Unique Biological Characteristics of Patients with High Gleason Score and Localized/Locally Advanced Prostate Cancer Using an In Silico Translational Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition for Patients with Localized/Locally Advanced PCa
2.2. Pathway Enrichment Analysis via GSVA
2.3. Estimation of Tumor Microenviornment Components
2.4. Assessment of Genomic Instability and Mutation Burden
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. GS Levels Were Associated with Patient Prognosis in Localized/Locally Advanced PCa
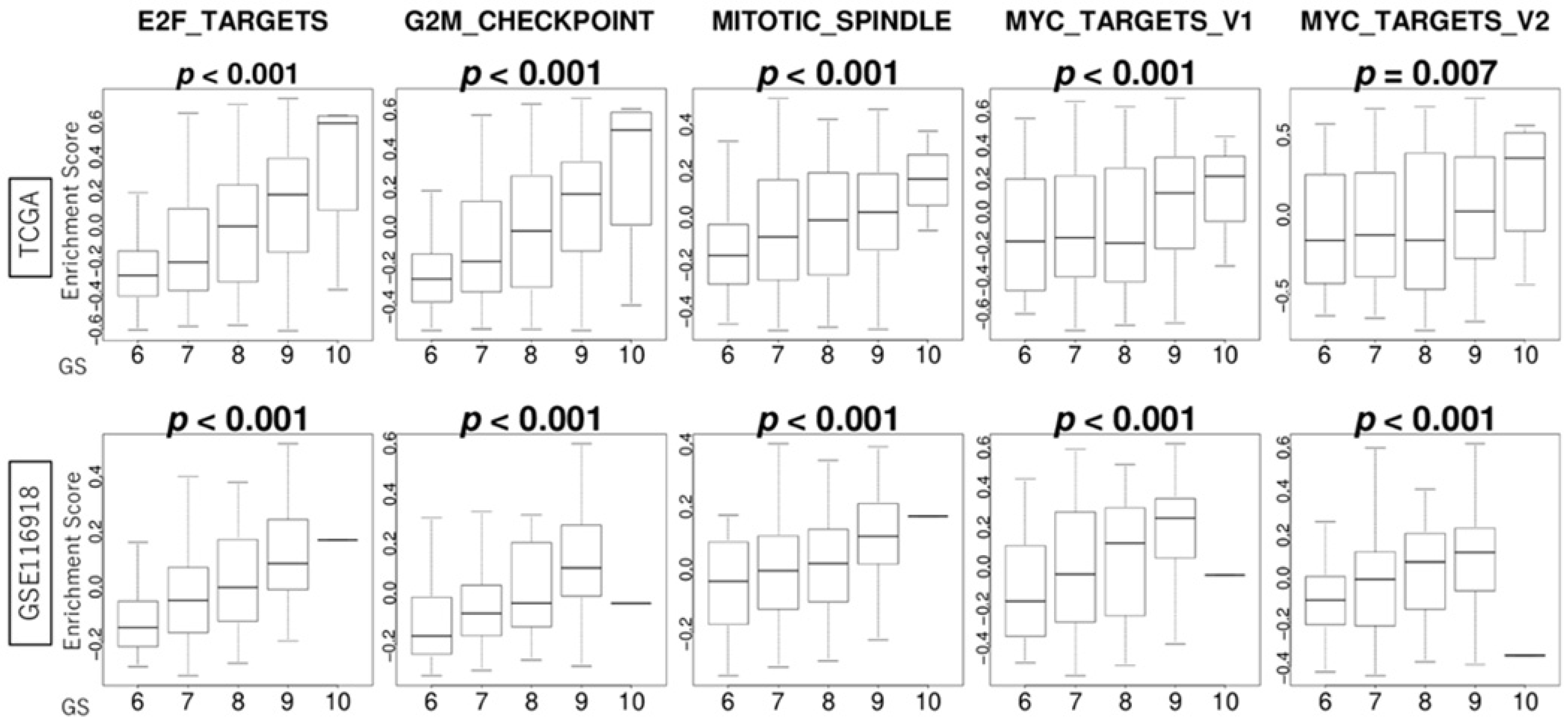
3.3. GS Levels Were Correlated with the Activity Levels of Cell Proliferation-Related Gene Sets
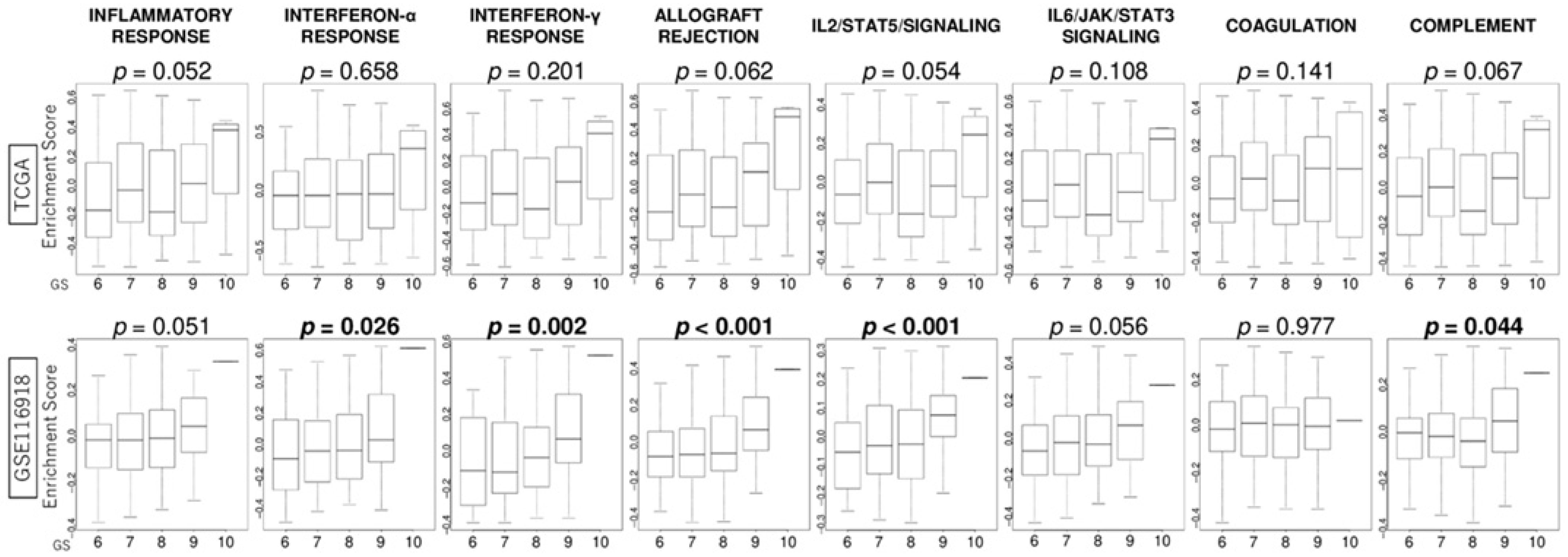
3.4. GS Levels Were Associated with the Activity Levels of Immunity-Related Gene Sets
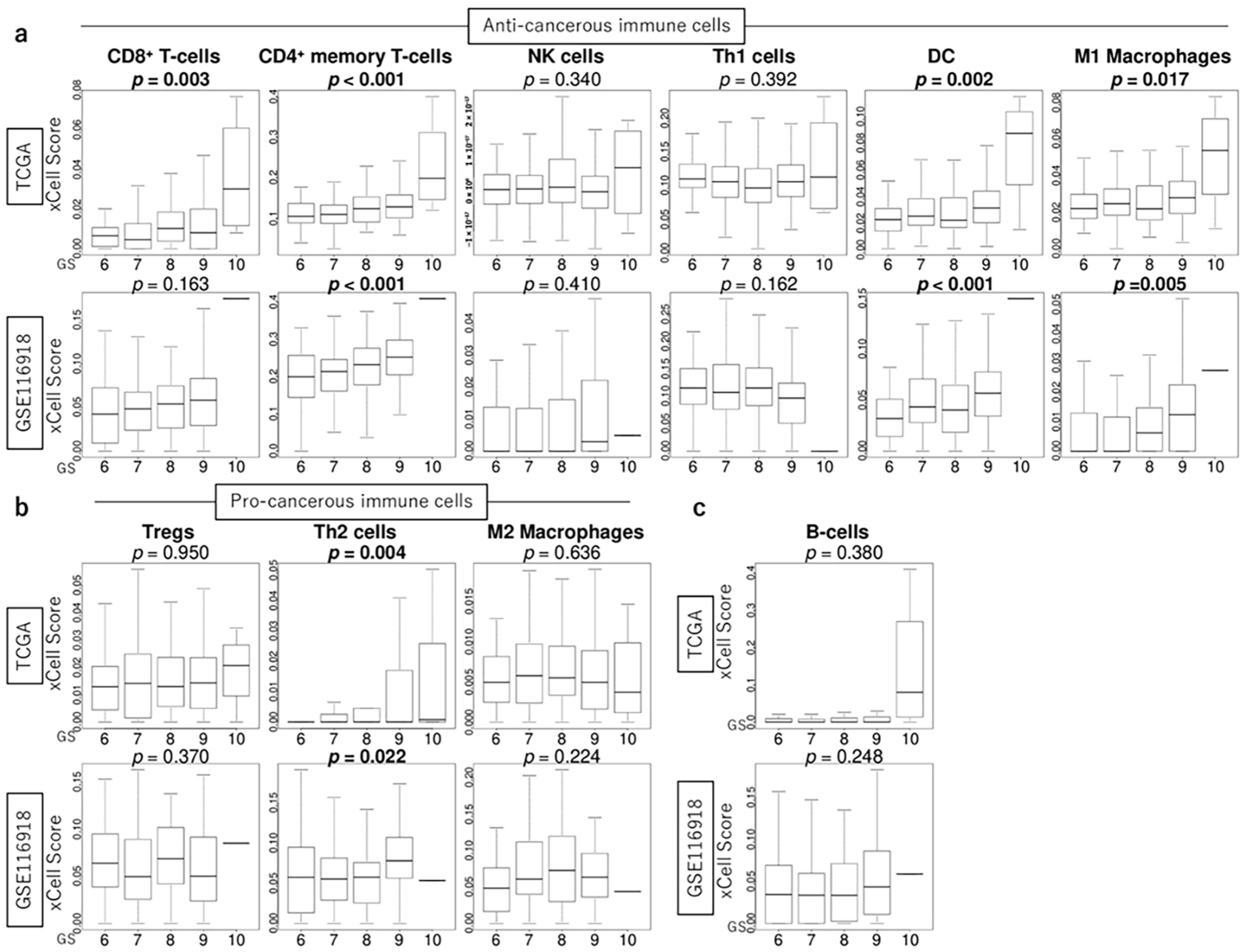
3.5. GS Levels Were Associated with the Infiltration Fraction of Several Immune Cells in the TME of PCa
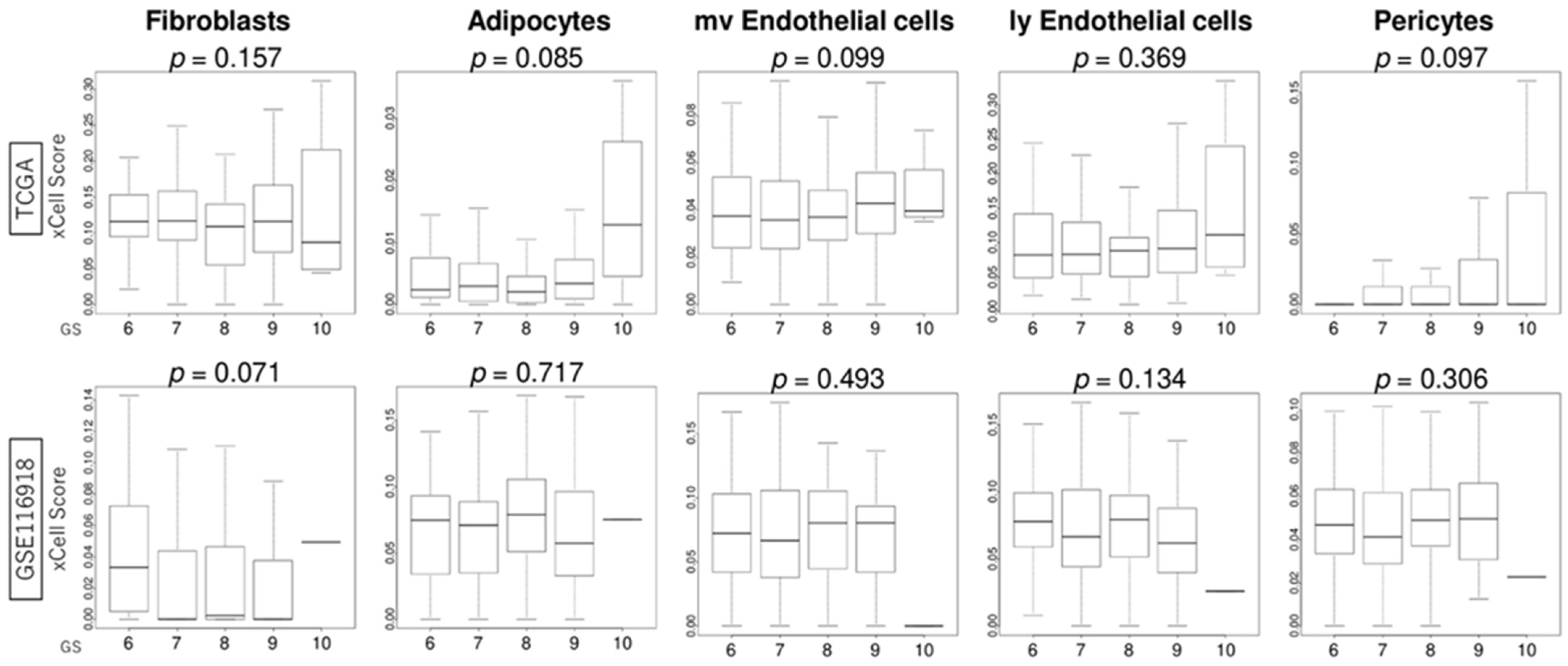
3.6. GS Levels Were Not Associated with the Infiltration Fraction Rate of Stromal Cells
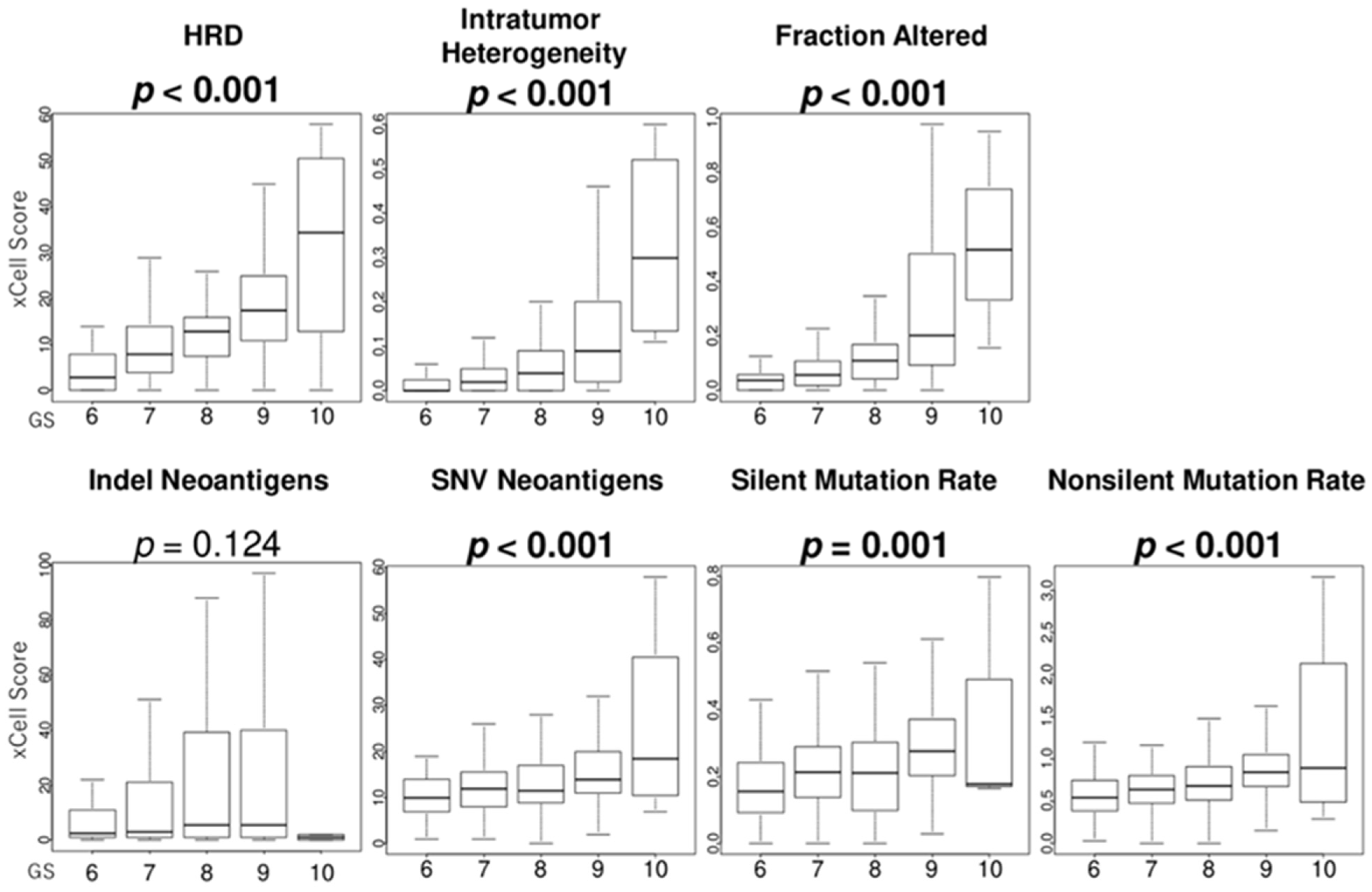
3.7. GS Levels Were Associated with the Score Levels of Homologous Recombination Defeciency (HRD), Intratumor Heterogeneity, Fraction Alteration, and Mutation Load
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GS | Gleason score |
| PCa | prostate cancer |
| TME | tumor microenvironment |
| BCR-FS | biochemical recurrence-free survival |
| OS | overall survival |
| MFS | metastasis-free survival |
| RP | radical prostatectomy |
| IL2 | interleukin 2 |
| IL6 | interleukin 6 |
| NK | natural killer |
| Th1 | T helper type 1 |
| DC | dendritic cell |
| Tregs | regulatory T cells |
| Th2 | T helper type 2 |
| HRD | homologous recombination defect |
| SNV | single-nucleotide variation |
References
- Schafer, E.J.; Jemal, A.; Wiese, D.; Sung, H.; Kratzer, T.B.; Islami, F.; Dahut, W.L.; Knudsen, K.E. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur. Urol. 2023, 84, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Higashi, T.; Inoue, T. Urological cancer statistics on incidence from 1975 to 2019 and mortality from 1958 to 2022 in Japan. Int. J. Clin. Oncol. 2024, 29, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, T.; van den Bergh, R.C.N.; Arfi, N.; Gross, T.; Moris, L.; Briers, E.; Cumberbatch, M.; De Santis, M.; Tilki, D.; Fanti, S.; et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur. Urol. 2019, 75, 967–987. [Google Scholar] [CrossRef] [PubMed]
- Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother. Rep. 1966, 50, 125–128. [Google Scholar] [PubMed]
- Humphrey, P.A. Gleason grading and prognostic factors in carcinoma of the prostate. Mod. Pathol. 2004, 17, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.; Uchida, K.; Hirokawa, Y.; Shiraishi, T.; Onishi, T.; Sasaki, T.; Inoue, T.; Watanabe, M. Short-term prognosis of low-risk prostate cancer patients is favorable despite the presence of pathological prognostic factors: A retrospective study. BMC Urol. 2023, 23, 174. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Hayakawa, K.; Horiguchi, G.; Murashita, J.; Shiraishi, T.; Fujimoto, S.; Miyashita, M.; Saito, Y.; Gabata, Y.; Sako, S.; et al. Screening for Predictive Factors of Efficacy of Second-Generation Androgen Receptor Axis-Targeted Agents in Patients with High-Risk Metastatic Hormone-Sensitive Prostate Cancer. Prostate 2025, 85, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sekito, S.; Kageyama, T.; Sugino, Y.; Sasaki, T. Roles of the PARP Inhibitor in BRCA1 and BRCA2 Pathogenic Mutated Metastatic Prostate Cancer: Direct Functions and Modification of the Tumor Microenvironment. Cancers 2023, 15, 2662. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Karanika, S.; Yang, G.; Wang, J.; Park, S.; Broom, B.M.; Manyam, G.C.; Wu, W.; Luo, Y.; Basourakos, S.; et al. Androgen receptor inhibitor-induced "BRCAness" and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal. 2017, 10, eaam7479. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Liu, Y.; Yao, Y.; Yang, T.; Zhang, H.; Yang, X.; Huang, R.; Zhou, W.; Pan, X.; Cui, X. Advances in sequencing and omics studies in prostate cancer: Unveiling molecular pathogenesis and clinical applications. Front. Oncol. 2024, 14, 1355551. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Bizzarri, F.P.; Filomena, G.B.; Marino, F.; Iacovelli, R.; Ciccarese, C.; Boccuto, L.; Ragonese, M.; Gavi, F.; Rossi, F.; et al. Relationship Between Loss of Y Chromosome and Urologic Cancers: New Future Perspectives. Cancers 2024, 16, 3766. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Karoii, D.; Bavandi, S.; Djamali, M.; Abroudi, A.S. Exploring the interaction between immune cells in the prostate cancer microenvironment combining weighted correlation gene network analysis and single-cell sequencing: An integrated bioinformatics analysis. Discov. Oncol. 2024, 15, 513. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, R.; Wang, J.; Luo, Z. Precision molecular insights for prostate cancer prognosis: Tumor immune microenvironment and cell death analysis of senescence-related genes by machine learning and single-cell analysis. Discov. Oncol. 2024, 15, 487. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tuo, Z.; Chen, X.; Wang, H.; Lyu, Z.; Li, G. Identification of cell differentiation trajectory-related gene signature to reveal the prognostic significance and immune landscape in prostate cancer based on multiomics analysis. Heliyon 2024, 10, e27628. [Google Scholar] [CrossRef] [PubMed]
- Jhun, M.A.; Geybels, M.S.; Wright, J.L.; Kolb, S.; April, C.; Bibikova, M.; Ostrander, E.A.; Fan, J.B.; Feng, Z.; Stanford, J.L. Gene expression signature of Gleason score is associated with prostate cancer outcomes in a radical prostatectomy cohort. Oncotarget 2017, 8, 43035–43047. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.H.; Fu, W.; Wang, H.; Baras, A.S.; Lotan, T.L.; Antonarakis, E.S. Prevalence of DNA repair gene mutations in localized prostate cancer according to clinical and pathologic features: Association of Gleason score and tumor stage. Prostate Cancer Prostatic Dis. 2019, 22, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Y. Comprehensive Analysis and Identification of an Immune-Related Gene Signature with Prognostic Value for Prostate Cancer. Int. J. Gen. Med. 2021, 14, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Yimamu, Y.; Yang, X.; Chen, J.; Luo, C.; Xiao, W.; Guan, H.; Wang, D. The Development of a Gleason Score-Related Gene Signature for Predicting the Prognosis of Prostate Cancer. J. Clin. Med. 2022, 11, 7164. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Q.; Mohyeddin, A.; Liu, Y.; Liu, Z.; Ge, J.; Zhang, B.; Shi, G.; Wang, W.; Wu, D.; et al. Identification of the Key Genes Involved in the Tumorigenesis and Prognosis of Prostate Cancer. Comput. Math. Methods Med. 2022, 2022, 5500416. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Aurelian, J.; Mocanu, M.M. Analysis of the Gene Networks and Pathways Correlated with Tissue Differentiation in Prostate Cancer. Int. J. Mol. Sci. 2024, 25, 3626. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, M.; Zhao, Y.; Zhang, Y. Establishment of a prognostic risk model for prostate cancer based on Gleason grading and cuprotosis related genes. J. Cancer Res. Clin. Oncol. 2024, 150, 376. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Zhang, H.; Wang, M.; Ping, H. UQCRB and LBH are correlated with Gleason score progression in prostate cancer: Spatial transcriptomics and experimental validation. Comput. Struct. Biotechnol. J. 2024, 23, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Lu, Y.; Li, X.; Wei, Y.; Ye, D.; Wei, G.; Zhu, Y. Targeting the tumor microenvironment, a new therapeutic approach for prostate cancer. Prostate Cancer Prostatic Dis. 2024, 28, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Oshi, M.; Roy, A.M.; Yachi, T.; Nara, M.; Yamada, K.; Matsuura, O.; Hashizume, T.; Endo, I.; Takabe, K. E2F target score is associated with cell proliferation and survival of patients with hepatocellular carcinoma. Surgery 2023, 174, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Oshi, M.; Asaoka, M.; Yan, L.; Benesch, M.G.K.; Khoury, T.; Nagahashi, M.; Miyoshi, Y.; Endo, I.; Ishikawa, T.; et al. Intratumoral Tumor Infiltrating Lymphocytes (TILs) are Associated with Cell Proliferation and Better Survival But Not Always with Chemotherapy Response in Breast Cancer. Ann. Surg. 2023, 278, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Lyons, C.A.; Walker, S.M.; McQuaid, S.; Hynes, S.O.; Mitchell, D.M.; Pang, B.; Logan, G.E.; McCavigan, A.M.; O’Rourke, D.; et al. Validation of a Metastatic Assay using biopsies to improve risk stratification in patients with prostate cancer treated with radical radiation therapy. Ann. Oncol. 2018, 29, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, S.M. The BRCAness Landscape of Cancer. Cells 2022, 11, 3877. [Google Scholar] [CrossRef] [PubMed]
- Diaz de la Guardia-Bolivar, E.; Barrios-Rodriguez, R.; Zwir, I.; Jimenez-Moleon, J.J.; Del Val, C. Identification of novel prostate cancer genes in patients stratified by Gleason classification: Role of antitumoral genes. Int. J. Cancer 2022, 151, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Blessin, N.C.; Yang, C.; Mandelkow, T.; Raedler, J.B.; Li, W.; Bady, E.; Simon, R.; Vettorazzi, E.; Lennartz, M.; Bernreuther, C.; et al. Automated Ki-67 labeling index assessment in prostate cancer using artificial intelligence and multiplex fluorescence immunohistochemistry. J. Pathol. 2023, 260, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Vlajnic, T.; Brunner, P.; Eppenberger-Castori, S.; Rentsch, C.A.; Zellweger, T.; Bubendorf, L. High Inter- and Intratumoral Variability of Ki67 Labeling Index in Newly Diagnosed Prostate Cancer with High Gleason Scores. Pathobiology 2022, 89, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Zhang, T.; Ma, L. Correlation analysis between immune-related genes and cell infiltration revealed prostate cancer immunotherapy biomarkers linked to T cells gamma delta. Sci. Rep. 2023, 13, 2459. [Google Scholar] [CrossRef] [PubMed]

| Cohort | TCGA | GSE116918 |
|---|---|---|
| Treatment | Radical prostatectomy | Radiation therapy |
| Number of patients | 493 | 248 |
| Median age (range) years at surgery | 61 (41–78) | 68 (48–79) |
| Clinical T stage at diagnosis (%) | ||
| cT1/T2/T3/T4/Unknown | 175/172/52/2/92 (35.5/35/10/0.5/19) | 51/76/92/4/25 (21/31/37/1/10) |
| Biopsy GS (%) | ||
| 6 | 45 (9) | 42 (17) |
| 7 | 245 (50) | 99 (40) |
| 8 | 62 (12) | 52 (21) |
| 9 | 137 (28) | 54 (21.5) |
| 10 | 4 (1) | 1 (0.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyachi, S.; Oshi, M.; Sasaki, T.; Endo, I.; Makiyama, K.; Inoue, T. Unique Biological Characteristics of Patients with High Gleason Score and Localized/Locally Advanced Prostate Cancer Using an In Silico Translational Approach. Curr. Oncol. 2025, 32, 409. https://doi.org/10.3390/curroncol32070409
Miyachi S, Oshi M, Sasaki T, Endo I, Makiyama K, Inoue T. Unique Biological Characteristics of Patients with High Gleason Score and Localized/Locally Advanced Prostate Cancer Using an In Silico Translational Approach. Current Oncology. 2025; 32(7):409. https://doi.org/10.3390/curroncol32070409
Chicago/Turabian StyleMiyachi, Shiori, Masanori Oshi, Takeshi Sasaki, Itaru Endo, Kazuhide Makiyama, and Takahiro Inoue. 2025. "Unique Biological Characteristics of Patients with High Gleason Score and Localized/Locally Advanced Prostate Cancer Using an In Silico Translational Approach" Current Oncology 32, no. 7: 409. https://doi.org/10.3390/curroncol32070409
APA StyleMiyachi, S., Oshi, M., Sasaki, T., Endo, I., Makiyama, K., & Inoue, T. (2025). Unique Biological Characteristics of Patients with High Gleason Score and Localized/Locally Advanced Prostate Cancer Using an In Silico Translational Approach. Current Oncology, 32(7), 409. https://doi.org/10.3390/curroncol32070409






