Insights into the Prognostic Efficacy of the Geriatric Nutritional Risk Index for Nasopharyngeal Carcinoma in the Era of Volumetric Modulated Arc Therapy: A Nomogram for Predicting Long-Term Survival Outcomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. GNRI Measurement
2.3. Radiotherapy
2.4. Chemotherapy
2.5. Follow-Up
2.6. Statistical Methods
3. Results
3.1. Treatment Outcomes
3.2. The Optimal Threshold for the GNRI
3.3. Patient Characteristics According to the Optimal Threshold for the GNRI
3.4. Survival Analysis
3.5. Nomogram Construction
3.6. Internal Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GNRI | Geriatric nutritional risk index |
| VMAT | Volumetric modulated arc therapy |
| PTV | Primary tumor volume |
| NPC | Nasopharyngeal carcinoma |
| ROC | Receiver operating characteristic |
| OS | Overall survival |
| C-index | Concordance index |
| DCA | Decision curve analysis |
| Alb | Albumin |
| BMI | Body mass index |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- He, L.; Xiao, J.; Wei, Z.; He, Y.; Wang, J.; Guan, H.; Mu, X.; Peng, X. Toxicity and dosimetric analysis of nasopharyngeal carcinoma patients undergoing radiotherapy with IMRT or VMAT: A regional center’s experience. Oral Oncol. 2020, 109, 104978. [Google Scholar] [CrossRef]
- Nanda, S.; Parida, S.; Ahirwar, M.K. A Dosimetric Comparison of Volumetric-modulated Arc Therapy and IMRT for Cochlea-sparing Radiation Therapy in Locally Advanced Nasopharyngeal Cancer. J. Med. Phys. 2023, 48, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Tang, L.L.; Mao, Y.P.; Zhou, G.Q.; Qi, Z.Y.; Liu, L.Z.; Lin, A.H.; Liu, M.Z.; Ma, J.; Sun, Y. Clinical outcomes of volume-modulated arc therapy in 205 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. PLoS ONE 2015, 10, e0129679. [Google Scholar] [CrossRef]
- Gomez-Millan Barrachina, J.; Jerez Sainz, I.; Perez Rozos, A.; Ramirez Ros, J.C.; Toledo Serrano, M.D.; Lupiañez Perez, Y.; Medina Carmona, J.A. Potential advantages of volumetric arc therapy in head and neck cancer. Head Neck 2015, 37, 909–914. [Google Scholar] [CrossRef]
- Anamalayil, S.J.; Teo, B.K.; Lin, A.; Lustig, R.A.; Ahn, P.H. Effects of full-neck volumetric-modulated arc therapy vs split-field intensity-modulated head and neck radiation therapy on low neck targets and structures. Br. J. Radiol. 2016, 89, 1062. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, Y.; Liang, Z.; Dassarath, M.; Wang, L.; Jin, L.; Chen, L.; Dong, J.; Price, R.A.; Ma, C.M.; et al. Volumetric-modulated arc therapy for oropharyngeal carcinoma: Adosimetric and delivery efficiency comparison with staticfield IMRT. Phys. Med. 2015, 31, 54–59. [Google Scholar] [CrossRef]
- Lu, S.H.; Cheng, J.C.; Kuo, S.H.; Lee, J.J.; Chen, L.H.; Wu, J.K.; Chen, Y.H.; Chen, W.Y.; Wen, S.Y.; Chong, F.C.; et al. Volumetric modulated arc therapy for nasopharyngeal carcinoma: A dosimetric comparison with TomoTherapy and step-and-shoot IMRT. Radiother. Oncol. 2012, 104, 324–330. [Google Scholar] [CrossRef]
- Grinstead, C.; Yoon, S.L. Geriatric Nutritional Risk Index (GNRI) and Survival in Pancreatic Cancer: A Retrospective Study. Nutrients 2025, 17, 509. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, S.W.; Chiang, Y.S. Prognostic role of geriatric nutritional risk index (GNRI) and controlling nutritional status (CONUT) on outcomes in patients with head and neck cancer: A systematic review and meta-analysis. BMC Cancer 2025, 25, 242. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, D.; Cui, F.; Qian, G.; Li, J.; Wen, J.; Jia, M.; Kang, Y.; Rong, Y.; Zhang, W.; et al. Prognostic value of the geriatric nutritional risk index in patients with non-metastatic clear cell renal cell carcinoma: A propensity score matching analysis. Nutr. J. 2024, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, W.; Wang, C.; Hu, Y. The Prognostic Value of Pretreatment Geriatric Nutritional Risk Index in Esophageal Cancer:A Meta-Analysis. Nutr. Cancer 2022, 74, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, Y.; Gong, D.; Fan, Y. Predictive Value of Geriatric Nutritional Risk Index in Patients with Colorectal Cancer: A Meta-Analysis. Nutr. Cancer 2023, 75, 24–32. [Google Scholar] [CrossRef]
- Shen, F.; Ma, Y.; Guo, W.; Li, F. Prognostic Value of Geriatric Nutritional Risk Index for Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lung 2022, 200, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rao, H.; Chen, N.; Li, R.; Chen, D.; Jiang, H. Geriatric Nutritional Risk Index (GNRI) and Prognostic Nutritional Index (PNI) Before Treatment as the Predictive Indicators for Bone Metastasis in Prostate Cancer Patients. Int. J. Gen. Med. 2025, 18, 2703–2713. [Google Scholar] [CrossRef]
- Tang, Q.N.; Qiu, H.Z.; Sun, X.Q.; Guo, S.S.; Liu, L.T.; Wen, Y.F.; Liu, S.L.; Xie, H.J.; Liang, Y.J.; Sun, X.S.; et al. Geriatric nutritional risk index as an independent prognostic factor in locally advanced nasopharyngeal carcinoma treated using radical concurrent chemoradiotherapy: A retrospective cohort study. Ann. Transl. Med. 2021, 9, 532. [Google Scholar] [CrossRef]
- Shoji, F.; Matsubara, T.; Kozuma, Y.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; Toyokawa, G.; Okamoto, T.; Maehara, Y. Preoperative geriatric nutritional risk index: A predictive and prognostic factor in patients with pathological stage I non-small cell lung cancer. Surg. Oncol. 2017, 26, 483–488. [Google Scholar] [CrossRef]
- Kanemasa, Y.; Shimoyama, T.; Sasaki, Y.; Hishima, T.; Omuro, Y. Geriatric nutritional risk index as a prognostic factor in patients with diffuse large B cell lymphoma. Ann. Hematol. 2018, 97, 999–1007. [Google Scholar] [CrossRef]
- Lin, X.; Wang, B.; Zheng, F.; Fei, Z.; Chen, C. Prognostic Relevance of Change in Body Mass Index in Patients With Nasopharyngeal Carcinoma Undergoing Volumetric Modulated Arc Therapy: A Retrospective Study. Cancer Control. 2022, 29, 10732748221126935. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, B.; Zheng, F.; Fei, Z.; Chen, C. The effect of primary tumor volume on the prognosis of nasopharyngeal carcinoma in era of volumetric modulated arc therapy: A propensity score matched cohort study. Braz. J. Otorhinolaryngol. 2023, 89, 374–382. [Google Scholar] [CrossRef]
- Fan, Y.; Yin, G. Concordance index: Surrogacy of progression-free survival for overall survival. Contemp. Clin. Trials 2021, 104, 106353. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.R.; Gu, X.Q.; Chen, H.Y.; Gu, J.; Pan, Z.G. Development and validation of a nomogram to predict frailty progression in nonfrail chinese community-living older adults. J. Am. Med. Dir. Assoc. 2021, 22, 2571–2578.e4. [Google Scholar] [CrossRef] [PubMed]
- Hoogeman, M.S.; Nuyttens, J.J.; Levendag, P.C.; Heijmen, B.J. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 609–618. [Google Scholar] [CrossRef]
- Hall, E.J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1–7. [Google Scholar] [CrossRef]
- Hall, E.J.; Wuu, C.S. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 83–88. [Google Scholar] [CrossRef]
- Patil, V.M.; Kapoor, R.; Chakraborty, S.; Ghoshal, S.; Oinam, A.S.; Sharma, S.C. Dosimetric risk estimates of radiation-induced malignancies after intensity modulated radiotherapy. J. Cancer Res. Ther. 2010, 6, 442–447. [Google Scholar] [CrossRef]
- Gao, J.; Qian, T.L.; Tao, C.Z.; Zhang, Y.H.; Zhou, Y.; Yang, J.; He, J.; Wang, R.; Zhou, P.J. SmartArc-based volumetric modulated arc therapy can improve the middle ear, vestibule and cochlea sparing for locoregionally advanced nasopharyngeal carcinoma: A dosimetric comparison with step-and-shoot intensity-modulated radiotherapy. Br. J. Radiol. 2015, 88, 20150052. [Google Scholar] [CrossRef]
- Hirahara, N.; Tajima, Y.; Fujii, Y.; Kaji, S.; Kawabata, Y.; Hyakudomi, R.; Yamamoto, T.; Taniura, T. Prediction of postoperative complications and survival after laparoscopic gastrectomy using preoperative Geriatric Nutritional Risk Index in elderly gastric cancer patients. Surg. Endosc. 2021, 35, 1202–1209. [Google Scholar] [CrossRef]
- Zeng, Q.; Shen, L.J.; Guo, X.; Guo, X.M.; Qian, C.N.; Wu, P.H. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer 2016, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Kubo, N.; Sakurai, K.; Tamura, T.; Toyokawa, T.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Ohira, M. The impact of geriatric nutritional risk index on surgical outcomes after esophagectomy in patients with esophageal cancer. Esophagus 2019, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- de Pinho, N.B.; Martucci, R.B.; Rodrigues, V.D.; D’Almeida, C.A.; Thuler, L.C.S.; Saunders, C.; Jager-Wittenaar, H.; Peres, W.A.F. Malnutrition associated with nutrition impact symptoms and localization of the disease: Results of a multicentric research on oncological nutrition. Clin. Nutr. 2019, 38, 1274–1279. [Google Scholar] [CrossRef] [PubMed]

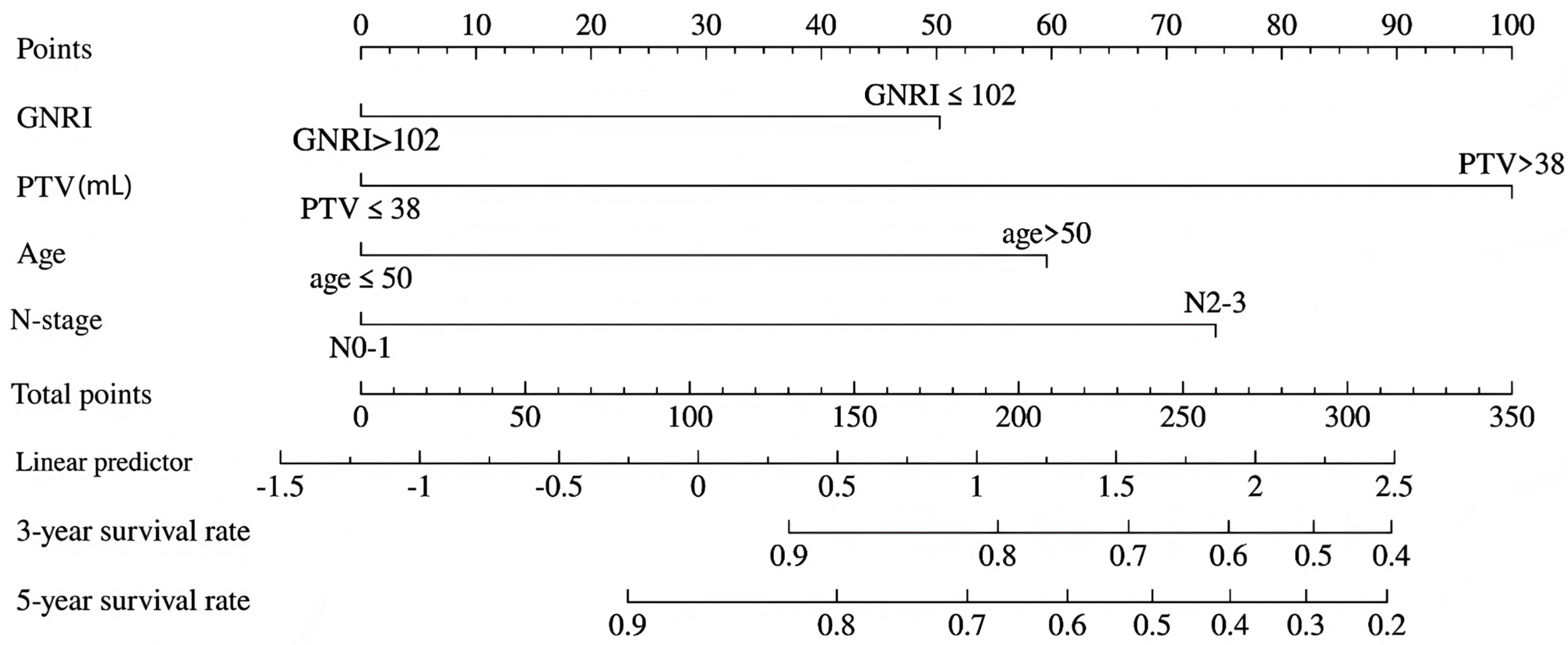
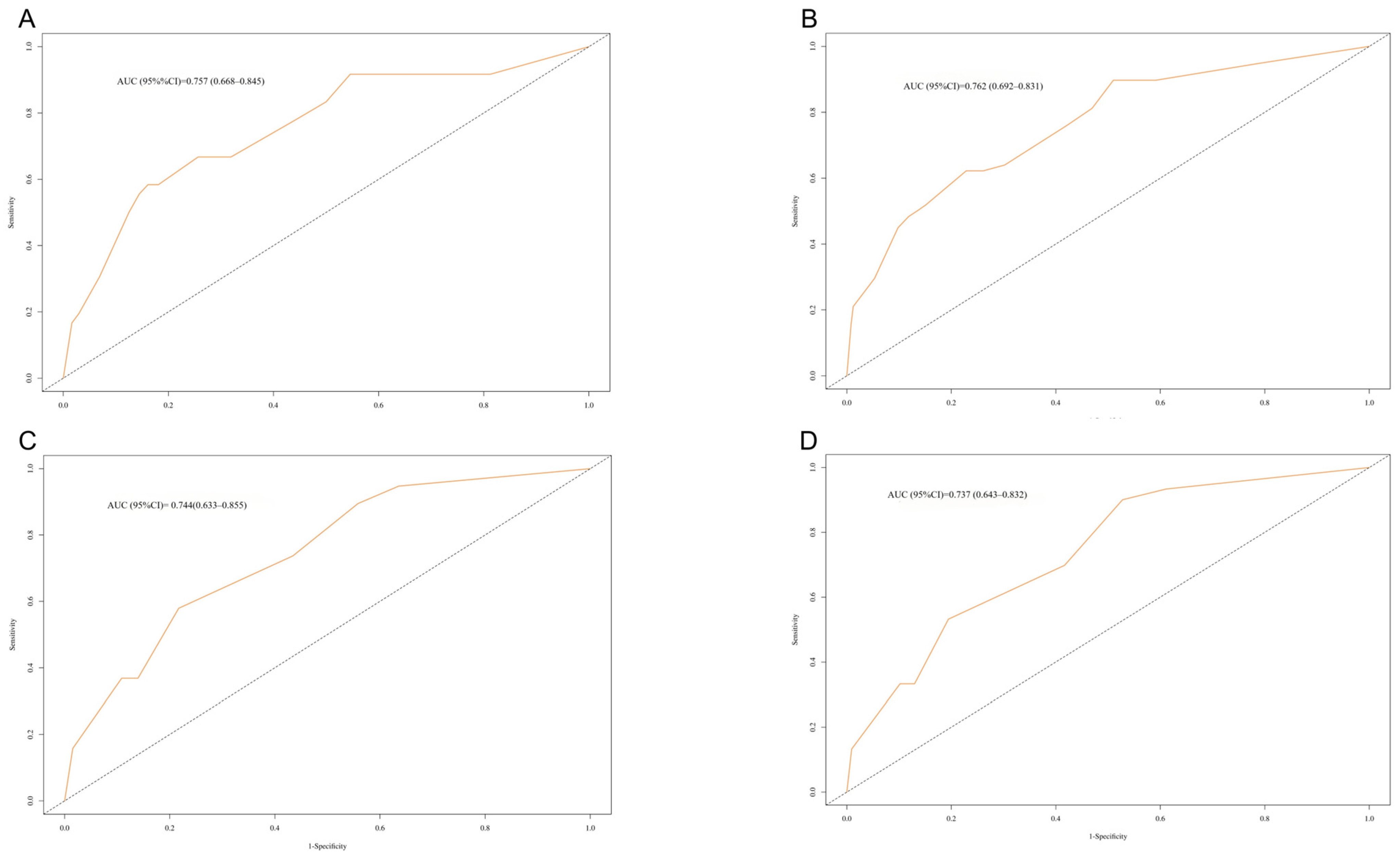


| Variables | Training Cohort | Validation Cohort | p | |
|---|---|---|---|---|
| Number | 348 | 150 | ||
| Gender | male | 269 (77.3%) | 114 (76%) | 0.752 |
| female | 79 (22.7%) | 36 (24%) | ||
| Age | ≤50 y | 217 (62.4%) | 106 (70.7%) | 0.075 |
| >50 y | 131 (37.6%) | 44 (29.3%) | ||
| N-stage | N0–1 | 264 (75.9%) | 105 (70%) | 0.171 |
| N2–3 | 84 (24.1%) | 45 (30%) | ||
| T-stage | T1–2 | 128 (36.8%) | 60 (40%) | 0.497 |
| T3–4 | 220 (63.2%) | 90 (60%) | ||
| KPS score | ≤80 | 11 (3.2%) | 8 (5.3%) | 0.246 |
| 90 | 337 (96.8%) | 142 (94.7%) | ||
| Con-CT | Yes | 81 (23.3%) | 41 (27.3%) | 0.334 |
| No | 267 (76.7%) | 109 (72.7%) | ||
| Int | Yes | 26 (7.5%) | 8 (5.3%) | 0.385 |
| No | 322 (92.5%) | 142 (94.7%) | ||
| Boost | Yes | 97 (27.9%) | 53 (35.3%) | 0.096 |
| No | 251 (72.1%) | 97 (64.7%) | ||
| GNRI | ≤102 | 200 (57.5%) | 75 (50%) | 0.124 |
| >102 | 148 (42.5%) | 75 (50%) | ||
| Alb (g/L) | ≤40 | 147 (42.2%) | 59 (39.3%) | 0.545 |
| >40 | 201 (57.8%) | 91 (60.7%) | ||
| BMI (Kg/m2) | ≤22 | 148 (42.5%) | 64 (42.7%) | 0.977 |
| >22 | 200 (57.5%) | 86 (57.3%) | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| GNRI | ||||
| GNRI > 102 | 1.00 (Reference) | 1.00 (Reference) | ||
| GNRI ≤ 102 | 2.24 (1.27–3.96) | 0.005 | 1.81 (1.02–3.22) | 0.044 |
| PTV | ||||
| PTV > 38 mL | 1.00 (Reference) | 1.00 (Reference) | ||
| PTV ≤ 38 mL | 0.25 (0.15–0.42) | <0.001 | 0.31 (0.18–0.52) | <0.001 |
| Age | ||||
| age > 50 | 1.00 (Reference) | 1.00 (Reference) | ||
| age ≤ 50 | 0.48 (0.29–0.79) | 0.004 | 0.50 (0.30–0.82) | 0.006 |
| Sex | ||||
| Female | 1.00 (Reference) | |||
| Male | 1.83 (0.90–3.72) | 0.093 | ||
| T-stage | ||||
| T1–2 | 1.00 (Reference) | 1.00 (Reference) | ||
| T3–4 | 2.11 (1.16–3.82) | 0.014 | 1.40 (0.48–2.51) | 0.812 |
| N-stage | ||||
| N0–1 | 1.00 (Reference) | 1.00 (Reference) | ||
| N2–3 | 2.79 (1.69–4.62) | <0.001 | 2.40 (1.44–4.00) | <0.001 |
| KPS | ||||
| KPS 90 | 1.00 (Reference) | |||
| KPS ≤ 80 | 1.50 (0.47–4.79) | 0.493 | ||
| Con CT | ||||
| No | 1.00 (Reference) | |||
| Yes | 0.88 (0.48–1.63) | 0.688 | ||
| Int | ||||
| Int < 5 | 1.00 (Reference) | |||
| Int ≥ 5 | 0.85 (0.31–2.34) | 0.752 | ||
| Boost | ||||
| No | 1.00 (Reference) | |||
| Yes | 1.30 (0.77–2.19) | 0.333 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Wang, W.; Ding, J.; Fei, Z.; Chen, C. Insights into the Prognostic Efficacy of the Geriatric Nutritional Risk Index for Nasopharyngeal Carcinoma in the Era of Volumetric Modulated Arc Therapy: A Nomogram for Predicting Long-Term Survival Outcomes. Curr. Oncol. 2025, 32, 372. https://doi.org/10.3390/curroncol32070372
Lin X, Wang W, Ding J, Fei Z, Chen C. Insights into the Prognostic Efficacy of the Geriatric Nutritional Risk Index for Nasopharyngeal Carcinoma in the Era of Volumetric Modulated Arc Therapy: A Nomogram for Predicting Long-Term Survival Outcomes. Current Oncology. 2025; 32(7):372. https://doi.org/10.3390/curroncol32070372
Chicago/Turabian StyleLin, Xiang, Wei Wang, Jianming Ding, Zhaodong Fei, and Chuanben Chen. 2025. "Insights into the Prognostic Efficacy of the Geriatric Nutritional Risk Index for Nasopharyngeal Carcinoma in the Era of Volumetric Modulated Arc Therapy: A Nomogram for Predicting Long-Term Survival Outcomes" Current Oncology 32, no. 7: 372. https://doi.org/10.3390/curroncol32070372
APA StyleLin, X., Wang, W., Ding, J., Fei, Z., & Chen, C. (2025). Insights into the Prognostic Efficacy of the Geriatric Nutritional Risk Index for Nasopharyngeal Carcinoma in the Era of Volumetric Modulated Arc Therapy: A Nomogram for Predicting Long-Term Survival Outcomes. Current Oncology, 32(7), 372. https://doi.org/10.3390/curroncol32070372





