Role of Circulating Tumor DNA in Adapting Immunotherapy Approaches in Breast Cancer
Simple Summary
Abstract
1. Introduction
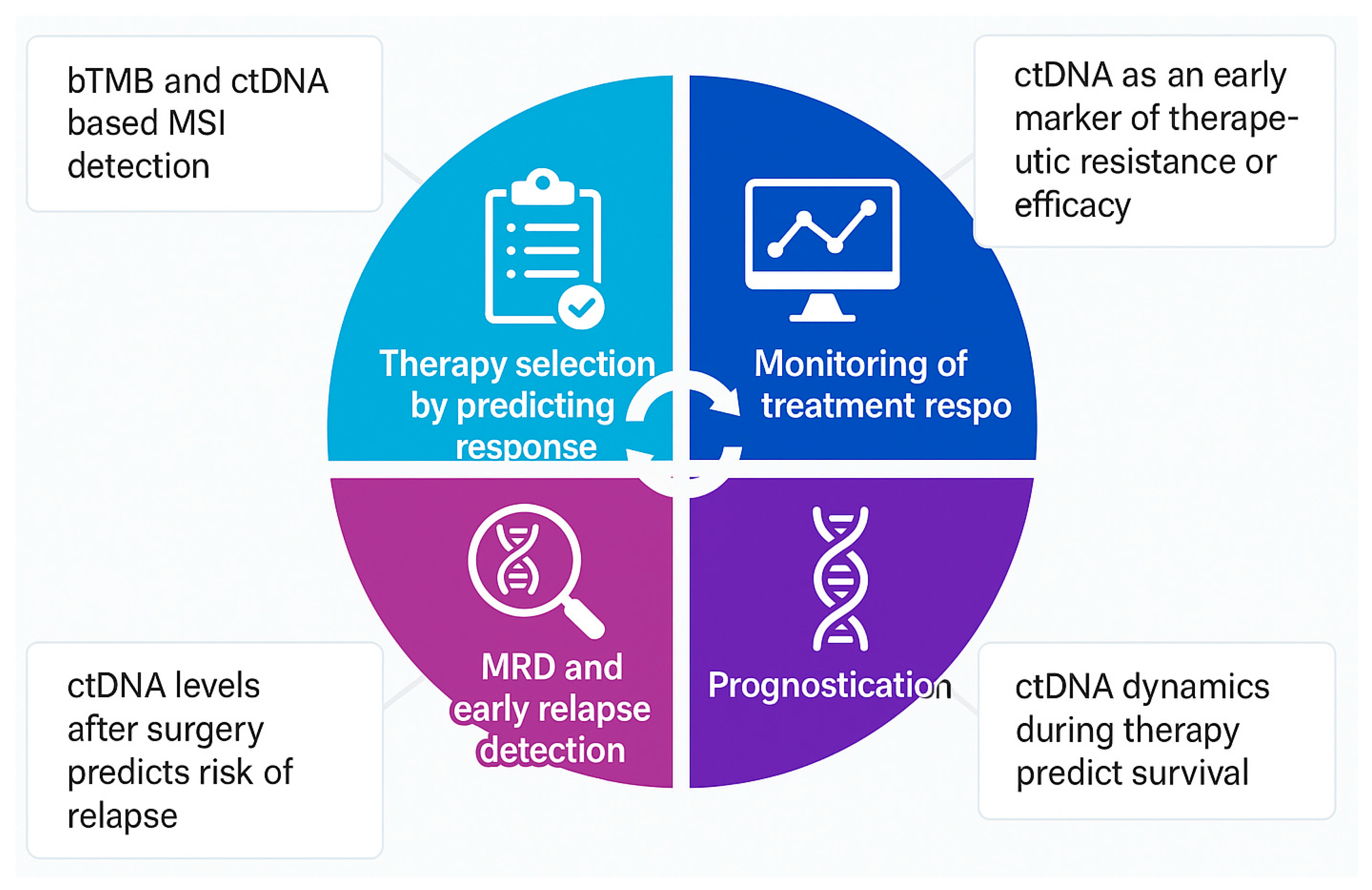
1.1. Approach to ctDNA Detection and Interpretation
1.2. Predicting Response to Immunotherapy
1.3. Monitoring Response to Immunotherapy
1.4. Minimal Residual Disease (MRD) and Early Relapse Detection
1.5. Assessment of Prognosis
1.6. Current Challenges
2. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Loi, S.; Salgado, R.; Curigliano, G.; Romero Díaz, R.I.; Delaloge, S.; Rojas García, C.I.; Kok, M.; Saura, C.; Harbeck, N.; Mittendorf, E.A.; et al. Neoadjuvant Nivolumab and Chemotherapy in Early Estrogen Receptor-Positive Breast Cancer: A Randomized Phase 3 Trial. Nat. Med. 2025, 31, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; O’Shaughnessy, J.; Liu, Z.; McArthur, H.; Schmid, P.; Cortes, J.; Harbeck, N.; Telli, M.L.; Cescon, D.W.; Fasching, P.A.; et al. Pembrolizumab and Chemotherapy in High-Risk, Early-Stage, ER+/HER2− Breast Cancer: A Randomized Phase 3 Trial. Nat. Med. 2025, 31, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Johnson, A.; Sklar, J.; Lindeman, N.I.; Moore, K.; Ganesan, S.; Lovly, C.M.; Perlmutter, J.; Gray, S.W.; Hwang, J.; et al. Somatic Genomic Testing in Patients With Metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2022, 40, 1231–1258. [Google Scholar] [CrossRef]
- Henry, N.L.; Somerfield, M.R.; Dayao, Z.; Elias, A.; Kalinsky, K.; McShane, L.M.; Moy, B.; Park, B.H.; Shanahan, K.M.; Sharma, P.; et al. Biomarkers for Systemic Therapy in Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 3205–3221. [Google Scholar] [CrossRef]
- Yue, D.; Liu, W.; Chen, C.; Zhang, T.; Ma, Y.; Cui, L.; Gu, Y.; Bei, T.; Zhao, X.; Zhang, B.; et al. Circulating Tumor DNA Predicts Neoadjuvant Immunotherapy Efficacy and Recurrence-Free Survival in Surgical Non-Small Cell Lung Cancer Patients. Transl. Lung Cancer Res. 2022, 11, 263–276. [Google Scholar] [CrossRef]
- Chan, W.Y.; Lee, J.H.; Stewart, A.; Diefenbach, R.J.; Gonzalez, M.; Menzies, A.M.; Blank, C.; Scolyer, R.A.; Long, G.V.; Rizos, H. Circulating Tumour DNA Dynamics Predict Recurrence in Stage III Melanoma Patients Receiving Neoadjuvant Immunotherapy. J. Exp. Clin. Cancer Res. 2024, 43, 238. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Swigart, L.B.; Wu, H.-T.; Hirst, G.L.; Yau, C.; Wolf, D.M.; Tin, A.; Salari, R.; Shchegrova, S.; Pawar, H.; et al. Circulating Tumor DNA in Neoadjuvant-Treated Breast Cancer Reflects Response and Survival. Ann. Oncol. 2021, 32, 229–239. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating Mutant DNA to Assess Tumor Dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- El Messaoudi, S.; Rolet, F.; Mouliere, F.; Thierry, A.R. Circulating Cell Free DNA: Preanalytical Considerations. Clin. Chim. Acta 2013, 424, 222–230. [Google Scholar] [CrossRef]
- Gydush, G.; Nguyen, E.; Bae, J.H.; Blewett, T.; Rhoades, J.; Reed, S.C.; Shea, D.; Xiong, K.; Liu, R.; Yu, F.; et al. Massively Parallel Enrichment of Low-Frequency Alleles Enables Duplex Sequencing at Low Depth. Nat. Biomed. Eng. 2022, 6, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, A.C.; Warburton, L.; Al-Ogaili, Z.; Celliers, L.; Calapre, L.; Pereira, M.R.; Khattak, M.A.; Meniawy, T.M.; Millward, M.; Ziman, M.; et al. Correlation between Circulating Tumour DNA and Metabolic Tumour Burden in Metastatic Melanoma Patients. BMC Cancer 2018, 18, 726. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and Future Perspectives of Liquid Biopsies in Genomics-Driven Oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Newman, A.M.; Bratman, S.V.; To, J.; Wynne, J.F.; Eclov, N.C.W.; Modlin, L.A.; Liu, C.L.; Neal, J.W.; Wakelee, H.A.; Merritt, R.E.; et al. An Ultrasensitive Method for Quantitating Circulating Tumor DNA with Broad Patient Coverage. Nat. Med. 2014, 20, 548–554. [Google Scholar] [CrossRef]
- Martins, I.; Ribeiro, I.P.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Melo, J.B.; Carreira, I.M. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, L.; Wang, X.; Chen, X.; Cao, P.; Yang, Z.; Wen, Q. Liquid Biopsy Biomarkers to Guide Immunotherapy in Breast Cancer. Front. Immunol. 2023, 14, 1303491. [Google Scholar] [CrossRef]
- Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Hylton, N.M.; Lou, B.; Mansi, T.; Pusztai, L. The Way of the Future: Personalizing Treatment Plans Through Technology. Am. Soc. Clin. Oncol. Educ. Book. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Razavi, P.; Li, B.T.; Brown, D.N.; Jung, B.; Hubbell, E.; Shen, R.; Abida, W.; Juluru, K.; De Bruijn, I.; Hou, C.; et al. High-Intensity Sequencing Reveals the Sources of Plasma Circulating Cell-Free DNA Variants. Nat. Med. 2019, 25, 1928–1937. [Google Scholar] [CrossRef]
- Amato, O.; Giannopoulou, N.; Ignatiadis, M. Circulating Tumor DNA Validity and Potential Uses in Metastatic Breast Cancer. NPJ Breast Cancer 2024, 10, 21. [Google Scholar] [CrossRef]
- Valenza, C.; Saldanha, E.F.; Gong, Y.; De Placido, P.; Gritsch, D.; Ortiz, H.; Trapani, D.; Conforti, F.; Cremolini, C.; Peters, S.; et al. Circulating Tumor DNA Clearance as a Predictive Biomarker of Pathologic Complete Response in Patients with Solid Tumors Treated with Neoadjuvant Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Ann. Oncol. 2025, 36, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Gong, Y.; Zhang, Y.; Lu, Y.; Wang, C.; Yao, R.; Li, P.; Guan, Y.; Wang, J.; et al. Clinical Factors Associated with Circulating Tumor DNA (ctDNA) in Primary Breast Cancer. Mol. Oncol. 2019, 13, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Magbanua, M.J.M.; Gumusay, O.; Kurzrock, R.; van ‘t Veer, L.J.; Rugo, H.S. Immunotherapy in Breast Cancer and the Potential Role of Liquid Biopsy. Front. Oncol. 2022, 12, 802579. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO Recommendations on the Use of Circulating Tumour DNA Assays for Patients with Cancer: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Willis, J.; Lefterova, M.I.; Artyomenko, A.; Kasi, P.M.; Nakamura, Y.; Mody, K.; Catenacci, D.V.T.; Fakih, M.; Barbacioru, C.; Zhao, J.; et al. Validation of Microsatellite Instability Detection Using a Comprehensive Plasma-Based Genotyping Panel. Clin. Cancer Res. 2019, 25, 7035–7045. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Jain, E.; Cohen, O.; Kim, D.; Buendia-Buendia, J.; Winer, E.; Lin, N.; Tolaney, S.M.; Wagle, N. Prevalence and Mutational Determinants of High Tumor Mutation Burden in Breast Cancer. Ann. Oncol. 2020, 31, 387–394. [Google Scholar] [CrossRef]
- Araujo, D.V.; Wang, A.; Torti, D.; Leon, A.; Marsh, K.; McCarthy, A.; Berman, H.; Spreafico, A.; Hansen, A.R.; Razak, A.-A.; et al. Applications of Circulating Tumor DNA in a Cohort of Phase I Solid Tumor Patients Treated With Immunotherapy. JNCI Cancer Spectr. 2021, 5, pkaa122. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Li, T.; Reddy, S.; Emens, L.A.; Overmoyer, B.; Lange, P.; Dilullo, M.K.; Attaya, V.; Kimmel, J.; Winer, E.P.; et al. Abstract GS2-10: Nimbus: A Phase 2 Trial of Nivolumab plus Ipilimumab for Patients with Hypermutated Her2-Negative Metastatic Breast Cancer (MBC). Cancer Res. 2022, 82, GS2–GS10. [Google Scholar] [CrossRef]
- Prasad, V.; Kaestner, V.; Mailankody, S. Cancer Drugs Approved Based on Biomarkers and Not Tumor Type—FDA Approval of Pembrolizumab for Mismatch Repair-Deficient Solid Cancers. JAMA Oncol. 2018, 4, 157–158. [Google Scholar] [CrossRef]
- Cheng, A.S.; Leung, S.C.Y.; Gao, D.; Burugu, S.; Anurag, M.; Ellis, M.J.; Nielsen, T.O. Mismatch Repair Protein Loss in Breast Cancer: Clinicopathological Associations in a Large British Columbia Cohort. Breast Cancer Res. Treat. 2020, 179, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.J.; Goodman, A.M.; Kato, S.; Ellison, C.K.; Daniels, G.A.; Kim, L.; Nakashe, P.; McCarthy, E.; Mazloom, A.R.; McLennan, G.; et al. Genome-Wide Sequencing of Cell-Free DNA Identifies Copy-Number Alterations That Can Be Used for Monitoring Response to Immunotherapy in Cancer Patients. Mol. Cancer Ther. 2019, 18, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kümmel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized Circulating Tumor DNA Analysis as a Predictive Biomarker in Solid Tumor Patients Treated with Pembrolizumab. Nat. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, B.; Su, M.; Yuan, C.; Liu, C.; Wang, X.; Song, X.; Li, C.; Wang, F.; Ma, J.; et al. Construction of a Risk Stratification Model Integrating ctDNA to Predict Response and Survival in Neoadjuvant-Treated Breast Cancer. BMC Med. 2023, 21, 493. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Wolf, D.; Renner, D.; Shchegrova, S.; Swigart, L.B.; Yau, C.; Hirst, G.; Wu, H.-T.; Kalashnikova, E.; Tin, A.; et al. Abstract PD9-02: Personalized ctDNA as a Predictive Biomarker in High-Risk Early Stage Breast Cancer (EBC) Treated with Neoadjuvant Chemotherapy (NAC) with or without Pembrolizumab (P). Cancer Res. 2021, 81, PD9-02. [Google Scholar] [CrossRef]
- Dang, D.K.; Park, B.H. Circulating Tumor DNA: Current Challenges for Clinical Utility. J. Clin. Investig. 2022, 132, e154941. [Google Scholar] [CrossRef]
- Garcia-Murillas, I.; Chopra, N.; Comino-Méndez, I.; Beaney, M.; Tovey, H.; Cutts, R.J.; Swift, C.; Kriplani, D.; Afentakis, M.; Hrebien, S.; et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol. 2019, 5, 1473–1478. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Hancock, B.A.; Solzak, J.P.; Brinza, D.; Scafe, C.; Miller, K.D.; Radovich, M. Next-Generation Sequencing of Circulating Tumor DNA to Predict Recurrence in Triple-Negative Breast Cancer Patients with Residual Disease after Neoadjuvant Chemotherapy. npj Breast Cancer 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Turner, N.C.; Swift, C.; Jenkins, B.; Kilburn, L.; Coakley, M.; Beaney, M.; Fox, L.; Goddard, K.; Garcia-Murillas, I.; Proszek, P.; et al. Results of the C-TRAK TN Trial: A Clinical Trial Utilising ctDNA Mutation Tracking to Detect Molecular Residual Disease and Trigger Intervention in Patients with Moderate- and High-Risk Early-Stage Triple-Negative Breast Cancer. Ann. Oncol. 2023, 34, 200–211. [Google Scholar] [CrossRef]
- Chiu, J.; Su, F.; Joshi, M.; Masuda, N.; Ishikawa, T.; Aruga, T.; Zarate, J.P.; Babbar, N.; Balbin, O.A.; Yap, Y.-S. Potential Value of ctDNA Monitoring in Metastatic HR + /HER2 - Breast Cancer: Longitudinal ctDNA Analysis in the Phase Ib MONALEESASIA Trial. BMC Med. 2023, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.K.L.; Solovieff, N.; Joshi, M.; Im, S.-A.; Bianchi, G.V.; de la Cruz-Merino, L.; Jerusalem, G.H.M.; Sonke, G.S.; Nusch, A.; Beck, J.T.; et al. On-Treatment (Tx) Dynamic Circulating Tumor DNA Changes (∆ctDNA) Associated with Progression-Free Survival (PFS) and Overall Survival (OS) of Patients (Pts) with HR+/HER2− Advanced Breast Cancer (ABC) in MONALEESA-3 (ML-3). J. Clin. Oncol. 2024, 42, 1012. [Google Scholar] [CrossRef]
- Stover, D.G.; Parsons, H.A.; Ha, G.; Freeman, S.S.; Barry, W.T.; Guo, H.; Choudhury, A.D.; Gydush, G.; Reed, S.C.; Rhoades, J.; et al. Association of Cell-Free DNA Tumor Fraction and Somatic Copy Number Alterations With Survival in Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Johnston, S.R.D.; Arteaga, C.L.; Graff, S.L.; Chandarlapaty, S.; Goetz, M.P.; Desmedt, C.; Sasano, H.; Liu, D.; Rodrik-Outmezguine, V.; et al. Prognostic Utility of ctDNA Detection in the monarchE Trial of Adjuvant Abemaciclib plus Endocrine Therapy (ET) in HR+, HER2−, Node-Positive, High-Risk Early Breast Cancer (EBC). J. Clin. Oncol. 2024, 42, LBA507. [Google Scholar] [CrossRef]
| Study | Design/Endpoints | Type of Breast Cancer | ctDNA Test | Key Findings |
|---|---|---|---|---|
| INSPIRE | Phase 2 study in five cohorts of advanced solid tumors treated with pembrolizumab | TNBC (n = 11) out of total 94 patients | Amplicon-based personalised bespoke ctDNA | Improved OS/PFS in patients with lowering or clearing of ctDNA on treatment |
| I-SPY2 | Adaptive phase 2 clinical trial in high-risk stage 2–3 breast cancer testing with addition of multiple new agents to neoadjuvant chemotherapy including pembrolizumab | N = 138 (TNBC: 61, HR+/HER2−: 77) | Personalised ctDNA | ctDNA dynamics during neoadjuvant treatment predictive of pCR, metastatic recurrence, and death |
| Valenza et al. [21] | Meta-analysis of phase 1–3 clinical trials investigating ctDNA clearance and pCR in solid tumors treated with neoadjuvant immunotherapy | 380 patients including breast cancer | Tumor-informed approach or tumor-naïve approach | Lack of ctDNA clearance may identify patients unlikely to have a pCR. The confirmatory power of ctDNA clearance is limited by low specificity and high heterogeneity due to the variability of the assays, and warrants further study |
| cTRAK-TN | Phase 2 clinical trial assessing utility of prospective ctDNA surveillance in TNBC and activity of pembrolizumab in patients with ctDNA+ residual disease | 161 patients with TNBC | Personalized droplet PCR ctDNA assay | First study to assess the clinical utility of ctDNA in guiding therapy in TNBC; ctDNA detection associated with high risk of metastatic disease. |
| Trial | Phase of Study | Stage and Type of Breast Cancer | Description | Role of ctDNA |
|---|---|---|---|---|
| APOLLO (NCT 04501523) | Phase 2 RCT | Early-stage TNBC | Patients treated with neoadjuvant chemotherapy and positive ctDNA after surgery are randomized to receive boost therapy (tislelizumab + capecitabine) vs. standard of care | ctDNA-based randomization |
| Artemis (NCT 04803539) | Phase 2 RCT | Early-stage TNBC | Investigates the benefit of boost therapy (Capecitabine + apatinib + camrelizumab) vs. standard of care (Capecitabine alone) in patients with positive ctDNA after surgery | ctDNA-based randomization |
| neoBREASTIM (NCT06067061) | Single-arm Phase 2 | Early-stage TNBC | Evaluates novel, biomarker-driven combination of atezolizumab + RP1 oncolytic immunotherapy in neoadjuvant setting | ctDNA-based treatment continuation |
| PERSEVERE (NCT04849364) | Phase 2 RCT | Early-stage TNBC | Patients with residual disease after neoadjuvant treatment are assigned to one of the three arms | Along with a genomic biomarker assay, ctDNA is used as a biomarker for treatment selection |
| RESPONSE (NCT05020860) | Phase 2 parallel-arm | Early-stage TNBC | Study correlates early clinical response to pathological outcome with neoadjuvant systemic therapy | Correlation of ctDNA dynamics with clinical and pathological response |
| ASPRIA (NCT04434040) | Phase 2 single-arm | Early-stage TNBC | Study investigates the combination of atezolizumab + Sacituzumab govitecan in reducing recurrence risk in patients with residual disease and positive ctDNA in blood | ctDNA-based treatment selection |
| GIM 25 CAPT (NCT05266937) | Phase 2 single-arm | Metastatic TNBC | Studies combination of atezolizumab, paclitaxel, and carboplatin as first-line treatment in PDL-1-positive metastatic TNBC | Longitudinal ctDNA analysis during treatment |
| PAveMenT | Phase 1b | Androgen receptor-positive metastatic TNBC | Investigates combination of palbociclib + avelumab in this setting | Exploration of ctDNA as biomarker of response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Pezo, R.C. Role of Circulating Tumor DNA in Adapting Immunotherapy Approaches in Breast Cancer. Curr. Oncol. 2025, 32, 373. https://doi.org/10.3390/curroncol32070373
Kumar S, Pezo RC. Role of Circulating Tumor DNA in Adapting Immunotherapy Approaches in Breast Cancer. Current Oncology. 2025; 32(7):373. https://doi.org/10.3390/curroncol32070373
Chicago/Turabian StyleKumar, Sudhir, and Rossanna C. Pezo. 2025. "Role of Circulating Tumor DNA in Adapting Immunotherapy Approaches in Breast Cancer" Current Oncology 32, no. 7: 373. https://doi.org/10.3390/curroncol32070373
APA StyleKumar, S., & Pezo, R. C. (2025). Role of Circulating Tumor DNA in Adapting Immunotherapy Approaches in Breast Cancer. Current Oncology, 32(7), 373. https://doi.org/10.3390/curroncol32070373




