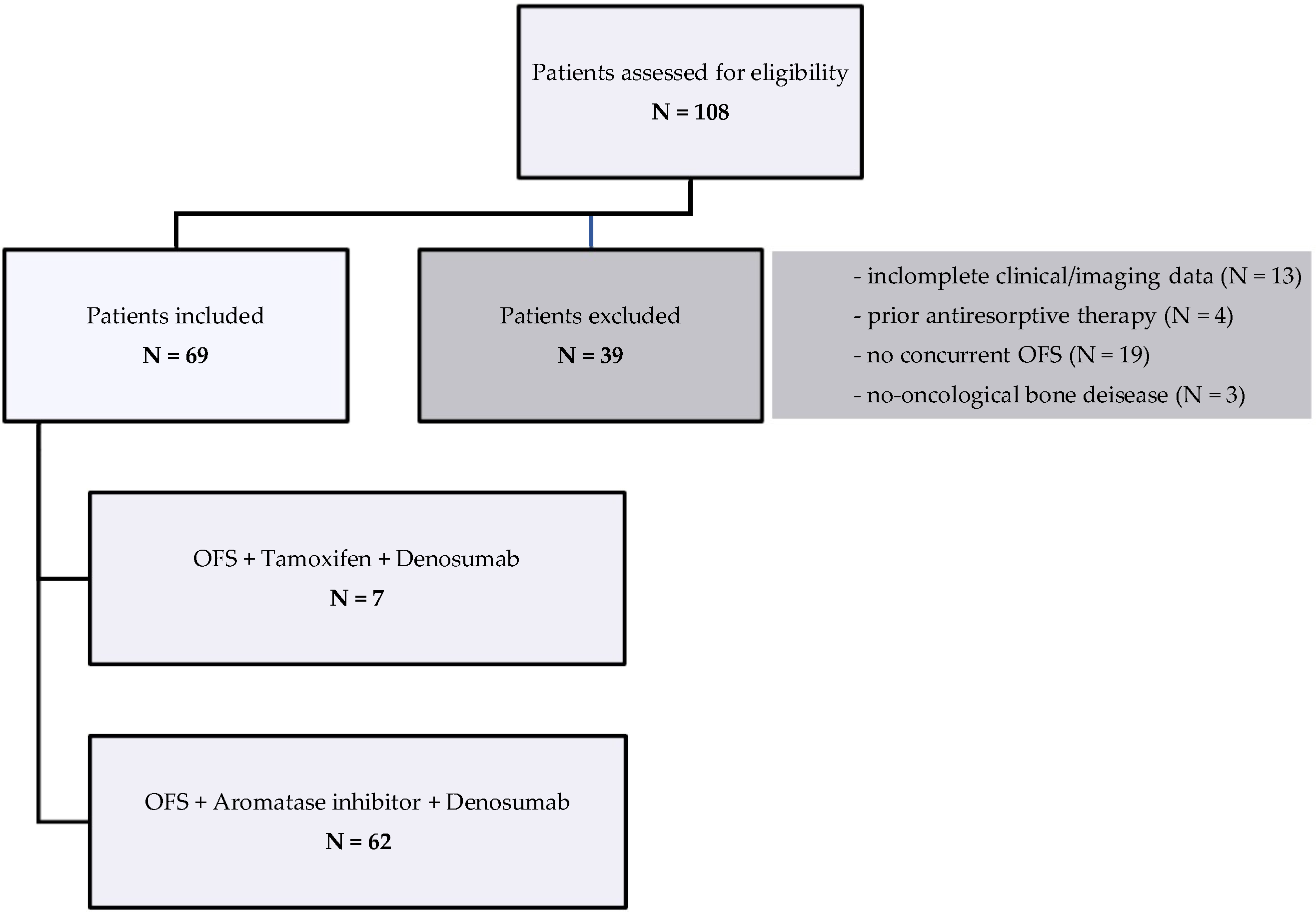
2. Materials and Methods
- Age between 18 and 55 years at the start of therapy;
- Diagnosis of early-stage and hormone receptor-positive BC;
- Confirmed premenopausal status at the time of diagnosis;
- Patients in therapy with adjuvant ET with OFS plus aromatase inhibitor or tamoxifen in combination with denosumab administered every 6 months (mo);
- Administration of denosumab at least twice;
- Availability and accessibility of baseline and follow-up clinical and laboratory data, such as BMD assessed by dual-energy X-ray absorptiometry (DEXA) or bone turnover markers as serum C-terminal telopeptide of type I collagen (CTX);
- Minimum follow-up of at least 12 mo.
- Exclusion criteria were as follows:
- Age over 55 years at the start of therapy;
- Adjuvant ET with aromatase inhibitor or tamoxifen in monotherapy, without concurrent OFS;
- Incomplete or unavailable clinical or imaging data for efficacy and safety analysis;
- Pre-existing non-oncological bone diseases (e.g., osteomalacia);
- Previous exposure to bisphosphonates or other antiresorptive agents prior to start of denosumab.
3. Results
3.1. Patients’ Characteristics
3.2. Adherence to Therapy and Complications
3.2.1. Endocrine Therapy
3.2.2. Denosumab Therapy
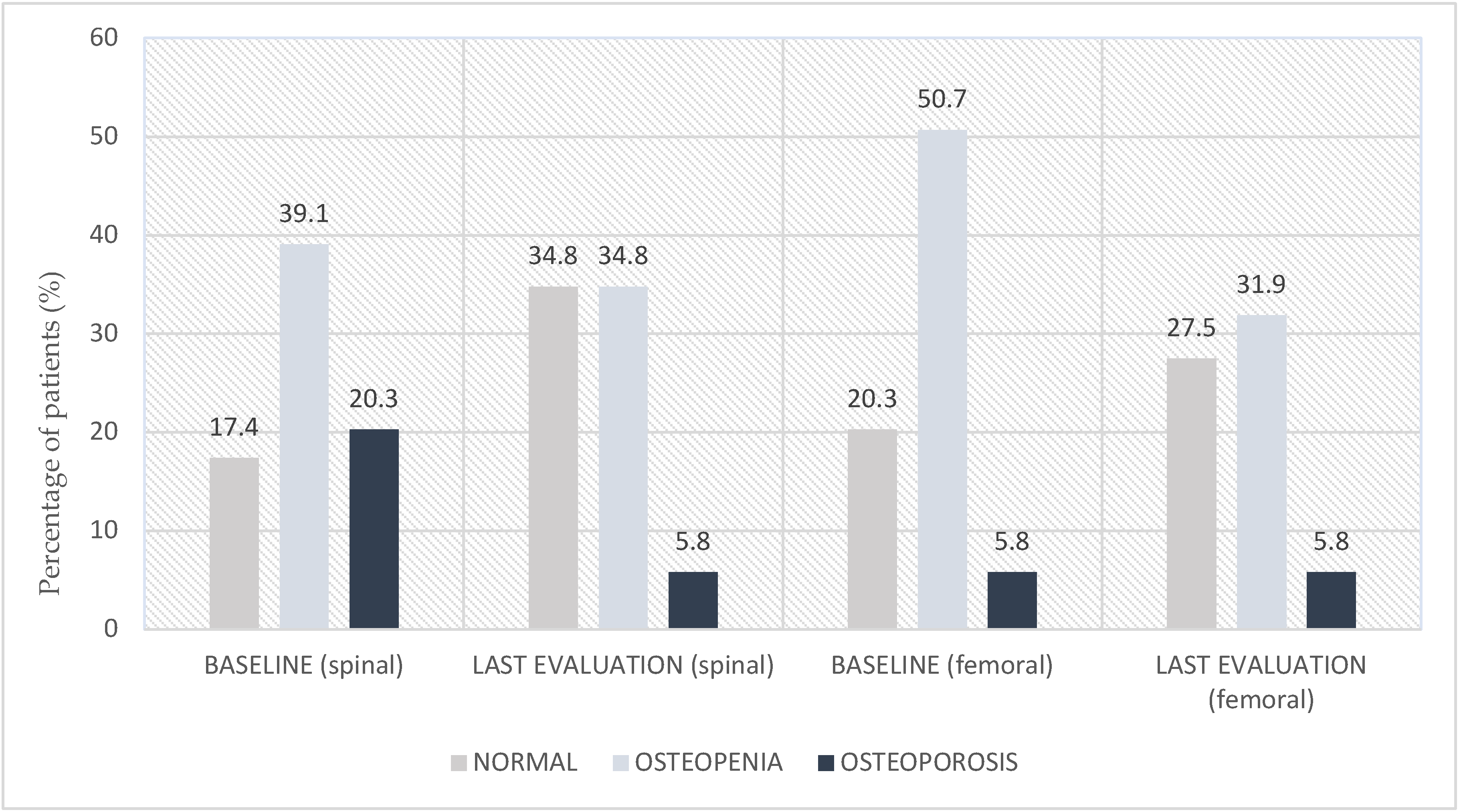
3.3. Bone Density and CTX Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ET | Endocrine Therapy |
| eBC | Early Breast Cancer |
| BC | Breast Cancer |
| BMD | Bone Mineral Density |
| CTX | C-terminal telopeptide of type I collagen |
| DEXA | Dual-Energy X-ray Absorptiometry |
| BMI | Body Mass Index |
| OFS | Ovarian Function Suppression |
| aLHRH | agonist of Luteinizing Hormone-Releasing Hormone |
| RT | Radiotherapy |
| ChT | Chemotherapy |
| ONJ | Osteonecrosis of the Jaw |
| SERM | Selective Estrogen Receptor Modulator |
| OS | Overall Survival |
| mo | Months |
References
- Italian Association of Medical Oncology (AIOM). Numbers of Cancer in Italy 2024. Available online: https://www.aiom.it/wp-content/uploads/2024/12/2024_NDC-def.pdf (accessed on 24 July 2025).
- AIOM. AIOM Guideline. Available online: https://www.aiom.it/linee-guida-aiom/ (accessed on 24 July 2025).
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.; Bergh, J.; Burstein, H.; Cardoso, M.; Carey, L.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Kerr, A.J.; Dodwell, D.; McGale, P.; Holt, F.; Duane, F.; Mannu, G.; Darby, S.C.; Taylor, C.W. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat. Rev. 2022, 105, 102375. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Fitzal, F.; Rinnerthaler, G.; Steger, G.G.; Greil-Ressler, S.; Balic, M.; Heck, D.; Jakesz, R.; Thaler, J.; Egle, D.; et al. Duration of Adjuvant Aromatase-Inhibitor Therapy in Postmenopausal Breast Cancer. N. Engl. J. Med. 2021, 385, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Del Mastro, L.; Mansutti, M.; Bisagni, G.; Ponzone, R.; Durando, A.; Amaducci, L.; Campadelli, E.; Cognetti, F.; Frassoldati, A.; Michelotti, A.; et al. Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1458–1467. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99 Pt. A, 11–15. [Google Scholar] [CrossRef]
- Eriksen, E.F. Normal and pathological remodeling of human trabecular bone: Three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr. Rev. 1986, 7, 379–408. [Google Scholar] [CrossRef]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef]
- Lu, L.; Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: The role and mechanisms of estrogen. J. Endocrinol. 2023, 259, e230116. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Härkönen, P.L. Estrogen and bone metabolism. Maturitas 1996, 23, S65–S69. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Diel, I.J. Osteoporosis due to cancer treatment: Pathogenesis and management. J. Clin. Oncol. 2000, 18, 1570–1593. [Google Scholar] [CrossRef]
- Cauley, J.A. Public health impact of osteoporosis. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1243–1251. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, B.H.; Min, S.Y.; Chae, S. Effects of Anticancer Therapy on Osteoporosis in Breast Cancer Patients: A Nationwide Study Using Data from the National Health Insurance Service-National Health Information Database. J. Clin. Med. 2025, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Birtolo, M.F.; Pedersini, R.; Palermo, A.; Vena, W.; Morenghi, E.; Cristofolini, G.; Presciuttini, B.; Tabacco, G.; Naciu, A.M.; Pigni, S.; et al. Bone-active drugs in premenopausal women with breast cancer under hormone-deprivation therapies. Eur. J. Endocrinol. 2024, 191, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Periañez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant denosumab in early breast cancer (D-CARE): An international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Frantal, S.; Pfeiler, G.; Steger, G.G.; Egle, D.; Greil, R.; Fitzal, F.; Wette, V.; Balic, M.; Haslbauer, F.; et al. Long-Term Outcomes of Adjuvant Denosumab in Breast Cancer. NEJM Evid. 2022, 1, EVIDoa2200162. [Google Scholar] [CrossRef]
- Adams, A.; Jakob, T.; Huth, A.; Monsef, I.; Ernst, M.; Kopp, M.; Caro-Valenzuela, J.; Wöckel, A.; Skoetz, N.; Cochrane Breast Cancer Group. Bone-modifying agents for reducing bone loss in women with early and locally advanced breast cancer: A network meta-analysis. Cochrane Database Syst. Rev. 2024, 7, CD013451. [Google Scholar] [CrossRef]
- AIFA. Nota 79. Available online: https://www.aifa.gov.it/nota-79 (accessed on 24 July 2025).
- Delmas, P.D. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J. Clin. Densitom. 2008, 11, 325–338. [Google Scholar] [CrossRef]
- Ellis, G.K.; Bone, H.G.; Chlebowski, R.; Paul, D.; Spadafora, S.; Smith, J.; Fan, M.; Jun, S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 2008, 26, 4875–4882. [Google Scholar] [CrossRef]
- Coleman, R.; Zhou, Y.; Jandial, D.; Cadieux, B.; Chan, A. Bone Health Outcomes from the International, Multicenter, Randomized, Phase 3, Placebo-Controlled D-CARE Study Assessing Adjuvant Denosumab in Early Breast Cancer. Adv. Ther. 2021, 38, 4569–4580. [Google Scholar] [CrossRef]
- Waqas, K.; Lima Ferreira, J.; Tsourdi, E.; Body, J.J.; Hadji, P.; Zillikens, M.C. Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre- and postmenopausal women with early-stage breast cancer. J. Bone Oncol. 2021, 28, 100355. [Google Scholar] [CrossRef]
- Ramchand, S.K.; Cheung, Y.M.; Yeo, B.; Grossmann, M. The effects of adjuvant endocrine therapy on bone health in women with breast cancer. J. Endocrinol. 2019, 241, R111–R124. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Langdahl, B.; Cohen-Solal, M.; Aubry-Rozier, B.; Eriksen, E.F.; Guañabens, N.; Obermayer-Pietsch, B.; Ralston, S.H.; Eastell, R.; Zillikens, M.C. Discontinuation of Denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone 2017, 105, 11–17. [Google Scholar] [CrossRef]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, M.L.; A Morrisey, M.; Becker, D.J.; Gary, L.C.; Curtis, J.R.; Saag, K.G.; Yun, H.; Matthews, R.; Smith, W.; Taylor, A.; et al. Health care expenditures associated with skeletal fractures among Medicare beneficiaries, 1999–2005. J. Bone Miner. Res. 2009, 24, 2050–2055. [Google Scholar] [CrossRef]
- Thomas, T.; Tubach, F.; Bizouard, G.; Crochard, A.; Maurel, F.; Perrin, L.; Collin, C.; Roux, C.; Paccou, J. The Economic Burden of Severe Osteoporotic Fractures in the French Healthcare Database: The FRACTOS Study. J. Bone Miner. Res. 2022, 37, 1811–1822. [Google Scholar] [CrossRef]
- Willers, C.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Borgström, F.; Kanis, J.A.; The SCOPE Review Panel of the IOF. Osteoporosis in Europe: A compendium of country-specific reports. Arch. Osteoporos. 2022, 17, 23. [Google Scholar] [CrossRef]
- Williams, S.A.; Chastek, B.; Sundquist, K.; Barrera-Sierra, S.; Leader, D.; Weiss, R.J.; Wang, Y.; Curtis, J.R. Economic burden of osteoporotic fractures in US managed care enrollees. Am. J. Manag. Care 2020, 26, e142–e149. [Google Scholar] [CrossRef]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran. 2020, 34, 154. [Google Scholar] [CrossRef]
| Variable | Assessment | Description |
|---|---|---|
| Demographics | ||
| Age | years | at baseline |
| Smoking habit | current/never/former | collected; baseline |
| BMI | kg/m2 | at baseline |
| Bone health parameters | ||
| BMD | DEXA scan | at baseline; during follow-up |
| Blood vitamin D level | ng/mL | at baseline |
| Vitamin D intake | yes/no | at baseline; during follow-up |
| Comorbidities | medical history | e.g., hypertension, diabetes… |
| Cancer-related parameters | ||
| Tumor stage | TNM 1 | at diagnosis |
| Type of surgery | mastectomy/quadrantectomy | - |
| Systemic therapy | ||
| ET type | OFS + aromatase inhibitor or tamoxifen | current |
| CDK4/6 inhibitor use | yes/no | concomitant |
| Anti-HER2 agent use | yes/no | concomitant |
| ChT | yes/no | prior or concomitant |
| RT | yes/no | prior or concomitant |
| Bone turnover markers | ||
| Serum CTX level | ng/mL | at baseline; during follow-up |
| Safety | ||
| Bone fractures | yes/no | number and site |
| ONJ | yes/no | confirmed by oral surgeon |
| Adherence to therapy | ||
| ET | adherence rate | during follow-up |
| Denosumab | adherence rate | during follow-up |
| Characteristics | Patients N (%) | |
|---|---|---|
| Patients’ characteristics | ||
| Age | <50 | 61 (88.4) |
| ≥50 | 8 (11.6) | |
| Median age | 45 | |
| Current | 2 (2.9) | |
| Smoking habits | Former | 8 (11.6) |
| Never | 59 (85.5) | |
| <25 | 52 (75.4) | |
| BMI | 25–29 | 9 (13) |
| ≥30 | 4 (5.8) | |
| Missing | 4 (5.8) | |
| <20 | 6 (8.7) | |
| Blood vitamin D level | 20–40 | 33 (47.8) |
| >40 | 23 (33.3) | |
| Missing | 7 (10.2) | |
| Vitamin D intake | Yes | 30 (43.5) |
| No | 39 (56.5) | |
| I | 35 (50.8) | |
| Tumor stage | II | 24 (34.8) |
| III | 5 (7.2) | |
| Unknown | 5 (7.2) | |
| Surgery | Quadrantectomy | 43 (62.3) |
| Mastectomy | 26 (37.7) | |
| ChT | Yes | 44 (63.8) |
| No | 25 (36.2) | |
| Anti-HER2 agents | Yes | 10 (14.5) |
| No | 59 (85.5) | |
| CDK4/6 inhibitors | Yes | 4 (5.8) |
| No | 65 (94.2) | |
| RT | Yes | 38 (55) |
| No | 31 (45) | |
| Recurrence | Yes | 1 (1.5) |
| No | 68 (98.5) | |
| Survival status | ||
| Alive | 63 (91.3) | |
| Died | 1 (1.5) | |
| Lost to follow-up | 5 (7.2) | |
| ET | ||
| Type | OFS * + aromatase inhibitor | 62 (89.9) |
| OFS * + tamoxifen | 7 (10.1) | |
| Ended | 10 (14.5) | |
| Time | Ongoing | 59 (85.5) |
| Median time | 56 mo | |
| No | 31 (45) | |
| Bone fractures | Yes | 0 (0) |
| No | 69 (100) | |
| Denosumab therapy | ||
| Ended | 11 (16) | |
| Time | Ongoing | 58 (84) |
| Median time | 33 mo | |
| Yes | 62 (89.9) | |
| Adherence | No | 4 (5.8) |
| Missing | 3 (4.3) | |
| ONJ | Yes | 1 (1.4) |
| No | 68 (98.6) | |
| Missing | 3 (4.3) | |
| BMD and bone turnover marker | ||
| T-score ≤ −2.5 | 14 (20.3) | |
| Spinal DEXA scan (at baseline) | T-score > −2.5 and ≤−1.0 | 27 (39.1) |
| T-score > −1.0 | 12 (17.4) | |
| Unknown | 16 (23.2) | |
| T-score ≤ −2.5 | 4 (5.8) | |
| Femoral DEXA scan (at baseline) | T-score > −2.5 and ≤−1.0 | 35 (50.7) |
| T-score > −1.0 | 14 (20.3) | |
| Unknown | 16 (23.2) | |
| T-score ≤ −2.5 | 4 (5.8) | |
| Spinal DEXA scan (at last follow-up) | T-score > −2.5 and ≤−1.0 | 24 (34.8) |
| T-score > −1.0 | 17 (34.8) | |
| Unknown | 24 (24.6) | |
| T-score ≤ −2.5 | 4 (5.8) | |
| Femoral DEXA scan (at last follow-up) | T-score > −2.5 and ≤−1.0 | 22 (31.9) |
| T-score > −1.0 | 19 (27.5) | |
| Unknown | 24 (34.8) | |
| ≤0.5 | 16 (23.2) | |
| Serum CTX level (at baseline) | > 0.5 and ≤1.0 | 4 (5.8) |
| >1.0 | 5 (7.2) | |
| Unknown | 44 (63.8) | |
| ≤0.5 | 10 (14.5) | |
| Serum CTX level (at last follow-up) | >0.5 and ≤1.0 | 3 (4.3) |
| >1.0 | 1 (1.5) | |
| Unknown | 55 (79.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotondi, A.; Frescura, V.; Arcuri, G.; Garufi, G.; Pontolillo, L.; Mastrantoni, L.; Di Monte, E.; Maliziola, N.; Fucile, M.A.; Salvatori, F.; et al. Bone Health and Endocrine Therapy with Ovarian Function Suppression in Premenopausal Early Breast Cancer: A Real-Life Monocenter Experience with Denosumab. Curr. Oncol. 2025, 32, 421. https://doi.org/10.3390/curroncol32080421
Rotondi A, Frescura V, Arcuri G, Garufi G, Pontolillo L, Mastrantoni L, Di Monte E, Maliziola N, Fucile MA, Salvatori F, et al. Bone Health and Endocrine Therapy with Ovarian Function Suppression in Premenopausal Early Breast Cancer: A Real-Life Monocenter Experience with Denosumab. Current Oncology. 2025; 32(8):421. https://doi.org/10.3390/curroncol32080421
Chicago/Turabian StyleRotondi, Angelachiara, Valentina Frescura, Giorgia Arcuri, Giovanna Garufi, Letizia Pontolillo, Luca Mastrantoni, Elena Di Monte, Noemi Maliziola, Maria Antonia Fucile, Francesca Salvatori, and et al. 2025. "Bone Health and Endocrine Therapy with Ovarian Function Suppression in Premenopausal Early Breast Cancer: A Real-Life Monocenter Experience with Denosumab" Current Oncology 32, no. 8: 421. https://doi.org/10.3390/curroncol32080421
APA StyleRotondi, A., Frescura, V., Arcuri, G., Garufi, G., Pontolillo, L., Mastrantoni, L., Di Monte, E., Maliziola, N., Fucile, M. A., Salvatori, F., Mondello, R., Poli, I., Oliva, G. R., Mongelli, G., Palazzo, A., Fabi, A., Bria, E., Tortora, G., & Orlandi, A. (2025). Bone Health and Endocrine Therapy with Ovarian Function Suppression in Premenopausal Early Breast Cancer: A Real-Life Monocenter Experience with Denosumab. Current Oncology, 32(8), 421. https://doi.org/10.3390/curroncol32080421








