Evaluating the Impact of Common Non-Oncologic Medication Use During Radiotherapy in Patients with High-Risk Prostate Cancer
Abstract
1. Introduction
2. Methods
Statistics
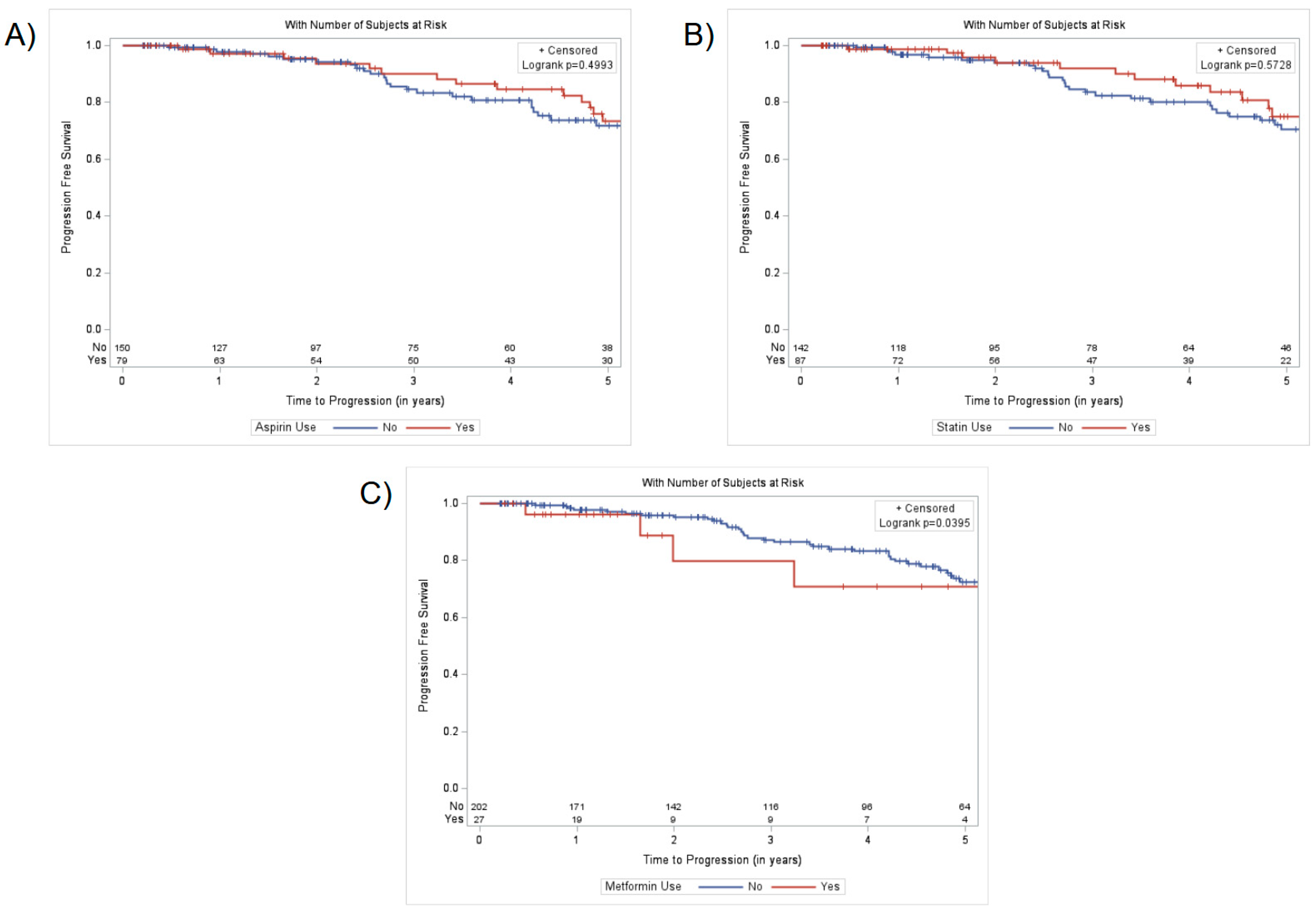
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pruthi, R.S.; Wallen, E.M. Cyclooxygenase-2: A therapeutic target for prostate cancer. Clin. Genitourin. Cancer 2005, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Lee, Y.H.; Koo, K.C. Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 8540. [Google Scholar] [CrossRef] [PubMed]
- Demir, U.; Koehler, A.; Schneider, R.; Schweiger, S.; Klocker, H. Metformin anti-tumor effect via disruption of the MID1 translational regulator complex and AR downregulation in prostate cancer cells. BMC Cancer 2014, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Tong, D.; Parmar, H.; Hasenmayer, D.; Yuan, W.; Zhang, D.; Jiang, J. Metformin represses androgen-dependent and androgen-independent prostate cancers by targeting androgen receptor. Prostate 2015, 75, 1187–1196. [Google Scholar] [CrossRef]
- Hoque, A.; Chen, H.; Xu, X.C. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 2008, 17, 88–94. [Google Scholar] [CrossRef]
- Longo, J.; Hamilton, R.J.; Masoomian, M.; Khurram, N.; Branchard, E.; Mullen, P.J.; Elbaz, M.; Hersey, K.; Chadwick, D.; Ghai, S.; et al. A pilot window-of-opportunity study of preoperative fluvastatin in localized prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 630–637. [Google Scholar] [CrossRef]
- Murtola, T.J.; Syvälä, H.; Tolonen, T.; Helminen, M.; Riikonen, J.; Koskimäki, J.; Pakarainen, T.; Kaipia, A.; Isotalo, T.; Kujala, P.; et al. Atorvastatin Versus Placebo for Prostate Cancer Before Radical Prostatectomy-A Randomized, Double-blind, Placebo-controlled Clinical Trial. Eur. Urol. 2018, 74, 697–701. [Google Scholar] [CrossRef]
- Mascan, B.; Marignol, L. Aspirin in the Management of Patients with Prostate Cancer Undergoing Radiotherapy: Friend or Foe? Anticancer Res. 2018, 38, 1897–1902. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Mason, M.D.; Clarke, N.W.; Anderson, J.; Dearnaley, D.P.; Dwyer, J.; Jovic, G.; Ritchie, A.W.; Russell, J.M.; et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: First results from the STAMPEDE multiarm, multistage, randomised controlled trial. Lancet Oncol. 2012, 13, 549–558. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, S.; Li, T.; Liu, R. Could aspirin be a lifesaver for prostate cancer patients in prostate cancer-specific mortality?: An update systematic review and meta-analysis. BMC Cancer 2019, 19, 1186. [Google Scholar] [CrossRef]
- Joshua, A.M.; Armstrong, A.; Crumbaker, M.; Scher, H.I.; de Bono, J.; Tombal, B.; Hussain, M.; Sternberg, C.N.; Gillessen, S.; Carles, J.; et al. Statin and metformin use and outcomes in patients with castration-resistant prostate cancer treated with enzalutamide: A meta-analysis of AFFIRM, PREVAIL and PROSPER. Eur. J. Cancer 2022, 170, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.G.; Lim, B.; Yun, S.C.; Lim, J.H.; Hong, J.H.; Kim, C.S. Adjuvant Low-dose Statin Use after Radical Prostatectomy: The PRO-STAT Randomized Clinical Trial. Clin. Cancer Res. 2021, 27, 5004–5011. [Google Scholar] [CrossRef] [PubMed]
- Morgans, A.K.; Chen, Y.H.; Jarrard, D.F.; Carducci, M.; Liu, G.; Eisenberger, M.; Plimack, E.R.; Bryce, A.; Garcia, J.A.; Dreicer, R.; et al. Association between baseline body mass index and survival in men with metastatic hormone-sensitive prostate cancer: ECOG-ACRIN CHAARTED E3805. Prostate 2022, 82, 1176–1185. [Google Scholar] [CrossRef]
- Downer, M.K.; Allard, C.B.; Preston, M.A.; Gaziano, J.M.; Stampfer, M.J.; Mucci, L.A.; Batista, J.L. Regular Aspirin Use and the Risk of Lethal Prostate Cancer in the Physicians’ Health Study. Eur. Urol. 2017, 72, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Stopsack, K.H.; Gonzalez-Feliciano, A.G.; Peisch, S.F.; Downer, M.K.; Gage, R.A.; Finn, S.; Lis, R.T.; Graff, R.E.; Pettersson, A.; Pernar, C.H.; et al. A Prospective Study of Aspirin Use and Prostate Cancer Risk by TMPRSS2:ERG Status. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1231–1233. [Google Scholar] [CrossRef]
- Skriver, C.; Dehlendorff, C.; Borre, M.; Brasso, K.; Larsen, S.B.; Dalton, S.O.; Norgaard, M.; Pottegard, A.; Hallas, J.; Sorensen, H.T.; et al. Use of Low-Dose Aspirin and Mortality After Prostate Cancer Diagnosis: A Nationwide Cohort Study. Ann. Intern. Med. 2019, 170, 443–452. [Google Scholar] [CrossRef]
- Kang, M.; Ku, J.H.; Kwak, C.; Kim, H.H.; Jeong, C.W. Effects of Aspirin, Nonsteroidal Anti-inflammatory Drugs, Statin, and COX2 Inhibitor on the Developments of Urological Malignancies: A Population-Based Study with 10-Year Follow-up Data in Korea. Cancer Res. Treat. 2018, 50, 984–991. [Google Scholar] [CrossRef]
- Harshman, L.C.; Wang, X.; Nakabayashi, M.; Xie, W.; Valenca, L.; Werner, L.; Yu, Y.; Kantoff, A.M.; Sweeney, C.J.; Mucci, L.A.; et al. Statin Use at the Time of Initiation of Androgen Deprivation Therapy and Time to Progression in Patients with Hormone-Sensitive Prostate Cancer. JAMA Oncol. 2015, 1, 495–504. [Google Scholar] [CrossRef]
- Raittinen, P.V.H.; Syvala, H.; Tammela, T.L.J.; Hakkinen, M.R.; Ilmonen, P.; Auriola, S.; Murtola, T.J. Atorvastatin induces adrenal androgen downshift in men with prostate cancer: A post Hoc analysis of a pilot adaptive Randomised clinical trial. EBioMedicine 2021, 68, 103432. [Google Scholar] [CrossRef]
- Peltomaa, A.I.; Talala, K.; Taari, K.; Tammela, T.L.J.; Auvinen, A.; Murtola, T.J. Statin use and outcomes of oncological treatment for castration-resistant prostate cancer. Sci. Rep. 2023, 13, 18866. [Google Scholar] [CrossRef]
- Khan, S.; Chang, S.H.; Seyerle, A.A.; Wang, M.; Hicks, V.; Drake, B.F. Post-diagnostic metformin and statin use and risk of biochemical recurrence in Veterans diagnosed with prostate cancer. Prostate 2023, 83, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; An, Y.; Liu, C.Q.; Xu, J.Z.; Zhong, X.Y.; Zeng, N.; Sun, J.X.; Xia, Q.D.; Wang, S.G. Association of Statin Use with the Risk of Incident Prostate Cancer: A Meta-Analysis and Systematic Review. J. Oncol. 2022, 2022, 7827821. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Sun, J.X.; Xu, M.Y.; Liu, C.Q.; Xu, J.Z.; Zhong, X.Y.; Hu, J.; Xia, Q.D.; Hu, H.L.; Wang, S.G. Statin Use Is Associated with Better Prognosis of Patients with Prostate Cancer after Definite Therapies: A Systematic Review and Meta-Analysis of Cohort Studies. J. Oncol. 2022, 2022, 9275466. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.; Gray, P.K.; Hahn, N.; Hayes, J.; Myers, L.J.; Carney-Doebbeling, C.; Sweeney, C.J. Presence of the metabolic syndrome is associated with shorter time to castration-resistant prostate cancer. Ann. Oncol. 2011, 22, 801–807. [Google Scholar] [CrossRef]
- Lee, C.S.; Lam, S.Y.; Liu, A.; Sison, C.; Zhu, X.H. A Retrospective Study of the Effect of Metformin on Patients with Metastatic Prostate Cancer. Clin. Med. Insights Oncol. 2023, 17, 11795549231152073. [Google Scholar] [CrossRef]
- Mahalingam, D.; Hanni, S.; Serritella, A.V.; Fountzilas, C.; Michalek, J.; Hernandez, B.; Sarantopoulos, J.; Datta, P.; Romero, O.; Pillai, S.M.A.; et al. Utilizing metformin to prevent metabolic syndrome due to androgen deprivation therapy (ADT): A randomized phase II study of metformin in non-diabetic men initiating ADT for advanced prostate cancer. Oncotarget 2023, 14, 622–636. [Google Scholar] [CrossRef]
- Spratt, D.E.; Zhang, C.; Zumsteg, Z.S.; Pei, X.; Zhang, Z.; Zelefsky, M.J. Metformin and prostate cancer: Reduced development of castration-resistant disease and prostate cancer mortality. Eur. Urol. 2013, 63, 709–716. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Shaikh, T.; Ruth, K.; Sharda, P.; Hayes, S.B.; Sobczak, M.L.; Hallman, M.A.; Smaldone, M.C.; Chen, D.Y.; Horwitz, E.M. Prostate Cancer Patients With Unmanaged Diabetes or Receiving Insulin Experience Inferior Outcomes and Toxicities After Treatment With Radiation Therapy. Clin. Genitourin. Cancer 2017, 15, 326–335.e323. [Google Scholar] [CrossRef]
- Fleshner, N.E.; Bernardino, R.M.; Lajkosz, K.; Saad, F.; Izawa, J.; Drachenberg, D.; Saranchuk, J.W.; Tanguay, S.; Rendon, R.A.; Leveridge, M.; et al. A randomized, double-blind, placebo-controlled trial of metformin in reducing progression among men on expectant management for low-risk prostate cancer: The MAST (Metformin Active Surveillance Trial) study. J. Clin. Oncol. 2024, 42, LBA5002. [Google Scholar] [CrossRef]
| Variable | Total (n = 237) |
|---|---|
| Age Median [IQR] (min, max) | 66 [59, 73] (43, 87) |
| Age (binary) | |
| <65 | 98 (41.35%) |
| 65+ | 139 (58.65%) |
| Race/Ethnicity | |
| Non-Hispanic White | 90 (37.97%) |
| Non-Hispanic African American/Black | 57 (24.05%) |
| Non-Hispanic Asian | 18 (7.59%) |
| Hispanic | 8 (3.38%) |
| Unknown | 64 (27.00%) |
| PSA at Diagnosis (n = 236) Median [IQR] (min, max) | 16.19 [7.69, 34.95] (1.78, 315.00) |
| PSA at Diagnosis (categorized) (n = 236) | |
| <10 | 79 (33.47%) |
| 10–20 | 55 (23.31%) |
| >20 | 102 (43.22%) |
| T Stage (n = 236) | |
| T1 | 104 (44.07%) |
| T2 | 85 (36.02%) |
| T3 | 40 (16.95%) |
| T4 | 7 (2.97%) |
| N Stage (n = 231) | |
| N0 | 219 (94.81%) |
| N1 | 12 (5.19%) |
| Gleason Score (n = 236) | |
| 6 | 11 (4.66%) |
| 7 | 47 (19.92%) |
| 8 | 90 (38.14%) |
| 9 | 78 (33.05%) |
| 10 | 10 (4.24%) |
| ADT with Radiation | |
| Yes | 224 (94.51%) |
| No | 13 (5.49%) |
| ADT Duration (n = 228) * | |
| ≤6 months ** | 53 (23.25%) |
| >6 months | 175 (76.75%) |
| ADT Duration (n = 215) *** Median [IQR] (min, max) | 20 [9, 24] (1, 50) |
| KPS-Stratified | |
| 0 | 174 (73.42%) |
| 1+ | 14 (5.91%) |
| Missing | 49 (20.68%) |
| Aspirin Use | |
| Yes | 82 (34.60%) |
| No | 155 (65.40%) |
| Statin Use | |
| Yes | 88 (37.13%) |
| No | 149 (62.87%) |
| Metformin Use | |
| Yes | 29 (12.24%) |
| No | 208 (87.76%) |
| Obesity (BMI ≥ 30) | |
| Yes | 85 (35.86%) |
| No | 152 (64.14%) |
| Type of Definitive RT | |
| Dose-escalated IMRT | 222 (93.67%) |
| IMRT + brachytherapy boost | 15 (6.33%) |
| Receipt of Pelvic RT | |
| Yes | 144 (60.76%) |
| No | 93 (39.24%) |
| RT Prescription Dose to Prostate (Gy) (n = 222) **** Median [IQR] (min, max) | 78 [77.4, 79.2] (42, 80) |
| Total # of RT Fractions (n = 222) **** Median [IQR] (min, max) | 42 [39, 44] (21, 44) |
| Variable | Crude HR (95% CIs) | p-Value |
|---|---|---|
| Age (in years) 1-year increase | 0.98 (0.95–1.01) | 0.2468 |
| Age (binary) | 0.9787 | |
| <65 | Reference | |
| ≥65 | 1.01 (0.58–1.75) | |
| Race/Ethnicity | 0.6364 | |
| Non-Hispanic White | Reference | |
| Non-Hispanic African American/Black | 0.91 (0.46–1.80) | 0.7904 |
| Non-Hispanic Asian | 0.43 (0.10–1.80) | 0.2460 |
| Hispanic | 2.07 (0.48–8.92) | 0.3312 |
| Unknown | 0.94 (0.47–1.90) | 0.8690 |
| Race/Ethnicity (binary) | 0.9184 | |
| Non-Hispanic African American/Black | 0.97 (0.52–1.81) | |
| Other | Reference | |
| PSA at Diagnosis | 0.0347 | |
| <20 | Reference | |
| ≥20 | 1.81 (1.04–3.12) | |
| T Stage | 0.0216 | |
| T1–2 | Reference | |
| T3–4 | 2.00 (1.11–3.60) | |
| N Stage | 0.0101 | |
| N0 | Reference | |
| N1 | 3.46 (1.34–8.91) | |
| Gleason Score | 0.0856 | |
| 6–7 | Reference | |
| ≥8 | 1.95 (0.91–4.17) | |
| ADT with Radiation | 0.2484 | |
| Yes | Reference | |
| No | 0.31 (0.04–2.27) | |
| ADT Duration | 0.6174 | |
| ≤ 6 months | Reference | |
| >6 months | 0.85 (0.44–1.62) | |
| KPS-Stratified | 0.4074 | |
| 0 | Reference | |
| ≥1 | 1.02 (0.31–3.30) | 0.9798 |
| Unknown | 0.63 (0.32–1.25) | 0.1844 |
| Aspirin Use | 0.5001 | |
| Yes | 0.82 (0.46–1.46) | |
| No | Reference | |
| Statin Use | 0.5733 | |
| Yes | 0.84 (0.47–1.52) | |
| No | Reference | |
| Metformin Use | 0.0443 | |
| Yes | 2.20 (1.02–4.72) | |
| No | Reference | |
| Obesity (BMI ≥ 30) | 0.1334 | |
| Yes | 1.52 (0.88–2.64) | |
| No | Reference | |
| Type of Definitive RT | 0.2976 | |
| Dose-escalated IMRT | Reference | |
| IMRT + brachytherapy boost | 1.64 (0.65–4.16) | |
| Receipt of Pelvic RT | 0.0747 | |
| Yes | Reference | |
| No | 0.57 (0.30–1.06) |
| Variable | Adjusted HR (95% CIs) | p-Value |
|---|---|---|
| Aspirin Use | 0.7147 | |
| Yes | 0.90 (0.49–1.62) | |
| No | Reference | |
| PSA at Diagnosis | 0.0056 | |
| <20 | Reference | |
| ≥20 | 2.30 (1.28–4.14) | |
| T Stage | 0.0329 | |
| T1–2 | Reference | |
| T3–4 | 1.95 (1.06–3.61) | |
| Gleason Score | 0.0077 | |
| 6–7 | Reference | |
| ≥8 | 3.00 (1.34–6.74) | |
| Race/Ethnicity | 0.4275 | |
| Non-Hispanic African American/Black | 1.30 (0.68–2.51) | |
| Other | Reference |
| Variable | Adjusted HR (95% CIs) | p-Value |
|---|---|---|
| Statin Use | 0.3975 | |
| Yes | 0.77 (0.42–1.41) | |
| No | Reference | |
| PSA at Diagnosis | 0.0051 | |
| <20 | Reference | |
| ≥20 | 2.30 (1.28–4.11) | |
| T Stage | 0.0288 | |
| T1–2 | Reference | |
| T3–4 | 1.98 (1.07–3.65) | |
| Gleason Score | 0.0056 | |
| 6–7 | Reference | |
| ≥8 | 3.17 (1.40–7.16) | |
| Race/Ethnicity | 0.3965 | |
| Non-Hispanic African American/Black | 1.33 (0.69–2.56) | |
| Other | Reference |
| Variable | Adjusted HR (95% CIs) | p-Value |
|---|---|---|
| Metformin Use | 0.0361 | |
| Yes | 2.46 (1.06–5.72) | |
| No | Reference | |
| PSA at Diagnosis | 0.0022 | |
| <20 | Reference | |
| ≥20 | 2.52 (1.40–4.55) | |
| T Stage | 0.0261 | |
| T1–2 | Reference | |
| T3–4 | 2.01 (1.09–3.71) | |
| Gleason Score | 0.0187 | |
| 6–7 | Reference | |
| ≥8 | 2.65 (1.18–5.99) | |
| Race/Ethnicity | 0.8221 | |
| Non-Hispanic African American/Black | 1.08 (0.54–2.16) | |
| Other | Reference |
| Variable | Adjusted HR (95% CIs) | p-Value |
|---|---|---|
| Aspirin Use | 0.9138 | |
| Yes | 1.04 (0.55–1.94) | |
| No | Reference | |
| Statin Use | 0.2845 | |
| Yes | 0.70 (0.36–1.35) | |
| No | Reference | |
| Metformin Use | 0.0158 | |
| Yes | 2.77 (1.21–6.32) | |
| No | Reference | |
| PSA at Diagnosis | 0.0029 | |
| <20 | Reference | |
| ≥20 | 2.50 (1.37–4.58) | |
| T Stage | 0.0220 | |
| T1–2 | Reference | |
| T3–4 | 2.06 (1.11–3.82) | |
| Gleason Score | 0.0127 | |
| 6–7 | Reference | |
| ≥8 | 2.76 (1.24–6.15) |
| Variable | Adjusted HR (95% CIs) | p-Value |
|---|---|---|
| Metformin Use | 0.0476 | |
| Yes | 2.30 (1.01–5.22) | |
| No | Reference | |
| PSA at Diagnosis | 0.0012 | |
| <20 | Reference | |
| ≥20 | 2.73 (1.49–5.03) | |
| T Stage | 0.0358 | |
| T1–2 | Reference | |
| T3–4 | 1.93 (1.05–3.56) | |
| Gleason Score | 0.0148 | |
| 6–7 | Reference | |
| ≥8 | 2.70 (1.22–6.01) | |
| Obesity (BMI ≥ 30) | 0.1919 | |
| Yes | 1.48 (0.82–2.66) | |
| No | Reference |
| Variable | Adjusted HR (95% CIs) | p-Value |
|---|---|---|
| Metformin Use | 0.0243 | |
| Yes | 2.51 (1.13–5.60) | |
| No | Reference | |
| PSA at Diagnosis | 0.0010 | |
| <20 | Reference | |
| ≥20 | 2.81 (1.52–5.19) | |
| T Stage | 0.0330 | |
| T1–2 | Reference | |
| T3–4 | 1.98 (1.06–3.72) | |
| Gleason Score | 0.0077 | |
| 6–7 | Reference | |
| ≥8 | 3.05 (1.34–6.94) | |
| ADT Duration | 0.2351 | |
| ≤6 months | Reference | |
| >6 months | 0.66 (0.33–1.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perlow, H.K.; Khullar, K.; Kumar, R.; Sasmal, S.; Nakamoto, K.; Gokun, Y.; Eckstein, J.; Young, R.; Diaz, D.A.; Martin, D.; et al. Evaluating the Impact of Common Non-Oncologic Medication Use During Radiotherapy in Patients with High-Risk Prostate Cancer. Curr. Oncol. 2025, 32, 353. https://doi.org/10.3390/curroncol32060353
Perlow HK, Khullar K, Kumar R, Sasmal S, Nakamoto K, Gokun Y, Eckstein J, Young R, Diaz DA, Martin D, et al. Evaluating the Impact of Common Non-Oncologic Medication Use During Radiotherapy in Patients with High-Risk Prostate Cancer. Current Oncology. 2025; 32(6):353. https://doi.org/10.3390/curroncol32060353
Chicago/Turabian StylePerlow, Haley K., Karishma Khullar, Ritesh Kumar, Sonya Sasmal, Kent Nakamoto, Yevgeniya Gokun, Jacob Eckstein, Rebekah Young, Dayssy A. Diaz, Douglas Martin, and et al. 2025. "Evaluating the Impact of Common Non-Oncologic Medication Use During Radiotherapy in Patients with High-Risk Prostate Cancer" Current Oncology 32, no. 6: 353. https://doi.org/10.3390/curroncol32060353
APA StylePerlow, H. K., Khullar, K., Kumar, R., Sasmal, S., Nakamoto, K., Gokun, Y., Eckstein, J., Young, R., Diaz, D. A., Martin, D., Collier, K. A., Meng, L., Parikh, R. R., Clinton, S., & Wang, S.-J. (2025). Evaluating the Impact of Common Non-Oncologic Medication Use During Radiotherapy in Patients with High-Risk Prostate Cancer. Current Oncology, 32(6), 353. https://doi.org/10.3390/curroncol32060353





