Surgical Management of Desmoid Tumors—Patient Selection, Timing, and Approach
Simple Summary
Abstract
1. Introduction
1.1. Mutational Drivers and Clinical Implications
1.2. Paradigm Shift: Active Surveillance as Frontline Approach
1.3. Optimizing Outcomes: The Importance of Multidisciplinary Management in Referral Centers
- (a)
- Persistent tumor growth documented on follow-up imaging, defined as an increase in tumor size across three or more follow-up visits, or 24 months [18].
- (b)
- Impairment or threat to life, function or quality of life.
- (c)
- Worsening or progressive symptoms.
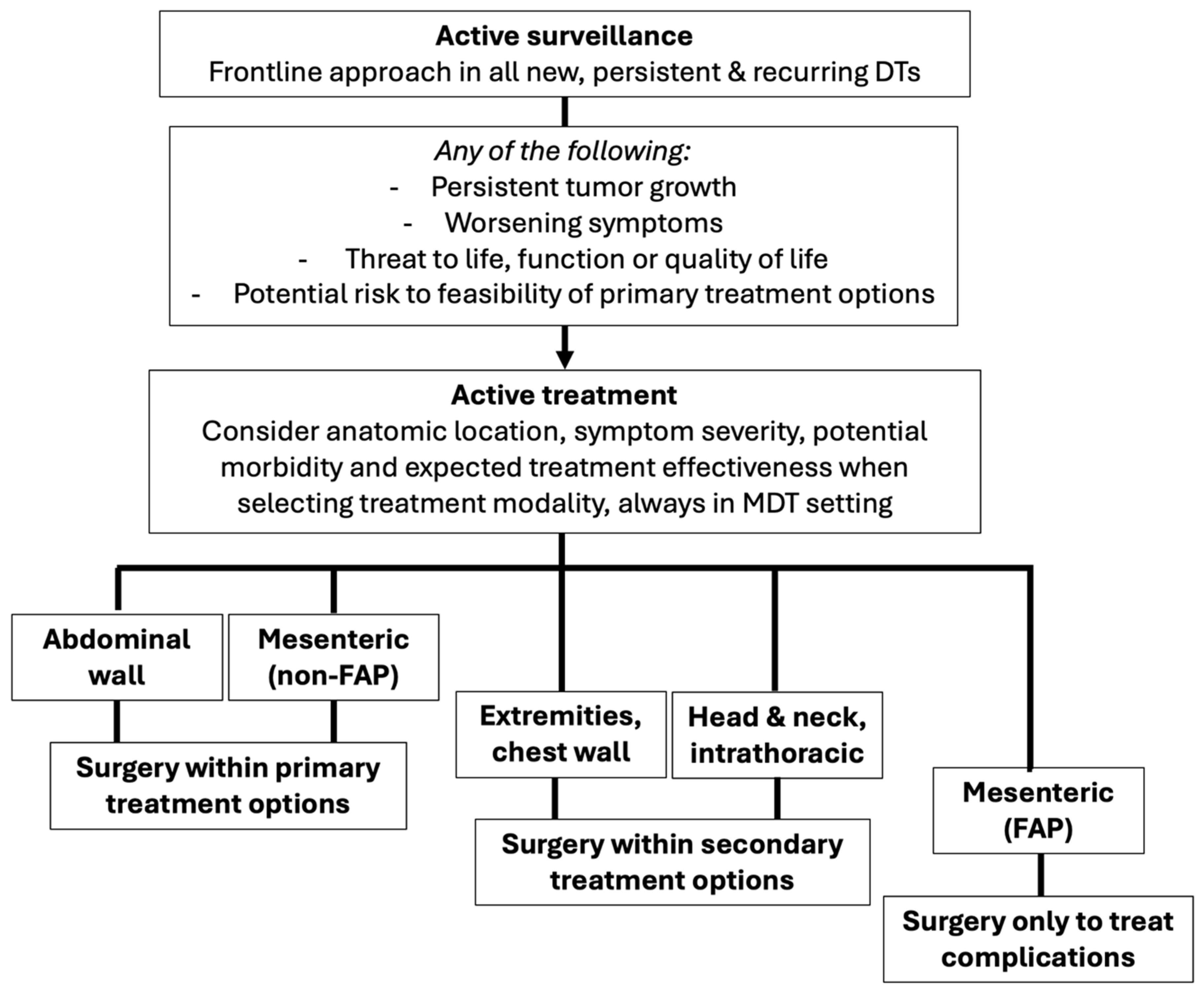
2. The Role of Surgical Management in Desmoid Tumors
- -
- Who? Anatomic location and FAP status are the main determinants of surgical eligibility, as they directly influence the risk of postoperative complications and long-term outcomes. In general, sporadic AW DTs derive the greatest benefit from resection, followed by sporadic mesenteric DTs.
- -
- When? Surgical resection must only be considered in case of sustained documented progression as defined above, given that approximately two-thirds of patients may experience spontaneous regression. All cases must be discussed in a multidisciplinary setting to ensure consideration of all available treatment options.
- -
- How? Resection should aim to achieve complete macroscopic excision with minimal to narrow margins while preserving function. Since LR rates are not significantly impacted by R0 vs. R1 margins, wider resections offer no added benefit and may increase the need for soft tissue reconstruction, associated surgical morbidity, and functional impairment [36].
2.1. Risk of Recurrence After Surgery
2.2. Patient-Related Outcomes
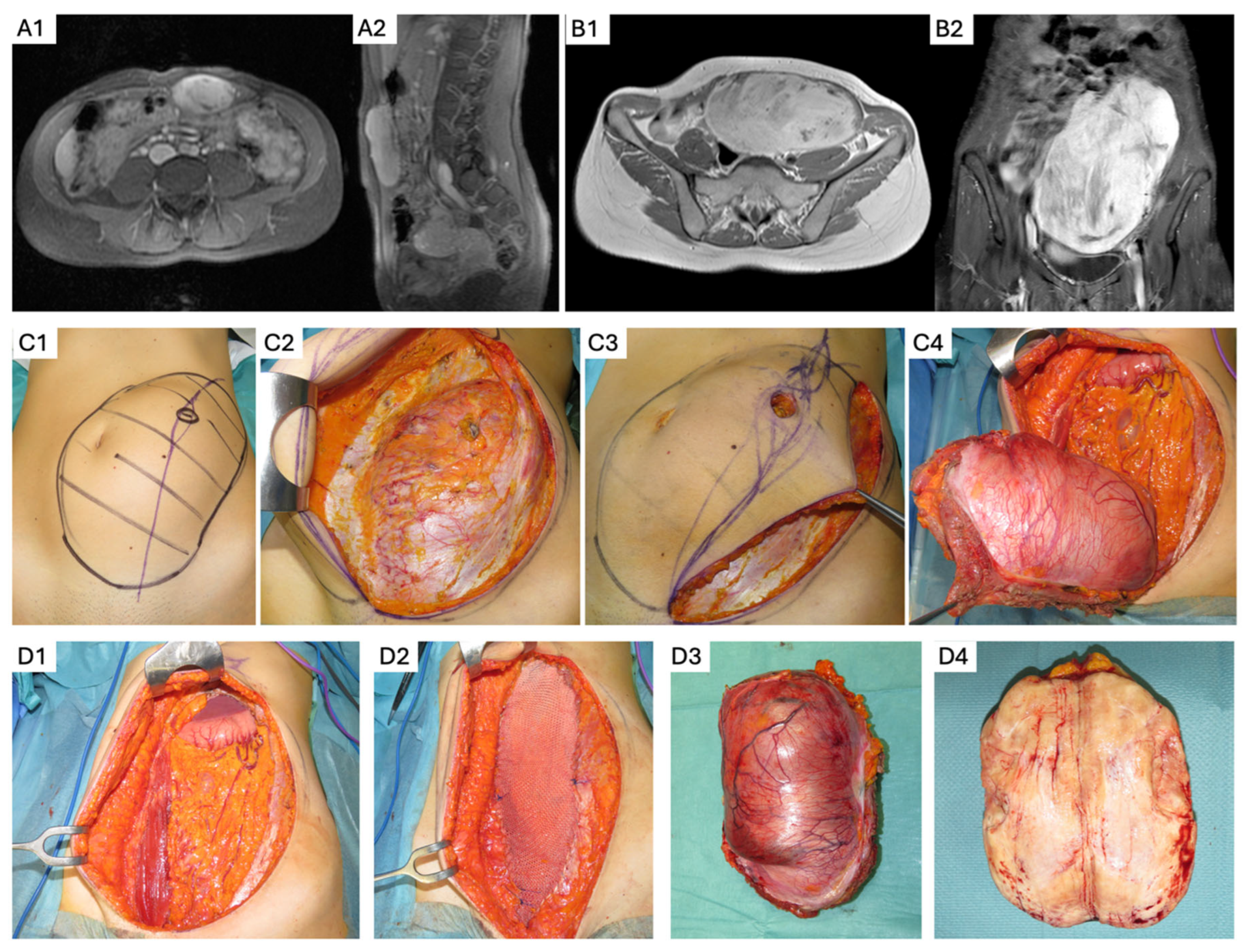
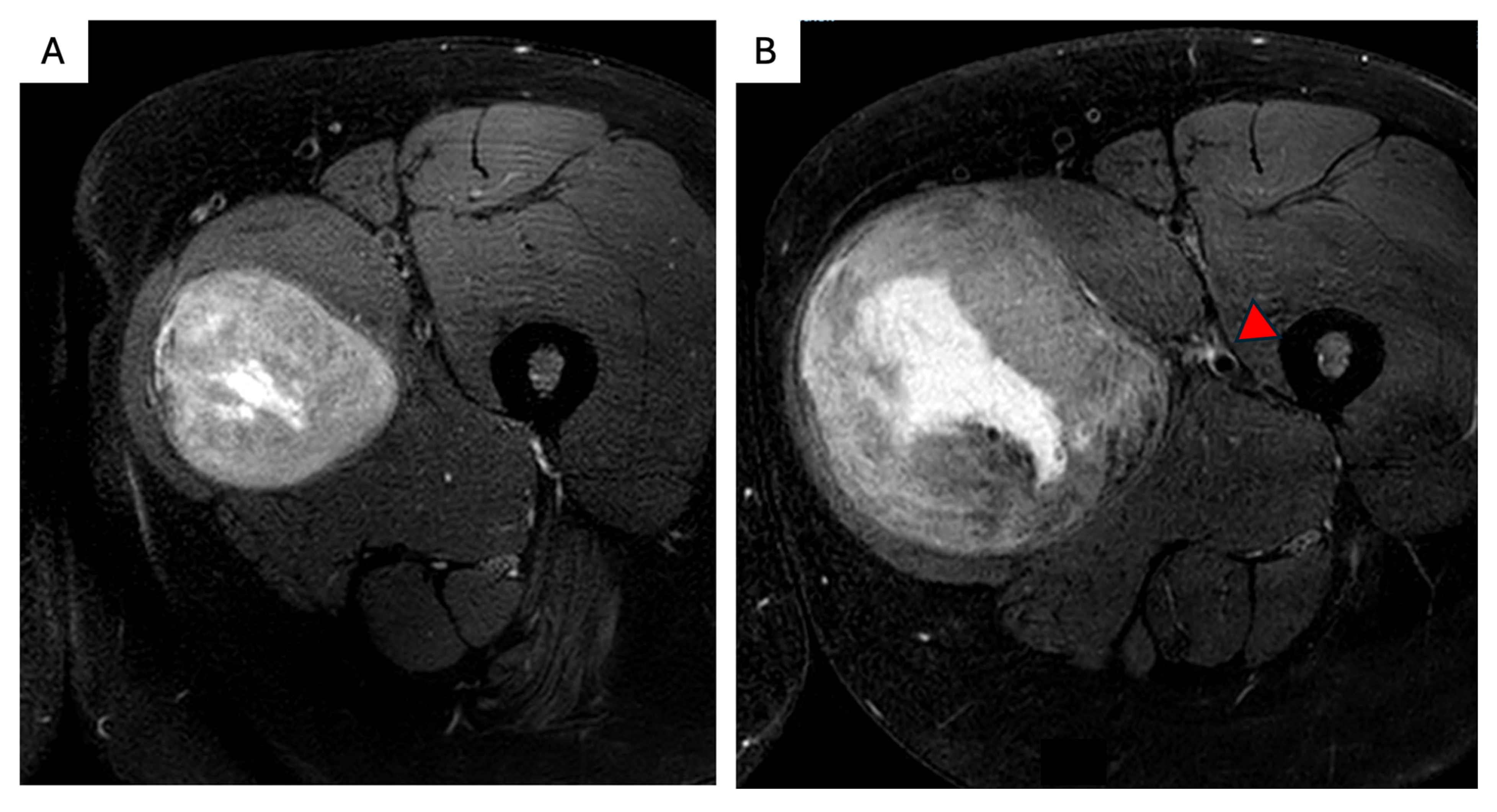
3. Abdominal Wall DTs
- -
- Whenever possible, overlying skin and subcutaneous adipose tissue should be preserved to decrease the need for cutaneous flap reconstruction. A fascia-preserving technique has been described by Nishida et al., who performed a marginal, macroscopically complete resection that reduces or eliminates the need for fascial resection and reconstruction, with a reported LR rate of 6.7% [45]. However, definitive data are lacking, and full-thickness AW resection remains the conventional approach when it can be performed with an acceptable functional impact.
- -
- If myofascial AW resection is required, reconstruction techniques should be selected based on the extent of the fascial defect, patients’ clinical characteristics, and risk of incisional hernia. When significant soft tissue or fascial resection is anticipated, multidisciplinary surgical planning—including collaboration with AW reconstruction or plastic surgery specialists—should be pursued to optimize functional and cosmetic outcomes. Expected postoperative results should be discussed preoperatively to align with patient expectations and priorities.
- -
- For smaller fascial defects, primary fascial closure with relaxing incisions may be sufficient and can be reinforced with an onlay or sublay mesh if needed [46]. In cases of larger myofascial defects, bridging mesh reconstruction may be required. The choice of mesh composition should be tailored to the risk of surgical site infection, wound contamination, and contact with IA contents [47].
- -
- In patients with potential future pregnancies, alternatives to mesh reconstruction should be considered to maximize future AW compliance. These strategies may include preoperative botulinum toxin A injection [48] to facilitate a tension-free, primary midline fascial closure with a reinforced tension-line suture technique [49]. If primary fascial closure is achieved, but there is a high risk of incisional hernia or non-midline closure, reinforcement with an onlay or sublay, slow-reabsorbing synthetic mesh may be appropriate [43,50].
- -
- When myofascial tissue reconstruction is required, advanced techniques such as component separation or autologous reconstruction with pedicled or free flaps may be indicated for full-thickness defects [51]. Alternatively, bridging mesh reconstruction with slow-reabsorbing synthetic mesh can be considered. Even in patients with permanent synthetic mesh reconstruction, future pregnancy is not contraindicated, though it carries a higher risk of pain during the third trimester [52] and chronic pain [53]. Close obstetric monitoring is recommended to ensure fetal and maternal well-being.
4. Desmoids and Pregnancy
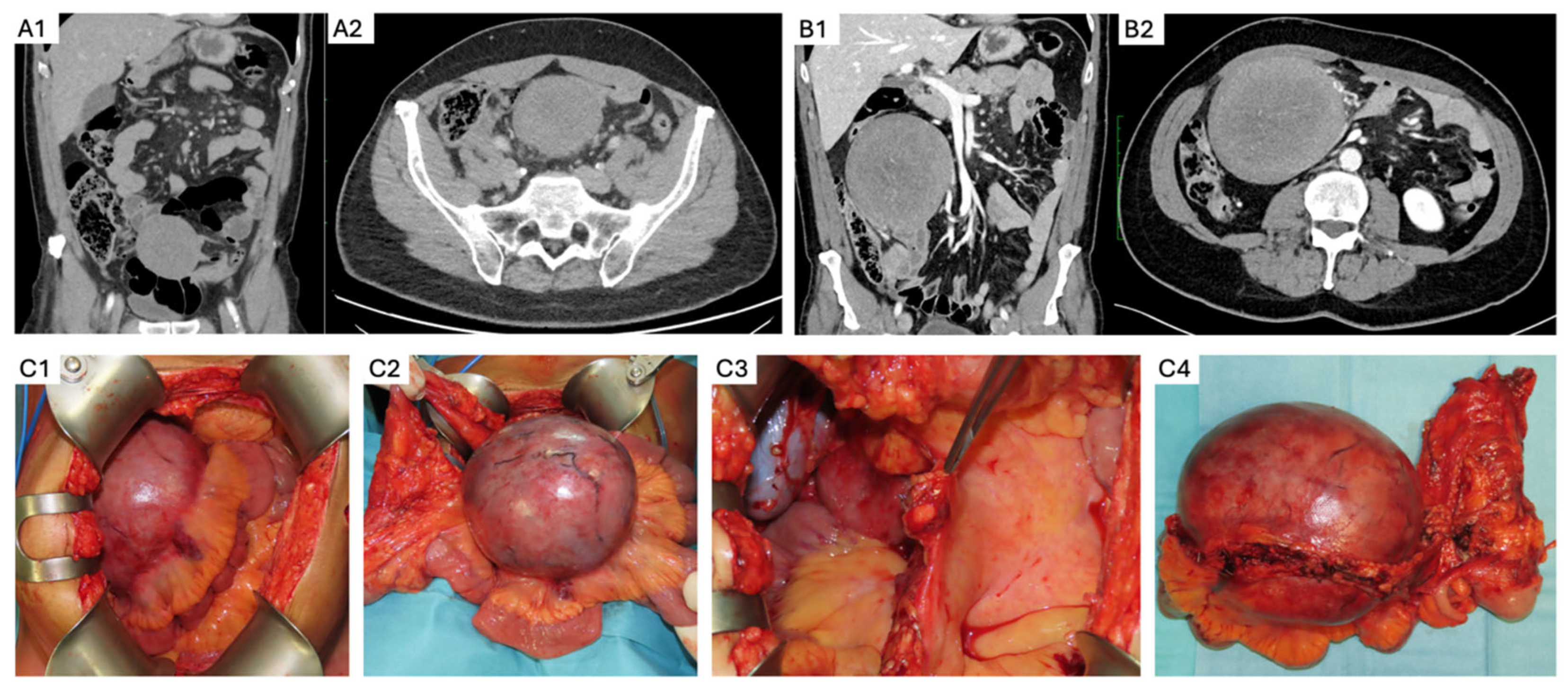
5. Sporadic Intra-Abdominal Desmoids
- -
- Elective resection as primary treatment. In patients with resectable primary disease and low anticipated morbidity, complete macroscopic clearance can be achieved (Figure 3) with favorable operative outcomes in specialist centers [62]. When assessing resectability, key considerations include mesenteric vasculature involvement, anticipated length of small bowel and/or colonic resection, risk of short gut syndrome, and additional visceral involvement. The potential for future tumor growth and associated complications must also be carefully weighed [20,59]. In case of prior incomplete resection and evidence of residual disease, AS is recommended due to the possibility of an indolent course [63].
- -
- PD or intolerance to systemic therapy. Surgery may be considered as an alternative treatment option for patients who develop treatment-limiting toxicity during systemic therapy, or in the case of PD, provided the tumor remains resectable with acceptable morbidity. This decision must be made in a multidisciplinary setting, considering the rate of progression, required extent of resection, current symptom burden, potential quality of life improvement, and availability of additional medical therapy options [34].
- -
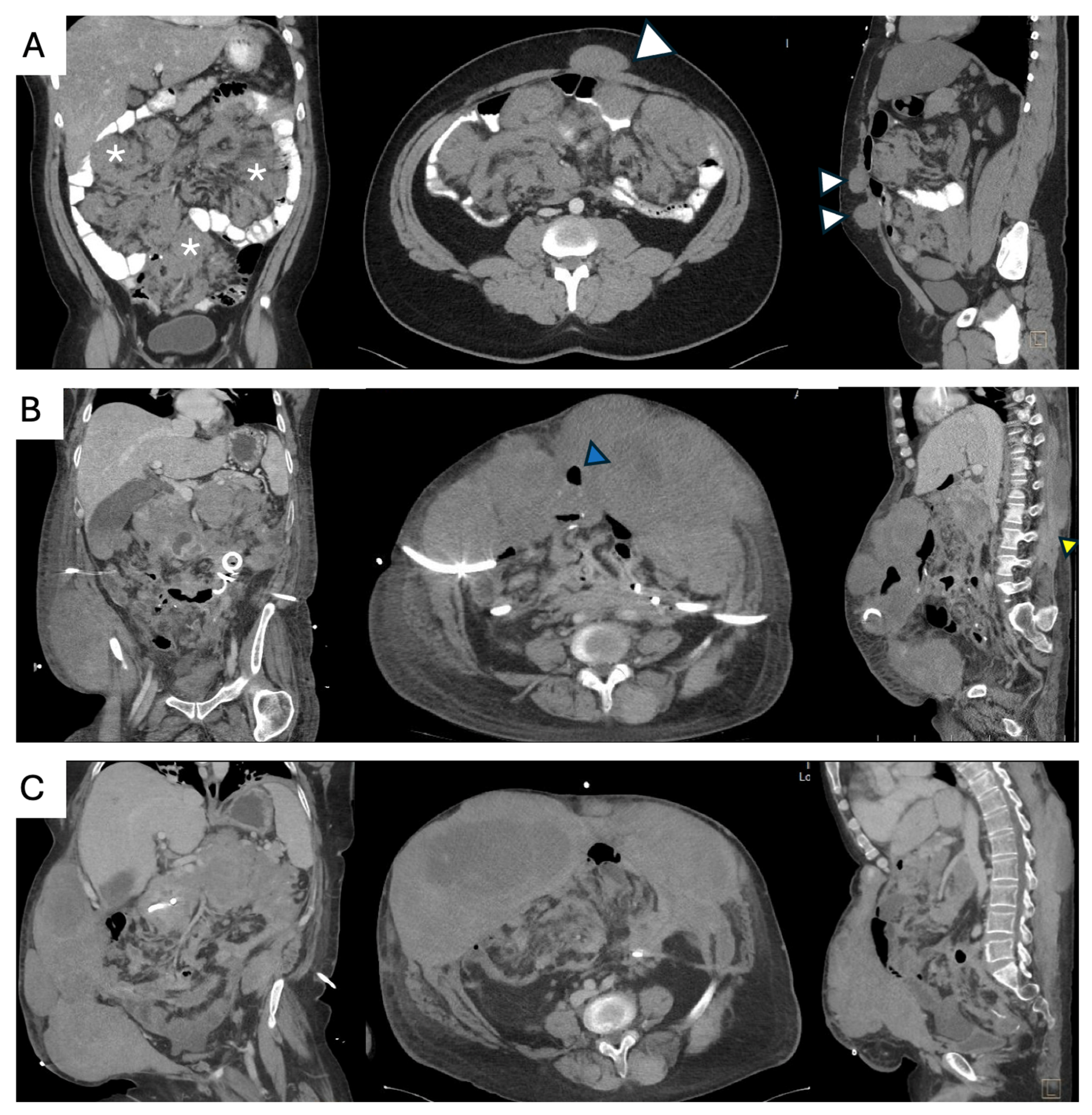
- Surgical management of complications. Sporadic IA DTs may be associated with complications such as bowel obstruction, perforation, bleeding, or intestinal ischemia in up to 10% of cases [64]. These complications can arise at initial presentation, during AS, or active treatment. Treatment should be guided by the patient’s clinical condition, type and severity of the complication, extent and resectability of the underlying disease, and compounded surgical morbidity. Management options include:
- ○
- Surgical treatment of complications with synchronous tumor resection, if complete macroscopic resection is feasible and potential morbidity—such as the anticipated length of remnant bowel—is acceptable. This approach should only be considered if the diagnosis of a sporadic IA DT has been established and FAP has been excluded prior to the complication.
- ○
- Surgical management of complications without tumor resection, aimed at stabilizing the patient clinically. This allows for further assessment of remnant bowel, postoperative symptom burden, functional status, extent of disease, and exclusion of FAP, all of which are critical for guiding primary treatment selection.
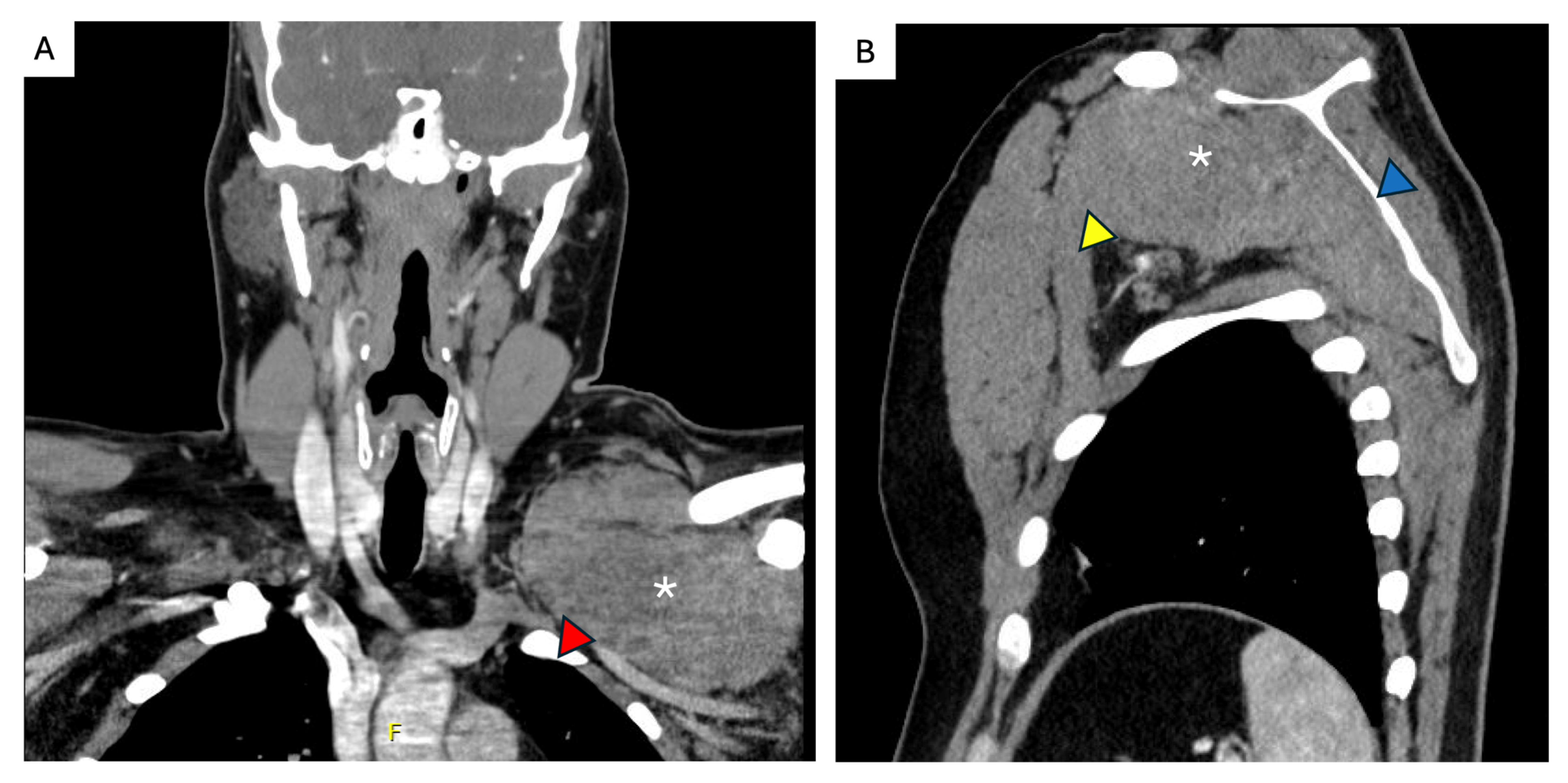
6. FAP-Associated Intraabdominal Desmoids
7. Unfavorable Locations
8. Follow up
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2020, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- de Pinieux, G.; Karanian-Philippe, M.; Loarer, F.L.; Guellec, S.L.; Chabaud, S.; Terrier, P.; Bouvier, C.; Battistella, M.; Neuville, A.; Robin, Y.-M.; et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS ONE 2021, 16, e0246958. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Bell, T.; Khan, S.; Tumminello, B.; Fernandez, M.M.; Heyes, C.; Oton, A.B. Desmoid Tumors: A Comprehensive Review. Adv. Ther. 2023, 40, 3697–3722. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, D.L.M.; Grünhagen, D.J.; Den Bakker, M.A.; Van Dalen, T.; Verhoef, C. Time Trends in the Incidence and Treatment of Extra-Abdominal and Abdominal Aggressive Fibromatosis: A Population-Based Study. Ann. Surg. Oncol. 2015, 22, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours. Soft Tissue and Bone Tumours, 5th ed.; IARC: Lyon, France, 2020; Available online: https://publications.iarc.fr/588 (accessed on 10 November 2021).
- Kasper, B.; Ströbel, P.; Hohenberger, P. Desmoid Tumors: Clinical Features and Treatment Options for Advanced Disease. Oncol. 2011, 16, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Lazar, A.J.F.; Tuvin, D.; Hajibashi, S.; Habeeb, S.; Bolshakov, S.; Mayordomo-Aranda, E.; Warneke, C.L.; Lopez-Terrada, D.; Pollock, R.E.; Lev, D. Specific Mutations in the β-Catenin Gene (CTNNB1) Correlate with Local Recurrence in Sporadic Desmoid Tumors. Am. J. Pathol. 2008, 173, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Federman, N. Molecular pathogenesis of desmoid tumor and the role of γ-secretase inhibition. NPJ Precis. Oncol. 2022, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, M.L.; Tonelli, F.; Quaresmini, D.; Lovero, D.; Della-Morte, D.; Silvestris, F.; Guadagni, F.; Palmirotta, R. Desmoid Tumors in Familial Adenomatous Polyposis. Anticancer Res. 2017, 37, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Durno, C.; Monga, N.; Bapat, B.; Berk, T.; Cohen, Z.; Gallinger, S. Does Early Colectomy Increase Desmoid Risk in Familial Adenomatous Polyposis? Clin. Gastroenterol. Hepatol. 2007, 5, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Provenzano, S.; Colombo, C.; Vitellaro, M.; Brunello, A.; Badalamenti, G.; Nannini, M.; Ibrahim, T.; Hohenberger, P.; Gasperoni, S.; et al. Familial adenomatosis polyposis–related desmoid tumours treated with low-dose chemotherapy: Results from an international, multi-institutional, retrospective analysis. ESMO Open 2020, 5, e000604. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Gantzer, J.; De Marini, P.; Garnon, J.; Koch, G.; Buy, X.; Autrusseau, P.-A.; Auloge, P.; Dalili, D.; Kurtz, J.-E.; et al. Sporadic Desmoid Tumours: Systematic Review with Reflection on the Role of Cryoablation. Cardiovasc. Interv. Radiol. 2022, 45, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Koskenvuo, L.; Peltomäki, P.; Renkonen-Sinisalo, L.; Gylling, A.; Nieminen, T.T.; Ristimäki, A.; Lepistö, A. Desmoid tumor patients carry an elevated risk of familial adenomatous polyposis. J. Surg. Oncol. 2016, 113, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Fiore, M.; Grignani, G.; Tolomeo, F.; Merlini, A.; Palassini, E.; Collini, P.; Stacchiotti, S.; Casali, P.G.; Perrone, F.; et al. A Prospective Observational Study of Active Surveillance in Primary Desmoid Fibromatosis. Clin. Cancer Res. 2022, 28, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Lev, D.; Kotilingam, D.; Wei, C.; Ballo, M.T.; Zagars, G.K.; Pisters, P.W.T.; Lazar, A.A.; Patel, S.R.; Benjamin, R.S.; Pollock, R.E. Optimizing Treatment of Desmoid Tumors. J. Clin. Oncol. 2007, 25, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Schut, A.-R.W.; Timbergen, M.J.M.; Van Broekhoven, D.L.M.; Van Dalen, T.; Van Houdt, W.J.; Bonenkamp, J.J.; Sleijfer, S.; Grunhagen, D.J.; Verhoef, C. A Nationwide Prospective Clinical Trial on Active Surveillance in Patients with Non-intraabdominal Desmoid-type Fibromatosis: The GRAFITI Trial. Ann. Surg. 2023, 277, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Eastley, N.; McCulloch, T.; Esler, C.; Hennig, I.; Fairbairn, J.; Gronchi, A.; Ashford, R. Extra-abdominal desmoid fibromatosis: A review of management, current guidance and unanswered questions. Eur. J. Surg. Oncol. 2016, 42, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Rimareix, F.; Mariani, L.; Domont, J.; Collini, P.; Le Péchoux, C.; Casali, P.G.; Le Cesne, A.; Gronchi, A.; Bonvalot, S. Desmoid-Type Fibromatosis: A Front-Line Conservative Approach to Select Patients for Surgical Treatment. Ann. Surg. Oncol. 2009, 16, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Cozic, N.; Le Cesne, A.; Blay, J.Y.; Penel, N.; Fau, M.; Chevreau, C.; Anract, P.; Waast, D.; Laurence, V.; et al. Initial Active Surveillance Strategy for Patients with Peripheral Sporadic Primary Desmoid-Type Fibromatosis: A Multicentric Phase II Observational Trial. Ann. Surg. Oncol. 2023, 30, 8653–8659. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Baldini, E.H.; Bonvalot, S.; Callegaro, D.; Cardona, K.; Colombo, C.; Corradini, N.; Crago, A.M.; Dei Tos, A.P.; Dileo, P.; et al. Current Management of Desmoid Tumors: A Review. JAMA Oncol. 2024, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Desai, A.; Coppola, S.; Le Péchoux, C.; Terrier, P.; Dômont, J.; Le Cesne, A. The treatment of desmoid tumors: A stepwise clinical approach. Ann. Oncol. 2012, 23, x158–x166. [Google Scholar] [CrossRef] [PubMed]
- Nathenson, M.J.; Hu, J.; Ratan, R.; Somaiah, N.; Hsu, R.; DeMaria, P.J.; Catoe, H.W.; Pang, A.; Subhawong, T.K.; Amini, B.; et al. Systemic Chemotherapies Retain Antitumor Activity in Desmoid Tumors Independent of Specific Mutations in CTNNB1 or APC: A Multi-institutional Retrospective Study. Clin. Cancer Res. 2022, 28, 4092–4104. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Mahoney, M.R.; Van Tine, B.A.; Ravi, V.; Attia, S.; Deshpande, H.A.; Gupta, A.A.; Milhem, M.M.; Conry, R.M.; Movva, S.; et al. Sorafenib for Advanced and Refractory Desmoid Tumors. N. Engl. J. Med. 2018, 379, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Toulmonde, M.; Pulido, M.; Ray-Coquard, I.; Andre, T.; Isambert, N.; Chevreau, C.; Penel, N.; Bompas, E.; Saada, E.; Bertucci, F.; et al. Pazopanib or methotrexate–vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): A non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol. 2019, 20, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.; Ratan, R.; Alcindor, T.; Schöffski, P.; Van Der Graaf, W.T.; Wilky, B.A.; Riedel, R.F.; Lim, A.; Smith, L.M.; Moody, S.; et al. Nirogacestat, a γ-Secretase Inhibitor for Desmoid Tumors. N. Engl. J. Med. 2023, 388, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Elnekave, E.; Ben Ami, E.; Shamai, S.; Peretz, I.; Tamir, S.; Bruckheimer, E.; Stemmer, A.; Erinjeri, J.; Abu Quider, A.; Seidensticker, M.; et al. Selective Intra-Arterial Doxorubicin Eluting Microsphere Embolization for Desmoid Fibromatosis: A Combined Prospective and Retrospective Study. Cancers 2022, 14, 5045. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.; Woodhead, G.; Hannallah, J.; Young, S. Role of the Interventional Radiologist in the Treatment of Desmoid Tumors. Life 2023, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Vora, B.M.K.; Munk, P.L.; Somasundaram, N.; Ouellette, H.A.; Mallinson, P.I.; Sheikh, A.; Abdul Kadir, H.; Tan, T.J.; Yan, Y.Y. Cryotherapy in extra-abdominal desmoid tumors: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0261657. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.-E.; Buy, X.; Deschamps, F.; Sauleau, E.; Bouhamama, A.; Toulmonde, M.; Honoré, C.; Bertucci, F.; Brahmi, M.; Chevreau, C.; et al. CRYODESMO-O1: A prospective, open phase II study of cryoablation in desmoid tumour patients progressing after medical treatment. Eur. J. Cancer 2021, 143, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Garnon, J.; Cazzato, R.L.; Autrusseau, P.-A.; Koch, G.; Weiss, J.; Gantzer, J.; Kurtz, J.-E.; Gangi, A. Desmoid fibromatosis: Interventional radiology (sometimes) to the rescue for an atypical disease. Br. J. Radiol. 2025, 98, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Keus, R.B.; Nout, R.A.; Blay, J.-Y.; De Jong, J.M.; Hennig, I.; Saran, F.; Hartmann, J.T.; Sunyach, M.P.; Gwyther, S.J.; Ouali, M.; et al. Results of a phase II pilot study of moderate dose radiotherapy for inoperable desmoid-type fibromatosis—An EORTC STBSG and ROG study (EORTC 62991–22998). Ann. Oncol. 2013, 24, 2672–2676. [Google Scholar] [CrossRef] [PubMed]
- Alman, B.; Attia, S.; Baumgarten, C.; Benson, C.; Blay, J.-Y.; Bonvalot, S.; Breuing, J.; Cardona, K.; Casali, P.G.; Van Coevorden, F.; et al. The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur. J. Cancer 2020, 127, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, D.L.M.; Deroose, J.P.; Bonvalot, S.; Gronchi, A.; Grünhagen, D.J.; Eggermont, A.M.M.; Verhoef, C. Isolated limb perfusion using tumour necrosis factor α and melphalan in patients with advanced aggressive fibromatosis. Br. J. Surg. 2014, 101, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.L.; Van Broekhoven, D.L.M.; Cates, J.M.M.; Bramer, W.M.; Nuyttens, J.J.; Gronchi, A.; Salas, S.; Bonvalot, S.; Grünhagen, D.J.; Verhoef, C. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br. J. Surg. 2017, 104, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Crago, A.M.; Denton, B.; Salas, S.; Dufresne, A.; Mezhir, J.J.; Hameed, M.; Gonen, M.; Singer, S.; Brennan, M.F. A Prognostic Nomogram for Prediction of Recurrence in Desmoid Fibromatosis. Ann. Surg. 2013, 258, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Casali, P.G.; Mariani, L.; Lo Vullo, S.; Colecchia, M.; Lozza, L.; Bertulli, R.; Fiore, M.; Olmi, P.; Santinami, M.; et al. Quality of Surgery and Outcome in Extra-Abdominal Aggressive Fibromatosis: A Series of Patients Surgically Treated at a Single Institution. J. Clin. Oncol. 2003, 21, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Timbergen, M.J.M.; Van De Poll-Franse, L.V.; Grünhagen, D.J.; Van Der Graaf, W.T.; Sleijfer, S.; Verhoef, C.; Husson, O. Identification and assessment of health-related quality of life issues in patients with sporadic desmoid-type fibromatosis: A literature review and focus group study. Qual. Life Res. 2018, 27, 3097–3111. [Google Scholar] [CrossRef] [PubMed]
- Penel, N.; Bonvalot, S.; Le Deley, M.; Italiano, A.; Tlemsani, C.; Pannier, D.; Leguillette, C.; Kurtz, J.; Toulmonde, M.; Thery, J.; et al. Pain in desmoid-type fibromatosis: Prevalence, determinants and prognosis value. Int. J. Cancer 2023, 153, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Atkinson, T.M.; Bell, T.; Daskalopoulou, C.; Griffiths, P.; Martindale, M.; Smith, L.M.; Lim, A. GOunder/Desmoid Tumor Research Foundation DEsmoid Symptom/Impact Scale (GODDESS©): Psychometric properties and clinically meaningful thresholds as assessed in the Phase 3 DeFi randomized controlled clinical trial. Qual. Life Res. 2023, 32, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Schut, A.-R.W.; Lidington, E.; Timbergen, M.J.M.; Younger, E.; Van Der Graaf, W.T.A.; Van Houdt, W.J.; Bonenkamp, J.J.; Jones, R.L.; Grünhagen, D.J.; Sleijfer, S.; et al. Development of a Disease-Specific Health-Related Quality of Life Questionnaire (DTF-QoL) for Patients with Desmoid-Type Fibromatosis. Cancers 2022, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.G.; Tzanis, D.; Messiou, C.; Benson, C.; Van Der Hage, J.A.; Fiore, M.; Bonvalot, S.; Hayes, A.J. The management of soft tissue tumours of the abdominal wall. Eur. J. Surg. Oncol. 2017, 43, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Chan, K.E.; Hayes, A.J.; Strauss, D.C. Surgical Outcomes following Resection for Sporadic Abdominal Wall Fibromatosis. Ann. Surg. Oncol. 2014, 21, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Hamada, S.; Sakai, T.; Ito, K.; Ikuta, K.; Urakawa, H.; Koike, H.; Imagama, S. Less-invasive fascia-preserving surgery for abdominal wall desmoid. Sci. Rep. 2021, 11, 19379. [Google Scholar] [CrossRef] [PubMed]
- Couto Netto, S.D.; Teixeira, F.; Menegozzo, C.A.M.; Albertini, A.; Akaishi, E.H.; Utiyama, E.M. Abdominal wall reconstruction after desmoid type fibromatosis radical resection: Case series from a single institution and review of the literature. Int. J. Surg. Case Rep. 2017, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Lak, K.L.; Goldblatt, M.I. Mesh Selection in Abdominal Wall Reconstruction. Plast. Reconstr. Surg. 2018, 142 (Suppl. 3), 99S–106S. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, S.A.; Elhage, S.A.; Salvino, M.J.; Sacco, J.M.; Heniford, B.T. State-of-the-art abdominal wall reconstruction and closure. Langenbecks Arch. Surg. 2023, 408, 60. [Google Scholar] [CrossRef] [PubMed]
- Wenzelberg, C.L.; Rogmark, P.; Ekberg, O.; Petersson, U. Reinforced tension-line suture after laparotomy: Early results of the Rein4CeTo1 randomized clinical trial. Br. J. Surg. 2024, 111, znae265. [Google Scholar] [CrossRef] [PubMed]
- Saiding, Q.; Chen, Y.; Wang, J.; Pereira, C.L.; Sarmento, B.; Cui, W.; Chen, X. Abdominal wall hernia repair: From prosthetic meshes to smart materials. Mater. Today Bio 2023, 21, 100691. [Google Scholar] [CrossRef] [PubMed]
- Roubaud, M.; Baumann, D. Flap Reconstruction of the Abdominal Wall. Semin. Plast. Surg. 2018, 32, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaeckers, E.; Stirler, V.; Raymakers, J.; Rakic, S. Pregnancy Following Laparoscopic Mesh Repair of Ventral Abdominal Wall Hernia. J. Soc. Laparoendosc. Surg. 2012, 16, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Oma, E.; Bisgaard, T.; Jorgensen, L.N.; Jensen, K.K. Nationwide Propensity-Score Matched Study of Mesh Versus Suture Repair of Primary Ventral Hernias in Women with a Subsequent Pregnancy. World J. Surg. 2019, 43, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Drabbe, C.; Van Der Graaf, W.T.A.; Husson, O.; Bonenkamp, J.J.; Verhoef, C.; Van Houdt, W.J. Pregnancy-associated desmoid fibromatosis: A Dutch multi-centre retrospective study. Eur. J. Surg. Oncol. 2023, 49, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Debaudringhien, M.; Blay, J.-Y.; Bimbai, A.-M.; Bonvalot, S.; Italiano, A.; Rousset-Jablonski, C.; Corradini, N.; Piperno-Neumann, S.; Chevreau, C.; Kurtz, J.-E.; et al. Association between recent pregnancy or hormonal contraceptive exposure and outcome of desmoid-type fibromatosis. ESMO Open 2022, 7, 100578. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Coppola, S.; Cannell, A.J.; Colombo, C.; Bertagnolli, M.M.; George, S.; Le Cesne, A.; Gladdy, R.A.; Casali, P.G.; Swallow, C.J.; et al. Desmoid-Type Fibromatosis and Pregnancy: A Multi-institutional Analysis of Recurrence and Obstetric Risk. Ann. Surg. 2014, 259, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Baumgarten, C.; Garcia, J.; Bonvalot, S.; Haas, R.; Haller, F.; Hohenberger, P.; Penel, N.; Messiou, C.; Van Der Graaf, W.T.; et al. An update on the management of sporadic desmoid-type fibromatosis: A European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann. Oncol. 2017, 28, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Ljevar, S.; Raut, C.P.; Personeni, G.; Rabih, M.; Gladdy, R.; Mercier, K.; Sulciner, M.; Rossi, E.; Tzanis, D.; et al. Impact and Safety of Pregnancy on Desmoid Fibromatosis Management in the Era of Active Surveillance. An International Multicenter Retrospective Observational Study. Eur. J. Cancer 2025, 222, 115474. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Zager, J.S.; Sondak, V.K. Multimodality management of desmoid tumors: How important is a negative surgical margin? J. Surg. Oncol. 2008, 98, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Crago, A.M.; Chmielecki, J.; Rosenberg, M.; O’Connor, R.; Byrne, C.; Wilder, F.G.; Thorn, K.; Agius, P.; Kuk, D.; Socci, N.D.; et al. Near universal detection of alterations in CTNNB1 and Wnt pathway regulators in desmoid-type fibromatosis by whole-exome sequencing and genomic analysis. Genes Chromosomes Cancer 2015, 54, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, D.L.M.; Grunhagen, D.J.; Verhoef, C. Abdominal Desmoid Tumors: Hands Off? Ann. Surg. Oncol. 2016, 23, 2128–2130. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Fitzgerald, J.E.F.; Thomas, J.M.; Hayes, A.J.; Strauss, D.C. Surgical resection for non-familial adenomatous polyposis-related intra-abdominal fibromatosis. Br. J. Surg. 2012, 99, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Burtenshaw, S.M.; Cannell, A.J.; McAlister, E.D.; Siddique, S.; Kandel, R.; Blackstein, M.E.; Swallow, C.J.; Gladdy, R.A. Toward Observation as First-line Management in Abdominal Desmoid Tumors. Ann. Surg. Oncol. 2016, 23, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; Fiore, M.; Provenzano, S.; Bertulli, R.; Ottini, A.; Colombo, C.; Vitellaro, M.; Greco, G.; Morosi, C.; Gronchi, A.; et al. Management of serious complications in intra-abdominal desmoid-type fibromatosis. Cancer Rep. 2021, 4, e1411. [Google Scholar] [CrossRef] [PubMed]
- Vitellaro, M.; Sala, P.; Signoroni, S.; Radice, P.; Fortuzzi, S.; Civelli, E.M.; Ballardini, G.; Kleiman, D.A.; Morrissey, K.P.; Bertario, L. Risk of desmoid tumours after open and laparoscopic colectomy in patients with familial adenomatous polyposis. Br. J. Surg. 2014, 101, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Latchford, A.R.; Sturt, N.J.H.; Neale, K.; Rogers, P.A.; Phillips, R.K.S. A 10-year review of surgery for desmoid disease associated with familial adenomatous polyposis. Br. J. Surg. 2006, 93, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Crago, A.; Gladdy, R.; Kasper, B. The Landmark Series: Desmoid. Ann. Surg. Oncol. 2021, 28, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Elshawy, M.; Smith, N.; Sommovilla, J.; Burke, C.A.; Vaidya, A.; Macaron, C.; Liska, D. Outcomes of intestinal transplantation for familial adenomatous polyposis-associated intra-abdominal desmoid disease. J. Gastrointest. Surg. 2025, 29, 102014. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.H.; Pollock, R.E. Intra-Abdominal and Abdominal Wall Desmoid Fibromatosis. Oncol. Ther. 2016, 4, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, P.; Scoccianti, G.; Schiavo, A.; Tortolini, V.; Wigley, C.; Muratori, F.; Matera, D.; Kukushkina, M.; Funovics, P.T.; Lingitz, M.-T.; et al. Extra-abdominal desmoid tumor fibromatosis: A multicenter EMSOS study. BMC Cancer 2021, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.; Gronchi, A. Desmoid tumours (extra-abdominal), a surgeon’s nightmare: The current philosophy for their treatment. Bone Jt. J. 2023, 105-B, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Eldweny, H.; Haddad, V.; Rimareix, F.; Missenard, G.; Oberlin, O.; Vanel, D.; Terrier, P.; Blay, J.Y.; Le Cesne, A.; et al. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur. J. Surg. Oncol. 2008, 34, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.D.; Hyder, O.; Mavros, M.N.; Turley, R.; Groeschl, R.; Firoozmand, A.; Lidsky, M.; Herman, J.M.; Choti, M.; Ahuja, N.; et al. Management and Recurrence Patterns of Desmoids Tumors: A Multi-institutional Analysis of 211 Patients. Ann. Surg. Oncol. 2012, 19, 4036–4042. [Google Scholar] [CrossRef] [PubMed]
- Timbergen, M.J.M.; Schut, A.-R.W.; Grünhagen, D.J.; Sleijfer, S.; Verhoef, C. Active surveillance in desmoid-type fibromatosis: A systematic literature review. Eur. J. Cancer 2020, 137, 18–29. [Google Scholar] [CrossRef] [PubMed]
| Clinical Presentation | Treatment Strategies |
|---|---|
| Systems of care Multidisciplinary team in sarcoma referral center | Therapeutic goals Optimize tumor control and improve quality of life |
Pathology diagnosis
| Active surveillance
|
Tumor characteristics
| Systemic treatment
|
Patient characteristics
| Locoregional therapies
|
FAP-related desmoids
| Surgical resection
|
Symptom burden
| Clinical trials
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazcano, C.S.; Gronchi, A. Surgical Management of Desmoid Tumors—Patient Selection, Timing, and Approach. Curr. Oncol. 2025, 32, 408. https://doi.org/10.3390/curroncol32070408
Lazcano CS, Gronchi A. Surgical Management of Desmoid Tumors—Patient Selection, Timing, and Approach. Current Oncology. 2025; 32(7):408. https://doi.org/10.3390/curroncol32070408
Chicago/Turabian StyleLazcano, Catherine Sarre, and Alessandro Gronchi. 2025. "Surgical Management of Desmoid Tumors—Patient Selection, Timing, and Approach" Current Oncology 32, no. 7: 408. https://doi.org/10.3390/curroncol32070408
APA StyleLazcano, C. S., & Gronchi, A. (2025). Surgical Management of Desmoid Tumors—Patient Selection, Timing, and Approach. Current Oncology, 32(7), 408. https://doi.org/10.3390/curroncol32070408





