Cannabidiol (CBD) and Colorectal Tumorigenesis: Potential Dual Modulatory Roles via the Serotonergic Pathway
Simple Summary
Abstract
1. Introduction
2. Cannabis, Cannabinoids, and Cannabidiol
2.1. Hemp vs. Marijuana
2.2. Cannabidiol vs. Tetrahydrocannabinol
2.3. Legislation of Cannabis (CBD and THC) Use
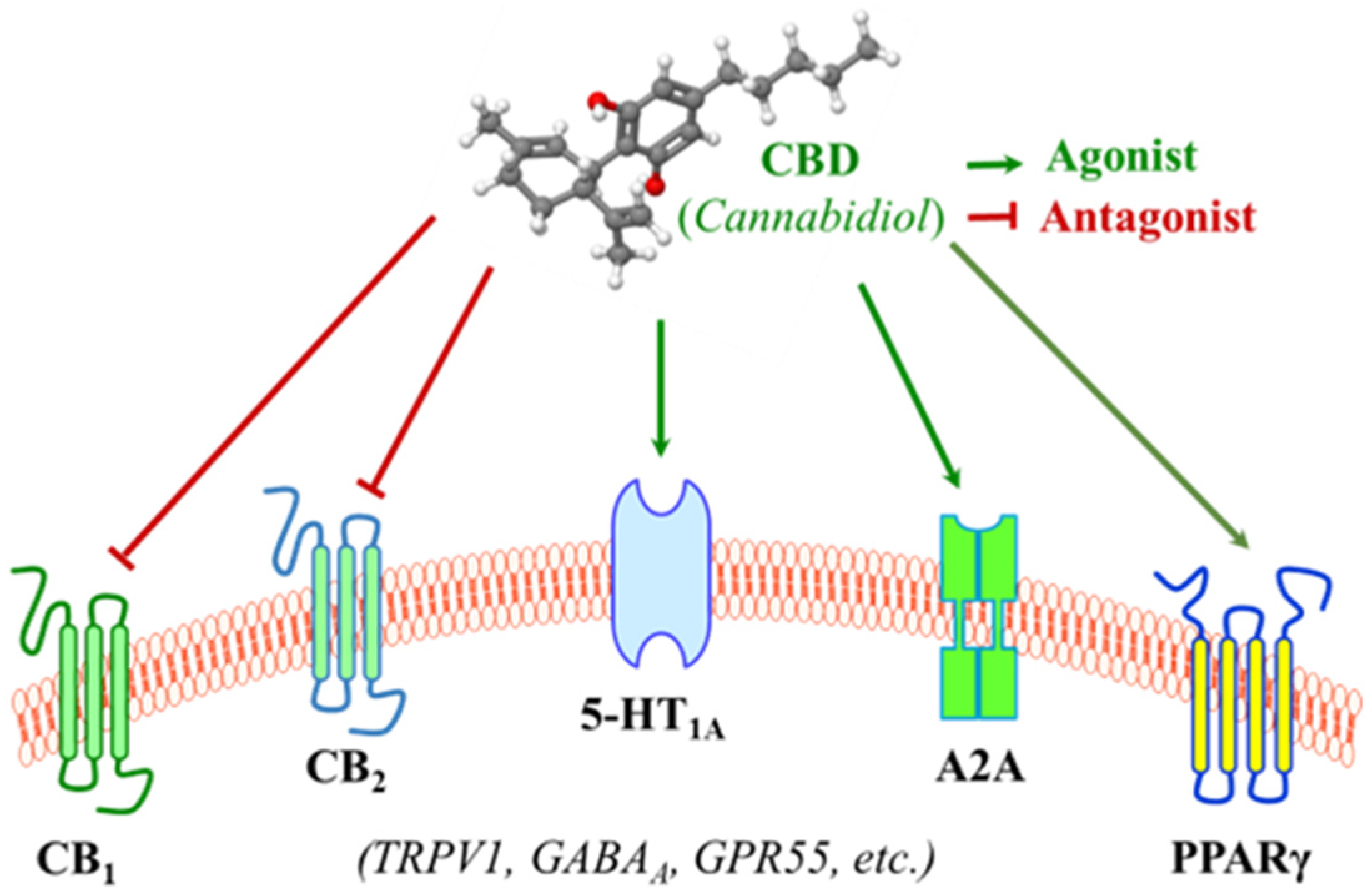
3. Molecular Actions by Cannabidiol: Effects on Membrane Receptors
3.1. Interaction with the Endocannabinoid System
3.2. Interaction with the Serotonergic System
3.3. Interactions with Membrane Receptors Involved in Inflammatory Regulation
4. Cannabidiol in the Context of Colorectal Cancer
4.1. Colorectal Cancer Landscape
4.2. Evidence of the Impact of CBD on Colorectal Tumorigenesis
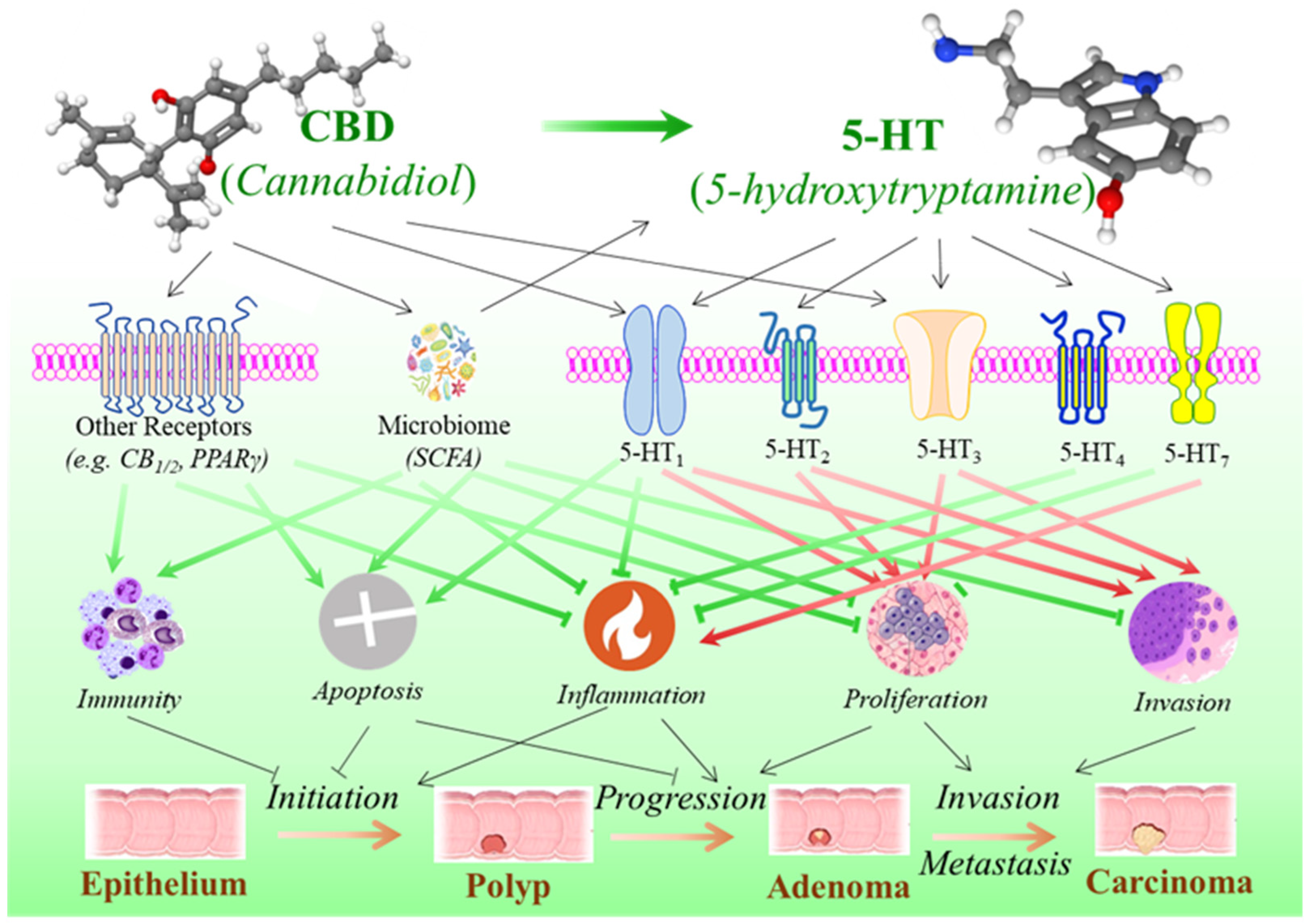
5. Cannabidiol, Serotonin Pathway, and the Development of Colorectal Cancer
5.1. Serotonergic System in the Gastrointestinal Tract
5.2. Dual Effects of Serotonin in Colorectal Tumorigenesis
5.3. The Potential Influence of CBD on the Development of Colorectal Cancer via Serotonin Pathway
6. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| TNBS | 2,4,6-trinitrobenzenesulfonic acid |
| 5-HT | 5-hydroxytryptamine |
| ACF | Aberrant crypt foci |
| ATF3/4 | Activating transcription factor 3, 4 |
| APC | Adenomatous polyposis coli |
| A2A | Adenosine receptor 2A |
| MAPK | Amitogen-activated protein kinase |
| AOM/DSS | Azoxymethane/dextran sodium sulfate |
| CBD | Cannabidiol |
| CBD-BDSs | Cannabidiol botanical drug substances |
| CB1 and CB2 | Cannabinoid receptor 1 and 2 |
| CHOP | C/EBP homologous protein |
| CNS | Central nervous system |
| CRC | Colorectal cancer |
| CDK2/4/6 | Cyclin-dependent kinase 2, 4, 6 |
| COX-2 | Cyclo-oxygenase-2 |
| DR5 | Death receptor 5 |
| THC | D9-tetrahydrocannabinol |
| ECs | Enterochromaffin cells |
| EMT | Epithelial–mesenchymal transition |
| GPR55 | G-protein-coupled receptor 55 |
| GABAA | γ-aminobutyric acid type A receptor |
| GI | Gastrointestinal tract |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| HIF-1α | Hypoxia-induced factor-1α |
| BiP | Immunoglobulin protein |
| IRE1α | Inositol-requiring enzyme 1α |
| NO | Nitric oxide |
| NOS3 | Nitric oxide synthase 3 |
| PPARγ | Peroxisome proliferator-activated receptor-gamma |
| eIF2α | Phosphorylated eukaryotic initiation factor 2α |
| PERK | Phosphorylated protein kinase RNA-like ER kinase |
| ROS | Reactive oxygen species |
| SSRIs | Serotonin reuptake inhibitors |
| SERTs | Serotonin transporters |
| SCFAs | Short-chain fatty acids |
| SOD | Superoxide dismutase |
| TGF-β | Transforming growth factor beta |
| TRPV | Transient receptor potential cation channel subfamily V |
| TPH | Tryptophan hydroxylase |
| VEGF | Vascular endothelial growth factor |
References
- U.S. Congress. Agriculture Improvement Act of 2018; Pub. L. No. 115-334, 132 Stat. 4490; United States Government Publishing Office: Washington, DC, USA, 2018.
- Rossheim, M.E.; LoParco, C.R.; Walker, A.; Livingston, M.D.; Trangenstein, P.J.; Olsson, S.; McDonald, K.K.; Yockey, R.A.; Luningham, J.M.; Kong, A.Y.; et al. Delta-8 THC Retail Availability, Price, and Minimum Purchase Age. Cannabis Cannabinoid Res. 2024, 9, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Geci, M.; Scialdone, M.; Tishler, J. The Dark Side of Cannabidiol: The Unanticipated Social and Clinical Implications of Synthetic Delta(8)-THC. Cannabis Cannabinoid Res. 2023, 8, 270–282. [Google Scholar] [CrossRef]
- Kirkpatrick, M.; O’Callaghan, F. Epilepsy and cannabis: So near, yet so far. Dev. Med. Child. Neurol. 2022, 64, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef]
- Del-Bel, E.; Barros-Pereira, N.; Moraes, R.P.; Mattos, B.A.; Alves-Fernandes, T.A.; Abreu, L.B.; Nascimento, G.C.; Escobar-Espinal, D.; Pedrazzi, J.F.C.; Jacob, G.; et al. A journey through cannabidiol in Parkinson’s disease. Int. Rev. Neurobiol. 2024, 177, 65–93. [Google Scholar]
- Lord, S.; Hardy, J.; Good, P. Does Cannabidiol Have a Benefit as a Supportive Care Drug in Cancer? Curr. Treat. Options Oncol. 2022, 23, 514–525. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Kappo, A.P. Anti-Cancer and Anti-Proliferative Potential of Cannabidiol: A Cellular and Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 5659. [Google Scholar] [CrossRef] [PubMed]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef]
- Lirio, P.H.C.; Gaspari, P.D.M.; Campos, A.C. Cannabidiol: Pharmacodynamics and pharmacokinetic in the context of neuropsychiatric disorders. Int. Rev. Neurobiol. 2024, 177, 11–27. [Google Scholar]
- Porter, B.; Marie, B.S.; Milavetz, G.; Herr, K. Cannabidiol (CBD) Use by Older Adults for Acute and Chronic Pain. J. Gerontol. Nurs. 2021, 47, 6–15. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Pharmacology, Clinical Effects, and Therapeutic Potential of Cannabinoids for Gastrointestinal and Liver Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Jensen, S.S.; Nikolajsen, G.N.; Bruun, H.Z.; Bhuller, R.; Hoeng, J. The therapeutic potential of purified cannabidiol. J. Cannabis Res. 2023, 5, 21. [Google Scholar] [CrossRef]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic activity of cannabinoids. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K. Cannabidiol (CBD) in Cancer Management. Cancers 2022, 14, 885. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, M.; Liu, C.; Zhang, H.; Yang, S.; An, J.; Qu, G.; Song, S.; Cao, Q. Research Progress on the Mechanism of the Antitumor Effects of Cannabidiol. Molecules 2024, 29, 1943. [Google Scholar] [CrossRef]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef]
- Guy, G.W.; Whittle, B.A.; Robson, P. The Medicinal Uses of Cannabis and Cannabinoids; Pharmaceutical Press: London, UK, 2004; pp. 74–116. ISBN 978-0-85369-517-2. [Google Scholar]
- Ren, G.; Zhang, X.; Li, Y.; Ridout, K.; Serrano-Serrano, M.L.; Yang, Y.; Liu, A.; Ravikanth, G.; Nawaz, M.A.; Mumtaz, A.S.; et al. Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Sci. Adv. 2021, 7, eabg2286. [Google Scholar] [CrossRef]
- Lambert, D.M. Cannabinoids in Nature and Medicine; Wiley-VCH: Weinheim, Germany, 2009; p. 20. ISBN 978-3-906390-56-7. [Google Scholar]
- ElSohly, M.A. Marijuana and the Cannabinoids; Humana Press: Totowa, NJ, USA, 2007; p. 8. ISBN 978-1-58829-456-2. [Google Scholar]
- Abyadeh, M.; Gupta, V.; Paulo, J.A.; Chitranshi, N.; Godinez, A.; Saks, D.; Hasan, M.; Amirkhani, A.; McKay, M.; Salekdeh, G.H.; et al. A Proteomic View of Cellular and Molecular Effects of Cannabis. Biomolecules 2021, 11, 1411. [Google Scholar] [CrossRef]
- Lambert, D.M.; Fowler, C.J. The endocannabinoid system: Drug targets, lead compounds, and potential therapeutic applications. J. Med. Chem. 2005, 48, 5059–5087. [Google Scholar] [CrossRef]
- Pertwee, R. Cannabinoids; Springer: Berlin/Heidelberg, Germany, 2005; p. 2. ISBN 978-3-540-22565-2. [Google Scholar]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef]
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2017, 1, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Emest, S. The Species Problem in Cannabis: Science & Semantics; Corpus: East Windsor, NJ, USA, 1979; Volume 2, ISBN 9780919217102. [Google Scholar]
- Erickson, B. USDA releases hemp production requirements. CEN Glob. Enterp. 2019, 97, 17. [Google Scholar] [CrossRef]
- Rizzo, G.; Storz, M.A.; Calapai, G. The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition. Foods 2023, 12, 3505. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.P.; Hindocha, C.; Green, S.F.; Bloomfield, M.A.P. Medicinal use of cannabis based products and cannabinoids. BMJ 2019, 365, l1141. [Google Scholar] [CrossRef]
- Murnion, B. Medicinal cannabis. Aust. Prescr. 2015, 38, 212–215. [Google Scholar] [CrossRef]
- Chowdhury, K.U.; Holden, M.E.; Wiley, M.T.; Suppiramaniam, V.; Reed, M.N. Effects of Cannabis on Glutamatergic Neurotransmission: The Interplay between Cannabinoids and Glutamate. Cells 2024, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Lisboa, J.R.; Guimaraes, F.S.; Gomes, F.V. Cannabidiol as an antipsychotic drug. Int. Rev. Neurobiol. 2024, 177, 295–317. [Google Scholar]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef]
- Busquets Garcia, A.; Soria-Gomez, E.; Bellocchio, L.; Marsicano, G. Cannabinoid receptor type-1: Breaking the dogmas. F1000Research 2016, 5, 990. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Scherma, M.; Masia, P.; Satta, V.; Fratta, W.; Fadda, P.; Tanda, G. Brain activity of anandamide: A rewarding bliss? Acta Pharmacol. Sin. 2018, 40, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, S.; Srebro, D.; Vujovic, K.S.; Vucetic, C.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Leinen, Z.J.; Mohan, R.; Premadasa, L.S.; Acharya, A.; Mohan, M.; Byrareddy, S.N. Therapeutic Potential of Cannabis: A Comprehensive Review of Current and Future Applications. Biomedicines 2023, 11, 2630. [Google Scholar] [CrossRef]
- Gabarin, A.; Yarmolinsky, L.; Budovsky, A.; Khalfin, B.; Ben-Shabat, S. Cannabis as a Source of Approved Drugs: A New Look at an Old Problem. Molecules 2023, 28, 7686. [Google Scholar] [CrossRef]
- Borowicz-Reutt, K.; Czernia, J.; Krawczyk, M. CBD in the Treatment of Epilepsy. Molecules 2024, 29, 1981. [Google Scholar] [CrossRef]
- Camilleri, M.; Zheng, T. Cannabinoids and the Gastrointestinal Tract. Clin. Gastroenterol. Hepatol. 2023, 21, 3217–3229. [Google Scholar] [CrossRef]
- Buckley, M.C.; Kumar, A.; Swaminath, A. Inflammatory Bowel Disease and Cannabis: A Practical Approach for Clinicians. Adv. Ther. 2021, 38, 4152–4161. [Google Scholar] [CrossRef]
- Thapa, D.; Warne, L.N.; Falasca, M. Pharmacohistory of Cannabis Use-A New Possibility in Future Drug Development for Gastrointestinal Diseases. Int. J. Mol. Sci. 2023, 24, 14677. [Google Scholar] [CrossRef]
- Hassan Almalki, W. A study of abnormal cannabidiols system-mediated cardiovascular protection in disrupted gut/brain axis associated depression. J. Biochem. Mol. Toxicol. 2021, 35, e22930. [Google Scholar] [CrossRef]
- Naya, N.M.; Kelly, J.; Hogwood, A.; Abbate, A.; Toldo, S. Therapeutic potential of cannabidiol (CBD) in the treatment of cardiovascular diseases. Expert. Opin. Investig. Drugs 2024, 33, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic. Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Darkovska-Serafimovska, M.; Serafimovska, T.; Arsova-Sarafinovska, Z.; Stefanoski, S.; Keskovski, Z.; Balkanov, T. Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases. J. Pain. Res. 2018, 11, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Guldager, M.B.; Chaves Filho, A.M.; Biojone, C.; Joca, S. Therapeutic potential of cannabidiol in depression. Int. Rev. Neurobiol. 2024, 177, 251–293. [Google Scholar]
- Simei, J.L.Q.; de Souza, J.D.R.; Lisboa, J.R.; Guimaraes, F.S.; Crippa, J.A.S. Cannabidiol in anxiety disorders: Current and future perspectives. Int. Rev. Neurobiol. 2024, 177, 205–234. [Google Scholar]
- Moreira, F.A.; de Oliveira, A.C.P.; Santos, V.R.; Moraes, M.F.D. Cannabidiol and epilepsy. Int. Rev. Neurobiol. 2024, 177, 135–147. [Google Scholar]
- Marques, B.L.; Campos, A.C. Cannabidiol and Alzheimer’s disease. Int. Rev. Neurobiol. 2024, 177, 121–134. [Google Scholar]
- Garza-Cervantes, J.A.; Ramos-Gonzalez, M.; Lozano, O.; Jerjes-Sanchez, C.; Garcia-Rivas, G. Therapeutic Applications of Cannabinoids in Cardiomyopathy and Heart Failure. Oxidative Med. Cell. Longev. 2020, 2020, 4587024. [Google Scholar] [CrossRef]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef]
- Dallabrida, K.G.; de Oliveira Bender, J.M.; Chade, E.S.; Rodrigues, N.; Sampaio, T.B. Endocannabinoid System Changes throughout Life: Implications and Therapeutic Potential for Autism, ADHD, and Alzheimer’s Disease. Brain Sci. 2024, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.; Dalle, S.; Mantini, D.; Koppo, K. Cannabidiol and brain function: Current knowledge and future perspectives. Front. Pharmacol. 2024, 14, 1328885. [Google Scholar] [CrossRef]
- HA, A.L.; Abuarab, S.F.; Salamah, H.M.; Ishqair, A.H.; Dwikat, M.F.; Nourelden, A.Z.; Qandil, A.N.; Barakat, Y.; Barakat, M. Cannabis and cancer: Unveiling the potential of a green ally in breast, colorectal, and prostate cancer. J. Cannabis Res. 2024, 6, 24. [Google Scholar]
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2016, 22, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Tao, R.; Li, C.; Jaffe, A.E.; Shin, J.H.; Deep-Soboslay, A.; Yamin, R.; Weinberger, D.R.; Hyde, T.M.; Kleinman, J.E. Cannabinoid receptor CNR1 expression and DNA methylation in human prefrontal cortex, hippocampus and caudate in brain development and schizophrenia. Transl. Psychiatry 2020, 10, 158. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB(2) receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Haney, M. Cannabis Use and the Endocannabinoid System: A Clinical Perspective. Am. J. Psychiatry 2022, 179, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Hill, M.N. Cannabis and Cannabinoids: From Synapse to Society. Neuropsychopharmacology 2017, 43, 1–3. [Google Scholar] [CrossRef]
- Sim-Selley, L.J. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit. Rev. Neurobiol. 2003, 15, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef]
- Boggs, D.L.; Nguyen, J.D.; Morgenson, D.; Taffe, M.A.; Ranganathan, M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Delta(9)-Tetrahydrocannabinol. Neuropsychopharmacology 2017, 43, 142–154. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Petitet, F.; Jeantaud, B.; Reibaud, M.; Imperato, A.; Dubroeucq, M.C. Complex pharmacology of natural cannabinoids: Evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998, 63, PL1–PL6. [Google Scholar] [CrossRef]
- Darmon, M.; Al Awabdh, S.; Emerit, M.B.; Masson, J. Insights into Serotonin Receptor Trafficking: Cell Membrane Targeting and Internalization. Prog. Mol. Biol. Transl. Sci. 2015, 132, 97–126. [Google Scholar]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Kannen, V.; Bader, M.; Sakita, J.Y.; Uyemura, S.A.; Squire, J.A. The Dual Role of Serotonin in Colorectal Cancer. Trends Endocrinol. Metab. 2020, 31, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Linge, R.; Jimenez-Sanchez, L.; Campa, L.; Pilar-Cuellar, F.; Vidal, R.; Pazos, A.; Adell, A.; Diaz, A. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Guimaraes, F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology 2008, 199, 223–230. [Google Scholar] [CrossRef]
- Gomes, F.V.; Reis, D.G.; Alves, F.H.; Correa, F.M.; Guimaraes, F.S.; Resstel, L.B. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J. Psychopharmacol. 2012, 26, 104–113. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Mayer, T.Z.; Simard, M.; Flamand, N.; Di Marzo, V. The Impact of the CB(2) Cannabinoid Receptor in Inflammatory Diseases: An Update. Molecules 2024, 29, 3381. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Blass, B.E.; Wang, H.; Grondin, J.A.; Banskota, S.; Korzekwa, K.; Ye, M.; Gordon, J.C.; Colussi, D.; Blattner, K.M.; et al. Novel 5-HT(7) receptor antagonists modulate intestinal immune responses and reduce severity of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 327, G57–G69. [Google Scholar] [CrossRef]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules 2023, 28, 5980. [Google Scholar] [CrossRef]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanus, L. Cannabidiol: An overview of some chemical and pharmacological aspects. Part I: Chemical aspects. Chem. Phys. Lipids 2002, 121, 35–43. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Szeles, L.; Torocsik, D.; Nagy, L. PPARgamma in immunity and inflammation: Cell types and diseases. Biochim. Biophys. Acta 2007, 1771, 1014–1030. [Google Scholar] [CrossRef]
- Sugiyama, H.; Nonaka, T.; Kishimoto, T.; Komoriya, K.; Tsuji, K.; Nakahata, T. Peroxisome proliferator-activated receptors are expressed in human cultured mast cells: A possible role of these receptors in negative regulation of mast cell activation. Eur. J. Immunol. 2000, 30, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wen, Y.; Lin, G.; Yu, S.; Wang, C.; Ye, W.; Zhang, J. Design, synthesis, and analgesia evaluation of novel Transient Receptor Potential Vanilloid 1 (TRPV1) agonists modified from Cannabidiol (CBD). Bioorg. Med. Chem. 2023, 90, 117379. [Google Scholar] [CrossRef]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABA(A) receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Ruffolo, G.; Gaeta, A.; Cannata, B.; Pinzaglia, C.; Aronica, E.; Morano, A.; Cifelli, P.; Palma, E. GABAergic Neurotransmission in Human Tissues Is Modulated by Cannabidiol. Life 2022, 12, 2042. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- World Health Organization. Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 12 August 2024).
- Zauber, A.G.; Lansdorp-Vogelaar, I.; Knudsen, A.B.; Wilschut, J.; van Ballegooijen, M.; Kuntz, K.M. Evaluating test strategies for colorectal cancer screening: A decision analysis for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2008, 149, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Holme, O.; Loberg, M.; Kalager, M.; Bretthauer, M.; Hernan, M.A.; Aas, E.; Eide, T.J.; Skovlund, E.; Schneede, J.; Tveit, K.M.; et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: A randomized clinical trial. JAMA 2014, 312, 606–615. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- de la Chapelle, A. Genetic predisposition to colorectal cancer. Nat. Rev. Cancer 2004, 4, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Al-Sohaily, S.; Biankin, A.; Leong, R.; Kohonen-Corish, M.; Warusavitarne, J. Molecular pathways in colorectal cancer. J. Gastroenterol. Hepatol. 2011, 27, 1423–1431. [Google Scholar] [CrossRef]
- Power, D.G.; Gloglowski, E.; Lipkin, S.M. Clinical genetics of hereditary colorectal cancer. Hematol. Oncol. Clin. N. Am. 2010, 24, 837–859. [Google Scholar] [CrossRef]
- Desai, T.K.; Barkel, D. Syndromic colon cancer: Lynch syndrome and familial adenomatous polyposis. Gastroenterol. Clin. N. Am. 2008, 37, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, W.F.; Bailey, C.J.; Bodmer, J.; Bussey, H.J.; Ellis, A.; Gorman, P.; Lucibello, F.C.; Murday, V.A.; Rider, S.H.; Scambler, P.; et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature 1987, 328, 614–616. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Nilbert, M.C.; Su, L.K.; Vogelstein, B.; Bryan, T.M.; Levy, D.B.; Smith, K.J.; Preisinger, A.C.; Hedge, P.; McKechnie, D.; et al. Identification of FAP locus genes from chromosome 5q21. Science 1991, 253, 661–665. [Google Scholar] [CrossRef]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of apoptosis by cannabinoids in prostate and colon cancer cells is phosphatase dependent. Anticancer. Res. 2011, 31, 3799–3807. [Google Scholar]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Pagano, E.; Cascio, M.G.; Pertwee, R.G.; Izzo, A.A. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014, 21, 631–639. [Google Scholar] [CrossRef]
- Kargl, J.; Andersen, L.; Hasenohrl, C.; Feuersinger, D.; Stancic, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br. J. Pharmacol. 2016, 173, 142–154. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G.; et al. Cannabidiol Enhances the Therapeutic Effects of TRAIL by Upregulating DR5 in Colorectal Cancer. Cancers 2019, 11, 642. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef]
- Honarmand, M.; Namazi, F.; Mohammadi, A.; Nazifi, S. Can cannabidiol inhibit angiogenesis in colon cancer? Comp. Clin. Pathol. 2019, 28, 165–172. [Google Scholar] [CrossRef]
- Cerretani, D.; Collodel, G.; Brizzi, A.; Fiaschi, A.I.; Menchiari, A.; Moretti, E.; Moltoni, L.; Micheli, L. Cytotoxic Effects of Cannabinoids on Human HT-29 Colorectal Adenocarcinoma Cells: Different Mechanisms of THC, CBD, and CB83. Int. J. Mol. Sci. 2020, 21, 5533. [Google Scholar] [CrossRef] [PubMed]
- Raup-Konsavage, W.M.; Carkaci-Salli, N.; Greenland, K.; Gearhart, R.; Vrana, K.E. Cannabidiol (CBD) Oil Does Not Display an Entourage Effect in Reducing Cancer Cell Viability in vitro. Med. Cannabis Cannabinoids 2020, 3, 95–102. [Google Scholar] [CrossRef]
- Lee, H.S.; Tamia, G.; Song, H.J.; Amarakoon, D.; Wei, C.I.; Lee, S.H. Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. Int. Immunopharmacol. 2022, 108, 108865. [Google Scholar] [CrossRef]
- Nkune, N.W.; Kruger, C.A.; Abrahamse, H. Synthesis of a novel nanobioconjugate for targeted photodynamic therapy of colon cancer enhanced with cannabidiol. Oncotarget 2022, 13, 156–172. [Google Scholar] [CrossRef]
- Feng, P.; Zhu, L.; Jie, J.; Yang, P.; Sheng, N.; Chen, X. Cannabidiol inhibits invasion and metastasis in colorectal cancer cells by reversing epithelial-mesenchymal transition through the Wnt/beta-catenin signaling pathway. J. Cancer Res. Clin. Oncol. 2022, 149, 3587–3598. [Google Scholar] [CrossRef]
- Wang, F.; Dezfouli, A.B.; Khosravi, M.; Sievert, W.; Stangl, S.; Schwab, M.; Wu, Z.; Steiger, K.; Ma, H.; Multhoff, G. Cannabidiol-induced crosstalk of apoptosis and macroautophagy in colorectal cancer cells involves p53 and Hsp70. Cell Death Discov. 2023, 9, 286. [Google Scholar] [CrossRef]
- Wang, F.; Bashiri Dezfouli, A.; Multhoff, G. The immunomodulatory effects of cannabidiol on Hsp70-activated NK cells and tumor target cells. Mol. Immunol. 2024, 174, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, V.; Ilnytskyy, Y.; Kovalchuk, O.; Kovalchuk, I. Transcriptome Analysis of Cisplatin, Cannabidiol, and Intermittent Serum Starvation Alone and in Various Combinations on Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 14743. [Google Scholar] [CrossRef]
- Wei, T.; Chen, L.; Shi, P.; Wang, C.; Peng, Y.; Yang, J.; Liao, X.; Yang, B.; Gao, C. Platinum (IV) drugs with cannabidiol inducing mitochondrial dysfunction and synergistically enhancing anti-tumor effects. J. Inorg. Biochem. 2024, 254, 112515. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Mohan, C.D.; Sethi, G.; Ahn, K.S. Cannabidiol activates MAPK pathway to induce apoptosis, paraptosis, and autophagy in colorectal cancer cells. J. Cell. Biochem. 2024, 125, e30537. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Szwaczko, K.; Dziuba, K.; Wiater, A. Exploring the Potential of Synthetic Cannabinoids: Modulation of Biological Activity of Normal and Cancerous Human Colon Epithelial Cells. Cells 2024, 13, 1616. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, L.; Wang, Y.; Deng, G.; Cao, X.; Ke, B.; Wu, X.; Gu, Y.; Cheng, H.; Xu, Q.; et al. Single-cell analyses reveal cannabidiol rewires tumor microenvironment via inhibiting alternative activation of macrophage and synergizes with anti-PD-1 in colon cancer. J. Pharm. Anal. 2023, 13, 726–744. [Google Scholar] [CrossRef]
- Sun, Q.; Bravo Iniguez, A.; Tian, Q.; Du, M.; Zhu, M.J. Dietary Cannabidiol Activates PKA/AMPK Signaling and Attenuates Chronic Inflammation and Leaky Gut in DSS-Induced Colitis Mice. Mol. Nutr. Food Res. 2024, 68, e2300446. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Wong, K.Y.; Janjua, T.I.; Martin, J.H.; Begun, J.; Popat, A. Cannabidiol Targets Colorectal Cancer Cells via Cannabinoid Receptor 2, Independent of Common Mutations. ACS Pharmacol. Transl. Sci. 2025, 8, 543–556. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 5859. [Google Scholar] [CrossRef]
- Orrego-Gonzalez, E.; Londono-Tobon, L.; Ardila-Gonzalez, J.; Polania-Tovar, D.; Valencia-Cardenas, A.; Velez-Van Meerbeke, A. Cannabinoid Effects on Experimental Colorectal Cancer Models Reduce Aberrant Crypt Foci (ACF) and Tumor Volume: A Systematic Review. Evid. Based Complement. Alternat Med. 2020, 2020, 2371527. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef]
- Jauhar, S.; Cowen, P.J.; Browning, M. Fifty years on: Serotonin and depression. J. Psychopharmacol. 2023, 37, 237–241. [Google Scholar] [CrossRef]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hortnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology 2017, 158, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Zifa, E.; Fillion, G. 5-Hydroxytryptamine receptors. Pharmacol. Rev. 1992, 44, 401–458. [Google Scholar] [CrossRef] [PubMed]
- Reeves, D.C.; Lummis, S.C. The molecular basis of the structure and function of the 5-HT3 receptor: A model ligand-gated ion channel (review). Mol. Membr. Biol. 2002, 19, 11–26. [Google Scholar] [CrossRef]
- Sjolund, K.; Sanden, G.; Hakanson, R.; Sundler, F. Endocrine cells in human intestine: An immunocytochemical study. Gastroenterology 1983, 85, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Beattie, D.T.; Smith, J.A. Serotonin pharmacology in the gastrointestinal tract: A review. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 181–203. [Google Scholar] [CrossRef]
- Coleman, N.S.; Foley, S.; Dunlop, S.P.; Wheatcroft, J.; Blackshaw, E.; Perkins, A.C.; Singh, G.; Marsden, C.A.; Holmes, G.K.; Spiller, R.C. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin. Gastroenterol. Hepatol. 2006, 4, 874–881. [Google Scholar] [CrossRef]
- Camilleri, M. Serotonin in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 53–59. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and cancer: What is the link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Mesnil, M. Serotonin and human cancer: A critical view. Biochimie 2019, 161, 46–50. [Google Scholar] [CrossRef]
- Balakrishna, P.; George, S.; Hatoum, H.; Mukherjee, S. Serotonin Pathway in Cancer. Int. J. Mol. Sci. 2021, 22, 1268. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, T.; Chen, Z.; Liu, B.; Fan, D.; Li, C.; Wu, J.; He, L.; Zhu, X.; Du, Y.; et al. 5-hydroxytryptamine produced by enteric serotonergic neurons initiates colorectal cancer stem cell self-renewal and tumorigenesis. Neuron 2022, 110, 2268–2282. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Xu, H.; Ji, Q.; Liu, X.; Zhou, L.; Song, H.; Zhou, X.; Xu, Y.; Chen, Z.; Cai, J.; et al. 5-hydroxytryptamine receptor (5-HT1DR) promotes colorectal cancer metastasis by regulating Axin1/beta-catenin/MMP-7 signaling pathway. Oncotarget 2015, 6, 25975–25987. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, A.; Bertoni, S.; Arcaro, V.; Saccani, F.; Grandi, A.; Vivo, V.; Cantoni, A.M.; Barocelli, E. Dual Role of Endogenous Serotonin in 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Colitis. Front. Pharmacol. 2016, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Ataee, R.; Ajdary, S.; Zarrindast, M.; Rezayat, M.; Hayatbakhsh, M.R. Anti-mitogenic and apoptotic effects of 5-HT1B receptor antagonist on HT29 colorectal cancer cell line. J. Cancer Res. Clin. Oncol. 2010, 136, 1461–1469. [Google Scholar] [CrossRef]
- Li, T.; Wei, L.; Zhang, X.; Fu, B.; Zhou, Y.; Yang, M.; Cao, M.; Chen, Y.; Tan, Y.; Shi, Y.; et al. Serotonin Receptor HTR2B Facilitates Colorectal Cancer Metastasis via CREB1-ZEB1 Axis-Mediated Epithelial-Mesenchymal Transition. Mol. Cancer Res. 2024, 22, 538–554. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Q.; Fan, Y.; Li, B.; Liu, X.; Hu, C. Dissecting the novel abilities of aripiprazole: The generation of anti-colorectal cancer effects by targeting Galphaq via HTR2B. Acta Pharm. Sin. B 2023, 13, 3400–3413. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Z.; Liu, J.; Zhou, C.; Chen, J. Downregulation of 5-hydroxytryptamine receptor 3A expression exerts an anticancer activity against cell growth in colorectal carcinoma cells in vitro. Oncol. Lett. 2018, 16, 6100–6108. [Google Scholar] [CrossRef]
- Li, T.; Fu, B.; Zhang, X.; Zhou, Y.; Yang, M.; Cao, M.; Chen, Y.; Tan, Y.; Hu, R. Overproduction of Gastrointestinal 5-HT Promotes Colitis-Associated Colorectal Cancer Progression via Enhancing NLRP3 Inflammasome Activation. Cancer Immunol. Res. 2021, 9, 1008–1023. [Google Scholar] [CrossRef]
- Spohn, S.N.; Bianco, F.; Scott, R.B.; Keenan, C.M.; Linton, A.A.; O’Neill, C.H.; Bonora, E.; Dicay, M.; Lavoie, B.; Wilcox, R.L.; et al. Protective Actions of Epithelial 5-Hydroxytryptamine 4 Receptors in Normal and Inflamed Colon. Gastroenterology 2016, 151, 933–944. [Google Scholar] [CrossRef]
- Guseva, D.; Holst, K.; Kaune, B.; Meier, M.; Keubler, L.; Glage, S.; Buettner, M.; Bleich, A.; Pabst, O.; Bachmann, O.; et al. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm. Bowel Dis. 2014, 20, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Bridle, B.W.; Ghia, J.E.; Wang, H.; Syed, S.N.; Manocha, M.M.; Rengasamy, P.; Shajib, M.S.; Wan, Y.; Hedlund, P.B.; et al. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J. Immunol. 2013, 190, 4795–4804. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; Hove, A.S.T.; Brul, S.; Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Resstel, L.B.; Tavares, R.F.; Lisboa, S.F.; Joca, S.R.; Correa, F.M.; Guimaraes, F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef]
- Yang, K.H.; Galadari, S.; Isaev, D.; Petroianu, G.; Shippenberg, T.S.; Oz, M. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J. Pharmacol. Exp. Ther. 2010, 333, 547–554. [Google Scholar] [CrossRef]
- Jenny, M.; Santer, E.; Pirich, E.; Schennach, H.; Fuchs, D. Delta9-tetrahydrocannabinol and cannabidiol modulate mitogen-induced tryptophan degradation and neopterin formation in peripheral blood mononuclear cells in vitro. J. Neuroimmunol. 2009, 207, 75–82. [Google Scholar] [CrossRef]
- Jenny, M.; Schrocksnadel, S.; Uberall, F.; Fuchs, D. The Potential Role of Cannabinoids in Modulating Serotonergic Signaling by Their Influence on Tryptophan Metabolism. Pharmaceuticals 2010, 3, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Funk, K.; Figueroa Barrientos, A.; Bailey, A.; Shrader, S.; Feng, W.; McClain, C.J.; Song, Z.H. The Modulatory Effects and Therapeutic Potential of Cannabidiol in the Gut. Cells 2024, 13, 1618. [Google Scholar] [CrossRef]
- Ibrahim, I.; Syamala, S.; Ayariga, J.A.; Xu, J.; Robertson, B.K.; Meenakshisundaram, S.; Ajayi, O.S. Modulatory Effect of Gut Microbiota on the Gut-Brain, Gut-Bone Axes, and the Impact of Cannabinoids. Metabolites 2022, 12, 1247. [Google Scholar] [CrossRef]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]

| References | Model | Dosage/Treatment | Molecular Actions | Hallmarks of Cancer |
|---|---|---|---|---|
| Sreevalsan et al., 2011 [114] | SW480 | Up to 15 μM CBD | Dependent on CB1 on CB2; ↑Several phosphatases. | ↓Cell proliferation; ↑Cell apoptosis. |
| Aviello et al., 2012 [115] | Caco-2 and HCT116 | 0.01–10 μM CBD | In a CB1-, TRPV1-, and PPARγ-antagonist manner. | ↓Cell proliferation; ↓Genome mutation. |
| Azoxymethane (AOM) CRC animal model | 1 and 5 mg/kg CBD by injection | ↑p-Akt (de-activation), NOS, COX2. | ↓Aberrant crypt foci (ACF), polyps, and tumor. | |
| Romano et al., 2014 [116] | HCT116 and DLD-1 | 1–5 μM CBD and CBD-BDS * | Dependent on CB1 and CB2. | ↓Cell proliferation. |
| Azoxymethane (AOM) CRC animal model | 5 μM CBD-BDS | N/A | ↓Aberrant crypt foci (ACF) and polyps. | |
| Male ICR mice (Xen: HCT116) | 5 μM CBD-BDS | N/A | ↓Tumor size. | |
| Kargl et al., 2016 [117] | HCT116 | 1 or 2.5 μM CBD | Dependent on GPR55. | ↓Adhesion and migration. |
| Jeong et al., 2019 [118] | HCT116 and DLD-1 | 6 μM CBD | Dependent on ROS and Noxa for apoptotic signaling. | ↓Cell viability. |
| BALB/c mice (Xen: HCT116) | 10–20 mg/kg CBD by injection | Dependent on Noxa. | ↓Tumor growth | |
| Kim et al., 2019 [119] | HCT116, HT29, and DLD-1 | 4 μM CBD | ↑CHOP, PERK, DR5. | ↓Cell viability; ↑Cell apoptosis. |
| Jeong et al., 2019 [120] | Oxaliplatin-resistant DLD-1 and colo205 | Up to 30 μM | ↓p-NOS3, ↓NO production, ↑Autophagy; ↓SOD; ↑ROS production. | ↓Cell proliferation; ↑Cell death. |
| BALB/c mice (Xen: colo205) | 10 mg/kg | ↓p-NOS3; ↓SOD; ↑Autophagy. | ↓Tumor growth. | |
| Honarmand et al., 2019 [121] | BALB/c mice (Xen: CT26) | 1–5 μM CBD | ↓VEGF, IL-6 and IL-8; ↓Malondialdehyde; ↑SOD, GPx, GR. | ↓Vasculature; ↓Tumor growth; ↓Metastasis. |
| Cerretani et al., 2020 [122] | HT-29 | 30 μM CBD | ↑Malondialdehyde; ↓GPx, GR. | ↓Cell viability. |
| Raup-Konsavage et al., 2020 [123] | SW480 and HCT116 | 10 μM pure CBD; 10 μM CBD oil ** | N/A | ↓Cell viability (pure CBD only). |
| Lee et al., 2022 [124] | SW620, SW480, HCT116, Caco-2 | 0–10 μM CBD | Dependent on CB2, but not CB1, TRPV, PPARγ; ↓Cyclin D1, Cyclin D3; ↓CDK2, CDK4, CDK6; ↑BiP, IRE1α, eIF2α, ATF3/4. | ↓Cell viability; ↓Cell proliferation; ↑Cell apoptosis. |
| Nkune et al., 2022 [125] | Caco-2 | 1 μM | ↑Photodamage. | ↓Cell viability. |
| Feng et al., 2022 [126] | HCT116, SW620, and DLD-1 | 3, 6, 12 μM CBD | ↓EMT; ↑E-cadherin; ↓N-cadherin, Snail, Vimentin, and HIF-1α; ↓Wnt-signaling. | ↓Cell proliferation; ↓Cell migration; ↓Cell invasion. |
| BALB/c mice (Xen: HCT116) | 10 and 15 mg/kg CBD | N/A | ↓Tumor volume. | |
| Wang et al., 2023 [127], 2024 [128] | HCT116 p53wt vs. p53mut | 5–20 μM CBD | Dependent on p53 and Hsp70; ↑ROS production; Trigger macroautophagy. | ↓Cell viability. |
| SCID mice (Xen: HCT116 p53wt or p53mut) | 20 mg/kg CBD by injection | Dependent on p53. | ↓Tumor growth. | |
| Cherkasova et al., 2023 [129] | HT29, HCT116 and LS-174T | 2–12 μM CBD | Altering TGF-β and MAPK signaling. | Altering cell metabolism. |
| Wei et al., 2024 [130] | HCT-116 | N/A | ↓SOD2/3 and ↑ROS; ↑Noxa; ↑Mitochondrial dysfunction. | ↑Cancer cell death. |
| Kim et al., 2024 [131] | HT-29, SW480, HCT-116 and HCT-15 | 30 μM CBD | ↑CHOP and ATF4; ↑ROS; ↑MAPK signaling; ↑Autophagy. | ↑Apoptosis and paraptosis. |
| Paduch et al., 2024 [132] | HT-29 and CCD 841 CoTr | 0–200 μg/mL | ↓Mitochondrial dehydrogenase activity; ↓Nitric oxide. | ↑Apoptosis. |
| Sun et al., 2024 [133] | C57BL/6 mice (Xen: MC38) | 10 mg/kg CBD by injection | ↓M2-like macrophages; ↑M1-like macrophages; ↓PI3K-Akt signaling. | ↑Immune function. |
| Sun et al., 2024 [134] | DSS-induced colitis model | 200 mg/kg CBD by dietary supplementation | ↓Macrophage infiltration; ↑PKA/AMPK signaling; ↓NLRP3 inflammasome activation. | ↓Disease activity index; ↓Inflammation and colitis symptoms. |
| Moniruzzaman et al., 2025 [135] | HCT116, HT-29, LS174T, and LS153 | 0–40 μg/mL | ↑CB2 activation; ↑Endoplasmic reticulum (ER) stress. | ↑Apoptosis; ↓Cell proliferation; ↓Cell migration; ↓Cell invasion. |
| Receptors | Gene Expression in GI 1 | Functions in Gut | Expressions in Colorectal Tumor | Functions Related to Colorectal Tumorigenesis 2 | |
|---|---|---|---|---|---|
| RNA | Protein | ||||
| 5-HT1(A, B, D, E, F) | Htr1a (low), Htr1b, Htr1d, Htr1e (low) | HTR1a, HTR1b (low), HTR1e | Intestinal motility; Immune protection. | ↑5-HT1(B, D, F) [152] ↑5-HT1D [153] | ↓/↑Inflammation and colitis (5-HT1A, A/AT) [154]; ↑CRC cell growth (5-HT1B, A) [155]; ↑Apoptosis, ↓Proliferation (5-HT1B, AT) [155]; ↑/↓Wnt signaling and metastasis (5-HT1D, A/AT) [153]. |
| 5-HT2(A, B, C) | Htr2a (low), Htr2b | HTR2b | Intestinal motility; Intestinal secretion; Immune protection. | ↑5-HT2B [156,157] | ↓Metastasis (5-HT2B, AT) [156]; ↓CRC cell growth (5-HT2B, AT) [157]. |
| 5-HT3(A, B, C, D, E) | Htr3a, Htr3c (low), Htr3e (low) | HTR3a, HTR3e | Intestinal motility; Intestinal secretion; Immune protection. | ↑5-HT3C [153] | ↑Apoptosis, ↓Proliferation and colony formation (5-HT3A, AT) [158]; ↑NLRP3 inflammasome (5-HT3A, A) [159]; ↓Tumor growth (5-HT3A, AT) [159]. |
| 5-HT4 | Htr4 | HTR4 | Intestinal motility; Intestinal secretion; Immune protection. | ↑5-HT4 [153] | ↓/↑Inflammation and colitis (5-HT4, A) [160]; ↑Barrier dysfunction (5-HT4, AT) [160]. |
| 5-HT5(A, B) | N/A | HTR5a | N/A | N/A | N/A |
| 5-HT6 | N/A | N/A | N/A | N/A | N/A |
| 5-HT7 | Htr7 (low) | HTR7 | Immune protection. | N/A | ↑Inflammation and colitis (5-HT7, AT) [161]; ↓Inflammation and colitis (5-HT7, AT) [162]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z. Cannabidiol (CBD) and Colorectal Tumorigenesis: Potential Dual Modulatory Roles via the Serotonergic Pathway. Curr. Oncol. 2025, 32, 375. https://doi.org/10.3390/curroncol32070375
Liu Z. Cannabidiol (CBD) and Colorectal Tumorigenesis: Potential Dual Modulatory Roles via the Serotonergic Pathway. Current Oncology. 2025; 32(7):375. https://doi.org/10.3390/curroncol32070375
Chicago/Turabian StyleLiu, Zhenhua. 2025. "Cannabidiol (CBD) and Colorectal Tumorigenesis: Potential Dual Modulatory Roles via the Serotonergic Pathway" Current Oncology 32, no. 7: 375. https://doi.org/10.3390/curroncol32070375
APA StyleLiu, Z. (2025). Cannabidiol (CBD) and Colorectal Tumorigenesis: Potential Dual Modulatory Roles via the Serotonergic Pathway. Current Oncology, 32(7), 375. https://doi.org/10.3390/curroncol32070375





