The Impact of Adjuvant Chemotherapy on Clinical Outcomes in Locally Advanced Rectal Cancer: A CHORD Consortium Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
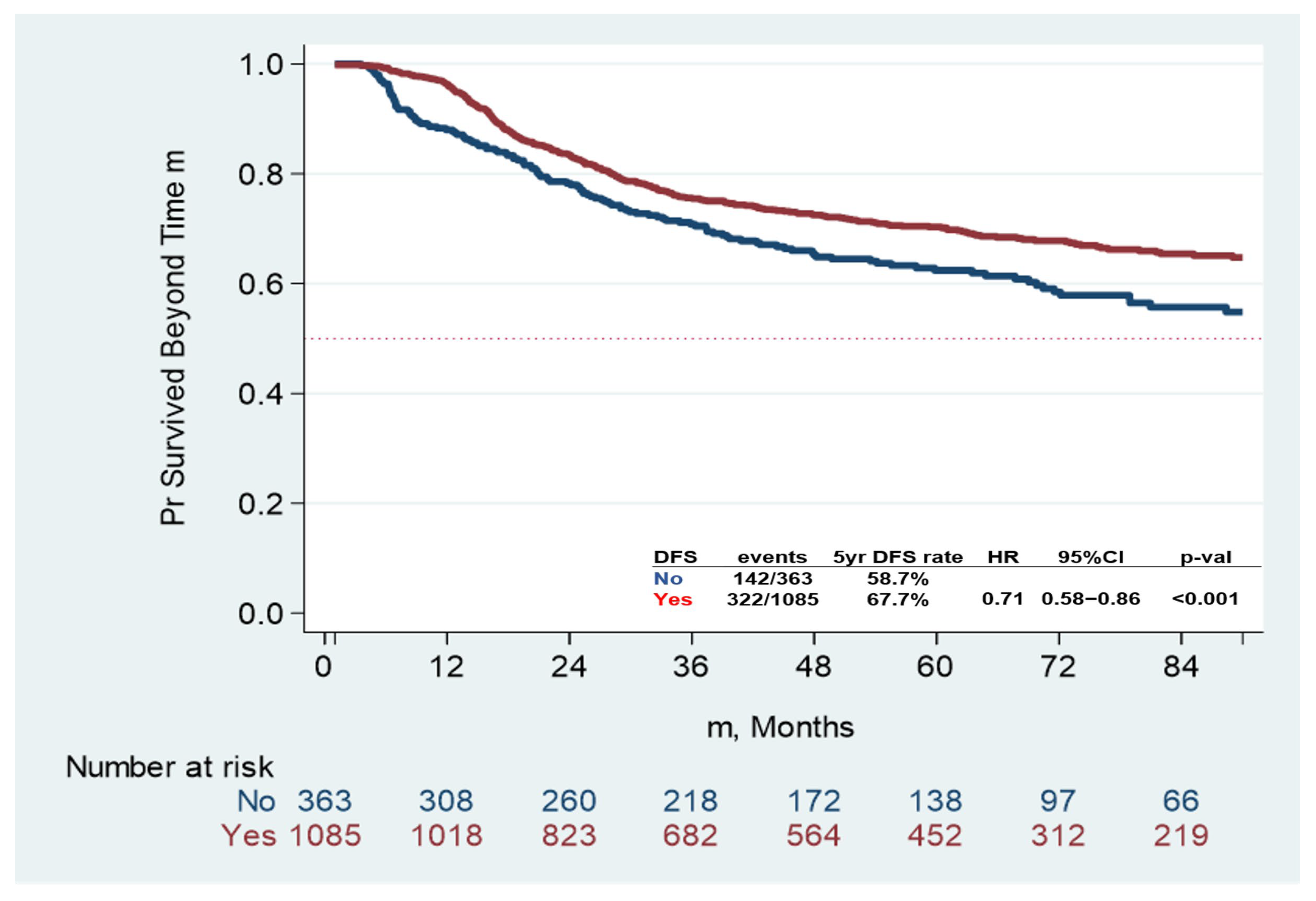
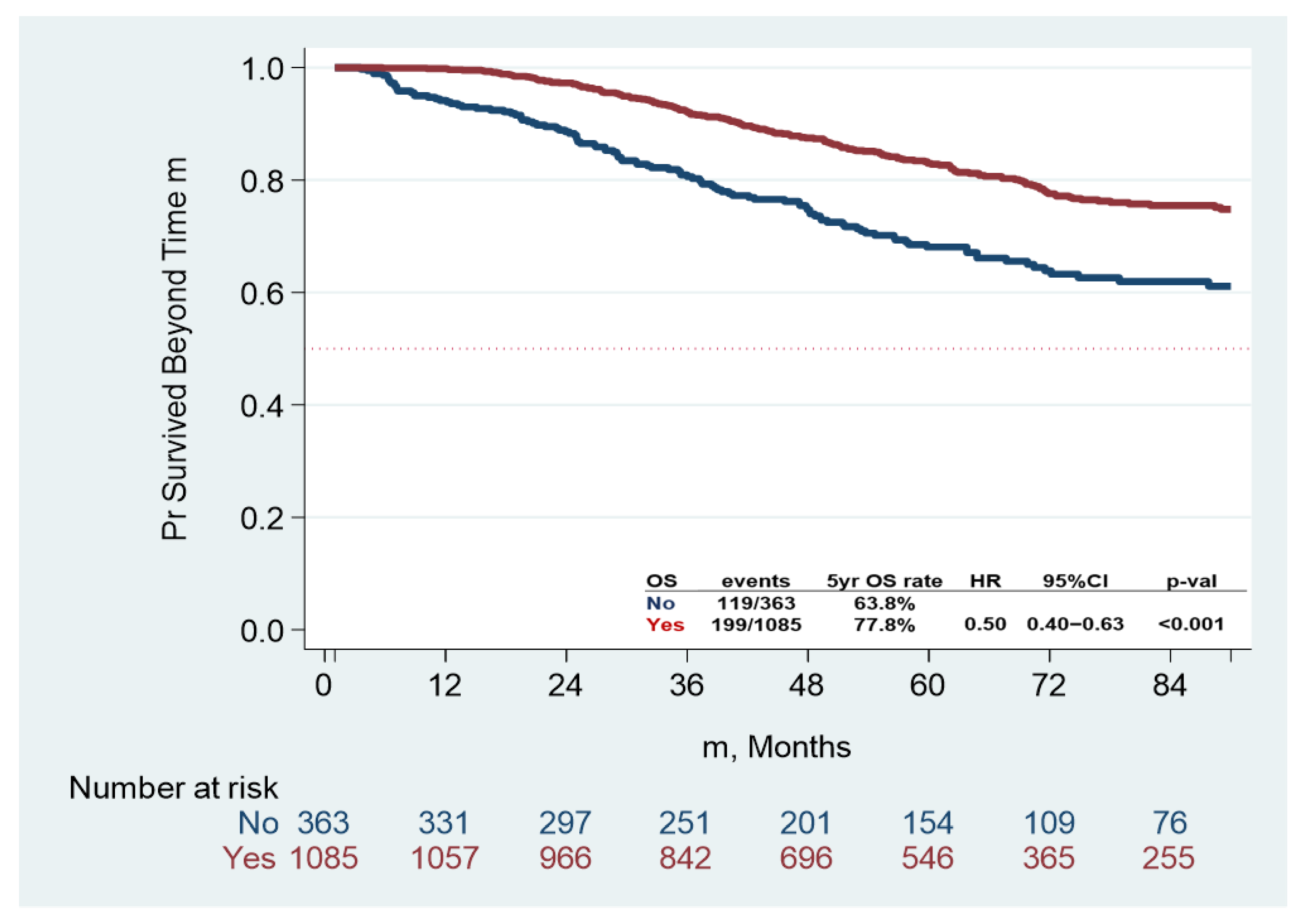
3.2. Clinical Outcomes
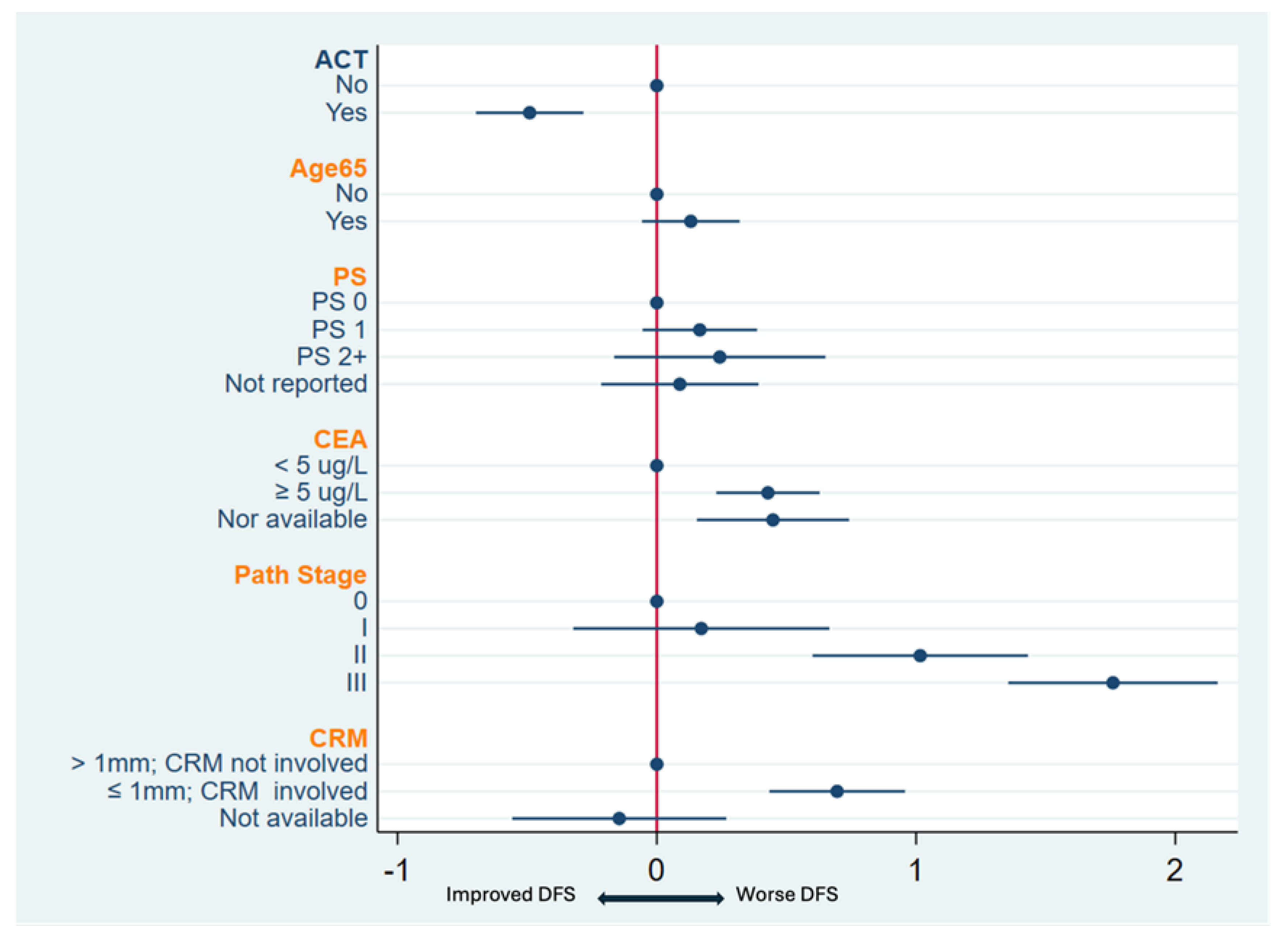
3.3. Predictors of DFS
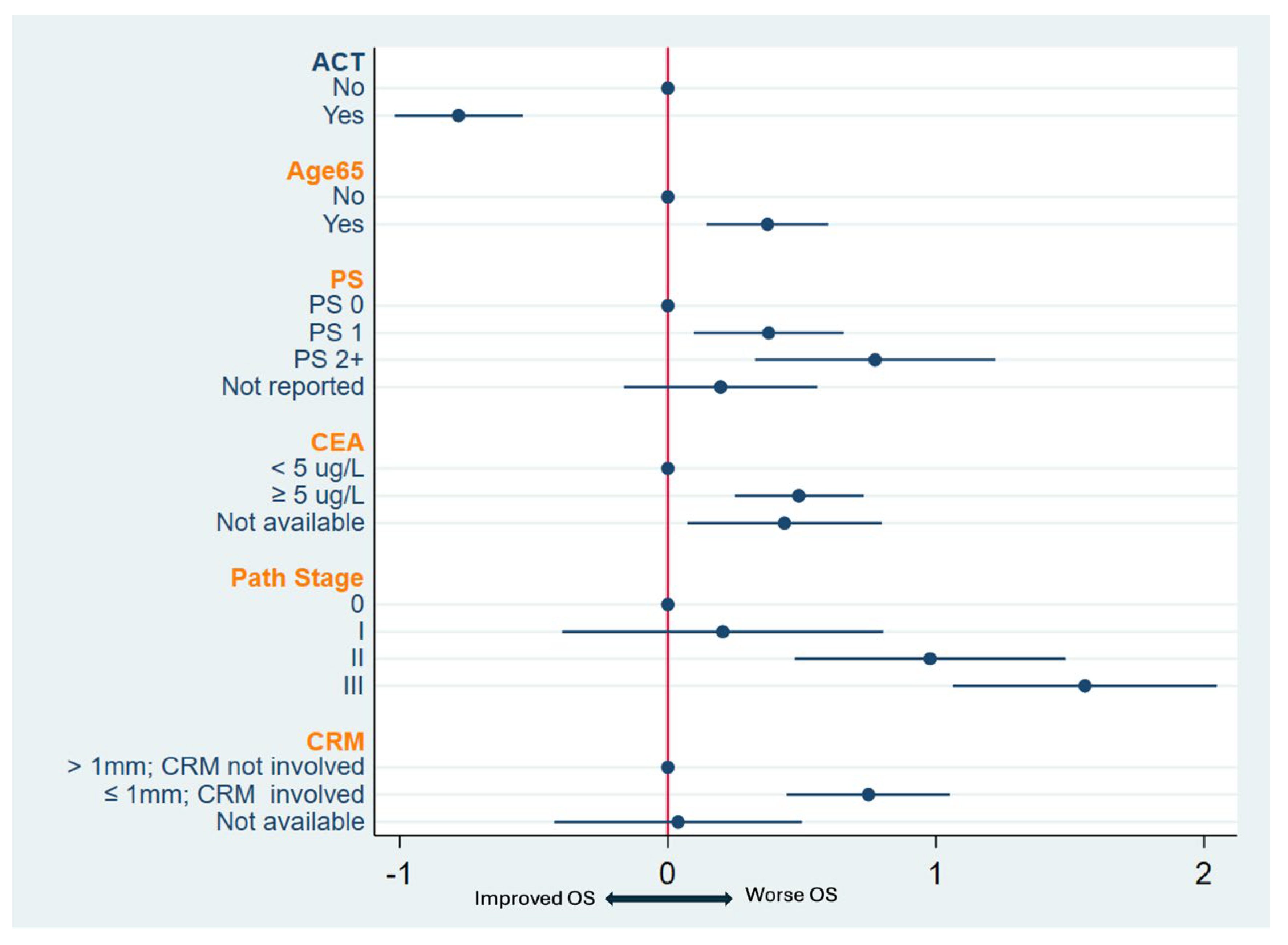
3.4. Predictors of OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Characteristic | Hazard Ratio (HR) | Confidence Interval (95% CI) |
|---|---|---|
| ACT (yes) | 0.60 | 0.49–0.74 |
| Age > 65 years | 1.14 | 0.94−1.37 |
| PS 1 | 1.17 | 0.94–1.46 |
| PS 2+ | 1.27 | 0.85–1.92 |
| PS not reported | 1.09 | 0.80–1.48 |
| CEA ≥ 5 µg/L | 1.52 | 1.25–1.86 |
| CEA not available | 1.55 | 1.15–2.07 |
| Pathology Stage 1 | 1.16 | 0.71–1.89 |
| Pathology Stage 2 | 2.68 | 1.78–4.04 |
| Pathology Stage 3 | 5.65 | 3.80–8.41 |
| CRM < 1 mm/not involved | 2.01 | 1.55–2.62 |
| CRM not available | 0.86 | 0.57–1.29 |
| Characteristic | Hazard Ratio (HR) | Confidence Interval (95% CI) |
|---|---|---|
| ACT (yes) | 0.46 | 0.36–0.58 |
| Age > 65 years | 1.45 | 1.16–1.82 |
| PS 1 | 1.46 | 1.10–1.93 |
| PS 2+ | 2.17 | 1.38–3.39 |
| PS not reported | 1.22 | 0.85–1.75 |
| CEA ≥ 5 µg/L | 1.63 | 1.28–2.08 |
| CEA not available | 1.55 | 1.08–2.22 |
| Pathology Stage 1 | 1.23 | 0.67–2.23 |
| Pathology Stage 2 | 2.66 | 1.61–4.41 |
| Pathology Stage 3 | 4.74 | 2.90–7.76 |
| Pathology Stage 4 | 71.08 | 9.22–547.72 |
| CRM < 1 mm/not involved | 2.11 | 1.56–2.86 |
| CRM not available | 1.04 | 0.65–1.65 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Gillis, J.; Demers, A.A.; Ellison, L.F.; Billette, J.-M.; Zhang, S.X.; Liu, J.L.; Woods, R.R.; Finley, C.; Fitzgerald, N.; et al. Projected Estimates of Cancer in Canada in 2024. CMAJ 2024, 196, E615–E623. [Google Scholar] [CrossRef]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The Rising Tide of Early-Onset Colorectal Cancer: A Comprehensive Review of Epidemiology, Clinical Features, Biology, Risk Factors, Prevention, and Early Detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Tamas, K.; Walenkamp, A.M.E.; De Vries, E.G.E.; Van Vugt, M.A.T.M.; Beets-Tan, R.G.; Van Etten, B.; De Groot, D.J.A.; Hospers, G.A.P. Rectal and Colon Cancer: Not Just a Different Anatomic Site. Cancer Treat. Rev. 2015, 41, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Mir, Z.M.; Yu, D.; Merchant, S.J.; Booth, C.M.; Patel, S.V. Management of Rectal Cancer in Canada: An Evidence-Based Comparison of Clinical Practice Guidelines. Can. J. Surg. 2020, 63, E27–E34. [Google Scholar] [CrossRef]
- Crawford, A.; Firtell, J.; Caycedo-Marulanda, A. How Is Rectal Cancer Managed: A Survey Exploring Current Practice Patterns in Canada. J. Gastrointest. Canc 2019, 50, 260–268. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Sauer, R.; Rödel, C.; Martus, P.; Hess, C.F.; Schmidberger, H. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Pettersson, D.; Holm, T.; Iversen, H.; Blomqvist, L.; Glimelius, B.; Martling, A. Preoperative Short-Course Radiotherapy with Delayed Surgery in Primary Rectal Cancer. Br. J. Surg. 2012, 99, 577–583. [Google Scholar] [CrossRef]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal Fractionation of Preoperative Radiotherapy and Timing to Surgery for Rectal Cancer (Stockholm III): A Multicentre, Randomised, Non-Blinded, Phase 3, Non-Inferiority Trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Fuentes, S.; Radu, C.; Frödin, J.-E.; Johansson, H.; Brandberg, Y.; Holm, T.; Glimelius, B.; Martling, A. Radiotherapy Regimens for Rectal Cancer: Long-Term Outcomes and Health-Related Quality of Life in the Stockholm III Trial. BJS Open 2021, 5, zrab137. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant Chemotherapy with FOLFIRINOX and Preoperative Chemoradiotherapy for Patients with Locally Advanced Rectal Cancer (UNICANCER-PRODIGE 23): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; Van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-Course Radiotherapy Followed by Chemotherapy before Total Mesorectal Excision (TME) versus Preoperative Chemoradiotherapy, TME, and Optional Adjuvant Chemotherapy in Locally Advanced Rectal Cancer (RAPIDO): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.-M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). JCO 2022, 40, 1681–1692. [Google Scholar] [CrossRef]
- Peeters, K.C.M.J.; Marijnen, C.A.M.; Nagtegaal, I.D.; Kranenbarg, E.K.; Putter, H.; Wiggers, T.; Rutten, H.; Pahlman, L.; Glimelius, B.; Leer, J.W.; et al. The TME Trial After a Median Follow-up of 6 Years: Increased Local Control But No Survival Benefit in Irradiated Patients With Resectable Rectal Carcinoma. Ann. Surg. 2007, 246, 693. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. JCO 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Ngan, S.Y.K.; Fisher, R.; Burmeister, B.H.; Mackay, J.; Goldstein, D.; Kneebone, A.; Schache, D.; Joseph, D.; McKendrick, J.; Leong, T.; et al. Promising Results of a Cooperative Group Phase II Trial of Preoperative Chemoradiation for Locally Advanced Rectal Cancer (TROG 9801). Dis. Colon. Rectum 2005, 48, 1389–1396. [Google Scholar] [CrossRef]
- Bregni, G.; Akin Telli, T.; Camera, S.; Deleporte, A.; Moretti, L.; Bali, A.M.; Liberale, G.; Holbrechts, S.; Hendlisz, A.; Sclafani, F. Adjuvant Chemotherapy for Rectal Cancer: Current Evidence and Recommendations for Clinical Practice. Cancer Treat. Rev. 2020, 83, 101948. [Google Scholar] [CrossRef]
- Sargent, D.; Sobrero, A.; Grothey, A.; O’Connell, M.J.; Buyse, M.; Andre, T.; Zheng, Y.; Green, E.; Labianca, R.; O’Callaghan, C.; et al. Evidence for Cure by Adjuvant Therapy in Colon Cancer: Observations Based on Individual Patient Data From 20,898 Patients on 18 Randomized Trials. JCO 2009, 27, 872–877. [Google Scholar] [CrossRef]
- Breugom, A.J.; Van Gijn, W.; Muller, E.W.; Berglund, Å.; Van Den Broek, C.B.M.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.H.; Marijnen, C.A.M.; et al. Adjuvant Chemotherapy for Rectal Cancer Patients Treated with Preoperative (Chemo)Radiotherapy and Total Mesorectal Excision: A Dutch Colorectal Cancer Group (DCCG) Randomized Phase III Trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Sainato, A.; Cernusco Luna Nunzia, V.; Valentini, V.; De Paoli, A.; Maurizi, E.R.; Lupattelli, M.; Aristei, C.; Vidali, C.; Conti, M.; Galardi, A.; et al. No Benefit of Adjuvant Fluorouracil Leucovorin Chemotherapy after Neoadjuvant Chemoradiotherapy in Locally Advanced Cancer of the Rectum (LARC): Long Term Results of a Randomized Trial (I-CNR-RT). Radiother. Oncol. 2014, 113, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Counsell, N.; Quirke, P.; Mortensen, N.; Maraveyas, A.; Meadows, H.M.; Ledermann, J.; Sebag-Montefiore, D. Chronicle: Results of a Randomised Phase III Trial in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiation Randomising Postoperative Adjuvant Capecitabine plus Oxaliplatin (XELOX) versus Control. Ann. Oncol. 2014, 25, 1356–1362. [Google Scholar] [CrossRef]
- Bosset, J.-F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.-J.; Bardet, E.; Beny, A.; Ollier, J.-C.; Bolla, M.; et al. Fluorouracil-Based Adjuvant Chemotherapy after Preoperative Chemoradiotherapy in Rectal Cancer: Long-Term Results of the EORTC 22921 Randomised Study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Breugom, A.J.; Swets, M.; Bosset, J.-F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; Van Den Broek, C.B.M.; et al. Adjuvant Chemotherapy after Preoperative (Chemo)Radiotherapy and Surgery for Patients with Rectal Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data. Lancet Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Coinu, A.; Lonati, V.; Barni, S. A Systematic Review and Meta-Analysis of Adjuvant Chemotherapy after Neoadjuvant Treatment and Surgery for Rectal Cancer. Int. J. Color. Dis. 2015, 30, 447–457. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.-H.; Kim, K.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.-Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. JCO 2019, 37, 3111–3123. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2024, 22, 366–375. [Google Scholar] [CrossRef]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of Locally Advanced Rectal Cancer: ASCO Guideline. JCO 2024, 42, 3355–3375. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Edge, S.B.; American Joint Committee on Cancer (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; ISBN 978-0-387-88440-0. [Google Scholar]
- Kapiteijn, E.; Marijnen, C.A.M.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.T.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.J.M.; et al. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Swedish Rectal Cancer Trial; Cedermark, B.; Dahlberg, M.; Glimelius, B.; Påhlman, L.; Rutqvist, L.; Wilking, N. Improved Survival with Preoperative Radiotherapy in Resectable Rectal Cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; Van De Velde, C.J. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer: 12-Year Follow-up of the Multicentre, Randomised Controlled TME Trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Fisher, B.; Wolmark, N.; Rockette, H.; Redmond, C.; Deutsch, M.; Wickerham, D.L.; Fisher, E.R.; Caplan, R.; Jones, J.; Lerner, H.; et al. Postoperative Adjuvant Chemotherapy or Radiation Therapy for Rectal Cancer: Results From NSABP Protocol R-011. JNCI J. Natl. Cancer Inst. 1988, 80, 21–29. [Google Scholar] [CrossRef]
- Thomas, P.R.M.; Lindblad, A.S. Adjuvant Postoperative Radiotherapy and Chemotherapy in Rectal Carcinoma: A Review of the Gastrointestinal Tumor Study Group Experience. Radiother. Oncol. 1988, 13, 245–252. [Google Scholar] [CrossRef]
- Sun, W.; Al-Rajabi, R.; Perez, R.O.; Abbasi, S.; Ash, R.; Habr-Gama, A. Controversies in Rectal Cancer Treatment and Management. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–11. [Google Scholar] [CrossRef]
- Quasar Collaborative Group. Adjuvant Chemotherapy versus Observation in Patients with Colorectal Cancer: A Randomised Study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef]
- Hong, Y.S.; Nam, B.-H.; Kim, K.; Kim, J.E.; Park, S.J.; Park, Y.S.; Park, J.O.; Kim, S.Y.; Kim, T.-Y.; Kim, J.H.; et al. Oxaliplatin, Fluorouracil, and Leucovorin versus Fluorouracil and Leucovorin as Adjuvant Chemotherapy for Locally Advanced Rectal Cancer after Preoperative Chemoradiotherapy (ADORE): An Open-Label, Multicentre, Phase 2, Randomised Controlled Trial. Lancet Oncol. 2014, 15, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin Added to Fluorouracil-Based Preoperative Chemoradiotherapy and Postoperative Chemotherapy of Locally Advanced Rectal Cancer (the German CAO/ARO/AIO-04 Study): Final Results of the Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, J.H.; Kim, S.H.; Um, J.W.; Korean Clinical Practice Guideline for Colon, Rectal Cancer Committee. Oxaliplatin-Based Adjuvant Chemotherapy Rather than Fluorouracil-Based Chemotherapy in Rectal Cancer Is More Efficient to Decrease Distant Metastasis and Increase Survival after Preoperative Chemoradiotherapy and Surgery: A Meta-Analysis. Int. J. Color. Dis. 2022, 37, 649–656. [Google Scholar] [CrossRef]
- Jiang, D.M.; Raissouni, S.; Mercer, J.; Kumar, A.; Goodwin, R.; Heng, D.Y.; Tang, P.A.; Doll, C.; MacLean, A.; Powell, E.; et al. Clinical Outcomes of Elderly Patients Receiving Neoadjuvant Chemoradiation for Locally Advanced Rectal Cancer. Ann. Oncol. 2015, 26, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.M.; Kennecke, H.F.; Renouf, D.J.; Lim, H.J.; Vickers, M.M.; Speers, C.H.; Cheung, W.Y. Effect of Adjuvant Chemotherapy on Stage II Rectal Cancer Outcomes After Preoperative Short-Course Radiotherapy. Clin. Color. Cancer 2016, 15, 352–359.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, J.; Kleppe, A.; Danielsen, H.E.; Kerr, D.J. Personalizing Adjuvant Therapy for Patients with Colorectal Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Kisakol, B.; Matveeva, A.; Salvucci, M.; Kel, A.; McDonough, E.; Ginty, F.; Longley, D.B.; Prehn, J.H.M. Identification of Unique Rectal Cancer-Specific Subtypes. Br. J. Cancer 2024, 130, 1809–1818. [Google Scholar] [CrossRef]
- Smolskas, E.; Mikulskytė, G.; Sileika, E.; Suziedelis, K.; Dulskas, A. Tissue-Based Markers as a Tool to Assess Response to Neoadjuvant Radiotherapy in Rectal Cancer—Systematic Review. Int. J. Mol. Sci. 2022, 23, 6040. [Google Scholar] [CrossRef]
- Chatila, W.K.; Kim, J.K.; Walch, H.; Marco, M.R.; Chen, C.-T.; Wu, F.; Omer, D.M.; Khalil, D.N.; Ganesh, K.; Qu, X.; et al. Genomic and Transcriptomic Determinants of Response to Neoadjuvant Therapy in Rectal Cancer. Nat. Med. 2022, 28, 1646–1655. [Google Scholar] [CrossRef]
- Sánchez-Vinces, S.; Duarte, G.H.B.; Messias, M.C.F.; Gatinoni, C.F.A.; Silva, A.A.R.; Sanches, P.H.G.; Martinez, C.A.R.; Porcari, A.M.; Carvalho, P.d.O. Rectal Cancer Tissue Lipidome Differs According to Response to Neoadjuvant Therapy. Int. J. Mol. Sci. 2023, 24, 11479. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Ni, F.; Tai, G.; Yu, C.; Jiang, X.; Wang, D. Research on Radiotherapy Related Genes and Prognostic Target Identification of Rectal Cancer Based on Multi-Omics. J. Transl. Med. 2023, 21, 856. [Google Scholar] [CrossRef]
- Wang, H.; Ji, D.; Tian, H.; Gao, Z.; Song, C.; Jia, J.; Cui, X.; Zhong, L.; Shen, J.; Gu, J. Predictive Value of Proteomic Markers for Advanced Rectal Cancer with Neoadjuvant Chemoradiotherapy. BMC Cancer 2022, 22, 868. [Google Scholar] [CrossRef]
- Wang, F.; Tan, B.F.; Poh, S.S.; Siow, T.R.; Lim, F.L.W.T.; Yip, C.S.P.; Wang, M.L.C.; Nei, W.; Tan, H.Q. Predicting Outcomes for Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiation with CT-Based Radiomics. Sci. Rep. 2022, 12, 6167. [Google Scholar] [CrossRef]
- Shin, J.; Seo, N.; Baek, S.-E.; Son, N.-H.; Lim, J.S.; Kim, N.K.; Koom, W.S.; Kim, S. MRI Radiomics Model Predicts Pathologic Complete Response of Rectal Cancer Following Chemoradiotherapy. Radiology 2022, 303, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Loft, M.; To, Y.H.; Gibbs, P.; Tie, J. Clinical Application of Circulating Tumour DNA in Colorectal Cancer. Lancet Gastroenterol. Hepatol. 2023, 8, 837–852. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, J.; Akazawa, N.; Hirata, K.; Kataoka, K.; Yokota, M.; Kato, K.; Kotaka, M.; Kagawa, Y.; Yeh, K.-H.; et al. ctDNA-Based Molecular Residual Disease and Survival in Resectable Colorectal Cancer. Nat. Med. 2024, 30, 3272–3283. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- van Rees, J.M.; Wullaert, L.; Grüter, A.A.J.; Derraze, Y.; Tanis, P.J.; Verheul, H.M.W.; Martens, J.W.M.; Wilting, S.M.; Vink, G.; van Vugt, J.L.A.; et al. Circulating Tumour DNA as Biomarker for Rectal Cancer: A Systematic Review and Meta-Analyses. Front. Oncol. 2023, 13, 1083285. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Wang, Y.; Li, L.; Christie, M.; Simons, K.; Elsaleh, H.; Kosmider, S.; Wong, R.; Yip, D.; et al. Serial Circulating Tumour DNA Analysis during Multimodality Treatment of Locally Advanced Rectal Cancer: A Prospective Biomarker Study. Gut 2019, 68, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.; Akiyoshi, T.; Sano, T.; Fukunaga, Y.; Noda, T.; Ueno, M.; Zembutsu, H. Serial Circulating Tumour DNA Analysis for Locally Advanced Rectal Cancer Treated with Preoperative Therapy: Prediction of Pathological Response and Postoperative Recurrence. Br. J. Cancer 2020, 123, 803–810. [Google Scholar] [CrossRef]
- Khakoo, S.; Carter, P.D.; Brown, G.; Valeri, N.; Picchia, S.; Bali, M.A.; Shaikh, R.; Jones, T.; Begum, R.; Rana, I.; et al. MRI Tumor Regression Grade and Circulating Tumor DNA as Complementary Tools to Assess Response and Guide Therapy Adaptation in Rectal Cancer. Clin. Cancer Res. 2020, 26, 183–192. [Google Scholar] [CrossRef]
- McDuff, S.G.R.; Hardiman, K.M.; Ulintz, P.J.; Parikh, A.R.; Zheng, H.; Kim, D.W.; Lennerz, J.K.; Hazar-Rethinam, M.; Van Seventer, E.E.; Fetter, I.J.; et al. Circulating Tumor DNA Predicts Pathologic and Clinical Outcomes Following Neoadjuvant Chemoradiation and Surgery for Patients With Locally Advanced Rectal Cancer. JCO Precis. Oncol. 2021, 5, PO.20–00220. [Google Scholar] [CrossRef]
- Al-Mansor, E.; Mahoney, M.; Chenard-Poirier, M.; Ramjeesingh, R.; Nair, V.; Kennedy, E.; Locke, G.; Welch, S.; Berry, S.; Couture, F.; et al. Eastern Canadian Gastrointestinal Cancer Consensus Conference 2023. Curr. Oncol. 2023, 30, 8172–8185. [Google Scholar] [CrossRef]
- Gill, S.; Ahmed, S.; Anderson, B.; Berry, S.; Lim, H.; Phang, T.; Sharma, A.; Solar Vasconcelos, J.P.; Gill, K.; Iqbal, M.; et al. Correction: Gill et al. Report from the 24th Annual Western Canadian Gastrointestinal Cancer Consensus Conference on Colorectal Cancer, Richmond, British Columbia, 28–29, October 2022. Curr. Oncol. 2023, 30, 7964–7983. Curr. Oncol. 2024, 31, 3252. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, e240029. [Google Scholar] [CrossRef] [PubMed]

| Adjuvant CT | |||||
|---|---|---|---|---|---|
| Characteristic | Total (N = 1448) | No (N = 363, 25.1%) | Yes (N = 1085, 74.9%) | p-Value | |
| Province, n (%) | AB | 606, 42% | 150, 41% | 456, 42% | <0.001 |
| BC | 252, 17% | 80, 22% | 172, 16% | ||
| NL | 188, 13% | 22, 6% | 166, 16% | ||
| ON | 402, 28% | 111, 31% | 291, 27% | ||
| Age, years | median (range) | 61 (22–92) | 66 (27–92) | 60 (22–86) | |
| ≥65, n (%) | 560, 39% | 188, 52% | 372, 34% | <0.001 | |
| Female, n (%) | 438, 30% | 111, 31% | 327, 30% | 0.874 | |
| BMI, kg/m2 | Underweight | 28, 1.93% | 5, 1.38% | 23, 2.12% | 0.001 |
| Normal weight | 432, 29.83% | 90, 24.79% | 243, 31.52% | ||
| Overweight | 519, 35.84% | 124, 34.16% | 395, 36.41% | ||
| Obese | 366, 25.28% | 104, 26.65% | 262, 24.15% | ||
| Unknown | 103, 7.11% | 40, 11.02% | 63, 5.81% | ||
| ECOG PS, n (%) | 0 | 673, 46% | 150, 41% | 523, 48% | 0.013 |
| 1 | 524, 36% | 133, 37% | 391, 36% | ||
| 2+ | 66, 5% | 25, 7% | 41, 4% | ||
| Unknown | 185, 13% | 55, 15% | 130, 12% | ||
| Distance from anal verge, cm | <5 | 478, 33% | 123, 34% | 355, 33% | 0.927 |
| 5–10 | 595, 41% | 149, 41% | 446, 41% | ||
| >10 | 273, 19% | 68, 19% | 205, 19% | ||
| Unknown | 102, 7% | 23, 6% | 79, 7% | ||
| Pretreatment CEA, ng/mL | <5 | 778, 54% | 194, 54% | 584, 54% | 0.107 |
| ≥5 | 497, 35% | 115, 32% | 382, 35% | ||
| Unknown | 173, 12% | 54, 15% | 119, 11% | ||
| Clinical stage, n (%) | II | 439, 30% | 108, 30% | 331, 31% | 0.787 |
| III | 1009, 70% | 255, 70.2% | 754, 69% | ||
| Radiation therapy dose, n (%) | <44 | 43, 3% | 23, 6% | 20, 2% | <0.001 |
| ≥44 to 46 | 248, 17% | 71, 20% | 177, 16% | ||
| ≥46 | 1157, 80% | 269, 74% | 888, 82% | ||
| Surgery type, n (%) | LAR | 737, 51% | 185, 51% | 552, 51% | 0.964 |
| APR | 665, 46% | 165, 45% | 500, 46% | ||
| PE | 35, 2% | 10, 3% | 25, 2% | ||
| Unknown | 11, 1% | 2, 1% | 8, 1% | ||
| Quirke grade, n (%) | Good | 677, 47% | 168, 46% | 509, 47% | 0.913 |
| Moderate | 177, 12% | 43, 12% | 134, 12% | ||
| Poor | 91, 6% | 21, 6% | 70, 6% | ||
| Not recorded | 503, 35% | 131, 36% | 372, 35% | ||
| Pathological grade, n (%) | 0 | 80, 22% | 172, 16% | 252, 17% | 0.060 |
| I | 67, 18% | 206, 19% | 273, 19% | ||
| II | 110, 30% | 327, 30% | 437, 30% | ||
| III | 106, 29% | 379, 34% | 485, 33% | ||
| IV | 0, 0% | 1, 0% | 1, 0% | ||
| Downstaged, n (%) | No | 640, 44% | 141, 39% | 499, 46% | 0.010 |
| Yes, not pCR | 556, 38% | 142, 39% | 414, 38% | ||
| pCR | 252, 17% | 80, 22% | 172, 16% | ||
| CRM, n (%) | >1 mm; CRM not involved | 1217, 84% | 306, 84% | 911, 84% | 0.153 |
| ≤1 mm; CRM involved | 112, 8% | 34, 9% | 78, 7% | ||
| Not available | 119, 8% | 23, 6% | 96, 9% | ||
| Local pelvic recurrence, n (%) | No | 1332, 92% | 332, 91% | 1000, 92% | 0.216 |
| Yes | 115, 8% | 30, 8% | 85, 7% | ||
| Distant recurrence, n (%) | No | 1132, 78% | 289, 80% | 843, 78% | 0.444 |
| Yes | 316, 21% | 74, 20% | 242, 22% | ||
| Dead, n (%) | No | 1130, 78% | 244, 67% | 886, 82% | <0.001 |
| Yes | 318, 22% | 119, 32% | 199, 18% | ||
| OS | DFS | ||||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | ||
| Adjuvant CT | No | 1.00 | 1.00 | ||||
| Yes | 0.50 | 0.4–0.63 | <0.001 | 0.71 | 0.58–0.86 | 0.001 | |
| Province | AB | 1.00 | 1.00 | ||||
| BC | 1.26 | 0.96–1.65 | 0.10 | 1.41 | 1.11–1.78 | 0.004 | |
| NL | 1.34 | 0.88–2.02 | 0.17 | 1.58 | 1.15–2.16 | 0.004 | |
| ON | 0.47 | 0.35–0.64 | <0.001 | 0.65 | 0.51–0.83 | <0.001 | |
| Age at diagnosis | <65 | 1.00 | 1.00 | ||||
| ≥65 | 1.67 | 1.34–2.08 | <0.001 | 1.30 | 1.08–1.56 | 0.005 | |
| ECOG PS | 0 | 1.00 | 1.00 | ||||
| 1 | 1.80 | 1.38–2.34 | <0.001 | 1.44 | 1.17–1.78 | 0.001 | |
| 2+ | 3.27 | 2.13–5.02 | <0.001 | 1.86 | 1.26–2.77 | 0.002 | |
| Unknown | 1.91 | 1.39–2.63 | <0.001 | 1.56 | 1.2–2.04 | 0.001 | |
| Distance from anal verge | <5 | 1.00 | 1.00 | ||||
| 5–10 | 0.89 | 0.69–1.14 | 0.35 | 0.92 | 0.74–1.14 | 0.434 | |
| >10 | 0.66 | 0.47–0.93 | 0.02 | 0.77 | 0.59–1.01 | 0.063 | |
| Unknown | 1.51 | 1.02–2.23 | 0.04 | 1.61 | 1.16–2.22 | 0.004 | |
| Pretreatment CEA | <5 | 1.00 | 1.00 | ||||
| (Ug/L) | ≥5 | 1.88 | 1.49–2.39 | <0.001 | 1.73 | 1.43–2.11 | <0.001 |
| Unknown | 1.38 | 0.97–1.96 | 0.07 | 1.42 | 1.07–1.89 | 0.015 | |
| RT dose | <44 Gy | 1.00 | 1.00 | ||||
| 44–46 Gy | 1.14 | 0.60–2.14 | 0.69 | 1.09 | 0.63–1.87 | 0.764 | |
| ≥46 Gy | 0.63 | 0.34–1.15 | 0.13 | 0.77 | 0.46–1.29 | 0.321 | |
| Type of surgery | LAR | 1.00 | 1.00 | ||||
| APR | 1.36 | 1.08–1.71 | 0.01 | 1.30 | 1.08–1.57 | 0.006 | |
| PE | 2.43 | 1.43–4.16 | <0.001 | 1.72 | 1.05–2.82 | 0.032 | |
| Not reported | 2.25 | 0.83–6.08 | 0.11 | 1.41 | 0.52–3.79 | 0.496 | |
| Quirke grade | Good | 1.00 | 1.00 | ||||
| Poor | 1.44 | 1.01–2.06 | 0.04 | 1.25 | 0.93–1.68 | 0.142 | |
| Moderate | 1.97 | 1.30–2.97 | <0.001 | 1.68 | 1.18–2.4 | 0.004 | |
| Not reported | 1.36 | 1.06–1.74 | 0.020 | 1.25 | 1.02–1.54 | 0.033 | |
| Pathological stage | 0 | 1.00 | 1.00 | ||||
| 1 | 1.23 | 0.68–2.22 | 0.50 | 1.23 | 0.76–2.01 | 0.4 | |
| 2 | 3.27 | 2.00–5.33 | <0.001 | 3.32 | 2.21–4.98 | <0.001 | |
| 3 | 5.43 | 3.38–8.72 | <0.001 | 6.53 | 4.42–9.65 | <0.001 | |
| 4 | 31.16 | 4.15–233.89 | <0.001 | 164.93 | 21.8–1248.04 | <0.001 | |
| CRM involved | No | 1.00 | 1.00 | ||||
| Yes | 3.18 | 2.37–4.25 | <0.001 | 3.10 | 2.41–4 | <0.001 | |
| Not reported | 1.03 | 0.67–1.59 | 0.90 | 0.86 | 0.5823323–1.26 | 0.426 | |
| pCR | No | 1.00 | 1.00 | ||||
| Yes | 0.28 | 0.18–0.45 | <0.001 | 0.26 | 0.18–0.38 | <0.001 | |
| Downstaged | No | 1.00 | 1.00 | ||||
| Yes | 0.38 | 0.30–0.48 | <0.001 | 0.32 | 0.26–0.39 | <0.001 | |
| Adjuvant CT | |||||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| DFS | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | |
| Pretreatment CEA | <5 | 1.00 | 1.00 | ||||
| (µg/L) | ≥5 | 1.67 | 1.15–2.43 | 0.007 | 1.47 | 1.16–1.86 | 0.002 |
| Unknown | 1.58 | 0.95–2.64 | 0.079 | 1.59 | 1.11–2.29 | 0.012 | |
| Pathological stage | 0 | 1.00 | 1.00 | ||||
| 1 | 1.02 | 0.46–2.27 | 0.954 | 1.40 | 0.73–2.65 | 0.308 | |
| 2 | 2.04 | 1.07–3.89 | 0.029 | 3.44 | 1.98–5.98 | 0.00 | |
| 3 | 5.77 | 3.11–10.70 | 0.00 | 6.42 | 3.73–11.05 | 0.00 | |
| CRM | No | 1.00 | |||||
| Yes | 1.85 | 1.18–2.89 | 0.007 | 2.05 | 1.48–2.83 | 0.00 | |
| Unknown | 0.78 | 0.38–1.64 | 0.517 | 0.88 | 0.53–1.46 | 0.617 | |
| Adjuvant CT | |||||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| OS | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | |
| Age at diagnosis | <65 yo | 1.00 | 1.00 | ||||
| ≥65 yo | 1.57 | 1.06–2.33 | 0.023 | 1.37 | 1.03–1.82 | 0.033 | |
| ECOG PS | 0 | 1.00 | 1.00 | ||||
| 1 | 1.15 | 0.72–1.83 | 0.551 | 1.52 | 1.07–2.16 | 0.02 | |
| 2+ | 1.93 | 0.98–3.78 | 0.056 | 2.51 | 1.36–4.64 | 0.003 | |
| Unknown | 1.21 | 0.66–2.20 | 0.535 | 1.19 | 0.75–1.89 | 0.464 | |
| Pretreatment CEA | <5 | 1.00 | 1.00 | ||||
| (ug/L) | ≥5 | 1.62 | 1.08–2.43 | 0.019 | 1.62 | 1.20–2.20 | 0.002 |
| Unknown | 1.36 | 0.75–2.46 | 0.316 | 1.65 | 1.04–2.64 | 0.035 | |
| Pathological stage | 0 | 1.00 | 1.00 | ||||
| 1 | 0.95 | 0.41–2.22 | 0.915 | 1.76 | 0.71–4.34 | 0.22 | |
| 2 | 1.51 | 0.75–3.05 | 0.247 | 4.57 | 2.07–10.09 | <0.001 | |
| 3 | 3.73 | 1.93–7.23 | <0.001 | 6.74 | 3.07–14.78 | <0.001 | |
| CRM | No | 1.00 | 1.00 | ||||
| Yes | 2.21 | 1.35–3.62 | 0.002 | 1.82 | 1.21–2.73 | 0.004 | |
| Unknown | 0.85 | 0.38–1.89 | 0.687 | 1.16 | 0.65–2.08 | 0.62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrokhi, K.; Marginean, H.; Al Ghamdi, A.; Al Mansor, E.; Dudani, S.; Goodwin, R.A.; Asmis, T.R.; Powell, E.; Tang, P.A.; Lee-Ying, R.; et al. The Impact of Adjuvant Chemotherapy on Clinical Outcomes in Locally Advanced Rectal Cancer: A CHORD Consortium Analysis. Curr. Oncol. 2025, 32, 371. https://doi.org/10.3390/curroncol32070371
Farrokhi K, Marginean H, Al Ghamdi A, Al Mansor E, Dudani S, Goodwin RA, Asmis TR, Powell E, Tang PA, Lee-Ying R, et al. The Impact of Adjuvant Chemotherapy on Clinical Outcomes in Locally Advanced Rectal Cancer: A CHORD Consortium Analysis. Current Oncology. 2025; 32(7):371. https://doi.org/10.3390/curroncol32070371
Chicago/Turabian StyleFarrokhi, Kaveh, Horia Marginean, Anas Al Ghamdi, Essa Al Mansor, Shaan Dudani, Rachel A. Goodwin, Timothy R. Asmis, Erin Powell, Patricia A. Tang, Richard Lee-Ying, and et al. 2025. "The Impact of Adjuvant Chemotherapy on Clinical Outcomes in Locally Advanced Rectal Cancer: A CHORD Consortium Analysis" Current Oncology 32, no. 7: 371. https://doi.org/10.3390/curroncol32070371
APA StyleFarrokhi, K., Marginean, H., Al Ghamdi, A., Al Mansor, E., Dudani, S., Goodwin, R. A., Asmis, T. R., Powell, E., Tang, P. A., Lee-Ying, R., & Vickers, M. M. (2025). The Impact of Adjuvant Chemotherapy on Clinical Outcomes in Locally Advanced Rectal Cancer: A CHORD Consortium Analysis. Current Oncology, 32(7), 371. https://doi.org/10.3390/curroncol32070371








