Using Machine Learning Approaches on Dynamic Patient-Reported Outcomes to Cluster Cancer Treatment-Related Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Mobile App
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APP | Application |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| CTCAE | Common Terminology Criteria for Adverse Event |
| DCTs | Decentralized Clinical Trials |
| ePROs | Electronic Patient-Reported Outcomes |
| ML | Machine Learning |
| SCs | Symptom Clusters |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Jiménez-Fonseca, P.; Galán-Moral, R.; Coca-Membribes, S.; Fernández-Montes, A.; Sorribes, E.; García-Torralba, E.; Puntí-Brun, L.; Gil-Raga, M.; Cano-Cano, J.; et al. Toxicities and Quality of Life during Cancer Treatment in Advanced Solid Tumors. Curr. Oncol. 2023, 30, 9205–9216. [Google Scholar] [CrossRef]
- de Góes Salvetti, M.; Sanches, M.B. Symptom Cluster: Management and Advanced Practices in Oncology Nursing. Rev. Esc. Enferm. USP 2022, 56, e20210452. [Google Scholar] [CrossRef]
- Kirkova, J.; Walsh, D.; Aktas, A.; Davis, M.P. Cancer Symptom Clusters: Old Concept but New Data. Am. J. Hosp. Palliat. Care 2010, 27, 282–288. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L. Cancer Symptom Cluster Management. Semin. Oncol. Nurs. 2016, 32, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; McGuire, D.B.; Tulman, L.; Barsevick, A.M. Symptom Clusters: Concept Analysis and Clinical Implications for Cancer Nursing. Cancer Nurs. 2005, 28, 270–282. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Le, L.W.; Zimmermann, C. Symptom Clusters in Patients with Advanced Cancers. Support. Care Cancer 2009, 17, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.T.; Butow, P.N.; Costa, D.S.J.; Lovell, M.R.; Agar, M. Symptom Clusters in Patients with Advanced Cancer: A Systematic Review of Observational Studies. J. Pain Symptom Manag. 2014, 48, 411–450. [Google Scholar] [CrossRef]
- Kirkova, J.; Aktas, A.; Walsh, D.; Davis, M.P. Cancer Symptom Clusters: Clinical and Research Methodology. J. Palliat. Med. 2011, 14, 1149–1166. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L.; Wieben, A.; Braithwaite, L.; Hopfensperger, K.; Kim, K.S.; Montgomery, K.; Reske, M.; Stevens, J. Characteristics of Cancer Symptom Clusters Reported through a Patient-Centered Symptom Cluster Assessment. West. J. Nurs. Res. 2022, 44, 662–674. [Google Scholar] [CrossRef]
- Dong, S.T.; Butow, P.N.; Tong, A.; Agar, M.; Boyle, F.; Forster, B.C.; Stockler, M.; Lovell, M.R. Patients’ Experiences and Perspectives of Multiple Concurrent Symptoms in Advanced Cancer: A Semi-Structured Interview Study. Support. Care Cancer 2016, 24, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.M.; Ameringer, S.; Linder, L.; Macpherson, C.F.; Elswick, R.K.; Luebke, J.M.; Stegenga, K. Using a Heuristic App to Improve Symptom Self-Management in Adolescents and Young Adults with Cancer. J. Adolesc. Young Adult Oncol. 2019, 8, 131–141. [Google Scholar] [CrossRef]
- Macpherson, C.F.; Linder, L.A.; Ameringer, S.; Erickson, J.; Stegenga, K.; Woods, N.F. Feasibility and Acceptability of an iPad Application to Explore Symptom Clusters in Adolescents and Young Adults with Cancer. Pediatr. Blood Cancer 2014, 61, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Salmani, H.; Nasiri, S.; Ahmadi, M. The Advantages, Disadvantages, Threats, and Opportunities of Electronic Patient-Reported Outcome Systems in Cancer: A Systematic Review. Digit. Health 2024, 10, 20552076241257146. [Google Scholar] [CrossRef]
- Trojan, A.; Kühne, C.; Kiessling, M.; Schumacher, J.; Dröse, S.; Singer, C.; Jackisch, C.; Thomssen, C.; Kullak-Ublick, G.A. Impact of Electronic Patient-Reported Outcomes on Unplanned Consultations and Hospitalizations in Patients with Cancer Undergoing Systemic Therapy: Results of a Patient-Reported Outcome Study Compared with Matched Retrospective Data. JMIR Form. Res. 2024, 8, e55917. [Google Scholar] [CrossRef]
- Avery, K.N.L.; Richards, H.S.; Portal, A.; Reed, T.; Harding, R.; Carter, R.; Bamforth, L.; Absolom, K.; O’Connell Francischetto, E.; Velikova, G.; et al. Developing a Real-Time Electronic Symptom Monitoring System for Patients after Discharge Following Cancer-Related Surgery. BMC Cancer 2019, 19, 463. [Google Scholar] [CrossRef]
- Holch, P.; Warrington, L.; Bamforth, L.C.A.; Keding, A.; Ziegler, L.E.; Absolom, K.; Hector, C.; Harley, C.; Johnson, O.; Hall, G.; et al. Development of an Integrated Electronic Platform for Patient Self-Report and Management of Adverse Events during Cancer Treatment. Ann. Oncol. 2017, 28, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Trojan, A.; Laurenzi, E.; Roth, S.; Kiessling, M.; Atassi, Z.; Kadvany, Y.; Mannhart, M.; Witschel, H.F.; Jüngling, S.; Kullak-Ublick, G.A.; et al. Towards an Early Warning System for Monitoring of Cancer Patients Using Hybrid Interactive Machine Learning. Front. Digit. Health 2024, 6, 1443987. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Wei, L.; Cui, S.; Luo, Y.; Ten Haken, R.K.; El Naqa, I. Machine Learning and Imaging Informatics in Oncology. Oncology 2018, 98, 344–362. [Google Scholar] [CrossRef]
- Mitchell, T. Machine Learning; MC. Graw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Owsiński, J.W.; Opara, K.; Stańczak, J.; Kacprzyk, J.; Zadrożny, S. Reverse Clustering: An Outline for a Concept and Its Use. Toxicol. Environ. Chem. 2017, 99, 1078–1095. [Google Scholar] [CrossRef]
- de Rooij, B.H.; Oerlemans, S.; van Deun, K.; Mols, F.; de Ligt, K.M.; Husson, O.; Ezendam, N.P.M.; Hoedjes, M.; van de Poll-Franse, L.V.; Schoormans, D. Symptom Clusters in 1330 Survivors of 7 Cancer Types from the PROFILES Registry: A Network Analysis. Cancer 2021, 127, 4665–4674. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Han, C.J.; Saligan, L.; Wallen, G.R. Comparing Symptom Clusters in Cancer Survivors by Cancer Diagnosis: A Latent Class Profile Analysis. Support. Care Cancer 2024, 32, 308. [Google Scholar] [CrossRef]
- Trojan, A.; Leuthold, N.; Thomssen, C.; Rody, A.; Winder, T.; Jakob, A.; Egger, C.; Held, U.; Jackisch, C. The Effect of Collaborative Reviews of Electronic Patient-Reported Outcomes on the Congruence of Patient-and Clinician-Reported Toxicity in Cancer Patients Receiving Systemic Therapy: Prospective, Multicenter, Observational Clinical Trial. J. Med. Internet Res. 2021, 23, e29271. [Google Scholar] [CrossRef] [PubMed]
- Trojan, A.; Roth, S.; Atassi, Z.; Kiessling, M.; Zenhaeusern, R.; Kadvany, Y.; Schumacher, J.; Kullak-Ublick, G.A.; Aapro, M.; Eniu, A. Comparison of the Real-World Reporting of Symptoms and Well-Being for the HER2-Directed Trastuzumab Biosimilar Ogivri With Registry Data for Herceptin in the Treatment of Breast Cancer: Prospective Observational Study (OGIPRO) of Electronic Patient-Reported Outcomes. JMIR Cancer 2024, 10, e54178. [Google Scholar] [CrossRef]
- Rudin, C. Stop Explaining Black Box Machine Learning Models for High Stakes Decisions and Use Interpretable Models Instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef]
- Kleppe, A. Area under the Curve May Hide Poor Generalisation to External Datasets. ESMO Open 2022, 7, 100429. [Google Scholar] [CrossRef]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver Operating Characteristic Curve Analysis in Diagnostic Accuracy Studies: A Guide to Interpreting the Area under the Curve Value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef]
- Trojan, A.; Huber, U.; Brauchbar, M.; Petrausch, U. Consilium Smartphone App for Real-World Electronically Captured Patient-Reported Outcome Monitoring in Cancer Patients Undergoing Anti-PD-L1-Directed Treatment. Case Rep. Oncol. 2020, 13, 491–496. [Google Scholar] [CrossRef]
- Li, N.; Wu, J.; Zhou, J.; Wu, C.; Dong, L.; Fan, W.; Zhang, J. Symptom Clusters Change Over Time in Patients With Lung Cancer During Perichemotherapy. Cancer Nurs. 2021, 44, 272. [Google Scholar] [CrossRef]
- Yang, X.; Bai, J.; Liu, R.; Wang, X.; Zhang, G.; Zhu, X. Symptom Clusters and Symptom Network Analysis during Immunotherapy in Lung Cancer Patients. Support. Care Cancer 2024, 32, 717. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Gu, L.; Liu, P.; Zhang, L.; Xu, H.; Qiu, Q.; Zhang, W. Symptom Clusters in Patients with Colorectal Cancer after Colostomy: A Longitudinal Study in Shanghai. J. Int. Med. Res. 2021, 49, 03000605211063105. [Google Scholar] [CrossRef]
- So, W.K.W.; Law, B.M.H.; Ng, M.S.N.; He, X.; Chan, D.N.S.; Chan, C.W.H.; McCarthy, A.L. Symptom Clusters Experienced by Breast Cancer Patients at Various Treatment Stages: A Systematic Review. Cancer Med. 2021, 10, 2531–2565. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial Intelligence in Radiology. Nat. Rev. Cancer 2018, 18, 500. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial Intelligence in Cancer Imaging: Clinical Challenges and Applications. CA A Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Silva, G.F.S.; Fagundes, T.P.; Teixeira, B.C.; Chiavegatto Filho, A.D.P. Machine Learning for Hypertension Prediction: A Systematic Review. Curr. Hypertens. Rep. 2022, 24, 523–533. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, N.; Ge, Y.; Zhang, L.; Oudkerk, M.; Xie, X. Development and Application of Artificial Intelligence in Cardiac Imaging. Br. J. Radiol. 2020, 93, 20190812. [Google Scholar] [CrossRef]
- Molloy, G.J.; Messerli-Bürgy, N.; Hutton, G.; Wikman, A.; Perkins-Porras, L.; Steptoe, A. Intentional and Unintentional Non-Adherence to Medications Following an Acute Coronary Syndrome: A Longitudinal Study. J. Psychosom. Res. 2014, 76, 430–432. [Google Scholar] [CrossRef]
- Sun, L.; Hu, J.; Zhou, S.; Huang, Z.; Ye, J.; Peng, H.; Yu, Z.; Yu, P. RicciNet: Deep Clustering via A Riemannian Generative Model. In Proceedings of the ACM Web Conference 2024, Singapore, 13–17 May 2024; Association for Computing Machinery: New York, NY, USA, 2024; pp. 4071–4082. [Google Scholar]
- Yang, Y.; Li, G.; Li, D.; Zhang, J.; Hu, P.; Hu, L. Integrating Fuzzy Clustering and Graph Convolution Network to Accurately Identify Clusters From Attributed Graph. IEEE Trans. Netw. Sci. Eng. 2025, 12, 1112–1125. [Google Scholar] [CrossRef]
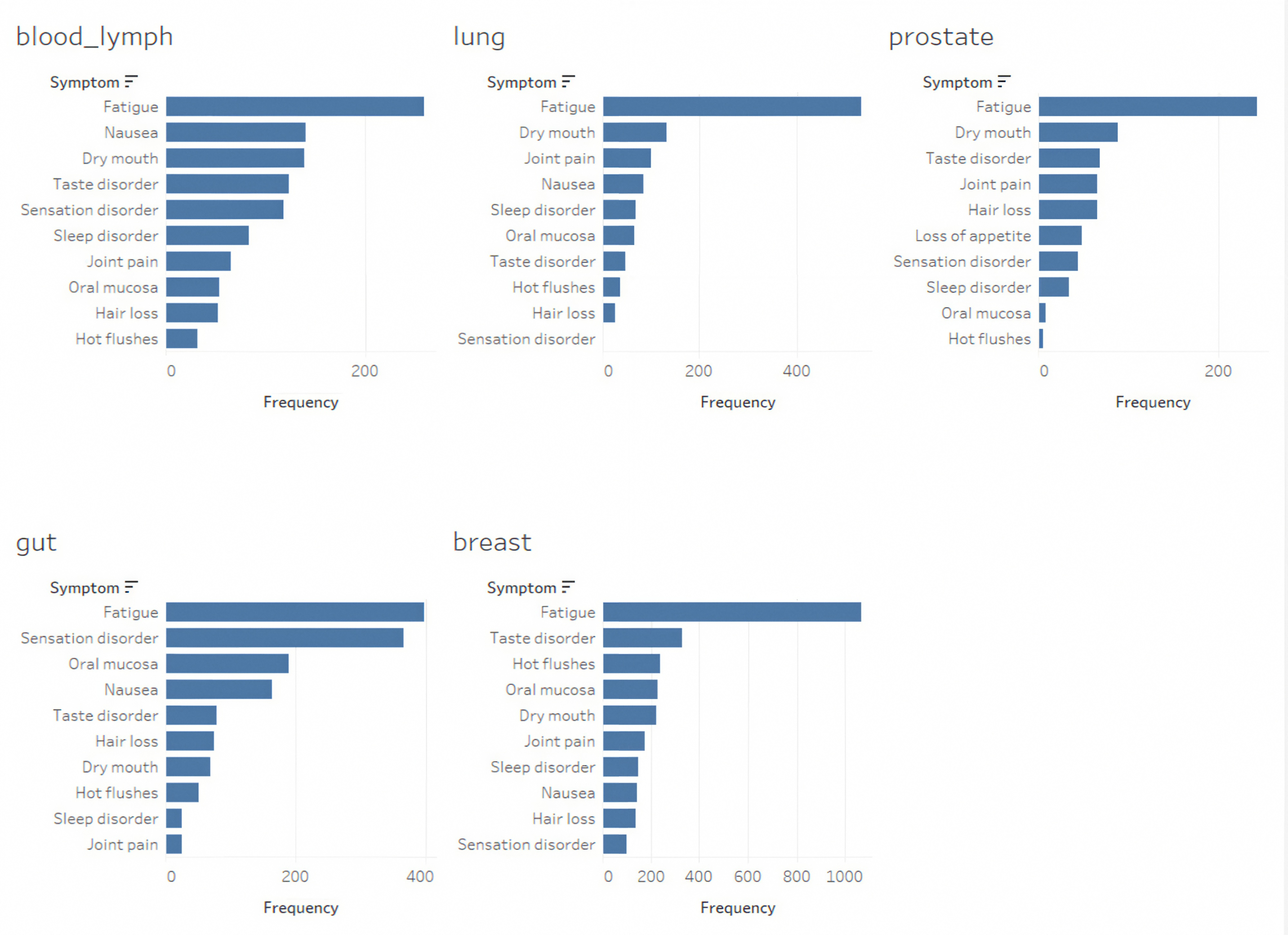
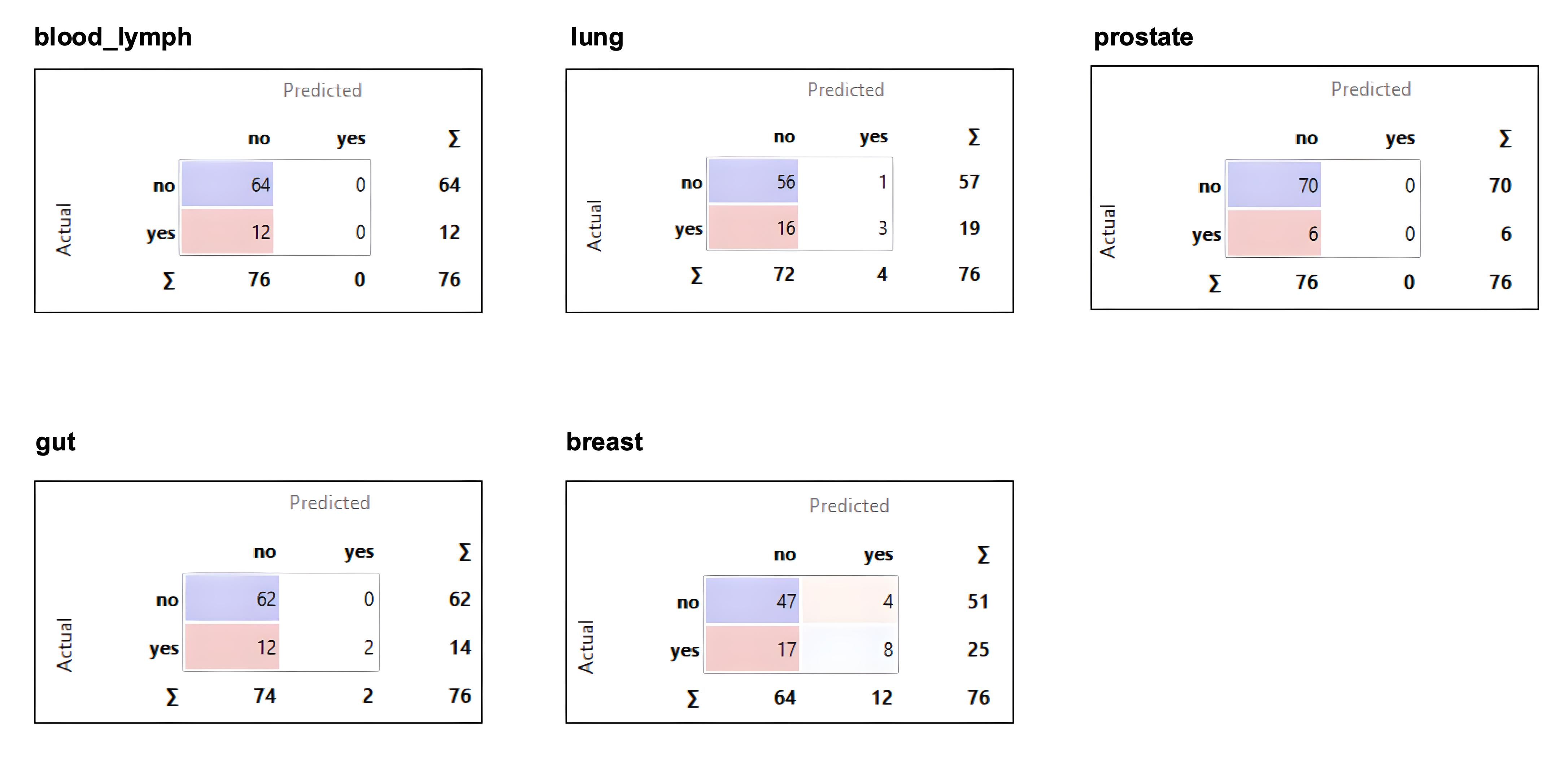
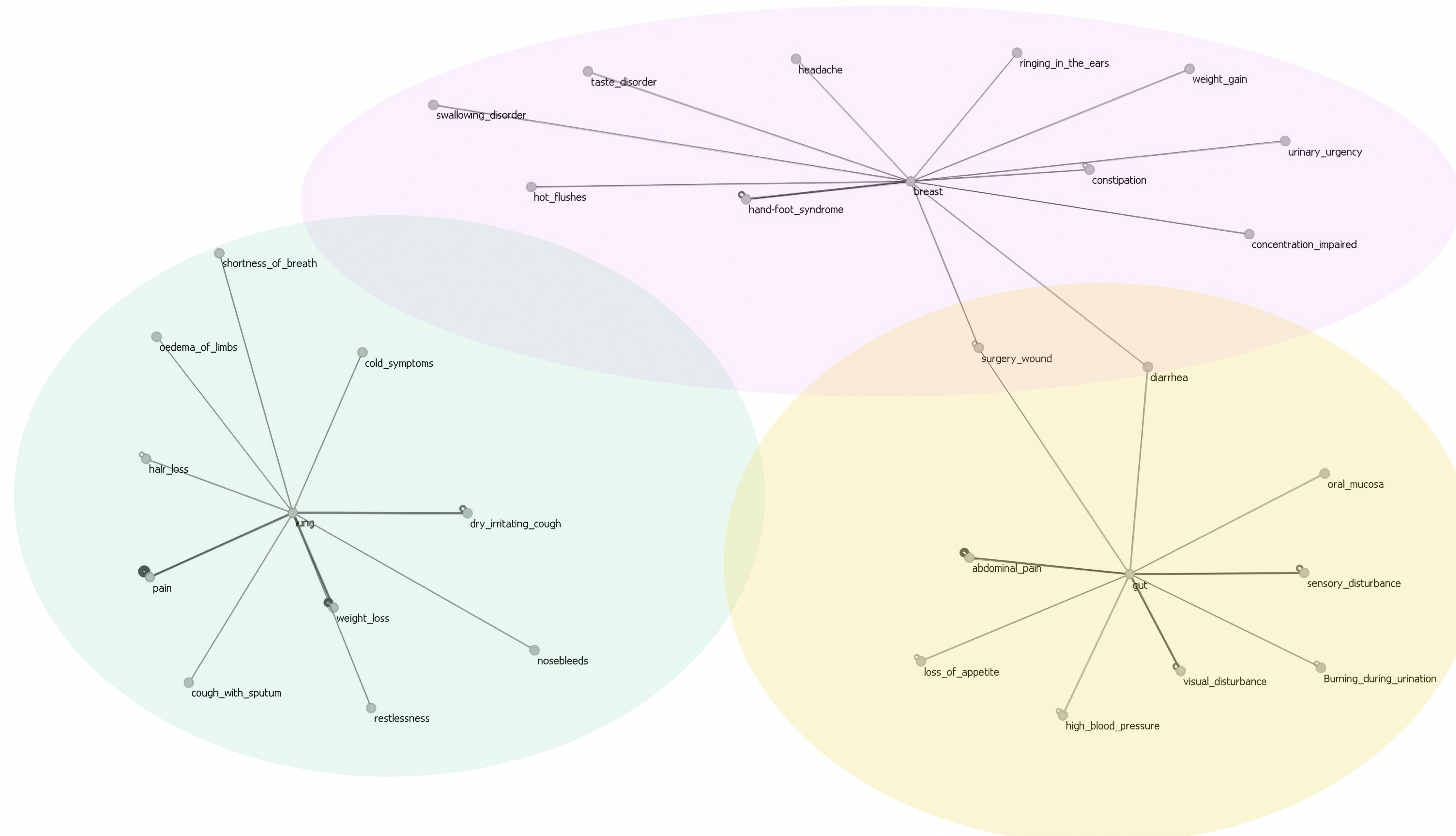
| Count | Percentage (%) | |
|---|---|---|
| Overall | 226 | 100 |
| Primary Tumor | ||
| Breast Cancer | 172 | 76.1 |
| Lung Cancer | 19 | 8.4 |
| Gut Cancer | 16 | 7.1 |
| Blood–lymph Cancer | 12 | 5.3 |
| Prostate Cancer | 7 | 3.1 |
| Gender | ||
| Male | 34 | 15 |
| Female | 191 | 84.5 |
| Diverse | 1 | 0.4 |
| Mean Age | 58.4 | |
| Selected Patients for Analysis | 60 | |
| Primary Tumor | ||
| Breast Cancer | 25 | 41.7 |
| Lung Cancer | 19 | 31.7 |
| Gut Cancer | 16 | 26.7 |
| Gender | ||
| Male | 15 | 25 |
| Female | 45 | 75 |
| Diverse | 0 | 0 |
| Mean Age | 50 |
| Most Frequently Applied Therapies | Percentage (%) |
|---|---|
| Herceptin/Perjeta +/− Docetaxel/Carboplatin | 16.3 |
| Antihormone +/− Everolimus o. CDK4/6-Inhibitor | 16.3 |
| Paclitaxel +/− Carboplatin | 12.1 |
| Docetaxel-Endoxan +/− Antihormon | 10.6 |
| EC-Paclitaxel | 9.9 |
| Checkpointinhibitor +/− Chemo | 8.5 |
| Capecitabine | 6.4 |
| EC-Docetaxel | 6.4 |
| Docetaxel/Carboplatin | 2.8 |
| Platine + Pemetrexed | 2.1 |
| FOLFIRI | 2.1 |
| CAPOX | 2.1 |
| Docetaxel | 1.4 |
| Platine + Etoposid | 1.4 |
| FOLFOX | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asper, N.; Witschel, H.F.; von Stockar, L.; Laurenzi, E.; Kolberg, H.C.; Vetter, M.; Roth, S.; Kullak-Ublick, G.; Trojan, A. Using Machine Learning Approaches on Dynamic Patient-Reported Outcomes to Cluster Cancer Treatment-Related Symptoms. Curr. Oncol. 2025, 32, 334. https://doi.org/10.3390/curroncol32060334
Asper N, Witschel HF, von Stockar L, Laurenzi E, Kolberg HC, Vetter M, Roth S, Kullak-Ublick G, Trojan A. Using Machine Learning Approaches on Dynamic Patient-Reported Outcomes to Cluster Cancer Treatment-Related Symptoms. Current Oncology. 2025; 32(6):334. https://doi.org/10.3390/curroncol32060334
Chicago/Turabian StyleAsper, Nora, Hans Friedrich Witschel, Louise von Stockar, Emanuele Laurenzi, Hans Christian Kolberg, Marcus Vetter, Sven Roth, Gerd Kullak-Ublick, and Andreas Trojan. 2025. "Using Machine Learning Approaches on Dynamic Patient-Reported Outcomes to Cluster Cancer Treatment-Related Symptoms" Current Oncology 32, no. 6: 334. https://doi.org/10.3390/curroncol32060334
APA StyleAsper, N., Witschel, H. F., von Stockar, L., Laurenzi, E., Kolberg, H. C., Vetter, M., Roth, S., Kullak-Ublick, G., & Trojan, A. (2025). Using Machine Learning Approaches on Dynamic Patient-Reported Outcomes to Cluster Cancer Treatment-Related Symptoms. Current Oncology, 32(6), 334. https://doi.org/10.3390/curroncol32060334








